Synthesis of K+ and Na+ Synthetic Sodalite Phases by Low-Temperature Alkali Fusion of Kaolinite for Effective Remediation of Phosphate Ions: The Impact of the Alkali Ions and Realistic Studies
Abstract
1. Introduction
2. Experimental Work
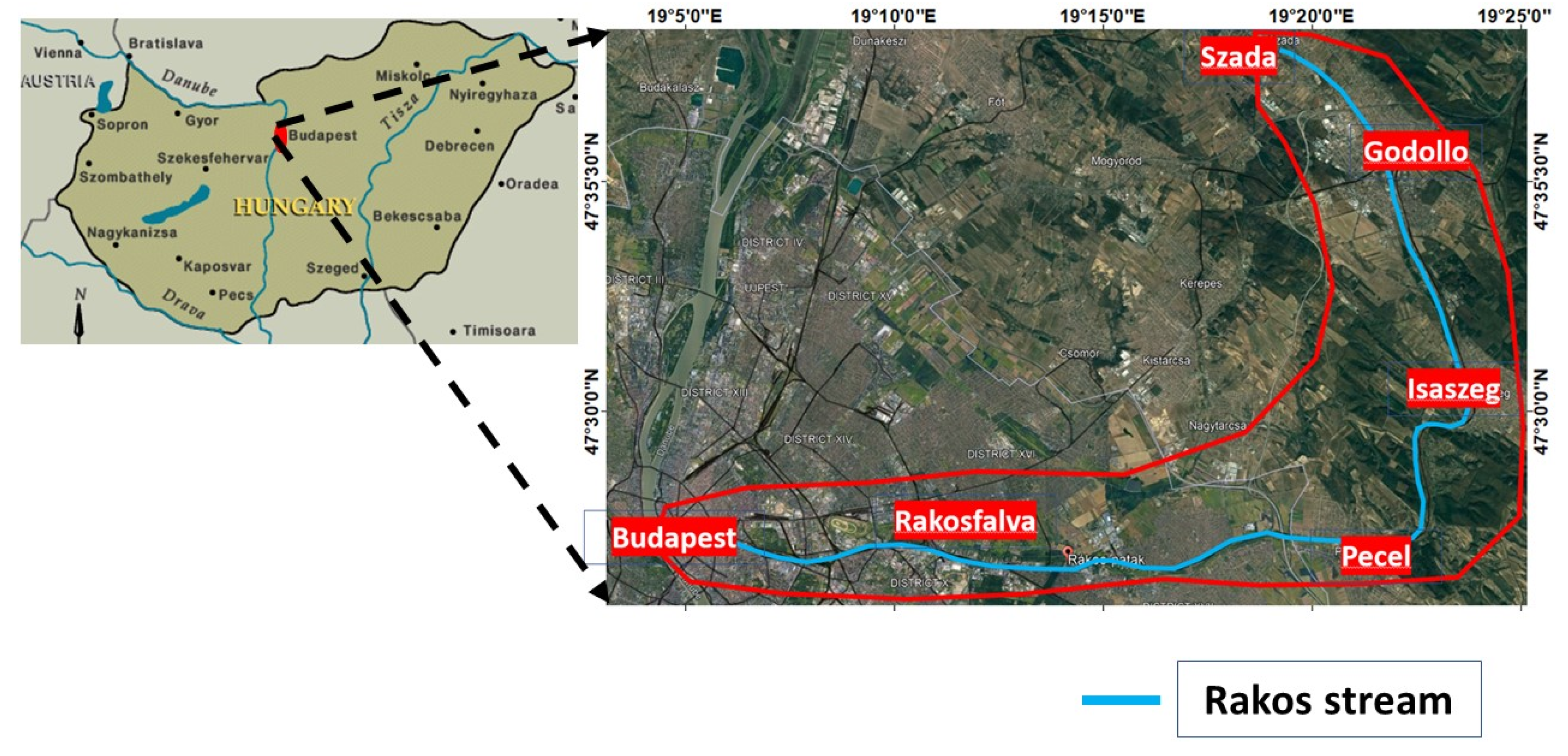
2.1. The Studied Area and Sampling
2.1.1. Location of Rákos Stream
2.1.2. The Water Sampling and Phosphate Content
2.2. Materials
2.3. Synthesis of Sodalite
2.4. Characterization Techniques
2.5. Batch Adsorption of PO43− from Aqueous Solutions and Real Water
2.6. Kinetic and Isotherm Studies
3. Results and Discussion
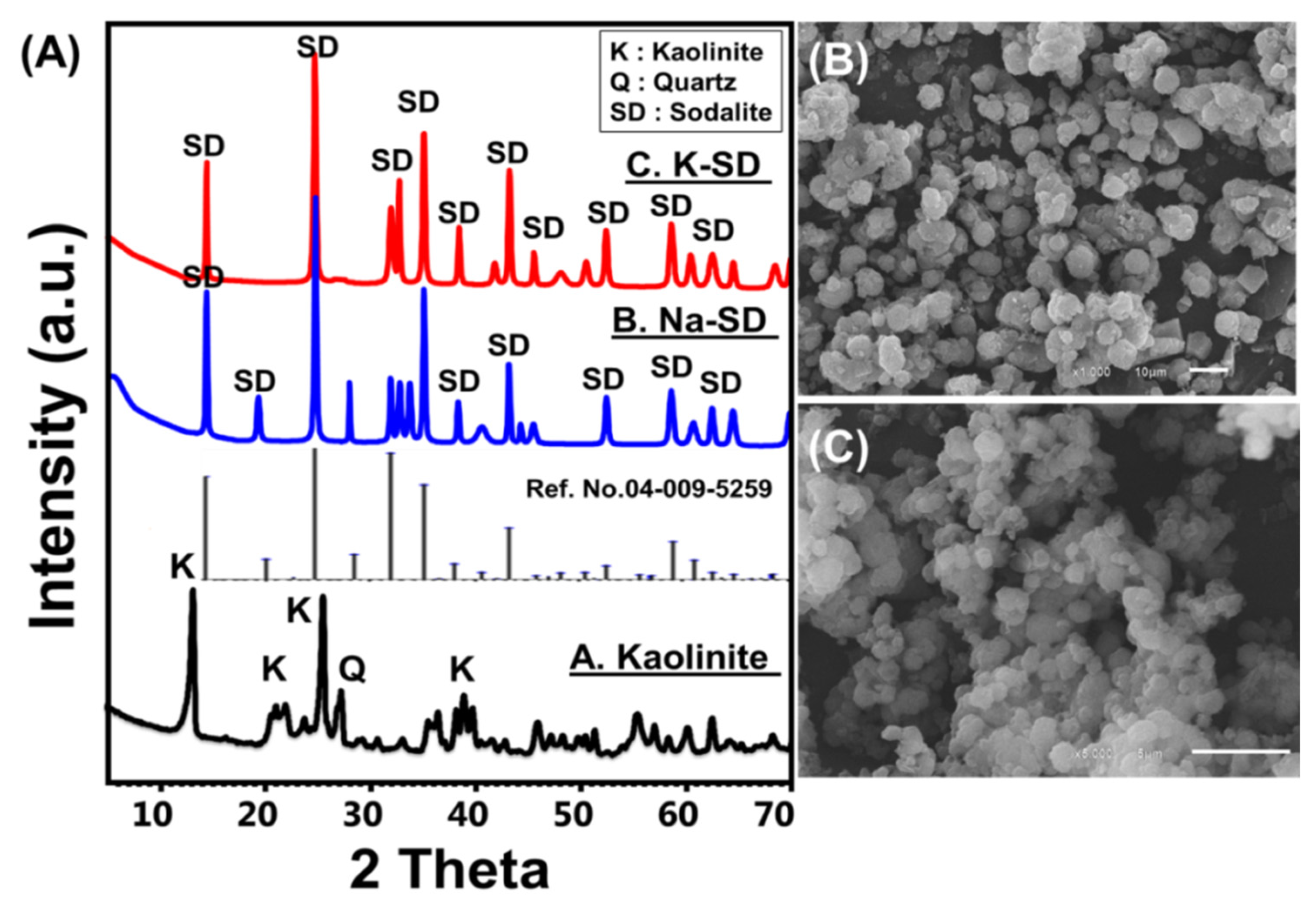
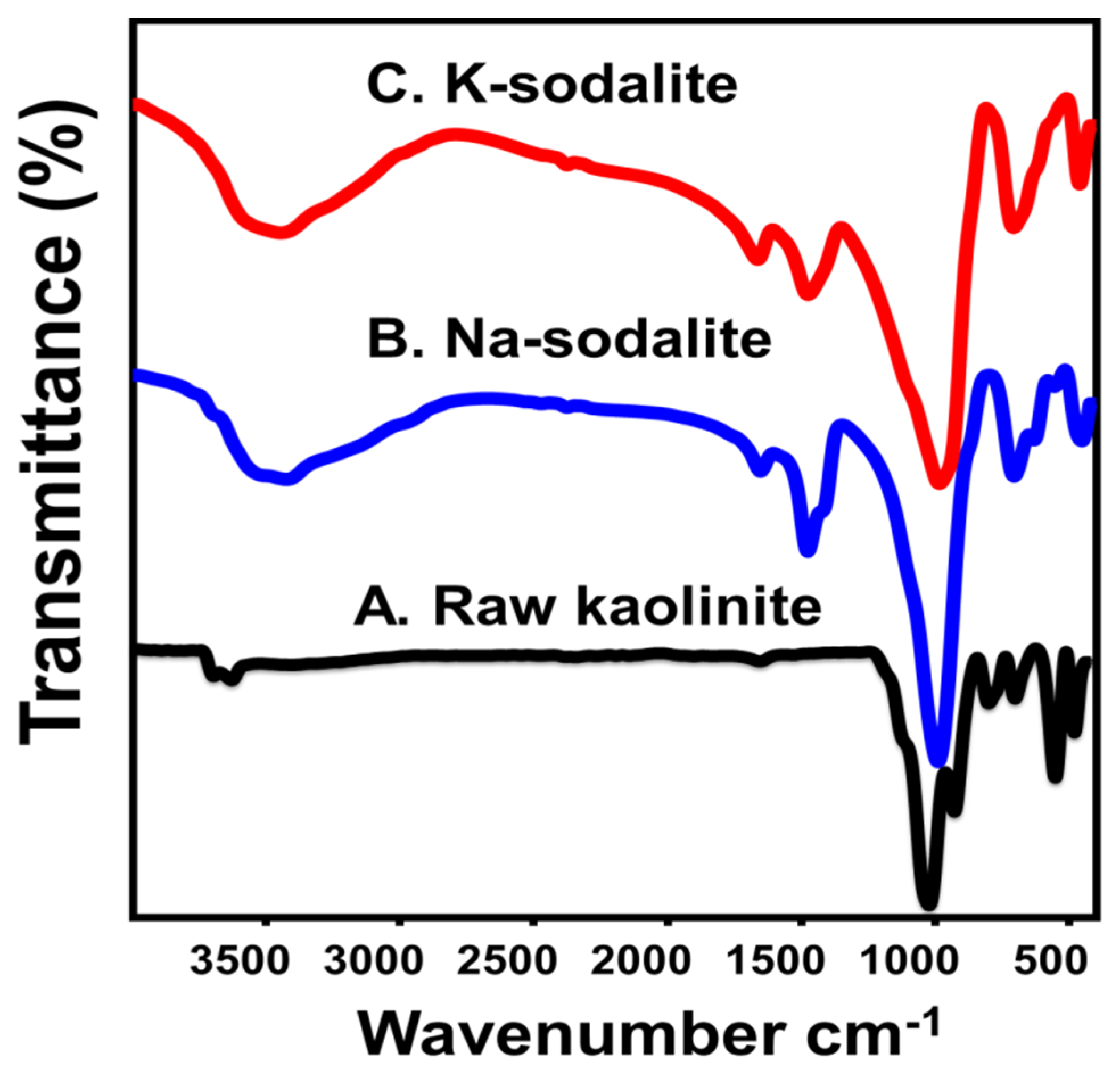
3.1. Characterization of the Sodalite Adsorbents
3.2. Adsorption Results
3.2.1. Effect of the Solution pH
3.2.2. Kinetic Studies
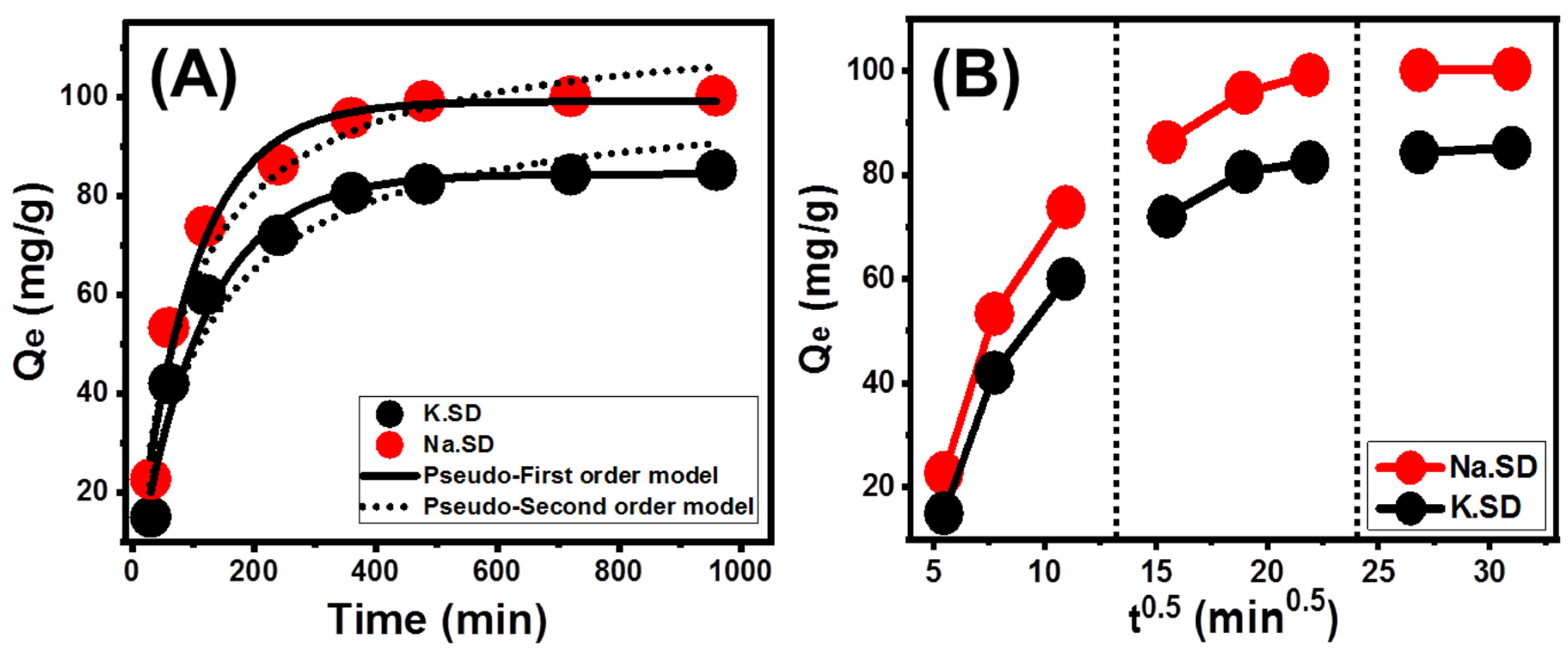
Effect of Contact Time
Kinetic Modeling
Intraparticle Diffusion Behavior
3.2.3. Equilibrium Studies
Effect of PO43− Concentration
Classic Isotherm Models
Advanced Equilibrium Studies
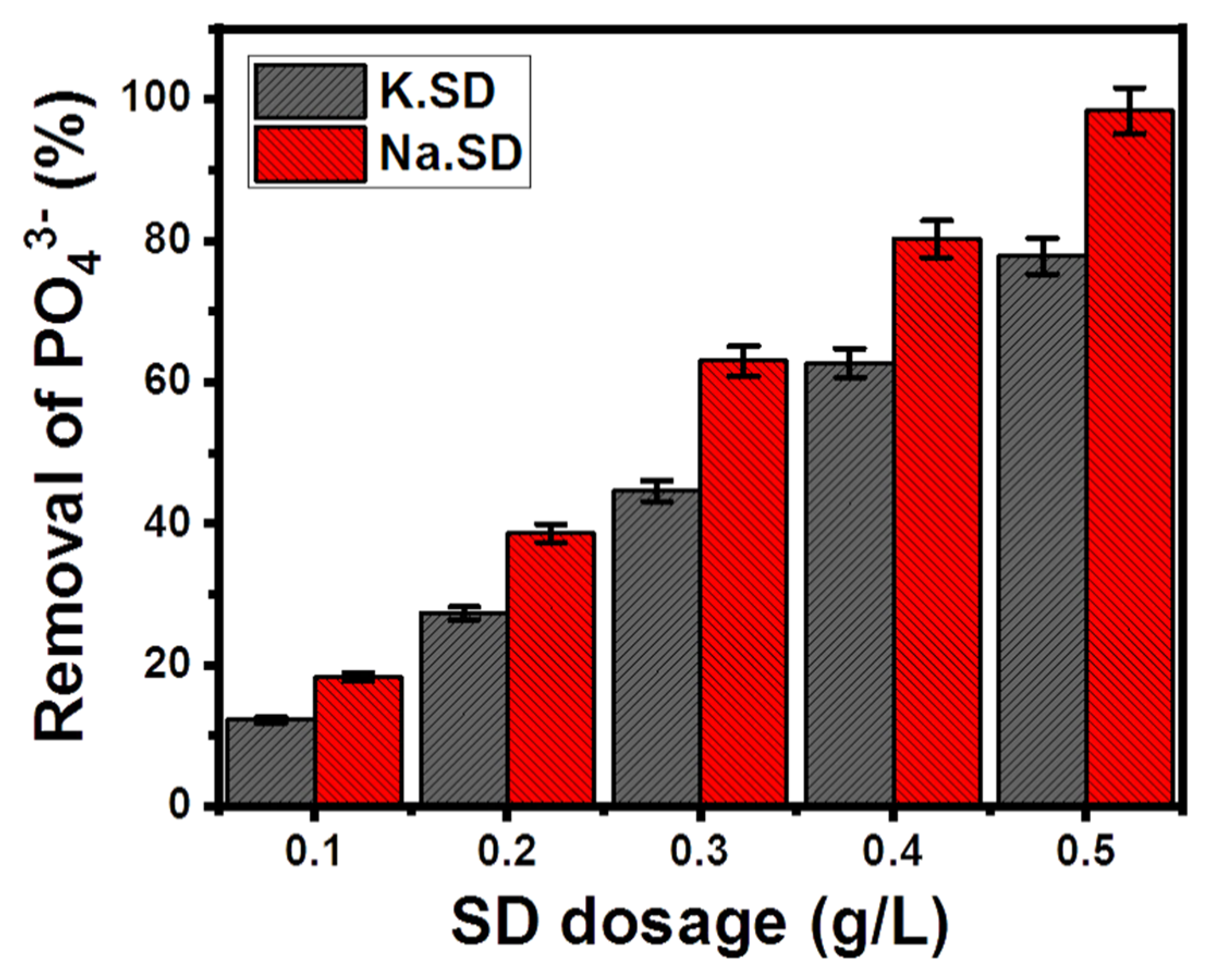
3.2.4. Effect of Dosages
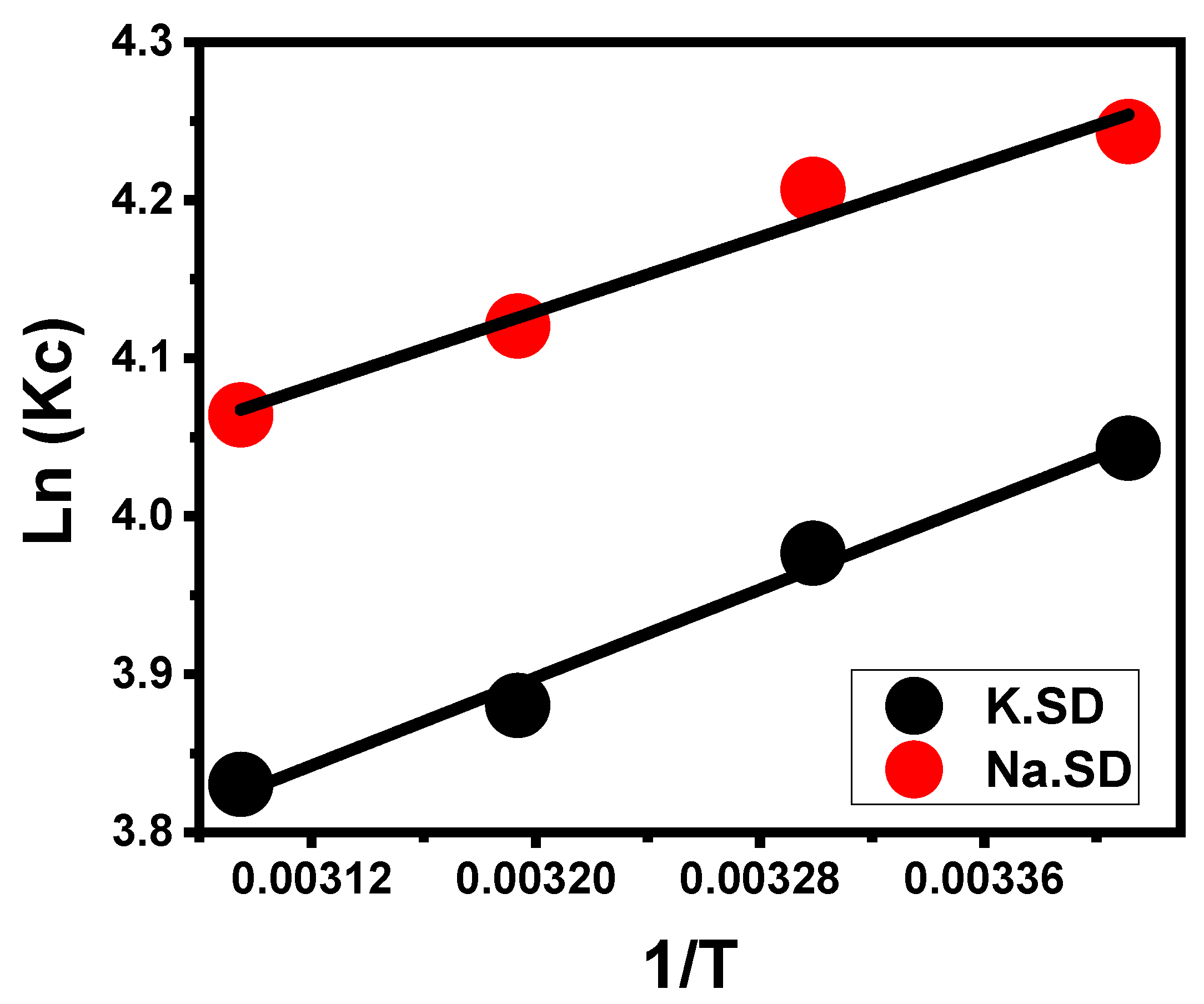
3.2.5. Thermodynamic Properties
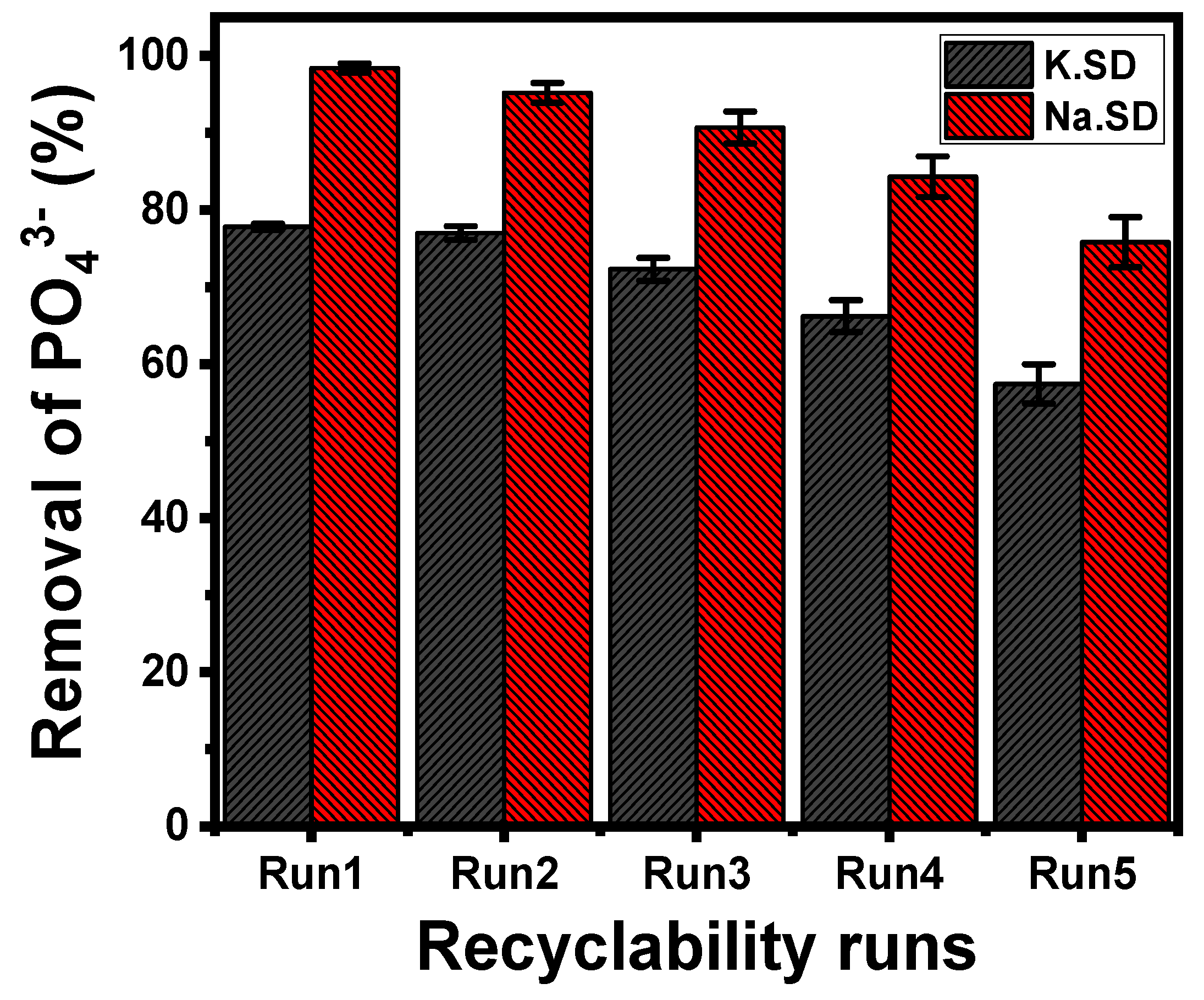
3.2.6. Recyclability
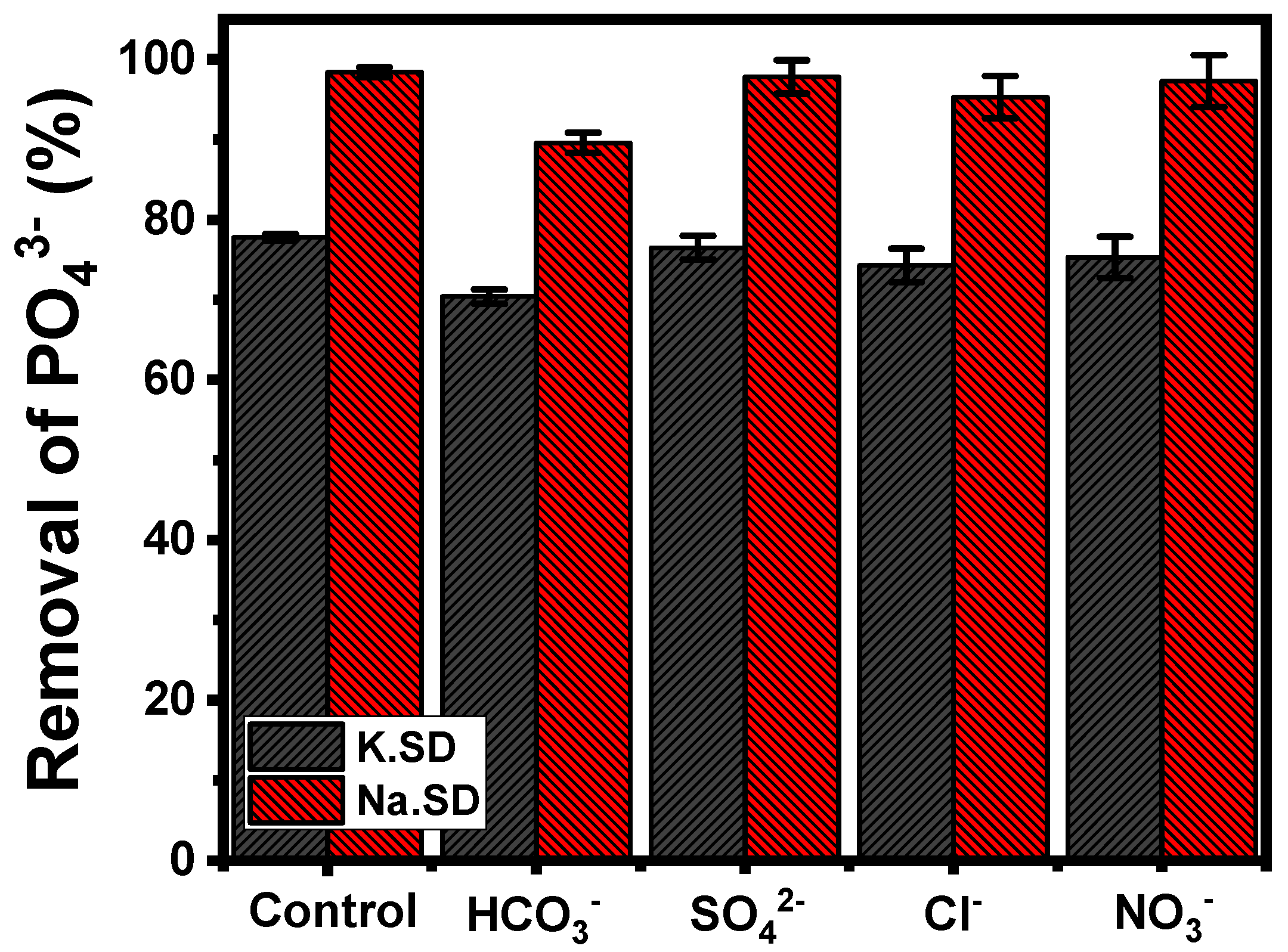
3.2.7. Effect of Coexisting Anions
3.2.8. Comparison Study
3.2.9. Realistic Study
3.2.10. Suggested Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, L.; Zeng, W.; Xu, L.; Huang, L.-Z. Green rusts as a new solution to sequester and stabilize phosphate in sediments under anoxic conditions and their implication for eutrophication control. Chem. Eng. J. 2020, 388, 124198. [Google Scholar] [CrossRef]
- Salam, M.A.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; AlHammadi, A.A.; Abukhadra, M.R. Synthesis of zeolite/geopolymer composite for enhanced sequestration of phosphate (PO43−) and ammonium (NH4+) ions; equilibrium properties and realistic study. J. Environ. Manag. 2021, 300, 113723. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, M.; Hu, F.; Qiu, F.; Dai, H.; Cao, Z. Facile fabrication of hollow biochar carbon-doped TiO2/CuO composites for the photocatalytic degradation of ammonia nitrogen from aqueous solution. J. Alloy. Compd. 2018, 770, 1055–1063. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Fedyna, M.; Marzec, M.; Szerement, J.; Panek, R.; Klimek, A.; Bajda, T.; Mierzwa-Hersztek, M. Copper ion-exchanged zeolite X from fly ash as an efficient adsorbent of phosphate ions from aqueous solutions. J. Environ. Chem. Eng. 2022, 10, 108567. [Google Scholar] [CrossRef]
- Deng, Y.; Li, M.; Zhang, Z.; Liu, Q.; Jiang, K.; Tian, J.; Zhang, Y.; Ni, F. Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar. J. Environ. Chem. Eng. 2021, 9, 105079. [Google Scholar] [CrossRef]
- Munar-Florez, D.A.; Varón-Cardenas, D.A.; Ramírez-Contreras, N.E.; García-Núñez, J.A. Adsorption of ammonium and phosphates by biochar produced from oil palm shells: Effects of production conditions. Results Chem. 2021, 3, 100119. [Google Scholar] [CrossRef]
- Boromisza, Z.; Illyés, Z.; Gergely, A.; Mészáros, S.; Monspart-Molnár, Z. Evaluation of the possibilities for stream restoration: Preassessment of the Váli-stream (Hungary). Landsc. Environ. 2016, 10, 26–44. [Google Scholar] [CrossRef]
- Asaoka, S.; Kawakami, K.; Saito, H.; Ichinari, T.; Nohara, H.; Oikawa, T. Adsorption of phosphate onto lanthanum-doped coal fly ash—Blast furnace cement composite. J. Hazard. Mater. 2020, 406, 124780. [Google Scholar] [CrossRef]
- Buyanjargal, A.; Kang, J.; Sleep, B.E.; Jeen, S.-W. Sequential treatment of nitrate and phosphate in groundwater using a permeable reactive barrier system. J. Environ. Manag. 2021, 300, 113699. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.; Eskandarloo, H.; Azizi, M.; Abbaspourrad, A. Synthesis of Highly Monodispersed, Stable, and Spherical NZVI of 20–30 nm on Filter Paper for the Removal of Phosphate from Wastewater: Batch and Column Study. ACS Sustain. Chem. Eng. 2018, 6, 11662–11676. [Google Scholar] [CrossRef]
- Van Truong, T.; Kim, D.-J. Phosphate removal using thermally regenerated Al adsorbent from drinking water treatment sludge. Environ. Res. 2021, 196, 110877. [Google Scholar] [CrossRef] [PubMed]
- Abali, M.; Ichou, A.A.; Zaghloul, A.; Sinan, F.; Zerbet, M. Removal of nitrate ions by adsorption onto micro-particles of shrimp-shells waste: Application to wastewater of infiltration-percolation process of the city of Agadir (Morocco). Mater. Today: Proc. 2020, 37, 3898–3904. [Google Scholar] [CrossRef]
- El-Sherbeeny, A.M.; Soliman, S.R.; AlHammadi, A.A.; Shim, J.-J.; Abukhadra, M.R. Insight into the CaO green decorated clinoptilolite as an effective adsorbent for nitrate and phosphate ions; equilibrium; kinetic, and safety studies. Surfaces Interfaces 2021, 27, 101568. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Meenakshi, S. Development of sodium alginate@ZnFe-LDHs functionalized beads: Adsorption properties and mechanistic behaviour of phosphate and nitrate ions from the aqueous environment. Environ. Chem. Ecotoxicol. 2020, 3, 42–50. [Google Scholar] [CrossRef]
- Lei, H.; Muhammad, Y.; Wang, K.; Yi, M.; He, C.; Wei, Y.; Fujita, T. Facile fabrication of metakaolin/slag-based zeolite microspheres (M/SZMs) geopolymer for the efficient remediation of Cs+ and Sr2+ from aqueous media. J. Hazard. Mater. 2021, 406, 124292. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Xu, Z.; Cao, X.; Song, J.; Huang, W.; Ge, X.; Wang, H. Adsorption of Nitrate and Ammonium from Water Simultaneously Using Composite Adsorbents Constructed with Functionalized Biochar and Modified Zeolite. Water Air Soil Pollut. 2021, 232, 1–19. [Google Scholar] [CrossRef]
- Jiao, Z.; Meng, Y.; He, C.; Yin, X.; Wang, X.; Wei, Y. One-pot synthesis of silicon-based zirconium phosphate for the enhanced adsorption of Sr (II) from the contaminated wastewater. Microporous Mesoporous Mater. 2021, 318, 111016. [Google Scholar] [CrossRef]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Li, B.; Xue, Q.; Wan, Y.; Liu, L.; Fu, J.; Yu, Y.; Wang, F.; Li, J.; Zhang, F.; Zhang, S.; et al. Substantial and rapid phosphorous adsorption by calcium modified mesoporous silicon micropheres. Chem. Eng. J. Adv. 2021, 7, 100124. [Google Scholar] [CrossRef]
- Kumar, I.A.; Jeyaseelan, A.; Viswanathan, N.; Naushad, M.; Valente, A.J. Fabrication of lanthanum linked trimesic acid as porous metal organic frameworks for effective nitrate and phosphate adsorption. J. Solid State Chem. 2021, 302, 122446. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total. Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Qi, Z.; Zhang, L.; Zhang, Y.; Huang, H.; Peng, Y. Designing ZIF-8/hydroxylated MWCNT nanocomposites for phosphate adsorption from water: Capability and mechanism. Chem. Eng. J. 2020, 394, 124992. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, J.; Go, G.-M.; Jang, B.; Cho, H.-B.; Choa, Y.-H. Removal performance and mechanism of anti-eutrophication anions of phosphate by CaFe layered double hydroxides. Appl. Surf. Sci. 2021, 548, 149157. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M. Effective decontamination of phosphate and ammonium utilizing novel muscovite/phillipsite composite; equilibrium investigation and realistic application. Sci. Total Environ. 2019, 667, 101–111. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2017, 159, 83–93. [Google Scholar] [CrossRef]
- Nasief, F.; Shaban, M.; Alamry, K.A.; Abu Khadra, M.R.; Khan, A.A.P.; Asiri, A.M.; El-Salam, H.A. Hydrothermal synthesis and mechanically activated zeolite material for utilizing the removal of Ca/Mg from aqueous and raw groundwater. J. Environ. Chem. Eng. 2021, 9, 105834. [Google Scholar] [CrossRef]
- Salam, M.A.; Abukhadra, M.R.; Mostafa, M. Effective decontamination of As(V), Hg(II), and U(VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: Adsorption behavior and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 13247–13260. [Google Scholar] [CrossRef]
- Simanjuntak, W.; Pandiangan, K.D.; Sembiring, Z.; Simanjuntak, A.; Hadi, S. The effect of crystallization time on structure, microstructure, and catalytic activity of zeolite-A synthesized from rice husk silica and food-grade aluminum foil. Biomass-Bioenergy 2021, 148, 106050. [Google Scholar] [CrossRef]
- Rios, C.A.; Williams, C.; Fullen, M.A. Nucleation and growth history of zeolite LTA synthesized from kaolinite by two different methods. Appl. Clay Sci. 2009, 42, 446–454. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Fedyna, M.; Marzec, M.; Panek, R.; Szerement, J.; Marcińska-Mazur, L.; Jarosz, R.; Bajda, T.; Franus, W.; Mierzwa-Hersztek, M. The influence of zeolite X ion-exchangeable forms and impregnation with copper nitrate on the adsorption of phosphate ions from aqueous solutions. J. Water Process. Eng. 2022, 50, 103299. [Google Scholar] [CrossRef]
- Capa-Cobos, L.F.; Jaramillo-Fierro, X.; González, S. Computational Study of the Adsorption of Phosphates as Wastewater Pollutant Molecules on Faujasites. Processes 2021, 9, 1821. [Google Scholar] [CrossRef]
- Chebbi, M.; Chibani, S.; Paul, J.-F.; Cantrel, L.; Badawi, M. Evaluation of volatile iodine trapping in presence of contaminants: A periodic DFT study on cation exchanged-faujasite. Microporous Mesoporous Mater. 2016, 239, 111–122. [Google Scholar] [CrossRef]
- AbuKhadra, M.R.; Basyouny, M.G.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Elgawad, A.E.E.A. Transesterification of commercial waste cooking oil into biodiesel over innovative alkali trapped zeolite nanocomposite as green and environmental catalysts. Sustain. Chem. Pharm. 2020, 17, 100289. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Salam, M.A.; Abukhadra, M.R. Effective retention of inorganic Selenium ions (Se (VI) and Se (IV)) using novel sodalite structures from muscovite; characterization and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 120, 116–126. [Google Scholar] [CrossRef]
- Fattahi, N.; Triantafyllidis, K.; Luque, R.; Ramazani, A. Zeolite-Based Catalysts: A Valuable Approach toward Ester Bond Formation. Catalysts 2019, 9, 758. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y.; Díaz, I. Conventional versus alkali fusion synthesis of zeolite A from low grade kaolin. Appl. Clay Sci. 2016, 132–133, 485–490. [Google Scholar] [CrossRef]
- Halász, G.; Szlepák, E.; Szilágyi, E.; Zagyva, A.; Fekete, I. Application of EU Water Framework Directive for monitoring of small water catchment areas in Hungary, II. Preliminary study for establishment of surveillance monitoring system for moderately loaded (rural) and heavily loaded (urban) catchment areas. Microchem. J. 2007, 85, 72–79. [Google Scholar] [CrossRef]
- Altoom, N.; Adlii, A.; Othman, S.I.; Allam, A.A.; Alqhtani, H.A.; Al-Otaibi, F.S.; Abukhadra, M.R. Synthesis and characterization of β-cyclodextrin functionalized zeolite-A as biocompatible carrier for Levofloxacin drug; loading, release, cytotoxicity, and anti-inflammatory studies. J. Solid State Chem. 2022, 312, 123280. [Google Scholar] [CrossRef]
- Sakızcı, M.; Özer, M. The characterization and methane adsorption of Ag-, Cu-, Fe-, and H-exchanged chabazite-rich tuff from Turkey. Environ. Sci. Pollut. Res. 2019, 26, 16616–16627. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum-decorated magnetite. J. Colloid Interface Sci. 2018, 530, 704–713. [Google Scholar] [CrossRef]
- Salehi, S.; Hosseinifard, M. Optimized removal of phosphate and nitrate from aqueous media using zirconium functionalized nanochitosan-graphene oxide composite. Cellulose 2020, 27, 8859–8883. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- El Qada, E. Kinetic Behavior of the Adsorption of Malachite Green Using Jordanian Diatomite as Adsorbent. Jordanian J. Eng. Chem. Ind. (JJECI) Res. Pap. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Shemy, M.H.; Khim, J.S.; Ajarem, J.S.; Rabie, A.M.; Abdelrahman, A.A.; Allam, A.A.; Salem, H.M.; Shaban, M.S. Insight into the Adsorption Properties of β-Cyclodextrin/Zeolite-A Structure for Effective Removal of Cd2+, PO43−, and Methyl Parathion; Kinetics and Advanced Equilibrium Studies. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1–15. [Google Scholar] [CrossRef]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 2020, 413, 127530. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Chen, J.; Zhang, X.; Chen, Y. Adsorption of Pb(II) on mesoporous activated carbons fabricated from water hyacinth using H3PO4 activation: Adsorption capacity, kinetic and isotherm studies. Appl. Surf. Sci. 2014, 293, 160–168. [Google Scholar] [CrossRef]
- Sayed, M.R.; Abukhadra, M.R.; Ahmed, S.A.; Shaban, M.; Javed, U.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Synthesis of advanced MgAl-LDH based geopolymer as a potential catalyst in the conversion of waste sunflower oil into biodiesel: Response surface studies. Fuel 2020, 282, 118865. [Google Scholar] [CrossRef]
- Ashraf, M.-T.; AlHammadi, A.A.; El-Sherbeeny, A.M.; Alhammadi, S.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; Characterization and advanced equilibrium studies. J. Mol. Liq. 2022, 360, 119573. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Bakry, B.M.; Adlii, A.; Yakout, S.M.; El-Zaidy, M.E. Facile conversion of kaolinite into clay nanotubes (KNTs) of enhanced adsorption properties for toxic heavy metals (Zn2+, Cd2+, Pb2+, and Cr6+) from water. J. Hazard. Mater. 2019, 374, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 111, 3973–3993. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Shahien, M.G.; Khan, A.A.P. Upgraded modified forms of bituminous coal for the removal of safranin-T dye from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 18135–18151. [Google Scholar] [CrossRef] [PubMed]
- Dawodu, F.A.; Akpomie, G.K.; Abuh, M.A. Equilibrium Isotherm Studies on the Batch Sorption of Copper (II) ions from Aqueous Solution unto Nsu Clay. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Dhaouadi, F.; Sellaoui, L.; Badawi, M.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Bonilla-Petriciolet, A.; Ben Lamine, A. Statistical physics interpretation of the adsorption mechanism of Pb2+, Cd2+ and Ni2+ on chicken feathers. J. Mol. Liq. 2020, 319, 114168. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; El-Sherbeeny, A.M.; AlHammadi, A.A.; Park, W.-H.; Abukhadra, M.R. Insight into the adsorption and oxidation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: Steric, energetic, and oxidation studies. Chem. Eng. J. 2021, 431, 134312. [Google Scholar] [CrossRef]
- Mostafa, M.; El-Meligy, M.A.; Sharaf, M.; Soliman, A.T.; AbuKhadra, M.R. Insight into chitosan/zeolite-A nanocomposite as an advanced carrier for levofloxacin and its anti-inflammatory properties; loading, release, and anti-inflammatory studies. Int. J. Biol. Macromol. 2021, 179, 206–216. [Google Scholar] [CrossRef]
- Seliem, M.K.; Komarneni, S.; Abu Khadra, M.R. Phosphate removal from solution by composite of MCM-41 silica with rice husk: Kinetic and equilibrium studies. Microporous Mesoporous Mater. 2015, 224, 51–57. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Lu, S.; Wu, D.; Zhang, Z.; Kong, H. Removal and recovery of phosphate from water by lanthanum hydroxide materials. Chem. Eng. J. 2014, 254, 163–170. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Nodeh, H.R.; Sereshti, H.; Afsharian, E.Z.; Nouri, N. Enhanced removal of phosphate and nitrate ions from aqueous media using nanosized lanthanum hydrous doped on magnetic graphene nanocomposite. J. Environ. Manag. 2017, 197, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Patra, B.S.; Baliarsingh, N.; Parida, K.M. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Zong, E.; Wei, D.; Wan, H.; Zheng, S.; Xu, Z.; Zhu, D. Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem. Eng. J. 2013, 221, 193–203. [Google Scholar] [CrossRef]
- Alshameri, A.; Yan, C.; Lei, X. Enhancement of phosphate removal from water by TiO2/Yemeni natural zeolite: Preparation, characterization and thermodynamic. Microporous Mesoporous Mater. 2014, 196, 145–157. [Google Scholar] [CrossRef]
- Sakulpaisan, S.; Vongsetskul, T.; Reamouppaturm, S.; Luangkachao, J.; Tantirungrotechai, J.; Tangboriboonrat, P. Titania-functionalized graphene oxide for an efficient adsorptive removal of phosphate ions. J. Environ. Manag. 2016, 167, 99–104. [Google Scholar] [CrossRef]
- Su, Y.; Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res. 2013, 47, 5018–5026. [Google Scholar] [CrossRef]
- Hamdi, N.; Srasra, E. Removal of phosphate ions from aqueous solution using Tunisian clays minerals and synthetic zeolite. J. Environ. Sci. 2012, 24, 617–623. [Google Scholar] [CrossRef]
- Lin, J.; Zhan, Y.; Wang, H.; Chu, M.; Wang, C.; He, Y.; Wang, X. Effect of calcium ion on phosphate adsorption onto hydrous zirconium oxide. Chem. Eng. J. 2016, 309, 118–129. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced phosphate removal by zeolite loaded with Mg–Al–La ternary (hydr)oxides from aqueous solutions: Performance and mechanism. Chem. Eng. J. 2018, 357, 33–44. [Google Scholar] [CrossRef]


| Kinetic Models | ||
|---|---|---|
| Model | Equation | Parameters |
| Pseudo-first-order | Qt (mg g−1) is the adsorbed ions at time (t), and K1 is the rate constant of the first-order adsorption (1 min−1) | |
| Pseudo-second-order | Qe is the quantity of adsorbed ions after equilibration (mg g−1), and K2 is the model rate constant (g mg−1 min−1). | |
| Classic Isotherm Models | ||
| Model | Equation | Parameters |
| Langmuir | Ce is the rest ions concentration (mg L−1), Qmax is the theoritical maximum adsorption capacity (mg g−1), and b is the Langmuir constant (L mg−1) | |
| Freundlich | KF (mg g−1) is the constant of Freundlich model related to the adsorption capacity and n is the constant of Freundlich model related to the adsorption intensities | |
| Dubinin–Radushkevich | β (mol2 KJ−2) is the D–R constant, ɛ (KJ2 mol−2) is the polanyil potential, and Qm is the adsorption capacity (mg/g) | |
| Advanced Isotherm Models | ||
| Model | Equation | Parameters |
| Monolayer model with one energy site (Model 1) | Q is the adsorbed quantities in mg g−1 n is the number of adsorbed ions per site Nm is the density of the effective receptor sites (mg g−1) Qo is the adsorption capacity at the saturation state in mg g−1 C1/2 is the concentration of the ions at half saturation stage in mg L−1 C1 and C2 are the concentrations of the ions at the half saturation stage for the first active sites and the second active sites, respectively n1 and n2 are the adsorbed ions per site for the first active sites and the second active sites, respectively | |
| Monolayer model with two energy sites (Model 2) | ||
| Double layer model with one energy site (Model 3) | ||
| Double layer model with two energy sites (Model 3) | ||
| Sample | Surface Area | Total Pore Volume | Average Pore Size | Cation-Exchange Capacity |
|---|---|---|---|---|
| Kaolinite | 10 m2 g−1 | 0.072 cm3 g−1 | 43.2 nm | ---- |
| K.SD | 217.6 m2 g−1 | 0.214 cm3 g−1 | 9.7 nm | 96.8 meq 100 g−1 |
| Na.SD | 232.4 m2 g−1 | 0.247 cm3 g−1 | 7.4 nm | 126.4 meq 100 g−1 |
| Kinetic Models | |||
|---|---|---|---|
| Model | Parameters | Values | |
| K.SD | Pseudo-first-order | K1 (min−1) | 0.0089 |
| Qe (Cal) (mg g−1) | 84.4 | ||
| R2 | 0.97 | ||
| X2 | 0.52 | ||
| Pseudo-second-order | k2 (mg g−1 min−1) | 8.94 × 10−5 | |
| Qe (Cal) (mg g−1) | 100.9 | ||
| R2 | 0.95 | ||
| X2 | 0.91 | ||
| Na.SD | Pseudo-first-order | K1 (1 min−1) | 0.010 |
| Qe (Cal) (mg g−1) | 99.09 | ||
| R2 | 0.98 | ||
| X2 | 0.34 | ||
| Pseudo-second-order | k2 (mg g−1 min−1) | 9.77 × 10−5 | |
| Qe (Cal) (mg g−1) | 115.79 | ||
| R2 | 0.96 | ||
| X2 | 0.66 | ||
| Isotherm Models | |||
|---|---|---|---|
| Classic Isotherm Models | |||
| K.SD | Langmuir model | Qmax (mg g−1) | 201.9 |
| b(L mg−1) | 0.0061 | ||
| R2 | 0.90 | ||
| X2 | 2.9 | ||
| Freundlich model | 1/n | 0.570 | |
| kF (mg g−1) | 5.37 | ||
| R2 | 0.84 | ||
| X2 | 4.74 | ||
| D-R model | β (mol2 KJ−2) | 0.0117 | |
| Qm (mg g−1) | 130.6 | ||
| R2 | 0.99 | ||
| X2 | 0.011 | ||
| E (KJ mol−1) | 6.5 | ||
| Na.SD | Langmuir model | Qmax (mg g−1) | 261.6 |
| b(L mg−1) | 0.0069 | ||
| R2 | 0.95 | ||
| X2 | 1.56 | ||
| Freundlich model | 1/n | 0.544 | |
| kF (mg g−1) | 8.19 | ||
| R2 | 0.87 | ||
| X2 | 3.44 | ||
| D-R model | β (mol2 KJ−2) | 0.01977 | |
| Qm (mg g−1) | 164.3 | ||
| R2 | 0.98 | ||
| X2 | 0.69 | ||
| E (KJ mol−1) | 7.15 | ||
| Advanced Isotherm model | |||
| Steric and Energetic Parameters | |||
| K.SD | R2 | 0.996 | |
| X2 | 0.0019 | ||
| n | 2.86 | ||
| Nm (mg g−1) | 44.4 | ||
| QSat (mg g−1) | 127.4 | ||
| C1/2 (mg L−1) | 65.24 | ||
| ΔE (kJ mol−1) | 17.72 | ||
| Na.SD | R2 | 0.997 | |
| X2 | 0.06 | ||
| n | 2.03 | ||
| Nm (mg g−1) | 86.1 | ||
| QSat (mg g−1) | 175.39 | ||
| C1/2 (mg L−1) | 68.57 | ||
| ΔE (kJ mol−1) | 18.04 | ||
| Thermodynamic Parameters | |||
|---|---|---|---|
| Parameters | Temperature | K.SD | Na.SD |
| ∆G° (kJ mol−1) | 293.13 | −9.85 | −10.34 |
| 303.13 | −10.02 | −10.60 | |
| 313.13 | −10.10 | −10.72 | |
| 323.13 | −10.29 | −10.91 | |
| ΔH° (kJ mol−1) | −5.78 | −4.89 | |
| ΔS° (J K−1mol−1) | 14.63 | 18.67 | |
| Adsorbents | Qmax (mg g−1) | References |
|---|---|---|
| MCM-41/Rice husk | 21 | [58] |
| Lanthanum hydroxides | 107.5 | [59] |
| Fe−Mn binary oxide | 36 | [60] |
| Mg(OH)2/ZrO2 | 87.2 | [47] |
| Biochar | 133 | [61] |
| La doping magnetic graphene | 116.28 | [62] |
| Mg/Al modified biochar | 56.12 | [5] |
| Calcined Mg-Al-LDHs | 40.78 | [63] |
| Zirconia/graphite oxide | 149.3 | [64] |
| Titanium modified zeolite | 37.60 | [65] |
| Titania/GO | 33.11 | [66] |
| ZrO2 nanoparticles | 99 | [67] |
| Kaolintic clay | 38.46 | [68] |
| Hydrous zirconium oxide | 51.8 | [69] |
| K.SD | 127.4 | This study |
| Na.SD | 175.39 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellucci, S.; Eid, M.H.; Fekete, I.; Péter, S.; Kovács, A.; Othman, S.I.; Ajarem, J.S.; Allam, A.A.; Abukhadra, M.R. Synthesis of K+ and Na+ Synthetic Sodalite Phases by Low-Temperature Alkali Fusion of Kaolinite for Effective Remediation of Phosphate Ions: The Impact of the Alkali Ions and Realistic Studies. Inorganics 2023, 11, 14. https://doi.org/10.3390/inorganics11010014
Bellucci S, Eid MH, Fekete I, Péter S, Kovács A, Othman SI, Ajarem JS, Allam AA, Abukhadra MR. Synthesis of K+ and Na+ Synthetic Sodalite Phases by Low-Temperature Alkali Fusion of Kaolinite for Effective Remediation of Phosphate Ions: The Impact of the Alkali Ions and Realistic Studies. Inorganics. 2023; 11(1):14. https://doi.org/10.3390/inorganics11010014
Chicago/Turabian StyleBellucci, Stefano, Mohamed Hamdy Eid, Ilona Fekete, Szűcs Péter, Attila Kovács, Sarah I. Othman, Jamaan S. Ajarem, Ahmed A. Allam, and Mostafa R. Abukhadra. 2023. "Synthesis of K+ and Na+ Synthetic Sodalite Phases by Low-Temperature Alkali Fusion of Kaolinite for Effective Remediation of Phosphate Ions: The Impact of the Alkali Ions and Realistic Studies" Inorganics 11, no. 1: 14. https://doi.org/10.3390/inorganics11010014
APA StyleBellucci, S., Eid, M. H., Fekete, I., Péter, S., Kovács, A., Othman, S. I., Ajarem, J. S., Allam, A. A., & Abukhadra, M. R. (2023). Synthesis of K+ and Na+ Synthetic Sodalite Phases by Low-Temperature Alkali Fusion of Kaolinite for Effective Remediation of Phosphate Ions: The Impact of the Alkali Ions and Realistic Studies. Inorganics, 11(1), 14. https://doi.org/10.3390/inorganics11010014








