Abstract
The review is devoted to the C–H functionalization of porphyrins. Porphyrins exhibit the properties of organic semiconductors, light energy converters, chemical and electrochemical catalysts, and photocatalysts. The review describes the iridium- and palladium-catalyzed direct functionalization of porphyrins, with more attention given to the results obtained in our laboratory. The development and improvement of synthetic methods that do not require preliminary modification of the substrate with various functional groups are extremely important for the preparation of new organic materials based on porphyrins. This makes it possible to simplify the synthetic procedure, to make the synthesis more economical, environmentally safe, and simple to perform.
1. Introduction
Most of the substances used in the industry are carbon-based compounds. These are medicines, pesticides, herbicides, plastics, fibers, dyes, explosives, fire extinguishing agents, and materials with various properties [1]. For most of the listed organic substances, it is necessary to compose a synthetic route in such a way that, on the one hand, minimizes the amounts of the reagents, and, on the other hand, avoids dangerous and harmful compounds, if possible. This approach has found its development within the framework of atom economy and green chemistry [2]. Therefore, the development of new methods for the synthesis of organic compounds is an urgent task. One of the directions in organic synthesis is C–H functionalization.
The possibility of introducing a new functional group into a molecule or the formation of a new C–C bond by C–H functionalization is a promising task due to the huge distribution of C–H bonds in organic compounds. The number of substrates is practically unlimited and includes: hydrocarbons, heterocyclic compounds, and synthetic and biological polymers, and the use of transition metals as reagents has opened up completely new opportunities in this field [3]. Methods of C–H functionalization require different reaction conditions and catalysts, including d- and f-metals in different oxidation states, and sometimes reactions take place only at high temperatures with specially selected bases and oxidizing agents. Despite the different conditions, these reactions are a development of the C-M/C-X cross-coupling method and therefore have significant advantages over the classical reaction. A large number of papers devoted to C–H functionalization have now been published. A wide variety of organic compounds have been modified [4,5,6,7,8,9,10,11,12,13]. C–H functionalization reactions are used, for example, for the synthesis of analogues of natural compounds, pharmaceuticals, organic materials of electronic components, etc. [14,15,16,17,18,19,20,21,22,23,24]. The modification of porphyrins and their analogues, taking into account their attractive semiconductor, photophysical, electrochemical, and other properties, is of particular interest [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
2. C–H-Functionalization on Porphyrins
A relatively small number of works have been devoted to the study of C–H functionalization in the field of porphyrin chemistry. This is due to the difficulties in detaching protons from the β- and meso-positions of porphyrins with the formation of a C-C and C-M bond without active groups. Currently, porphyrins containing such substituents as bromine, iodine, tosylate, and boron compounds are used for modification. In this regard, the development of new catalytic systems capable of activating hydrogen atoms in the β-, meso-positions of the porphyrin macrocycle, as well as in phenyl rings, followed by the formation of a new carbon-carbon and carbon-heteroatom bond, is an urgent task.
β-Selective C–H functionalization was first carried out by the group of Professor Osuka [41]. Various unsubstituted diarylporphyrins were subjected to iridium-catalyzed borylation [41,42] (Scheme 1). In all cases, the borylation proceeded selectively at the β-positions located near the meso-carbon, with yields up to 47%, and was not observed at the meso-positions [41]. Diborylated triarylporphyrins and tetraborylated diarylporphyrins were obtained under similar conditions, and further substitution was observed only in the β-position adjacent to the vacant meso-carbon atom [41]. Osuka and co-workers demonstrated the usefulness of porphyrinylboronates as building blocks for multiporphyrin structures [43].
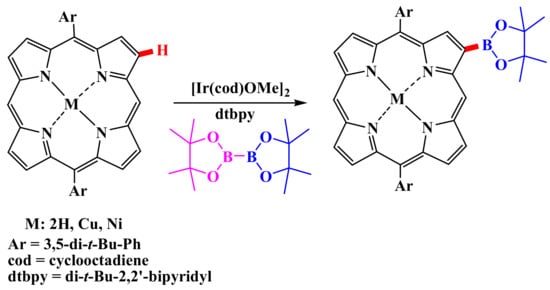
Scheme 1.
Iridium-catalyzed borylation of diaryl porphyrins [41,42].
β-borylation requires a catalytic amount of the iridium complex [Ir(OMe)(cod)]2 and the dtbpy ligand, as well as a stoichiometric amount of boron [44]. The study of the reaction mechanism showed that the introduction of iridium (III) at the C–H bond is the rate-determining step. The regioselectivity of β-borylation is explained only by the mutual steric hindrances that the reagent and substrate exert on each other [45,46].
The first example of a palladium-catalyzed reaction was the intramolecular cyclization of meso-phenyl porphyrins 1–4 orthoiodinated at the phenyl fragments by Boyle and Fox (Scheme 2) [47,48]. Both mono- (5–8, up to 44%) and doubly cyclized products (10, 11, up to 36%) were obtained in acceptable yields, but the authors failed to separate isomers 10 and 11. Similar reactions using ortho-bromophenylporphyrins could not be carried out.
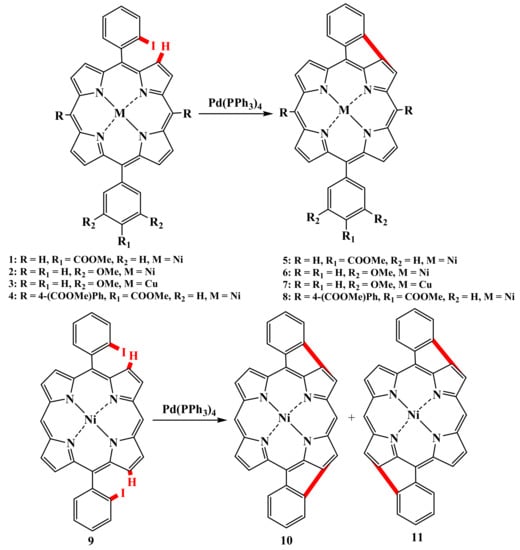
Scheme 2.
Palladium-catalyzed intramolecular cyclization of meso-phenyl porphyrins ortho-iodinated at the phenyl fragments [47,48].
An attempt to carry out the reaction of naphthyl triflate 12 with 1,1′-diborylferrocene under Suzuki reaction conditions did not lead to the expected double addition of porphyrin to ferrocene. As the only product in this reaction, naphthalene-condensed porphyrin 13 was isolated, which was formed due to intramolecular C–H arylation in 14% yield [49] (Scheme 3).
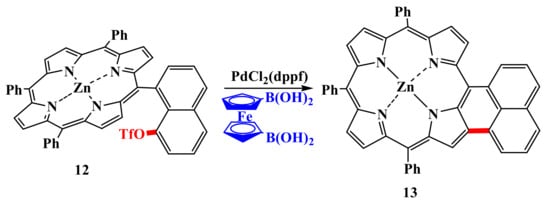
Scheme 3.
Scheme for the synthesis of condensed porphyrin by intramolecular C–H-arylation [49].
Similarly, an attempt by Osuka and colleagues to perform a Suzuki coupling of borylporphyrin 14 with 1,2-diiodobenzene gave phenylene-fused porphyrin 15, along with diporphyrin 16 (Scheme 4) [50]. It was found that the annulation involves the formation of meso-(o-iodophenyl)porphyrin and its subsequent intramolecular C–H arylation.
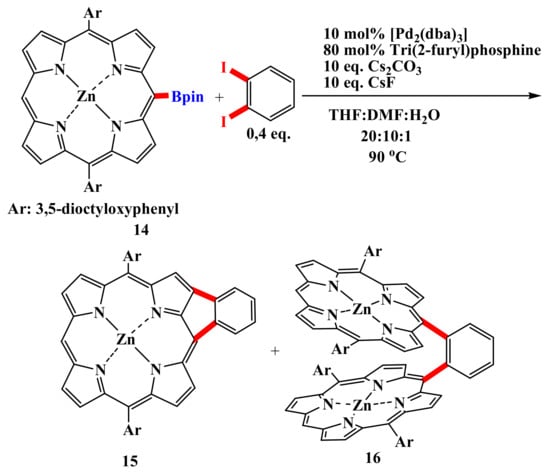
Scheme 4.
Synthesis of phenylene-condensed porphyrin along with diporphyrin [50].
Matsuo synthesized thiophene-fused porphyrins 19 (47%) and 21 (79%) using a palladium-catalyzed intramolecular C–H/C–Cl or C–H/C–Br reaction of porphyrins 18 and 20 (Scheme 5) [51].
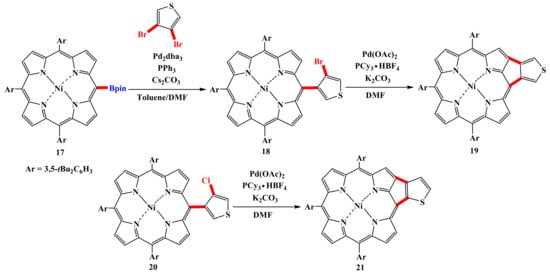
Scheme 5.
Synthesis thiophene-fused porphyrins using a palladium-catalyzed intramolecular C–H/C–Cl or C–H/C–Br reaction [51].
Arnold’s papers reported Heck-type coupling of nickel (II) meso-vinylporphyrinate with various meso-bromoporphyrins. In all cases, the only isolated product is the meso-β-linked porphyrin dimer [52]. Osuka et al. reported the formation of various cyclopentadiene-condensed porphyrins 26–35 in palladium-catalyzed reactions of the corresponding bromoporphyrins 22–25 with symmetrical alkynes in high yields of 69–87% (Scheme 6) [53].
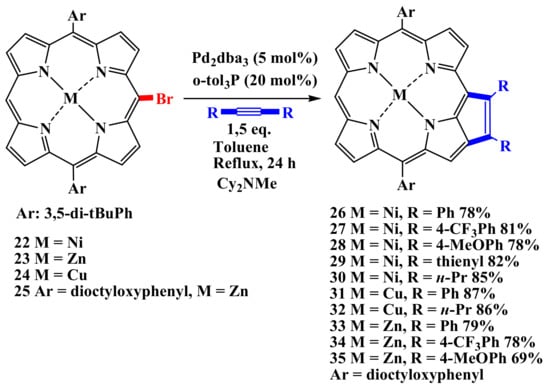
Scheme 6.
Synthesis of cyclopentadiene-condensed porphyrins (26–35) in palladium-catalyzed reactions [53].
The first example of intermolecular CH-arylation of porphyrins is the palladium-catalyzed dimerization of meso-bromoporphyrins (Scheme 7) carried out by Osuka’s group [54].
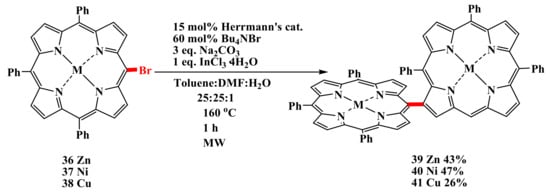
Scheme 7.
Palladium-catalyzed dimerization of meso-bromoporphyrins [54].
The palladium-catalyzed homocoupling reaction of meso-bromoporphyrins proceeds to form directly meso-β-linked diporphyrins in good yields and ideal regioselectivity. It should be noted that the C–H-functionalization of porphyrin proceeds to the most hindered β-position.
Osuka’s group described intermolecular CH-arylation of the porphyrin periphery with aryl bromides as a general and efficient method of arylation [55]. Palladium-catalyzed C–H-arylation of arenes, proceeding with the participation of pivalic acid [56,57,58,59,60], was used for the β,β’-diarylation of meso-unsubstituted porphyrin 42 (Scheme 8) [61].
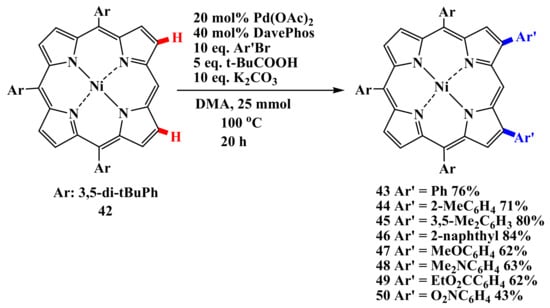
Scheme 8.
Palladium-catalyzed C–H-arylation of arenes [61].
Its scope is quite wide, although the introduction of heteroatom-substituted aryl groups requires some changes in the reaction conditions. The reaction is efficient for the diarylation of 5,15-triarylporphyrin 42 (Scheme 9) [61].
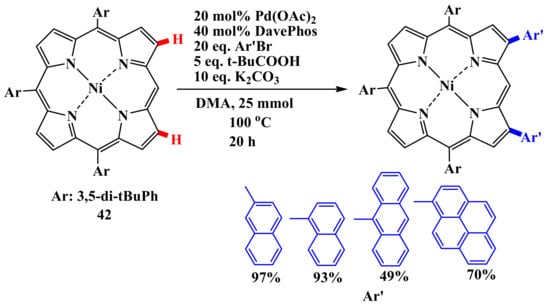
Scheme 9.
Diarylation of 5,15-triarylporphyrin [61].
Direct arylation is so efficient that even formylporphyrin 51 could be arylated (Scheme 10).
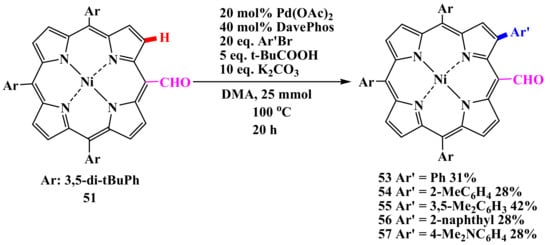
Scheme 10.
Reaction of direct formylporphyrin arylation.
Due to the moderate steric hindrance of the formyl group, arylation proceeded much more slowly to the β-position adjacent to the formyl group, giving monoarylated products 53–57 under similar reaction conditions. Diarylation requires an additional amount of palladium catalyst (Scheme 11). The remaining formyl group can undergo further transformations, as evidenced by the McMurry coupling. It should be noted that an attempt at β-borylation of formylporphyrin 58 failed due to the transformation of the formyl group under the action of iridium catalysis [60].
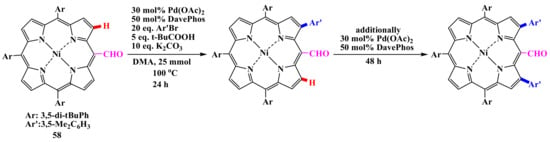
Scheme 11.
Direct formylporphyrin diarylation [60].
Direct β-arylation has advantages over β-borylation and subsequent cross-coupling [24,62,63,64,65,66,67,68,69,70,71,72,73,74,75] because it does not involve the undesirable process of protodeborylation. An example of the introduction of heterocyclic fragments into a porphyrin molecule is the work carried out in our laboratory [76] where arylation by heteroarenes containing “acidic” C–H bonds, such as mono-, di- and tetra-meso-bromophenyl-substituted porphyrins, were exposed. The reactions were carried out in the presence of three alternative catalytic systems: Pd(dba)2/DavePhos/Cs2CO3, Pd(PPh3)4/PivOH/K2CO3, and Pd(OAc)2/Cu(OAc)2/PPh3/K2CO3. The first catalytic system turned out to be successful in the reaction with benzoxazole, while the second was less efficient. The third catalytic system turned out to be the most versatile and made it possible to obtain the corresponding mono-, di-, tri-, and even tetra-arylated porphyrin derivatives (Scheme 12) [76]. Porphyrins 59 and 60 were successfully heterylated. Despite the more “acidic” C–H bond in the benzoxazole molecule, the best result was obtained when carrying out the reaction with 1,3,7-trimethylxanthine (96%) 67 and (95%) 68.
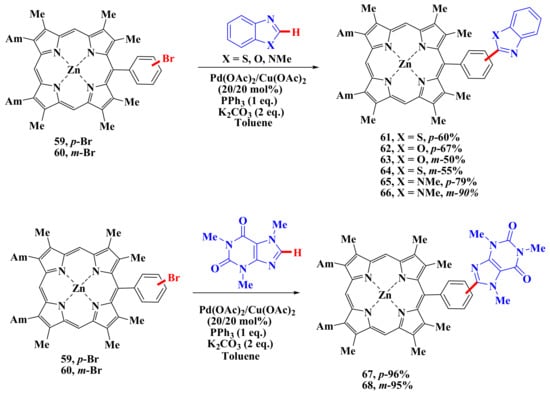
Scheme 12.
Direct β-arylation of porphyrins with catalytic system containing Pd(OAc)2/Cu(OAc)2/PPh3/K2CO3 [76].
The C–H functionalization method allows targeted synthesis of porphyrin compounds that meet the requirements of biomedicine. For example, porphyrins 70–72 were constructed for high-affinity binding to pathogen DNA based on the results of molecular docking. These porphyrins form π-π bonds with nitrogenous bases of one of the DNA strands in the helical stacking and H-bonds/π-π-bonds with the nitrogenous bases of the second DNA strand outside the helical stacking. The desired porphyrins 70–72 were obtained by C–H functionalization of porphyrin 69 using the above catalytic system (Scheme 13). Compounds 70–72 were also capable of high-affinity binding to the receptor-binding domain of the SARS-CoV-2 S protein. The S-protein complex is not capable of binding to the human-angiotensin-converting enzyme with porphyrins 70–72 in vitro [77].
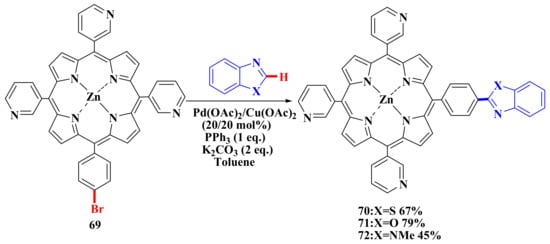
Scheme 13.
Synthesis of hetaryl substituted porphyrins [77].
Porphyrin 73 was modified with the same heteroarenes. As a result, heterocycle-substituted porphyrins were obtained in good yields (Scheme 14).
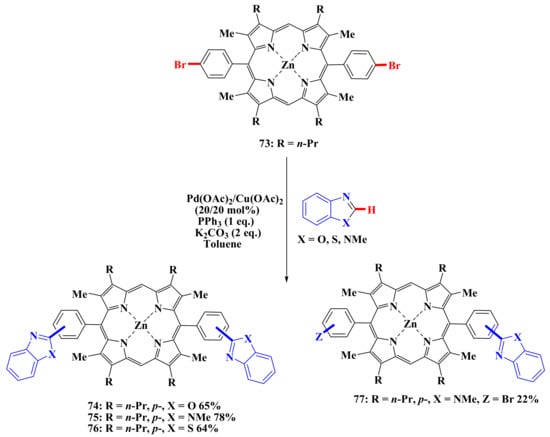
Scheme 14.
Scheme of porphyrins modification by heteroarenes.
To study the possibilities and limitations of this approach, we carried out reactions with porphyrin 78 using 8 equivalent of corresponding heteroarenes, 40 mol% Pd(OAc)2/Cu(OAc)2, 2.5 equivvalent PPh3 and 5 equivalent K2CO3 (Scheme 15) [76].
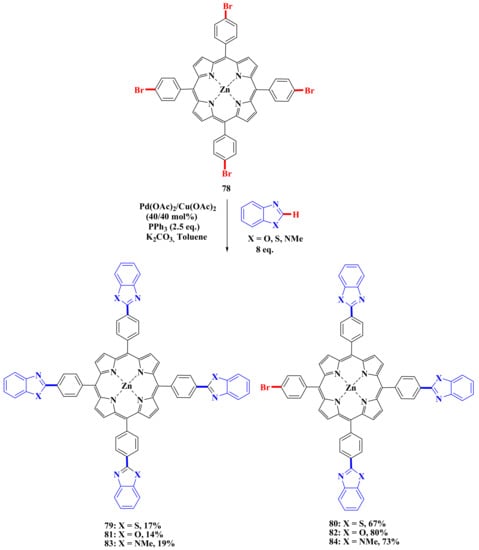
Scheme 15.
Scheme of porphyrin modification by heteroarenes [76].
It was found that triarylation products 80, 82, 84 are the main ones in reactions with all heteroarenes. Tetrasubstituted derivatives 79, 81, 83 were obtained in low yields (17% for benzothiazole, 14% for benzoxazole, and 20% for N-methylbenzimidazole). It should be noted that no elimination of the bromine atom was observed in these reactions, although this process took place in almost all the reactions with monobromoporphyrins described above. We also carried out the modification of 5,10,15,20-tetrakis(4′-bromophenyl)-21,23-dithioporphyrin using the same conditions as in the case of tetrabromoporphyrins. It was found that, in contrast to 5,10,15,20-tetrakis(4′-bromophenyl)porphyrin, where triheteryl-substituted porphyrins are the main reaction product, their dithio analog gives a tetrasubstitution product (Scheme 16) [78].
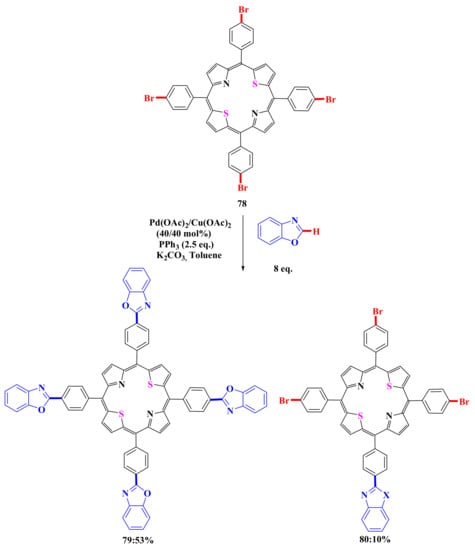
Scheme 16.
Scheme of porphyrins modification by heteroarenes [78].
The possibility of introducing meso-bromoporphyrins in arylation reactions with heteroarenes opens up an approach to another family of heteroarylated porphyrins studied using meso-dibromoporphyrin 81 as an example (Scheme 17). Reactions with benzothiazole and benzoxazole were carried out using 4 eq of corresponding heteroarenes, 20 mol % catalyst, 1 eq PPh3 and 2 equivalent K2CO3. As a result of studying this process, it was also found that benzothiazole is less reactive than benzoxazole in these reactions. In the reaction with benzothiazole, only the monoarylated derivative 82 was obtained in 24% yield. In the case of the reaction with benzoxazole, diaryl-substituted porphyrin 83 was isolated in 32% yield [76].
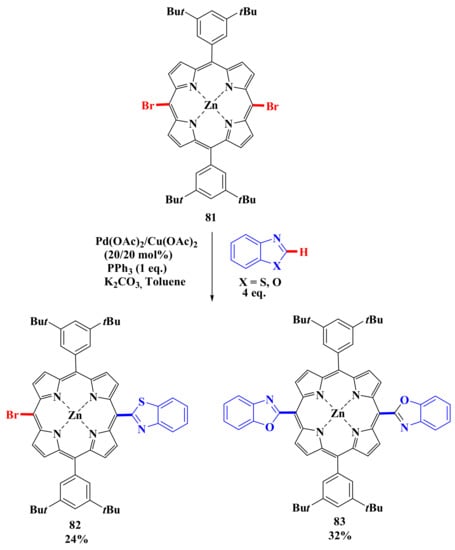
Scheme 17.
Scheme of porphyrins modification by heteroarenes [76].
One of the main disadvantages of the metal–catalytic functionalization of the C–H bond is the duration of the reactions. A recent work by Kumar and co-workers developed an efficient method for carrying out these reactions using microwave radiation to obtain a variety of heteroaromatic substituted porphyrins [79]. Optimal conditions consisted of using Pd(OAc)2 (10 mol.%), CuI (10 mol.%), and Cs2CO3 (2 mol. equivalent) in DMF at 120 °C under microwave irradiation for 10 min. Using this technique, various meso-heteroaromatic substituted porphyrins were synthesized (Scheme 18). This is promising, as it can significantly reduce the reaction time.
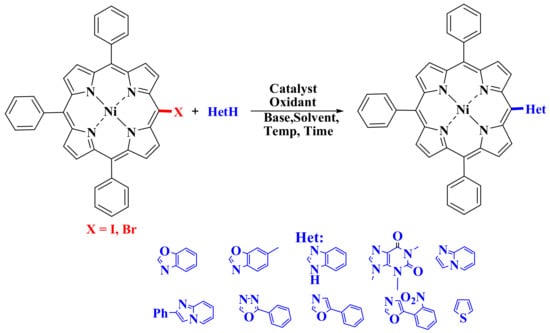
Scheme 18.
Scheme for the synthesis of heteroaromatic substituted porphyrins using microwave radiation [79].
We have studied the possibility of introducing heterocyclic residues into the β-position of the porphyrin core using a catalytic system based on Pd(OAc)2 and Cu(OAc)2 [76]. The reaction was carried out on a model porphyrin 84. It turned out that the reaction proceeds only with benzoxazole with a 5% yield of product 85. Almost quantitative elimination of the bromine atom is observed. Therefore, another catalytic system is required to modify the β-position (Scheme 19).
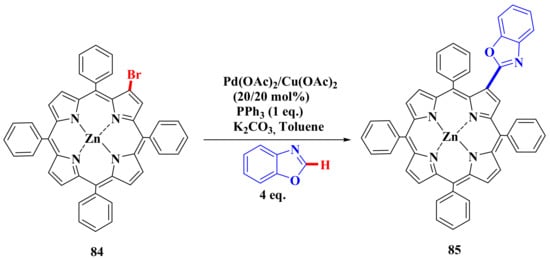
Scheme 19.
Scheme for the synthesis of heteroaromatic substituted porphyrins [76].
3. Conclusions
Metal-catalyzed C–H functionalization in the chemistry of porphyrins opens up new possibilities for the design of porphyrin compounds of a given structure for various practical compounds. Despite the fact that metal-catalytic C–H functionalization of porphyrins is currently not a widely used method for the synthesis of porphyrins, this method is promising, since it allows the synthesis of a wide range of porphyrins from a small number of available precursors. This review makes it possible to single out the most promising directions in the development of the method of metal-catalytic C–H activation for the functionalization of porphyrins.
The basis for the successful modification of porphyrins was the work on the C–H functionalization of various arenes and heteroarenes, for example, those presented in reviews [8,80,81,82]. We found that when the bromine atom is in the phenyl ring of the macroheterocycle, the best catalytic system is the system reported by Z.-Z. Huang [83,84]. It is based on the use of Pd (II) and Cu (II) acetates. In the case of iridium-catalyzed direct borylation of porphyrins, the work of Smith, Hartwig, Ishiyama, and Miyaura [85,86,87,88,89,90,91,92,93] served as the basis. When a bromine atom or a hydrogen atom in the beta position of the porphyrin macrocycle undergoes modification, the system based on the work of Fagnou [56,94] serves as an excellent catalytic system. When it is necessary to introduce heterocyclic residues into the meso position of the porphyrin macrocycle, the system developed by Kumar [79] is the best.
Author Contributions
Conceptualization, A.N.K., S.A.S. and N.S.L.; validation, A.N.K. and S.A.S.; formal analysis, A.N.K., S.A.S. and N.S.L.; data curation, A.N.K. and S.A.S.; writing—original draft preparation, A.N.K., S.A.S., N.S.L. and Y.A.G.; writing—review and editing, N.S.L. and Y.A.G.; visualization, A.N.K.; supervision, S.A.S.; funding acquisition, S.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Russian Science Foundation, grant No. 21-73-20140.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Am | amyl; |
| Ar | aryl; |
| Bu | butyl; |
| Cod | cyclooctadiene |
| DMF | N, N-dimethylformamide |
| Dtbpy | di-t-Bu-2,2′-bipyridyl |
| DavePhos | 2-Dicyclohexylphosphino-2′-(N, N-dimethylamino)biphenyl |
| Et | ethyl; |
| EtOH | ethanol |
| Herrmann’s catalyst | (2-methanidylphenyl)-bis(2-methylphenyl) phosphane palladium (II) diacetate |
| Me | methyl; |
| MeOH | methanol |
| Mes | mesyl |
| Ph | phenyl |
| Ph3P | triphenylphosphine; |
| Pr | propyl |
| PCy3 | tricyclohexylphosphine; |
| Py | pyridine |
| Pd(OAc)2 | palladium (II) acetate |
| PdCl2(dppf) | [1,1′-bis(diphenylphosphino)ferrocene] palladium (II) dichloride |
| Pd2(dba)3 | tris(dibenzylideneacetone) dipalladium (0) |
| THF | tetrahydrofuran |
| t-Bu | tert-butyl |
| t-BuCOOH | pivalic acid |
References
- Beletskaya, I.; Ananikov, V. The reasons organic chemistry is needed for in a well developed country. Russ. J. Org. Chem. 2015, 51, 145–147. [Google Scholar] [CrossRef]
- Han, B.; Wu, T. Green Chemistry and Chemical Engineering; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Goldman, A.; Ghosh, R. Handbook of CH Transformations: Applications in Organic Synthesis; Wiley-VCH: New York, NY, USA, 2005. [Google Scholar]
- Karlinskii, B.Y.; Ananikov, V.P. Catalytic C−H Functionalization of Unreactive Furan Cores in Bio-Derived Platform Chemicals. ChemSusChem 2021, 14, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; de Carvalho, R.L.; da Silva Júnior, E.N. The Different Facets of Metal-Catalyzed C−H Functionalization Involving Quinone Compounds. Chem. Rec. 2021, 21, 2604–2637. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Yarwood, S.J.; Barker, G. Recent Developments in C−H Functionalisation of Benzofurans and Benzothiophenes. Eur. J. Org. Chem. 2021, 2021, 1072–1102. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Dutta, S.; Maiti, D. Deciphering the Role of Silver in Palladium-Catalyzed C–H Functionalizations. ACS Catal. 2021, 11, 9702–9714. [Google Scholar] [CrossRef]
- Segawa, Y.; Maekawa, T.; Itami, K. Synthesis of Extended π-Systems through C–H Activation. Angew. Chem. Int. Ed. 2015, 54, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; McMullon, M.W.; Rieb, J.; Kuehn, F.E. C–H Bond Activation by f-Block Complexes. Angew. Chem. Int. Ed. 2015, 54, 82–100. [Google Scholar] [CrossRef]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition-metal-catalyzed direct arylation of (hetero) arenes by C–H bond cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9826. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative coupling between two hydrocarbons: An update of recent C–H functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef]
- Klussmann, M.; Sureshkumar, D. Catalytic oxidative coupling reactions for the formation of carbon-carbon bonds without carbon-metal intermediates. Synthesis 2011, 2011, 353–369. [Google Scholar] [CrossRef]
- Liu, C.; Jin, L.; Lei, A. Transition-metal-catalyzed oxidative cross-coupling reactions. Synlett 2010, 2010, 2527–2536. [Google Scholar]
- Huang, S.-T.; Hsei, I.-J.; Chen, C. Synthesis and anticancer evaluation of bis (benzimidazoles), bis (benzoxazoles), and benzothiazoles. Biorg. Med. Chem. 2006, 14, 6106–6119. [Google Scholar] [CrossRef] [PubMed]
- Rohini, R.; Reddy, P.M.; Shanker, K.; Hu, A.; Ravinder, V. Antimicrobial study of newly synthesized 6-substituted indolo [1, 2-c] quinazolines. Eur. J. Med. Chem. 2010, 45, 1200–1205. [Google Scholar] [CrossRef]
- Nadres, E.T.; Lazareva, A.; Daugulis, O. Palladium-catalyzed indole, pyrrole, and furan arylation by aryl chlorides. J. Org. Chem. 2011, 76, 471–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rohini, R.; Shanker, K.; Reddy, P.M.; Sekhar, V.C.; Ravinder, V. 6-Substituted Indolo [1, 2-c] quinazolines as New Antimicrobial Agents. Arch. Pharm. Int. J. Pharm. Med. Chem. 2009, 342, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Joucla, L.; Djakovitch, L. Transition Metal-Catalysed, Direct and Site-Selective N1-, C2-or C3-Arylation of the Indole Nucleus: 20 Years of Improvements. Adv. Synth. Catal. 2009, 351, 673–714. [Google Scholar] [CrossRef]
- Wang, Y.; Frett, B.; McConnell, N.; Li, H.-Y. Metal-free, efficient hydrazination of imidazo [1, 2-a] pyridine with diethyl azodicarboxylate in neutral media. Org. Biomol. Chem. 2015, 13, 2958–2964. [Google Scholar] [CrossRef]
- Odlo, K.; Fournier-Dit-Chabert, J.; Ducki, S.; Gani, O.A.; Sylte, I.; Hansen, T.V. 1,2,3-Triazole analogs of combretastatin A-4 as potential microtubule-binding agents. Biorg. Med. Chem. 2010, 18, 6874–6885. [Google Scholar] [CrossRef]
- Zhan, B.-B.; Jin, L.; Shi, B.-F. Palladium-catalyzed enantioselective C–H functionalization via C–H palladation. Trends Chem. 2022, 4, 220–235. [Google Scholar] [CrossRef]
- Zhang, L.; Ritter, T. A Perspective on Late-Stage Aromatic C–H Bond Functionalization. J. Am. Chem. Soc. 2022, 144, 2399–2414. [Google Scholar] [CrossRef]
- Xing, L.; Luscombe, C.K. Advances in applying C–H functionalization and naturally sourced building blocks in organic semiconductor synthesis. J. Mater. Chem. C 2021, 9, 16391–16409. [Google Scholar] [CrossRef]
- Yu, H.; Thiessen, A.N.; Hossain, M.A.; Kloberg, M.J.; Rieger, B.; Veinot, J.G. Thermally Induced Dehydrogenative Coupling of Organosilanes and H-Terminated Silicon Quantum Dots onto Germanane Surfaces. Chem. Mater. 2020, 32, 4536–4543. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Tyurin, V.S.; Uglov, A.; Stern, C.; Guilard, R. Survey of synthetic routes for synthesis and substitution in porphyrins. In Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine; World Scientific: Singapore, 2012; Volume 23, pp. 81–279. [Google Scholar]
- Sessler, J.L.; Wang, B.; Harriman, A. Photoinduced energy transfer in associated, but noncovalently-linked photosynthetic model systems. J. Am. Chem. Soc. 1995, 117, 704–714. [Google Scholar] [CrossRef]
- Milgrom, L.R.; Dempsey, P.J.; Yahioglu, G. 5,10,15,20-tetrakis (N-protected-imidazol-2-yl) porphyrins. Tetrahedron 1996, 52, 9877–9890. [Google Scholar] [CrossRef]
- Sengupta, K.; Chatterjee, S.; Samanta, S.; Dey, A. Direct observation of intermediates formed during steady-state electrocatalytic O2 reduction by iron porphyrins. Proc. Natl. Acad. Sci. USA 2013, 110, 8431–8436. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.M.; Lourenço, M.A.; Calvete, M.J.; Abreu, A.R.; Rosado, M.T.; Burrows, H.D.; Pereira, M.M. Synthesis of new metalloporphyrin triads: Efficient and versatile tripod optical sensor for the detection of amines. Inorg. Chem. 2011, 50, 7916–7918. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.M.; Burrell, A.K.; Officer, D.L.; Jolley, K.W. Porphyrins as light harvesters in the dye-sensitised TiO2 solar cell. Coord. Chem. Rev. 2004, 248, 1363–1379. [Google Scholar] [CrossRef]
- Wamser, C.C.; Kim, H.-S.; Lee, J.-K. Solar cells with porphyrin sensitization. Opt. Mater. 2003, 21, 221–224. [Google Scholar] [CrossRef]
- Schäferling, M.; Bäuerle, P. Porphyrin-functionalized oligo-and polythiophenes. J. Mater. Chem. 2004, 14, 1132–1141. [Google Scholar] [CrossRef]
- Borek, C.; Hanson, K.; Djurovich, P.I.; Thompson, M.E.; Aznavour, K.; Bau, R.; Sun, Y.; Forrest, S.R.; Brooks, J.; Michalski, L. Highly Efficient, Near-Infrared Electrophosphorescence from a Pt–Metalloporphyrin Complex. Angew. Chem. Int. Ed. 2007, 46, 1109–1112. [Google Scholar] [CrossRef]
- Suslick, K.S.; Chen, C.T.; Meredith, G.R.; Cheng, L.T. Push-pull porphyrins as nonlinear optical materials. J. Am. Chem. Soc. 1992, 114, 6928–6930. [Google Scholar] [CrossRef]
- Calvete, M.J. Near-infrared absorbing organic materials with nonlinear transmission properties. Int. Rev. Phys. Chem. 2012, 31, 319–366. [Google Scholar] [CrossRef]
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular electronic components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kang, S.W.; Harden, J.; Sun, Q.; Zhou, X.; Dai, L.; Jakli, A.; Kumar, S.; Li, Q. Nature-inspired light-harvesting liquid crystalline porphyrins for organic photovoltaics. Liq. Cryst. 2008, 35, 233–239. [Google Scholar] [CrossRef]
- Gianferrara, T.; Spagnul, C.; Alberto, R.; Gasser, G.; Ferrari, S.; Pierroz, V.; Bergamo, A.; Alessio, E. Towards matched pairs of porphyrin–ReI/99mTcI conjugates that combine photodynamic activity with fluorescence and radio imaging. ChemMedChem 2014, 9, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Barron, G.; Valentine, R.; Moseley, H.; Brancaleon, L.; Hill, C.; Woods, J. Porphyrin profile in four human cell lines after supplementation with 5-aminolaevulinic acid and its methyl ester. Photodiagn. Photodyn. Ther. 2013, 10, 654–663. [Google Scholar] [CrossRef]
- Koifman, O.I.; Ageeva, T.A.; Beletskaya, I.P.; Averin, A.D.; Yakushev, A.A.; Tomilova, L.G.; Dubinina, T.V.; Tsivadze, A.Y.; Gorbunova, Y.G.; Martynov, A.G. Macroheterocyclic compounds a key building block in new functional materials and molecular devices. Macroheterocycles 2020, 13, 311–467. [Google Scholar] [CrossRef]
- Hata, H.; Shinokubo, H.; Osuka, A. Highly regioselective Ir-Catalyzed β-borylation of porphyrins via C−H bond activation and construction of β−β-linked diporphyrin. J. Am. Chem. Soc. 2005, 127, 8264–8265. [Google Scholar] [CrossRef]
- Deng, Y.; Chang, C.; Nocera, D.G. Facile Synthesis of β-Derivatized Porphyrins—Structural Characterization of a β–β-Bis-Porphyrin. Angew. Chem. Int. Ed. 2000, 39, 1066–1068. [Google Scholar] [CrossRef]
- Evans, B.; Smith, K.M. Novel meso-substitution reactions of zinc (II) octaethylporphyrin. Tetrahedron Lett. 1977, 18, 3079–3082. [Google Scholar] [CrossRef]
- Shinokubo, H. Transition metal catalyzed borylation of functional π-systems. Proc. Jpn. Acad. Ser. B 2014, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.M.; Murphy, J.M.; Hapke, M.; Ishiyama, T.; Miyaura, N.; Hartwig, J.F. Mechanism of the mild functionalization of arenes by diboron reagents catalyzed by iridium complexes. Intermediacy and chemistry of bipyridine-ligated iridium trisboryl complexes. J. Am. Chem. Soc. 2005, 127, 14263–14278. [Google Scholar] [CrossRef] [PubMed]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and functionalization of porphyrins through organometallic methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Boyle, R.W. First examples of intramolecular Pd (0) catalysed couplings on ortho-iodinated meso-phenyl porphyrins. Chem. Commun. 2004, 11, 1322–1323. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Boyle, R.W. Synthetic routes to porphyrins bearing fused rings. Tetrahedron 2006, 43, 10039–10054. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Scaife, P.J.; Berber, G.; Hughes, D.L. Cofacial porphyrin−ferrocene dyads and a new class of conjugated porphyrin. Org. Lett. 2005, 7, 3413–3416. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Aratani, N.; Osuka, A. Facile synthesis and photophysical properties of 1, 2-phenylene-bridged porphyrin dimers. Tetrahedron Lett. 2009, 50, 3333–3337. [Google Scholar] [CrossRef]
- Mitsushige, Y.; Yamaguchi, S.; Lee, B.S.; Sung, Y.M.; Kuhri, S.; Schierl, C.A.; Guldi, D.M.; Kim, D.; Matsuo, Y. Synthesis of thieno-bridged porphyrins: Changing the antiaromatic contribution by the direction of the thiophene ring. J. Am. Chem. Soc. 2012, 134, 16540–16543. [Google Scholar] [CrossRef]
- Locos, O.B.; Arnold, D.P. The Heck reaction for porphyrin functionalisation: Synthesis of meso-alkenyl monoporphyrins and palladium-catalysed formation of unprecedented meso–β ethene-linked diporphyrins. Org. Biomol. Chem. 2006, 4, 902–916. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Mori, S.; Shinokubo, H.; Osuka, A. Facile Peripheral Functionalization of Porphyrins by Pd-Catalyzed [3 + 2] Annulation with Alkynes. Angew. Chem. Int. Ed. 2006, 45, 7972–7975. [Google Scholar] [CrossRef]
- Tokuji, S.; Yurino, T.; Aratani, N.; Shinokubo, H.; Osuka, A. Palladium-Catalyzed Dimerization of meso-Bromoporphyrins: Highly Regioselective meso–β Coupling through Unprecedented Remote C–H Bond Cleavage. Chem. Eur. J. 2009, 15, 12208–12211. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Tokuji, S.; Yorimitsu, H.; Osuka, A. Palladium-Catalyzed β-Selective Direct Arylation of Porphyrins. Angew. Chem. Int. Ed. 2011, 50, 8867–8870. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, M.; Fagnou, K. Palladium-catalyzed benzene arylation: Incorporation of catalytic pivalic acid as a proton shuttle and a key element in catalyst design. J. Am. Chem. Soc. 2006, 128, 16496–16497. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Y.; Gorelsky, S.I.; Stuart, D.R.; Campeau, L.-C.; Fagnou, K. Mechanistic analysis of azine N-oxide direct arylation: Evidence for a critical role of acetate in the Pd(OAc)2 precatalyst. J. Org. Chem. 2010, 75, 8180–8189. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, D.; Markiewicz, T.; Whipp, C.J.; Toderian, A.; Fagnou, K. Predictable and site-selective functionalization of poly (hetero) arene compounds by palladium catalysis. J. Org. Chem. 2011, 76, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the palladium-catalyzed (aromatic) C–H bond metalation–deprotonation mechanism spanning the entire spectrum of arenes. J. Org. Chem. 2012, 77, 658–668. [Google Scholar] [CrossRef]
- Tokuji, S.; Awane, H.; Yorimitsu, H.; Osuka, A. Direct Arylation of meso-Formyl Porphyrin. Chem. Eur. J. 2013, 19, 64–68. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tokuji, S.; Tanaka, T.; Yorimitsu, H.; Osuka, A. Direct Arylation of Porphyrins with π-Extended Aryl Bromides under Ligand-free Fagnou–Hartwig Conditions. Asian J. Org. Chem. 2013, 2, 320–324. [Google Scholar] [CrossRef]
- Chen, J.; Aratani, N.; Shinokubo, H.; Osuka, A. Post-Modification of meso–meso-Linked Porphyrin Arrays by Iridium and Rhodium Catalyses for Tuning of Energy Gap. Chem. Asian J. 2009, 4, 1126–1133. [Google Scholar] [CrossRef]
- Hisaki, I.; Hiroto, S.; Kim, K.S.; Noh, S.B.; Kim, D.; Shinokubo, H.; Osuka, A. Synthesis of Doubly β-to-β 1, 3-Butadiyne-Bridged Diporphyrins: Enforced Planar Structures and Large Two-Photon Absorption Cross Sections. Angew. Chem. 2007, 119, 5217–5220. [Google Scholar] [CrossRef]
- Song, J.; Jang, S.Y.; Yamaguchi, S.; Sankar, J.; Hiroto, S.; Aratani, N.; Shin, J.Y.; Easwaramoorthi, S.; Kim, K.S.; Kim, D. 2,5-Thienylene-Bridged Triangular and Linear Porphyrin Trimers. Angew. Chem. 2008, 120, 6093–6096. [Google Scholar] [CrossRef]
- Hiroto, S.; Hisaki, I.; Shinokubo, H.; Osuka, A. Synthesis of directly and doubly linked dioxoisobacteriochlorin dimers. J. Am. Chem. Soc. 2008, 130, 16172–16173. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Aratani, N.; Easwaramoorthi, S.; Kim, D.; Osuka, A. Meso-β doubly linked Zn (II) porphyrin trimers: Distinct anti-versus-syn effects on their photophysical properties. Org. Lett. 2009, 11, 3080–3083. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yoon, M.-C.; Lim, J.M.; Kim, P.; Aratani, N.; Nakamura, Y.; Ikeda, T.; Osuka, A.; Kim, D. Structural factors determining photophysical properties of directly linked zinc (II) porphyrin dimers: Linking position, dihedral angle, and linkage length. J. Phys. Chem. B 2009, 113, 10619–10627. [Google Scholar] [CrossRef]
- Song, J.; Kim, P.; Aratani, N.; Kim, D.; Shinokubo, H.; Osuka, A. Strategic Synthesis of 2,6-Pyridylene-Bridged β-to-β Porphyrin Nanorings through Cross-Coupling. Chem. Eur. J. 2010, 16, 3009–3012. [Google Scholar] [CrossRef]
- She, C.; Easwaramoorthi, S.; Kim, P.; Hiroto, S.; Hisaki, I.; Shinokubo, H.; Osuka, A.; Kim, D.; Hupp, J.T. Excess Polarizability Reveals Exciton Localization/Delocalization Controlled by Linking Positions on Porphyrin Rings in Butadiyne-Bridged Porphyrin Dimers. J. Phys. Chem. A 2010, 114, 3384–3390. [Google Scholar] [CrossRef]
- Song, J.; Aratani, N.; Kim, P.; Kim, D.; Shinokubo, H.; Osuka, A. Porphyrin “Lego Block” Strategy To Construct Directly meso–β Doubly Linked Porphyrin Rings. Angew. Chem. 2010, 122, 3699–3702. [Google Scholar] [CrossRef]
- Song, J.; Aratani, N.; Shinokubo, H.; Osuka, A. A porphyrin nanobarrel that encapsulates C60. J. Am. Chem. Soc. 2010, 132, 16356–16357. [Google Scholar] [CrossRef]
- Song, J.; Aratani, N.; Shinokubo, H.; Osuka, A. A β-to-β 2,5-thienylene-bridged cyclic porphyrin tetramer: Its rational synthesis and 1: 2 binding mode with C60. Chem. Sci. 2011, 2, 748–751. [Google Scholar] [CrossRef]
- Song, J.; Anabuki, S.; Aratani, N.; Shinokubo, H.; Osuka, A. A hexameric porphyrin triangle constructed by Suzuki–Miyaura Cross-coupling reaction. Chem. Lett. 2011, 40, 902–903. [Google Scholar] [CrossRef]
- Tokuji, S.; Maeda, C.; Yorimitsu, H.; Osuka, A. New Synthetic Strategy for Diporphyrins: Pinacol Coupling–Rearrangement. Chem. Eur. J. 2011, 17, 7154–7157. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Aratani, N.; Osuka, A. A doubly 2,6-pyridylene-bridged porphyrin–perylene–porphyrin triad. Chem. Commun. 2012, 48, 4317–4319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiselev, A.N.; Grigorova, O.K.; Averin, A.D.; Syrbu, S.A.; Koifman, O.I.; Beletskaya, I.P. Direct catalytic arylation of heteroarenes with meso-bromophenyl-substituted porphyrins. Beilstein J. Org. Chem. 2017, 13, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Gubarev, Y.A.; Lebedeva, N.S.; Yurina, E.S.; Syrbu, S.A.; Kiselev, A.N.; Lebedev, M.A. Possible therapeutic targets and promising drugs based on unsymmetrical hetaryl-substituted porphyrins to combat SARS-CoV-2. J. Pharm. Anal. 2021, 11, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Pukhovskaya, S.; Ivanova, Y.; Kiselev, A.; Fomina, N.; Syrbu, S. Synthesis, structure and basic properties of 5, 10, 15, 20-tetrakis [4′-(benzoxazole-2-yl) phenyl]-21, 23-dithiaporphyrin. J. Mol. Struct. 2021, 1238, 130406. [Google Scholar] [CrossRef]
- Khandagale, S.B.; Pilania, M.; Arun, V.; Kumar, D. Metal-catalyzed direct heteroarylation of C–H (meso) bonds in porphyrins: Facile synthesis and photophysical properties of novel meso-heteroaromatic appended porphyrins. Org. Biomol. Chem. 2018, 16, 2097–2104. [Google Scholar] [CrossRef]
- Seregin, I.V.; Gevorgyan, V. Direct transition metal-catalyzed functionalization of heteroaromatic compounds. Chem. Soc. Rev. 2007, 36, 1173–1193. [Google Scholar] [CrossRef] [PubMed]
- Roudesly, F.; Oble, J.; Poli, G. Metal-catalyzed CH activation/functionalization: The fundamentals. J. Mol. Catal. A Chem. 2017, 426, 275–296. [Google Scholar] [CrossRef]
- Verrier, C.; Lassalas, P.; Théveau, L.; Quéguiner, G.; Trécourt, F.; Marsais, F.; Hoarau, C. Recent advances in direct C–H arylation: Methodology, selectivity and mechanism in oxazole series. Beilstein J. Org. Chem. 2011, 7, 1584–1601. [Google Scholar] [CrossRef]
- Yan, X.-M.; Mao, X.-R.; Huang, Z.-Z. An efficient arylation of benzoazoles with aryl bromides by a practical palladium-copper cocatalytic system. Heterocycles 2011, 83, 1371–1376. [Google Scholar] [CrossRef]
- Huang, J.; Chan, J.; Chen, Y.; Borths, C.J.; Baucom, K.D.; Larsen, R.D.; Faul, M.M. A highly efficient palladium/copper cocatalytic system for direct arylation of heteroarenes: An unexpected effect of Cu (Xantphos) I. J. Am. Chem. Soc. 2010, 132, 3674–3675. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, T.; Miyaura, N. Transition metal-catalyzed borylation of alkanes and arenes via C–H activation. J. Organomet. Chem. 2003, 680, 3–11. [Google Scholar] [CrossRef]
- Ishiyama, T.; Miyaura, N. Metal-catalyzed reactions of diborons for synthesis of organoboron compounds. Chem. Rec. 2004, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, T. Transition metal-catalyzed direct borylation and silylation based on CH activation. J. Synth. Org. Chem. Jpn. 2005, 63, 440–452. [Google Scholar] [CrossRef]
- Hartwig, J.F. Borylation and silylation of C–H bonds: A platform for diverse C–H bond functionalizations. Acc. Chem. Res. 2012, 45, 864–873. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Tse, M.K.; Holmes, D.; Maleczka, R.E., Jr.; Smith, M.R., III. Remarkably selective iridium catalysts for the elaboration of aromatic CH bonds. Science 2002, 295, 305–308. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Iverson, C.N.; Smith, M.R. Steric and chelate directing effects in aromatic borylation. J. Am. Chem. Soc. 2000, 122, 12868–12869. [Google Scholar] [CrossRef]
- Ishiyama, T.; Nobuta, Y.; Hartwig, J.F.; Miyaura, N. Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent. Chem. Commun. 2003, 17, 2924–2925. [Google Scholar] [CrossRef]
- Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N.R.; Hartwig, J.F. Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate. J. Am. Chem. Soc. 2002, 124, 390–391. [Google Scholar] [CrossRef]
- Ishiyama, T.; Takagi, J.; Hartwig, J.F.; Miyaura, N. A Stoichiometric Aromatic C–H Borylation Catalyzed by Iridium (i)/2,2′-Bipyridine Complexes at Room Temperature. Angew. Chem. Int. Ed. 2002, 41, 3056–3058. [Google Scholar] [CrossRef]
- Lapointe, D.; Fagnou, K. Overview of the mechanistic work on the concerted metallation–deprotonation pathway. Chem. Lett. 2010, 39, 1118–1126. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).