Abstract
In this paper, it is experimentally proven that the generalized Peukert equation C(i,T) = Cm(T)/(1 + (i/i0(T))n(T)) is applicable to nickel–metal hydride batteries at any discharge currents, while the classical Peukert equation can be used only in a limited range of the discharge currents (approximately from 0.3 Cn to 3 Cn). In addition, the classical Peikert equation does not take into account the influence of the temperature of a battery on its released capacity. It is also proven that for the nickel–metal hydride batteries, the generalized Peukert equation heavily depends on battery temperature (via the parameters Cm(T), i0(T) and n(T)). The temperature dependencies of the parameters of the generalized Peukert equation and their physical meaning are also established. The obtained generalized Peukert equation, which considers the batteries’ temperature, can be used at any discharge current and temperature of the batteries.
1. Introduction
Batteries of various electrochemical systems are used in many technical devices, vehicles and airplanes. For the operation of any technical object containing batteries, it is very important to know the residual capacity of the batteries because the operation duration of the whole technical object depends on the residual capacity of the batteries. Currently, many methods are known for battery residual capacity assessment. However, the existing methods developed for the batteries’ residual capacity determination do not provide sufficient accuracy and reliability. Therefore, the problem of finding the batteries’ residual capacity has not yet been solved. This is due to the fact that the batteries’ residual capacity depends on many factors, including discharge current, and a battery’s temperature, lifespan, operating mode, etc.
One of the first and simplest methods for the batteries’ residual capacity assessment was estimating it by the open circuit voltage [1]. However, this method provides only an approximate estimate of the batteries’ residual capacity as in the mode, when the discharge current changes rapidly, the error is very large, up to 20% [2]. In addition, this method is absolutely inapplicable to batteries with a flat discharge curve, for example, lithium iron phosphate batteries. The method of counting ampere-hours on every discharge cycle is also widely used. Then, the residual battery capacity can be calculated as a difference between the ampere-hours spent on the previous full discharge cycle and the number of ampere-hours spent during this unfinished discharge cycle. However, at different dynamic discharge modes, this method gives an error of up to 10% [3].
The most accurate methods for assessing the residual battery capacity should be based on physical models using the known natural laws describing processes in batteries [4,5,6,7,8]. However, such models are rarely used in practice. The reason is that these models are very complicated and cannot be solved by the onboard computers of airplanes, vehicles, etc. Furthermore, these models contain various parameters describing the internal processes in batteries. Many of these parameters are either absolutely impossible to measure (for example, parameters describing processes running inside of a porous electrode) or the parameters’ findings require very sophisticated electrochemical and chemical experiments. It should also be noted that the physical models cannot describe thermal runaway [9,10], hydrogen accumulation in electrodes [11,12], or other poorly studied processes in batteries.
In [13], it was noted that battery models are the preference to be solved by the onboard computers of electric vehicles and airplanes for practical use. Moreover, the model parameters should be found using simple experiments without the batteries’ disassembling. Therefore, for practical application, only semi-empirical (analytical) models [14,15,16,17] or structural models [18,19,20] can be used.
An inherent part of any analytical model is empirical or semi-empirical dependencies. Often, analytical models use either the Kalman Filter [21,22] or the Peukert equation [23]. For the first time, a relationship between the battery released capacity and the discharge current was established by the Peukert equation [23], which was obtained for the lead–acid batteries. In many papers [13,24], it is written in the following form
where C is the capacity, i is the discharge current, A and n are the empiric constants.
This equation is not applicable to batteries of any electrochemical systems at very low discharge currents because at i → 0, C → ∞, which is impossible for any battery. In addition, the Peukert Equation (1) is not applicable to alkaline and lithium-ion batteries at medium- and high-discharge currents [17,24,25] because the experimental function C(i) for these batteries is both convex and concave at different discharge currents.
Meanwhile, in Equation (1), the function C(i) (n > 0) is always concave. In [25], it was proven experimentally that for alkaline and lithium-ion batteries, the Peukert Equation (1) is applicable only in the discharge currents range from approximately 0.2 Cn to 2–3 Cn (Cn is nominal battery capacity). It was exactly in this range of the discharge currents that the Peukert Equation (1) was used in the Hausmann model [13] for the batteries’ residual capacity assessment. Notably, the relative error in the batteries’ residual capacity estimation was less than 5%.
This study aimed to improve the Peukert equation so that it can be used for the nickel–metal hydride batteries at any temperature and discharge current.
2. Theory
The Haussman model [13] was developed to evaluate the batteries’ residual capacity in the case of their dynamic discharge mode, i.e., when the discharge currents vary greatly during discharging. In this model, the entire discharge time is divided into time intervals ∆t = 1 s. Within every time span ∆t = 1 s, the battery temperature and the discharge current can be considered constant. Consequently, within every time span ∆t = 1 s, it is possible to use the empiric dependencies C(i) and C(T) experimentally obtained at constant discharge currents and constant battery temperatures.
In [26], it is proven that the Hausmann model [13] uses the Peukert equation in the following form:
where Tref = 298 K is the reference temperature, and M, n, and β are the empiric constants.
Thus, the Peukert Equation (2) takes into account both the discharge current and the battery temperature as factors influencing the released battery capacity. In Equation (2), the first multiplier is the classical Peukert Equation (1) and the second multiplier is the empirical dependence of the released capacity on the battery temperature:
where Cmref is the maximum battery capacity at reference temperature Tref = 298 K.
The classical Peukert equation is applicable to lithium-ion and alkaline batteries only in the discharge currents range from 0.2 Cn to 2–3 Cn [25]; meanwhile, the generalized Peukert equation:
applies to any discharge currents [17,24,25,26]. In Equation (4), Cm is the top capacity of a battery, C(i0) = Cm/2, i.e., i0 is the discharge current, at which a battery released capacity is half the maximum capacity. Besides, for the Equation (4):
That is, in the standardized coordinates (C(i)/Cm, i/i0), the parameter n/4 is equal to the angle of inclination of the function C(i) (4) in the point i = i0. Hence, in Equation (4), all the parameters have a clear physical meaning, while in Equations (1) and (2), all the parameters are just empirical constants.
In addition, Equation (3) is applicable only in the limited temperature range, around the temperature value Tref [16,26]. Indeed, in Equation (3), C → 0 at T → 0. However, in real batteries, C → 0 at T → Tk, where Tk is a temperature value close to the electrolyte freezing temperature. In addition, in Equation (3), C → ∞ at T → ∞. However, in real batteries, the released capacity is always limited by the capacity obtained when the batteries are charged.
The experimental function C(T), applicable to lithium-ion batteries at any temperature, has the following form [16,26]:
Equation (6) corresponds to the above-noted boundary experimental data since C(Tk) = 0, and . Thus, at a temperature growth, the parameter K determines the maximum possible battery capacity (CmrefK).
In the Hausmann model [26], instead of Equations (1) and (3), Equations (4) and (6) were used. Upon that, in the estimation of the residual capacity of the lithium-ion batteries, the relative error decreased to 4%.
However, in [13,26], the authors believed that in Equation (4), only the maximum battery capacity depends on a temperature, i.e., Cm.
In this study, we check the temperature dependencies of all the parameters (Cm, i0, and n) of Equation (4) for the nickel–metal hydride batteries.
3. Experimental
For our experiments, we used the nickel–metal hydride batteries (RS PRO Rechargeable NI-MH); their nominal voltage is 1.2 V and the nominal capacity is Cn = 2.7 Ah. The standard charge rate is 260 mA ∗ 16 h.
For the batteries’ charging, we used the computer-controlled QL355P power supply. In order to discharge the batteries, a computer-controlled electronic load, BK Precision 8601, was used. The discharge was performed by a constant current down to the voltage 1 V. In the experiments, the discharge currents varied from 0.3 Cn to 10 Cn.
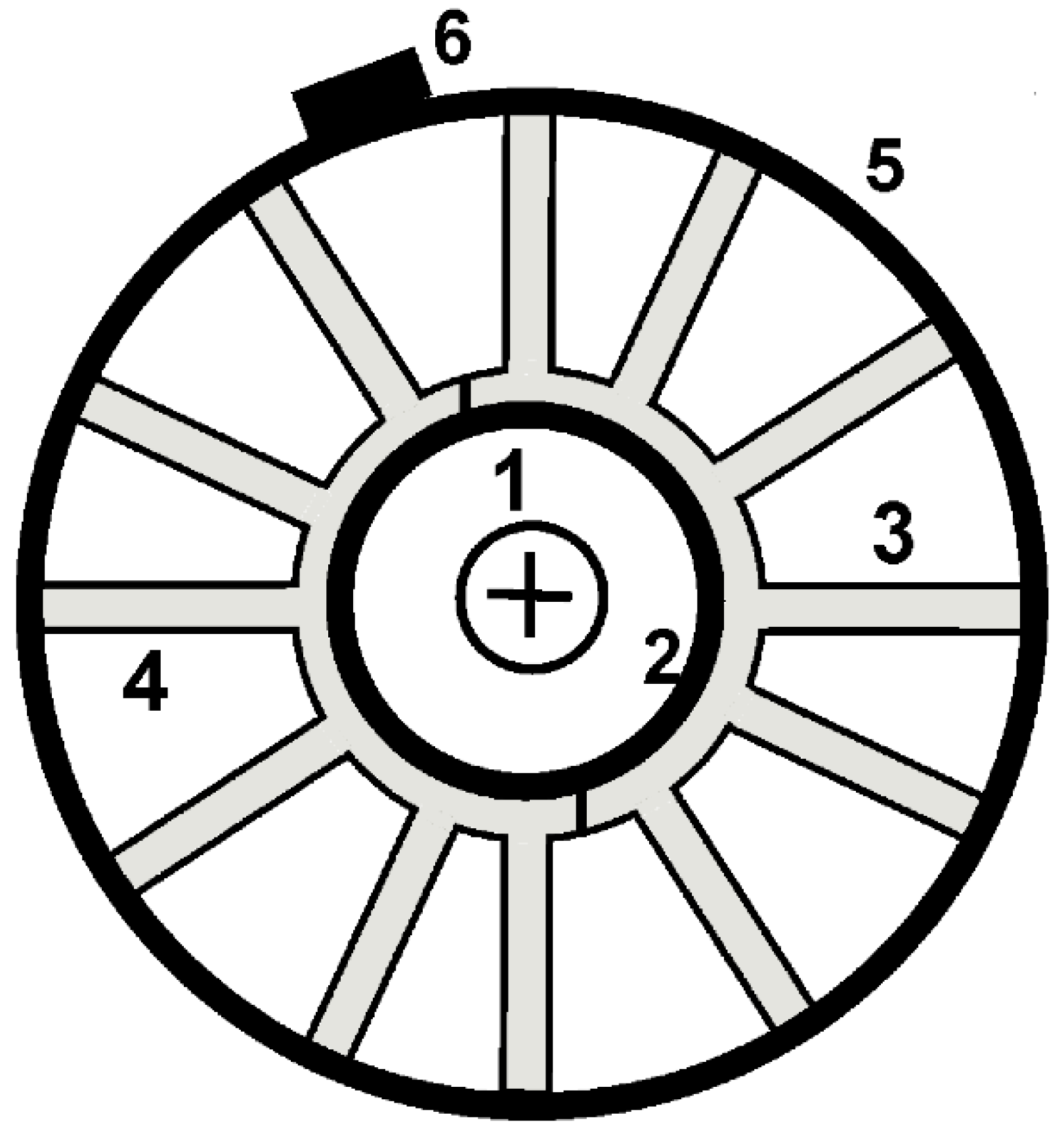
The experimental measurements were carried out at temperature values of 25 °C, 0 °C, −12 °C and −18 °C in a Binder MK240 climatic chamber. The temperature control of the batteries was carried out using three LM35 temperature sensors fixed in the upper, middle and lower parts of the batteries. In order to increase the heat exchange between the battery and the interior of the climatic chamber, custom heat-sinks were attached to the battery walls with f special clamps and a heat-conducting paste (Figure 1). In the climatic chamber, the temperature of the battery was maintained close to the value necessary to study the batteries; for this purpose, the LM35 temperature sensors were used.
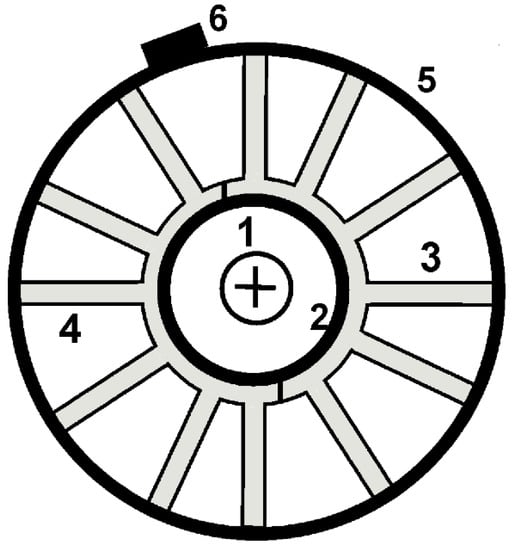
Figure 1.
Diagram of the setup (top view): (1) nickel–metal hydride battery, (2) heat-conducting paste, (3, 4) two halves of a modified heat sink, (5) clamp, and (6) screw of the clamp.
In order to collect more statistical data, we conducted all experiments simultaneously with five batteries. Based on the data obtained and using statistical methods, it was possible to more accurately calculate the value of the battery capacity (as an average value) and estimate the error of each measurement at a certain current and temperature.
The experimental measurements were included in the following stages.
Firstly, we performed five charge–discharge training cycles for ten batteries. The charging was carried out by the standard method described above. The discharging was performed by the current 0.2 Cn down to the voltage of 1 V. Then, among those ten batteries, five were selected with the lowest dispersion of the released capacity and further experimental studies were carried out with the selected five batteries.
Secondly, prior to changing of the discharge current, we performed three charge–discharge training cycles. This made it possible to avoid the mutual influence between the different charge–discharge cycles.
Thirdly, after each measurement at a certain discharge current, the obtained values of the released capacity were compared. If the released capacity of any battery differed by more than 5% from the average capacity of other batteries, this battery was replaced with another more stable one, and the experiment was repeated from the beginning.
Fourthly, to avoid the effect of battery aging, when changing the temperature of batteries, all the above three stages were repeated for a new group of batteries at the new temperature.
4. Results
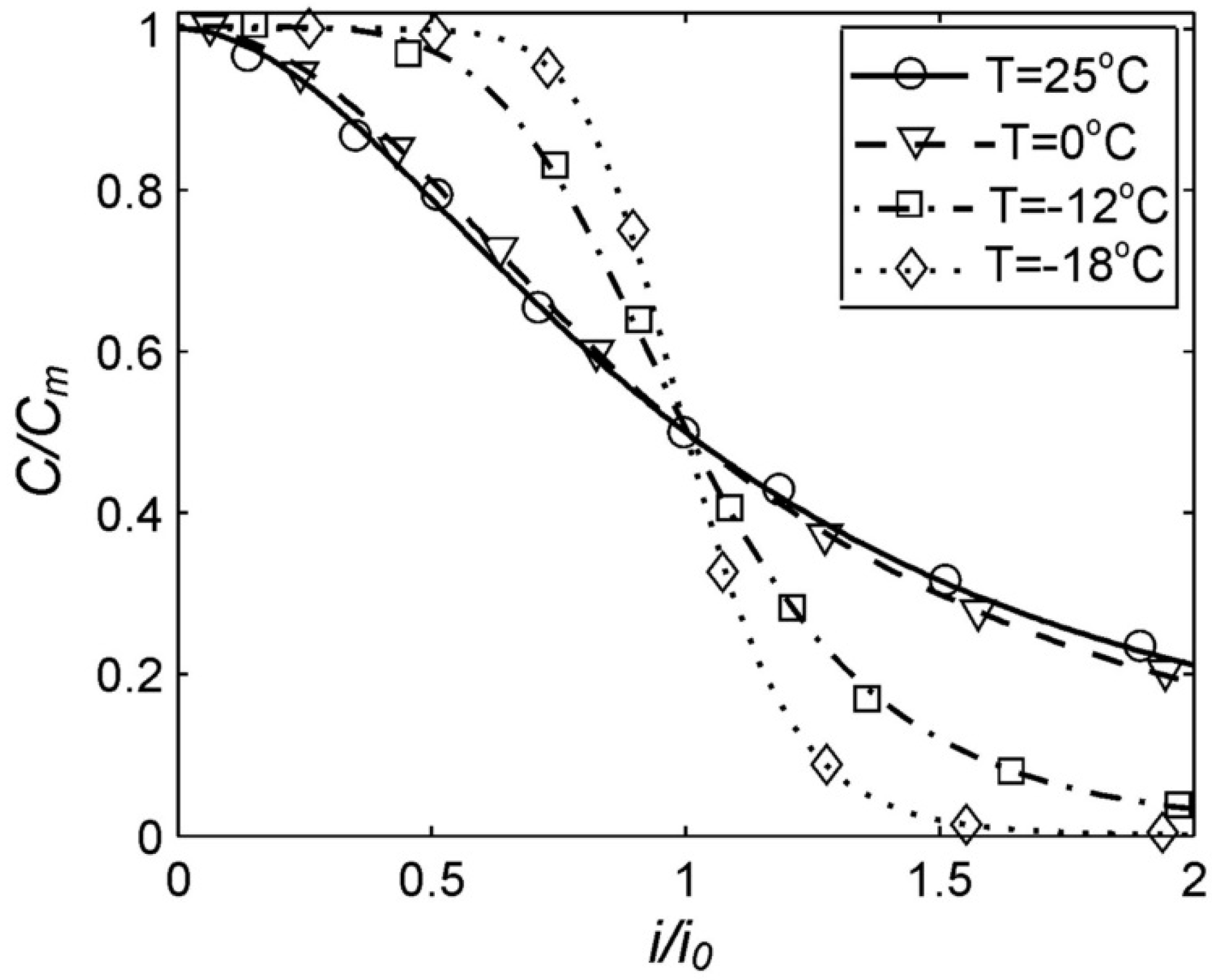
Figure 2 presents the experimental values obtained in the coordinates (C/Cm, i/i0). As the parameters (Cm, i0) are obtained for specific batteries by experiments, then in the ratios (C/Cm, i/i0), the small unavoidable random processes associated with both the battery manufacturing process and the measurement process will be compensated. More reliable experimental curves can be obtained with this method.
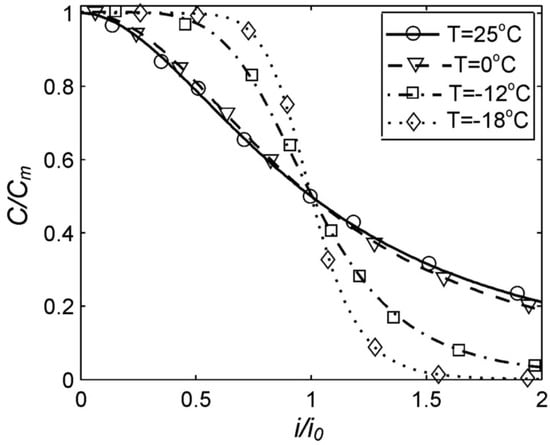
Figure 2.
Dependence of the capacity of RS PRO Rechargeable NI-MH batteries on the discharge current in standardized coordinates. Parameters i0 and Cm are taken from Table 1.
Using the obtained experimental data (Figure 2), with the aid of the least square method and the Levenberg–Marquardt optimization procedure, the optimal parameters were obtained for the generalized Peukert Equation (4) at different temperatures (Table 1).

Table 1.
Optimal parameter values for Equation (4) at different temperatures.
From the results (Table 1), it follows that all the parameters (Cm, i0, and n) of Equation (4) depend on the battery temperature and not exclusively the parameter Cm (as it was believed in the papers [13,26]). Hence, in the Hausmann model [13], when assessing the residual capacity of batteries, it is necessary to take into account the temperature dependence of all the parameters (Cm, i0, and n) of Equation (4).
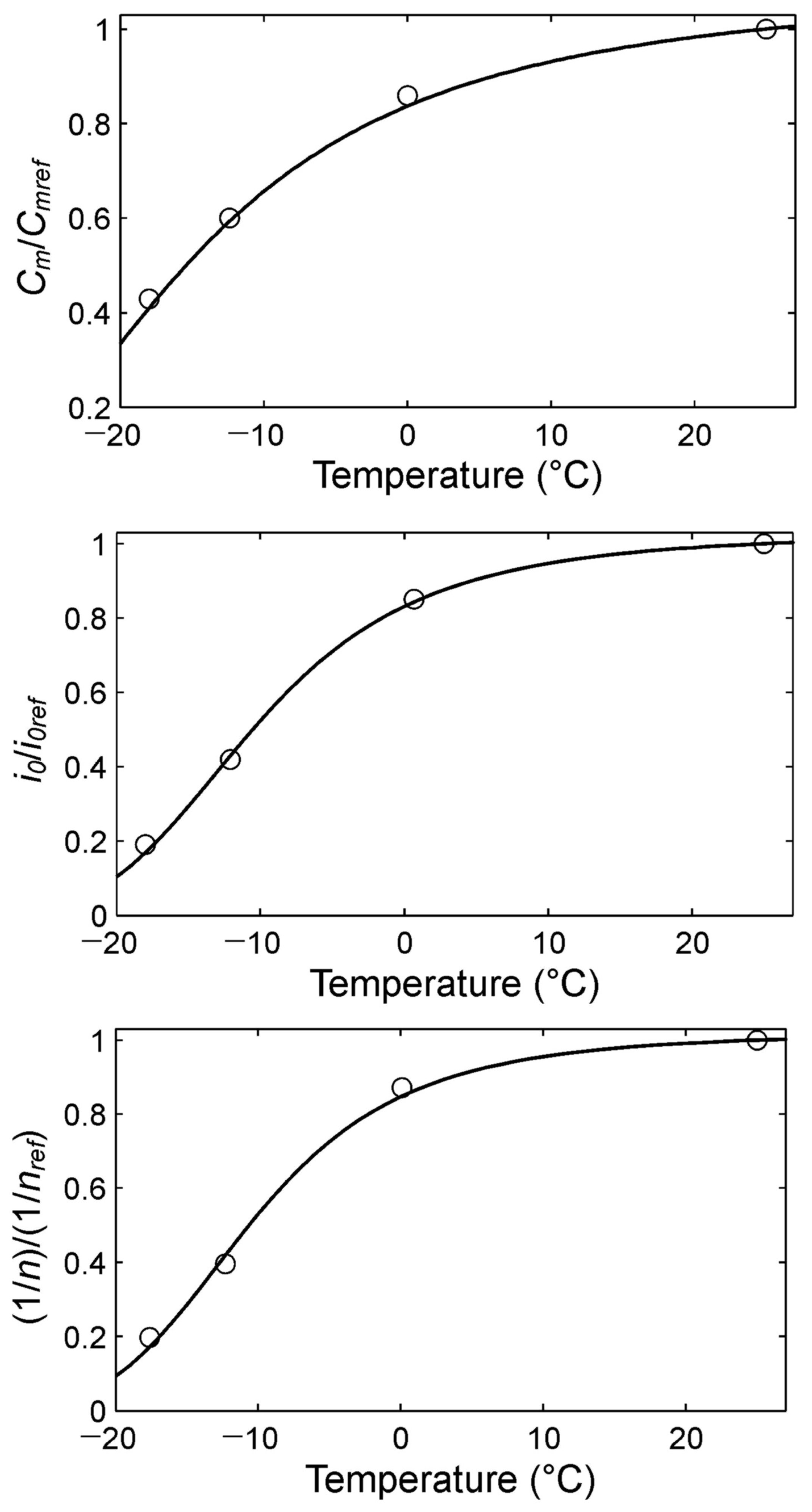
Figure 3 shows the dependence of the Equation (4) parameters on temperature values in standardized coordinates.
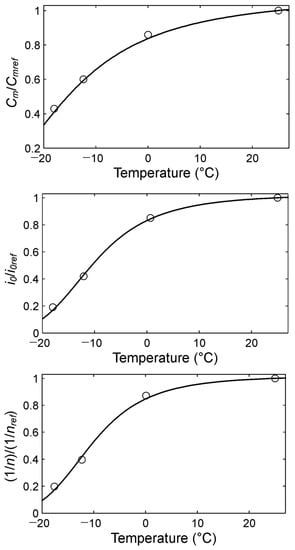
Figure 3.
Dependence of parameters Cm, i0, and n in Equation (4) on the temperature in standardized coordinates. Cmref, i0ref, and nref are parameter values at the temperature Tref = 298 K.
The dependence of the experimental values of the Equation (4) parameters on the temperature (Figure 3) has a qualitative form quite similar to that of Equation (6). Accordingly, the dependencies of all the parameters of Equation (4) on temperature can be summarized in one vectorial equation:
P = (Cm, i0, 1/n)
Table 1 shows the compliance of Equation (8) with the experimental data (Table 1). In doing this, we find the optimal parameters of Equation (8) using the least square method and the Levenberg–Marquardt optimization procedure.
The results are represented in Table 2.

Table 2.
Optimal parameter values for Equations (8).
5. Discussion
The experimental studies have shown that the classical Peukert Equation (2) (taking the temperature into account) has limited scope for nickel–metal hydride batteries in comparison with the generalized Peukert Equation (4) (with due account of Equation (8)).
Firstly, the Peukert Equation (1) is applicable to the nickel–metal hydride batteries approximately in the range of discharge currents from 0.3 Cn up to 3 Cn [27]. At discharge currents less than 0.3 Cn, the resulting value of the Peukert Equation (1) grows indefinitely, which does not correspond to the experimental data for any batteries. At an increase of the discharge currents to more than 3 Cn for the nickel–metal hydride batteries, the experimental function C(i) becomes convex and then concave (Figure 2). However, the Peukert Equation (1) always has a concave curve (when n > 0). Meanwhile, the generalized Peukert Equation (4) applies to the nickel–metal hydride batteries at any discharge current (Figure 2).
Secondly, the applicability of the Peukert Equation (2) (accounting for temperature is very limited (approximately by the range Tref ± 10 K (Figure 3)). The main reason for this is that for function (3), C(T) = 0 only at temperature T = 0. However, the studies showed that for the experimental functions (Figure 3), C(T) = 0 approximately at the temperature T = 240 K (Table 2). Therefore, in principle, function (3) cannot accurately approximate the experimental data in Figure 3 in the appropriately large temperature range. Meanwhile, Equation (8) from the generalized Peukert Equation (4) corresponds well to the experimental data at any temperature of the nickel–metal hydride batteries (Figure 3).
Thirdly, it should be noted that in Equation (4), all of the parameters have their clear physical meaning, whereas in Equations (1) and (2), all the parameters are simply empirical constants.
Thus, in the Hausmann model [13], it is necessary to use the more accurate generalized Peukert equation in the following form:
with a due account of Equation (8) for the functions Cm(T), i0(T), and n(T).
Our preliminary experimental studies showed that in the framework of the Hausmann model [13], the use of the generalized Peukert Equation (9) instead of the classical Peukert Equation (2) increases the accuracy of the estimation of a battery’s capacity by at least 10–15%. In using nickel–metal hydride batteries at very low or very high currents or at low temperatures, the use of Equation (9) in the Hausmann model [13] significantly increases the accuracy of estimating the residual capacity of batteries. This is the topic of our further research.
6. Conclusions
Nickel–metal hydride batteries are used widely for standby power of communication systems in photovoltaic systems, electric vehicles, etc. They are considered more environmentally friendly than nickel–cadmium batteries, which explains the need for reliable calculation models for nickel–metal hydride batteries. The Peukert equation is used in many battery models [2,28,29,30]. However, a number of factors must be considered when using the Peukert Equation (1). Firstly, for batteries at any discharge current, only the generalized Peukert Equation (9) can be used as the classical Peukert Equation (1), which has a limited scope. Secondly, in this paper, it was experimentally proven that the capacity of nickel–metal hydride batteries depends strongly on their temperature. Therefore, the generalized Peukert Equation (9) should be used to account for the dependencies of its parameters on the batteries’ temperature. In this case, Equation (9) applies at any temperature and discharge currents. However, in many models, the temperature of the batteries is considered [5,21,31]. This can result in a significant discrepancy between the calculated values and the experimental data.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Coleman, M.; Lee, C.K.; Zhu, C.; Hurley, W.G. State-of-charge determination from EMF voltage estimation: Using impedance, terminal voltage, and current for lead-acid and lithium-ion batteries. IEEE Trans. Ind. Electron. 2007, 54, 2550–2557. [Google Scholar] [CrossRef]
- Omar, N.; Daowd, M.; Van den Bossche, P.; Hegazy, O.; Smekens, J.; Coosemans, T.; van Mierlo, J. Rechargeable energy storage systems for plug-in hybrid electric vehicles—Assessment of electrical characteristics. Energies 2012, 5, 2952–2988. [Google Scholar] [CrossRef]
- Yazvinskaya, N.N.; Lipkin, M.S.; Galushkin, N.E.; Galushkin, D.N. Analysis of Peukert Generalized Equations Use for Estimation of Remaining Capacity of Automotive-Grade Lithium-Ion Batteries. Batteries 2022, 8, 118. [Google Scholar] [CrossRef]
- Chen, H.; Buston, J.E.H.; Gill, J.; Howard, D.; Williams, R.C.E.; Read, E.; Abaza, A.; Cooper, B.; Wen, J.X. Simplified Mathematical Model for Heating-Induced Thermal Runaway of Lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 010502. [Google Scholar] [CrossRef]
- Cugnet, M.; Laruelle, S.; Grugeon, S.; Sahut, B.; Sabatier, J.; Tarascon, J.-M.; Oustaloup, A. A mathematical model for the simulation of new and aged automotive lead-acid batteries. J. Electrochem. Soc. 2009, 156, A974–A985. [Google Scholar] [CrossRef]
- Arunachalam, H.; Onori, S.; Battiato, I. On Veracity of Macroscopic Lithium-Ion Battery Models. J. Electrochem. Soc. 2015, 162, A1940–A1951. [Google Scholar] [CrossRef]
- Fan, G.; Pan, K.; Canova, M.; Marcicki, J.; Yang, X.G. Modeling of Li-Ion cells for fast simulation of high C-rate and low temperature operations. J. Electrochem. Soc. 2016, 163, A666–A676. [Google Scholar] [CrossRef]
- Liu, S.; Dougal, R.A.; Weidner, J.W.; Gao, L. A simplified physics-based model for nickel hydrogen battery. J. Power Sources 2005, 141, 326–339. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Analytical model of thermal runaway in alkaline batteries. Int. J. Electrochem. Sci. 2018, 13, 1275–1282. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Mechanism of thermal runaway as a cause of Fleischmann-Pons effect. J. Electroanal. Chem. 2020, 870, 114237. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Pocket electrodes as hydrogen storage units of high-capacity. Int. J. Electrochem. Sci. 2017, 164, A2555–A2558. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Nickel-cadmium batteries with pocket electrodes as hydrogen energy storage units of high-capacity. J. Energy Storage 2021, 39, 102597. [Google Scholar] [CrossRef]
- Hausmann, A.; Depcik, C. Expanding the Peukert equation for battery capacity modeling through inclusion of a temperature dependency. J. Power Sources 2013, 235, 148–158. [Google Scholar] [CrossRef]
- Feng, F.; Lu, R.; Wei, G.; Zhu, C. Online estimation of model parameters and state of charge of LiFePO4 batteries using a novel open-circuit voltage at various ambient temperatures. Energies 2015, 8, 2950–2976. [Google Scholar] [CrossRef]
- Tremblay, O.; Dessaint, L.A. Experimental validation of a battery dynamic model for EV applications. World Electr. Veh. J. 2009, 3, 289–298. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Analysis of generalized Peukert’s equations for capacity calculation of lithium-ion cells. J. Electrochem. Soc. 2020, 167, 013535. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Models for Evaluation of capacitance of Batteries. Int. J. Electrochem. Sci. 2014, 9, 1911–1919. [Google Scholar]
- Zou, Y.; Hu, X.; Ma, H.; Li, S.E. Combined State of Charge and State of Health estimation over lithium-ion battery cell cycle lifespan for electric vehicles. J. Power Sources 2015, 273, 793–803. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Nonlinear Structural Model of the Battery. Int. J. Electrochem. Sci. 2014, 9, 6305–6327. [Google Scholar]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Model of Relaxation Processes in Batteries. ECS Electrochem. Lett. 2015, 4, A94–A96. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.; Sunwoo, M. State-of-charge estimation of lead-acid batteries using an adaptive extended Kalman filter. J. Power Sources 2009, 188, 606–612. [Google Scholar] [CrossRef]
- He, W.; Williard, N.; Chen, C.; Pecht, M. State of charge estimation for electric vehicles batteries using unscented Kalman filtering. Microelectron. Reliab. 2013, 53, 840–847. [Google Scholar] [CrossRef]
- Peukert, W. About the dependence of the capacity of the discharge current magnitude and lead acid batterie. Elektrotech. Z. 1897, 20, 287–288. [Google Scholar]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N.; Galushkina, I.A. Generalized Analytical Models of Batteries, Capacitance Dependence on Discharge Currents. Int. J. Electrochem. Sci. 2014, 9, 4429–4439. [Google Scholar]
- Galushkin, N.E.; Yazvinskaya, N.N.; Ruslyakov, D.V.; Galushkin, D.N. Analysis of Peukert and Liebenow Equations Use for Evaluation of Capacity Released by Lithium-Ion Batteries. Processes 2021, 9, 1753–1763. [Google Scholar]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Generalized analytical model for capacity evaluation of automotive-grade lithium batteries. J. Electrochem. Soc. 2015, 162, A308–A314. [Google Scholar] [CrossRef]
- Wu, G.; Lu, R.; Zhu, C.; Chan, C.C. Apply a Piece-wise Peukert’s Equation with Temperature Correction Factor to NiMH Battery State of Charge Estimation. J. Asian Electr. Veh. 2010, 8, 1419–1423. [Google Scholar] [CrossRef]
- Cugnet, M.G.; Dubarry, M.; Liaw, B.Y. Peuket’s Law of a Lead-Acid Battery Simulated by a Mathematical Model. ECS Trans. 2010, 25, 223–233. [Google Scholar] [CrossRef]
- Doerffel, D.; Sharkh, S.A. A critical review of using the Peukert equation for determining the remaining capacity of lead-acid and lithium-ion batteries. J. Power Sources 2006, 155, 395–400. [Google Scholar] [CrossRef]
- Omar, N.; van den Bossche, P.; Coosemans, T.; Mierlo, J.V. Peukert Revisited—Critical Appraisal and Need for Modification for Lithium-Ion Batteries. Energies 2013, 6, 5625–5641. [Google Scholar] [CrossRef]
- Larminie, J.; Lowry, J.; NetLibrary, I. Electric Vehicle Technology Explained; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).