Abstract
Vanadium is a hard, silver-grey transition metal found in at least 60 minerals and fossil fuel deposits. Its oxide and other vanadium salts are toxic to humans, but the toxic effects depend on the vanadium form, dose, exposure duration, and route of intoxication. Vanadium is used by some life forms as an active center in enzymes, such as the vanadium bromoperoxidase of ocean algae and nitrogenases of bacteria. The structure and biochemistry of vanadate resemble those of phosphate, hence vanadate can be regarded as a phosphate competitor in a variety of biochemical enzymes such as kinases and phosphatases. In this review, we describe the biochemical pathways regulated by vanadium compounds and their potential therapeutic benefits for a range of disorders including type 2 diabetes, cancer, cardiovascular disease, and microbial pathology.
1. Introduction
Vanadium was first discovered by the Mexican mineralogist Andrés Manuel del Rio in 1801 from a specimen of vanadinite, Pb5(VO4)3Cl. The element is named after ‘Vanadis’, the old Norse name for the Scandinavian goddess Freyja representing youth and beauty [1]. However, vanadium was erroneously identified as a chromium mineral until 1831, when the Swedish chemist Nil Gabriel Selfström was investigating steel brittleness in Taberg, Småland, Sweden [2,3]. Vanadium’s importance in biological systems has long been recognized, and vanadium-based catalysts are widely used in industry [4]. Many researchers, like the Pessoa, Kiss, and Garribba groups, have concentrated on vanadium complexes and published tens of articles on the topic, probably owing to the chemical diversity of vanadium complexes and their medicinal and biological significance [4,5,6,7,8,9,10,11,12,13,14].
Among the discovered elements, vanadium is the 20th most abundant chemical element on Earth [15] and is naturally occurring in different minerals including carnotite, patronite vanadinite, roscoelite, and vanadium-bearing magnetite. Vanadium is also naturally found in certain crude oils in the form of vanadium organic complexes and vanadium-carbonaceous deposits from the Cryogenian and Cambrian periods [5].
Chemically, vanadium exhibits a broad range of oxidation states from +5 to −3, which can be easily interconverted. Moreover, at maximum oxidation states, vanadium can be a very good Lewis’s acid. The multiple oxidation states of vanadium, along with its Lewis acid character, good oxophilicity, and ready hydrolysis, give it a rich reaction versatility compared with sulfur complexes and aggregated oxyanions, as well as broad applications of its by-products in catalysis and biology.
Similar to other transition metal complexes, such as copper [16], gold [17], iron [18], and zinc [19], vanadium complexes show a wide range of bioactivities. The interest in vanadium complexes in medicine started earlier than 1939 and rose gradually until the 1970s [11]. The number of published articles related to the promise of vanadium compounds/complexes in various therapeutic applications, including diabetes, microbial infections, and cancers, has increased rapidly since 1999 (Figure 1), which is mostly attributed to vanadium’s biological properties, many of which are shared with phosphates [20]. A recent study showed that vanadium (and its complexes with inorganic and organic ligands) plays an important role in lipid, DNA and protein synthesis, glucose transport and metabolism, and insulin-mimetic activity, and has mitogenic effects on different types of cells. It also shows therapeutic potential against RNA viruses including COVID-19 [21]. A series of in vitro investigations have demonstrated that oxidovanadium VO2+ and vanadate VO43− decrease lipolysis and stimulate glucose oxidation, glycogen synthase, glucose transport, and tyrosine phosphorylation in rat adipocytes [22]. Vanadates also promote glycogen synthase glucose uptake, glucose, and glycolysis oxidation in striated muscle [23,24]. However, vanadium is a highly toxic element that inhibits some biochemical processes.
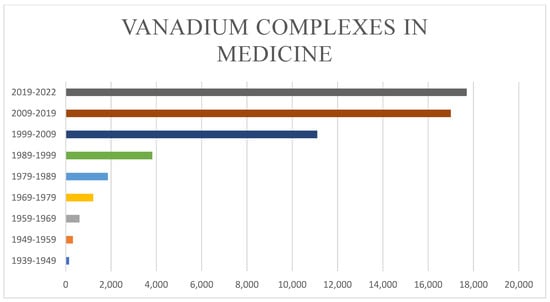
Figure 1.
Published studies using vanadium complexes in medical applications. Studies were found by searching the exact phrase “vanadium complexes in medicine” on Google Scholar (http://scholar.google.com (accessed on 22 March 2022)).
Finally, its multiple oxidation states and Lewis acid character make vanadium highly reactive and versatile for numerous industrial applications, such as the pigmentation of ceramics, glass manufacturing, coatings, and microelectronic devices [25]. The use of transition metal compounds in biomedical applications is under investigation because of their efficacy in lowering the toxicity of the free organic ligands and increasing their absorbance and stability [26,27]. For this purpose, vanadium is being investigated as a potential metallodrug for the treatment of cancer [28], diabetes [29], and infectious diseases [30].
In this review, we describe the role of vanadium in different biochemical processes and its potentially toxic effects. We especially focus on recent studies that examined the enzyme switching activity of vanadium compounds for the treatment of osteogenesis; type 2 diabetes mellitus (T2DM); cancer; and pathologies caused by viruses, microorganisms, and parasites.
2. Vanadium as an Enzyme Switch
Vanadium is necessary for the activity or functionality of proteins in many species on the planet. Some vanadium-using species have highly effective and specialized protein-dependent vanadium absorption and transport mechanisms [31]. Under physiological settings, vanadium compounds in biological systems can quickly interconvert, and multiple vanadium-containing moieties can form and bind to proteins. These interactions are crucial for the form that is transported in the circulation, cell uptake, inhibitory characteristics, and mode of action of essential and pharmacologically active vanadium species [6]. Vanadium complexes conduct biological activities through their ability to activate or inactive certain enzymes [11,32]. Vanadate (VO4)3− structure is remarkably like that of phosphate (PO4)−3 and has similar chemistry. For instance, (H2PO4)− has a pKa = 7.2, whereas (H2VO4)− has a pKa = 7.8 [30]. Phosphorous can typically be coordinated by more than five ligands, while vanadium can accept a maximum of six donor atoms. Nevertheless, both participate in esterification-type reactions, demonstrating that vanadate can be regarded as a phosphate competitor. Indeed, if vanadate replaces phosphate in an enzyme readily, it can form stable complexes with the enzyme target, thus inhibiting the enzyme [33]. Accordingly, some vanadium complexes activate and/or inhibit several enzymes, including phosphatases, ATPases, nucleases, and kinases, among others [30].
The ability of vanadium to activate enzymes is mostly attributed to complexing with the ligand. For instance, the anionic form of vanadium (VO4)−3 activates glucose-6-phosphate dehydrogenase in mammalian cells. Moreover, vanadate compounds activate the tyrosine kinases p56Ick and p59fyn [34,35]. They also activate protein kinase B (PKB) or Akt, as well as extracellular-signal-regulated kinases (ERKs), which are involved in antilipolytic and cell cycle regulation processes (Figure 2).
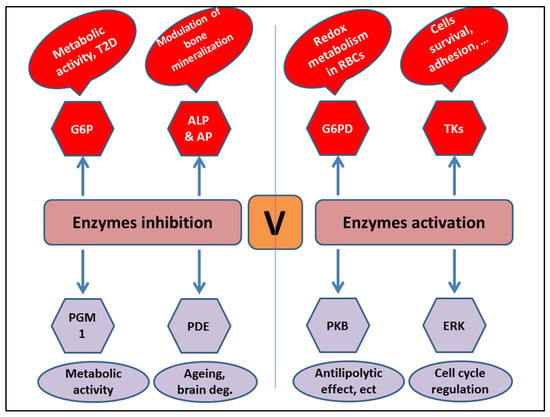
Figure 2.
Main enzyme targets of vanadium complexes.
Conversely, vanadium can inhibit several enzymes, such as alkaline phosphatase (ALP) and acid phosphatase (AP), both of which are involved in bone mineralization [36,37], as well as glucose-6-phosphatase (G6P) [38,39], phosphodiesterases [40,41], and phosphoglucomutase [42,43] (Figure 2). Together, these findings suggest that vanadium complexes control numerous biological processes.
Vanadium and its compounds stimulate the biosynthesis of glycogen and lipids in muscle, liver, and adipose tissues. The insulin-mimetic action of vanadium is mediated by the inhibition of gluconeogenesis through blocking of phosphoenol pyruvate carboxykinase (PEPCK) [33] and G6P [38] and inhibition of lipolytic pathways [22]. Vanadium reverses peripheral insulin resistance and improves glucose uptake by perpetuating the nitric oxide (NO)/cGMP/protein kinase (PKG) signaling pathway through inhibition of phosphodiesterases [40].
Vanadium inhibits protein tyrosine phosphatases (PTPs) by generating reactive oxygen species (ROS) [44], and suppresses the dephosphorylation of tyrosine residues of the beta subunit of insulin receptors [45]; this ability is due to the structural homology of the vanadate group with the phosphate group [46,47]. The inhibition of protein tyrosine phosphatase 1B (PTP-1B) results in the activation of insulin receptor and phosphorylation of insulin substrate 1 (IRS-1), leading to activation of the 3-phosphatidylinositol kinase/Akt protein kinase (PI3K-Akt) pathway [48], which in turn increases the number of glucose-type 4 (GLUT4) transporters, and thus their translocation [49]. Moreover, the inhibitory effect of vanadium on PTP results in the increased activity of insulin-like growth factors, which in turn leads to the stimulation of glycogenesis, impairment of the gluconeogenic pathway, and increased production of GLUT4 [50]. Furthermore, it has been reported that the interaction of vanadium with cell membranes results in the stabilization of vanadium complexes and produces changes in membrane proteins that may further contribute to the insulin-mimetic action [51].
3. Insulin-Mimetic Activity of Vanadium Compounds
Metabolic disorders lead to obesity, diabetes, and insulin resistance. T2DM accounts for approximately 90% of global diabetes cases (463 million in 2019; 578 million are estimated by 2030 [52]). It is a multifaceted disorder characterized by impaired insulin secretion, diminished cellular response to insulin, increased glucose production, and reduced peripheral glucose uptake [53]. Current drug therapies reverse the blood glucose increase, but do not prevent the disease progression.
The antihyperglycemic effect of vanadium was first reported in diabetic patients more than 100 years ago [54]. In vivo and in vitro studies have shown that vanadium compounds have metabolic effects on glycemic control in diabetic patients similar to that of insulin [55]. The role of the red blood cells and serum in vanadium transport, the role of GIT in vanadium absorption, and the role of cell components such as ATP and glutathione that exert redox reaction and complex formation with vanadium have been reported to integrate the vanadium insulin mimetic activity [56]. In addition, the binding liability of vanadium and blood components such as albumin and human serum apo-transferrin have been reported as potential transporters of vanadium to the target cells [57,58]. At a physiological ratio, the majority of the VO2+ ion is present as (VO) in human serum apo-transferrin and a little amount is present as (VO) in human serum albumin, according to research by Sanna et al. Transferrin is a stronger binder to vanadium than albumin in aqueous solution [7]. The complex bis(sallixinato) oxovanadium(IV) (Figure 3A) is a potent anti-diabetic agent, presented by the Adachi group [59]. They used both in vitro and in vivo studies to investigate the antidiabetic potency of bis(3-hydroxy-4-pyronato)oxovanadium(IV) and bis(allixinato)oxovanadium(IV) complexes with VO(O4). These complexes contain allixin, a garlic component high in in vitro insulin-mimetic activity in terms of both free fatty acid (FFA)-release inhibitory and glucose-uptake enhancing activity in isolated rat adipocytes. Second-generation pyrone derivatives, such as bis((5-hydroxy-4-oxo-4H-pyran-2-yl)methylbenzoatato)oxovanadium(IV) (BBOV), which are less toxic than bis (maltolato) oxovanadium (BMOV), have been developed and can be administered at 1000-fold lower doses than their first generation counterparts [60].
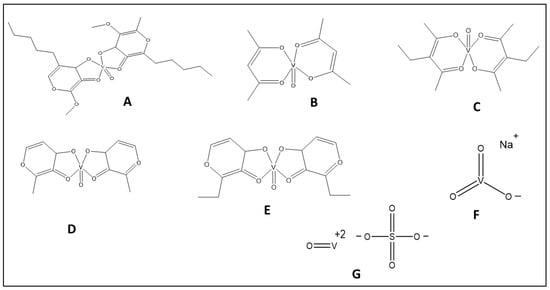
Figure 3.
The structure of vanadium complexes with insulin-mimetic activity. (A) Bis(sallixinato) oxovanadium. (B) Vanadyl acetylacetonate. (C) Vanadyl 3-ethylacetylacetonate. (D) Bis(maltolato) oxovanadium. (E) Bis(2-ethyl-3-hydroxy-4-pyronato)oxovanadium(IV). (F) Sodium metavanadate. (G) Vanadyl sulfate.
Vanadium compounds, such as vanadyl acetylacetonate (Vac; Figure 3B), vanadyl 3-ethylacetylacetonate (Vet; Figure 3C), and BMOV (Figure 3D), show better glycemic control and restoration of the hepatic glycolytic pathway by upregulating and downregulating the mRNA expression of glucokinase and L-type pyruvate kinase and phosphoenolpyruvate carboxykinase (PEPCK), respectively, compared with vanadium sulfate in diabetic rat hepatocytes [61]. Further, several other organic complexes of vanadium such as bis(2-ethyl-3-hydroxy-4-pyronato)oxovanadium(IV) (BEOV; Figure 3E) and bis(maltolato) oxovanadium also exhibited improved antidiabetic effects compared with inorganic salts against streptozocin (STZ)-induced diabetes in rats [62,63]. Although BEOV is the first vanadium complex that entered clinical trials, its testing was suspended because of renal problems in some of the treated patients [64].
The oral administration (125 mg/day) of sodium metavanadate, which refers to mixtures such as [H2VO4]−, [H2V2O7]2−, and [V4O12]4− (Figure 3F) [47], in insulin-dependent diabetes mellitus patients (IDDM) and non-insulin-dependent diabetes mellitus (NIDDM) patients for 2 weeks led to a 3.9-fold increase in the basal activity of mitogen-activated protein kinase (MAPK), a reduction in the cholesterol level in both groups, and a reduction in the insulin requirement in IDDM patients [65]. Another study showed that sodium metavanadate moderately decreased blood glucose levels in two out of three diabetic patients without adverse effects [55].
Rehder et al. tested the insulin mimetic characteristics of 41 vanadium compounds and found that these complexes exerted toxic effects at a dose of 1 mM. However, these compounds exerted insulin mimetic activity similar to or more potent than insulin with a non-toxic effect at the concentration of 0.01 mM or below [66]. In addition, insulin mimetic activity of three vanadium complexes, [((papy)(VO))2 μ-O)], [(glysal)VO(H2O)], and [(salam)2VO], has been examined. Over a pH range of 2–10, the speciation of complexes [((papy)(VO))2 μ-O)] and [(glysal)VO(H2O)] was investigated. Only complexes [(glysal)VO(H2O)] and [(salam)2VO] display an insulin-mimetic action, while [((papy)(VO))2 μ-O)] exhibits better stability over the entire pH range [67].
A randomized placebo-controlled clinical trial involving T2DM patients given 100 mg sodium monovanadate once a day for six weeks found no significant reduction in fasting blood glucose (FBS), glycosylated hemoglobin (HbA1C), total cholesterol (TC), or low-density lipoproteins (LDLs) [68]. On the other hand, vanadyl sulfate (Figure 3G) orally administered for 6 weeks in 16 T2DM patients lowered FBG, HbA1c, and total TC without any significant change in hemodynamic parameters. In addition, the treatment produced a significant increase in the insulin-mediated activation of insulin receptors, Shc, IRS-1 protein kinase, and PI3K, without an increase in insulin secretion [69]. Vanadyl sulfate has also been reported to improve carbohydrate and lipid metabolism [70] and hepatic and peripheral insulin sensitivity in NIDDM patients [71], except in nondiabetic individuals with obesity [72]. A recent study showed that vanadyl sulfate (25 mg/kg) not only produced a significant reduction in blood glucose and insulin secretion, but also alleviated oxidative stress and inflammatory markers in renal tissues of type 2 diabetic rats [73]. However, some studies reported negative effects of vanadium sulfate therapy. For example, 0.25 mg/kg and 1.2 mg/kg vanadyl sulfate for 24 weeks produced a dose-dependent reduction in blood glucose and insulin levels, but had a negative effect on the lipid profile, as evidenced by the significant decrease in high-density lipoprotein c (HDL-c) and increase in LDL-c and triacylglycerols (TGs) in healthy rats [74]. Another study showed that vanadyl sulfate administration (50 mg twice daily for 4 weeks) produced a significant increase in serum triglyceride without an improvement in insulin sensitivity in obese type 2 diabetic patients with impaired glucose tolerance compared with placebo [75]. Finally, Jakusch et al. have shown that vanadium ion exerts antidiabetic activity. However, the role of drug candidate ligands of the original vanadium complexes is that of a carrier function until the vanadium is taken up into the serum [12]. The breakdown of the initially neutral VO(IV) molecules and subsequent ternary complex formation with endogenous or foreign ligands in the organism significantly alters their ability to be absorbed. Transferrin plays a crucial function in transferring VO(IV) to the cell by displacing the carrier ligands from the VO(IV) molecules during transport in the bloodstream [76]. The dissociation of vanadium complexes prior to antidiabetic activity also supports the activity of the vanadium ion itself and limited the ability of the complexes to enhance the absorption of the free active metabolite, vanadium in the oxidation state (VV) [56].
4. Osteogenic Activity of Vanadium Compounds
In animals, vanadium is mainly stored in bones and has a positive effect on osteogenesis owing to its growth-factor-mimicking properties [77,78]. Therefore, vanadium compounds have been investigated for their osteogenic activity. Experimental studies on osteosarcoma epithelial (UMR106) cells showed that vanadium derivatives (vanadate, vanadyl, and vanadium peroxide complexes) improve cell differentiation and proliferation and enhance alkaline phosphatase enzyme activity [79].
Furthermore, vanadium derivatives stimulate two insulin signaling pathways, PI3K and MAPK, responsible for bone mineralization, bone density, and bone formation in mammalians [80]. Two bone-derived cell lines (Vertebra Sparus aurata; VSa13 and VSa16) treated with metavanadate and decavanadate (Figure 4A) solutions exhibited similar effects as treatments with insulin and IGF-1 in the short-term; namely, VSa13 cell proliferation and extracellular matrix mineralization inhibition [80].
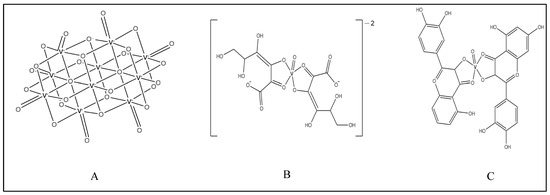
Figure 4.
Structures of vanadium complexes of (A) decavanadate, (B) 2,3-diketogulonic acid, and (C) quercetin.
Vanadyl(IV) complex of 2,3-diketogulonic acid (Figure 4B) was found to allow proliferation of UMR106, but not mouse-calvaria-derived cells (MC3T3E1) [81]. It also activated type-I collagen production in osteoblasts and activated P-ERK in a dose-dependent manner. The effects on the osteoblasts were partially inhibited by a PI3K inhibitor (wortmannin) and calcium channel blocker (nifedipine).
Nano vanadium dioxide (VO2) deposited on biomedical titanium demonstrated osteogenic activity in rat mesenchymal stem cells by regulating ROS levels [82].
In a recent study, the titanium aluminium vanadium (TiAl6V4) complex enhanced the osteogenic differentiation and adhesion of osteoblasts and mesenchymal stem and progenitor cells [83].
A vanadium complex with quercetin (Figure 4C) exhibited osteogenic effects by stimulating type I collagen production and ERK phosphorylation in a dose–response manner [84]. Vanadium-loaded collagen scaffolds have been fabricated for osteochondral tissue engineering purposes. These scaffolds exerted better adhesion, growth, osteoblastic, and chondrocytic differentiation compared with no loading in rat bone marrow progenitor cells [85].
5. Anticancer Potency
Vanadium compounds have various cancer treating properties, where different cancer pathways may be targeted, such as MAPK/ERK and PI3K/AKT, or caspase signaling pathways [86]. The anticancer mechanism includes defensive effects against chemical carcinogenesis and as inhibitors of cancer cell metastatic potential by reversing drug resistance and changing cellular adhesion [87]. Moreover, it could be the activation of xenobiotic enzymes, inhibition of PTPs and/or activation of protein tyrosine kinases, production of free radicals, and DNA cleavage [87].
The first vanadyl complexes shown to have anticancer activity against several cell lines were vanadocene dichloride and its derivatives, with IC50 values lower than cisplatin [88]. METVAN ([VO(SO4)(Me2phen)2], where Me2phen is 4,7-dimethyl-1,10-phenanthroline) also showed practical activity at low concentrations (micromolar or nanomolar) against many cell lines [89]. Moreover, antitumor properties of the V(IV)O complexes of flavonoids, such as quercetin, morin, chrysin, and silibinin, were found for osteosarcoma and breast cancer cell lines [20]. The antiproliferative activity against ovarian (A2780) and prostate (PC3) human cancer cells for three aroylhydrazones and the corresponding vanadium complexes (one oxido and two nonoxido compounds) were evaluated by Dinda’s group [90]. This work showed a significant inhibition of [VVO(L1)(OEt)] among the tested complexes. Moreover, the measured cytotoxicity values were better than the free ligand owing to the presence of several species such as VVO2, VIVO, and VIV in the aqueous solution, which gave more ability to bind to the model proteins, ubiquitin (Ub) and lysozyme (Lyz). In addition, four different vanadium species have been examined for their antiproliferative potency against two malignant melanoma cell lines, A375 and CN-mel cells [91]. These four species, namely, inorganic anion vanadate, [VIVO(1,2-dimethyl-3-hydroxy-4(1H)-pyridinonate)2], [VIVO(1-methyl-3-hydroxy-4(1H)-pyridinonate)2], and [VIVO(1-phenyl-2-methyl-3-hydroxy-4(1H)-pyridinonate)2], have exerted marked cytotoxic effects in a dose-dependent manner and showed IC50 values of 2.4 to 14 μM [91].
Schiff base oxidovanadium(IV) complexes were studied in vitro for their anti-cancer effects [92] on hepatocellular carcinoma (HepG2), human hepatic carcinoma cells (Hep3B), breast adenocarcinoma (MCF7), and colon carcinoma (HCT-116) cell lines. The DSHNVO complex (Figure 5A) showed a higher cytotoxic effect compared with the free ligand, with the biggest effect on MCF-7 [92]. The complexes presented in Figure 5B have considerable cytotoxicity on Hep3B and remain stable for 3 days in cell culture media [93].
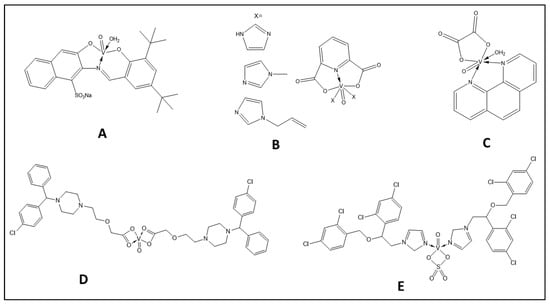
Figure 5.
The structure of some vanadium complexes with anticancer activity.
Recently, Nunes et al. has reported the potential anticancer effect of Copper(II) and oxidovanadium (IV) complexes of chromone Schiff bases that effectively produce genotoxic damage, elevate ROS production, and promote cell death by apoptosis in various human cancer cell lines (breast, brain, cervix, and ovary) preferentially compared with normal cells [94]
Mixed–ligand oxidovanadium(IV) compounds, such as complex ([VO(ox)(phen)(H2O)] (ox = oxalic acid dihydrates and phen = 1,10-phenanthroline)), show high cytotoxicity against SMMC-7721 and HepG2 cells with IC50 values of 5.34 and 29.07 μM, respectively [95] (Figure 5C).
Introducing metal ions reduces the toxicity of free drugs. Additionally, metal ions can enhance the lipophilicity of the drugs to penetrate the cell and increase their absorbance and stability [96]. Four approved drugs, sulfonamide (antibacterial), cetirizine (antihistamine), carbimazole (antithyroid), and lornoxicam (anti-inflammatory), have been used as ligands to form oxidovanadium(IV) complexes with potential anticancer activity in colon cancer cells (3IG7) [97]. Cetirizine-based oxidovanadium (IV) complex (Figure 5D) showed greater selectivity and potential than cisplatin, cleaving DNA with a predicted binding constant of Kb = 1.40 × 106 M−1.
Imidazole derivatives have remarkable pharmaceutical potencies. They can bind to DNA and proteins with multiple modes of interaction such as electrostatic, intercalation, and groove binding [98]. Basaleh et al. [99] used three imidazole derivatives to synthesize novel vanadyl-based drug complexes from clotrimazole (CTNZ), miconazole (MNZ), and pantoprazole (PNZ). The investigated complexes bind to DNA non-covalently, and the [VO(SO4)(MNZ)2] H2O complex (Figure 5E) has a promising binding constant. Remarkably, all obtained metal complexes strongly inhibited HepG-2 and human breast cancer (HCF-7) cell lines.
Mono-oxovanadium(V) complex with mixed ligands, tridentate hydrazine, and benzhydroxamic acid have shown significant inhibitory activity towards MCF-7 with an IC50 = 6.0 μM (cisplatin had IC50 = 22.8 μM under the same experimental condition) [100].
7. Vanadium Compounds’ Effect in the Cardiovascular System
Many studies have proposed different mechanisms for the cardioprotective effect of vanadium compounds. The intracellular responses to decavanadates and monomeric vanadium complexes are different [120]. However, monomeric vanadate is still released following decavanadate treatment in biological systems and will contribute to decavanadate-induced effects on PTPs [121,122]. There is an expanding repertoire of compounds containing monomeric vanadium coordinated by different classes of organic ligands, including but not limited to maltolato vanadium compounds, vandocenes, and peroxidovanadium complexes [123]. One key advantage of these larger complexes with organic ligands seems to be improved bioavailability in vivo compared with vanadate [55,62]. However, increasing evidence now suggests that these larger compounds often undergo a complex range of ligand exchange events once in circulation and/or in the cell, ultimately releasing uncomplexed vanadate as the active PTP-inhibiting moiety [124].
In general, the cardioprotective activity of vanadium compounds appears to be linked to their hypoglycemic, hypolipidemic, antiapoptotic, and antihypertensive properties. Furthermore, their inhibition of cardiac hypertrophy and control of vascular cell contraction may play an essential role [20]. Table 1 shows the effect of some vanadium compounds and their mode of action on different models.

Table 1.
Cardioprotective effects of some reported vanadium compounds.
8. Vanadium Toxicity
The cationic and anionic forms of vanadium are considered to be toxic, with the latter having higher toxicity [134]. Vanadium toxicity depends on the vanadate form, oxidation state, time, dose and route of intoxication [134,135]. Accumulation of vanadium may be fatal and lead to irreversible tissue damage due to the inhibition of enzymatic processes and oxidative stress, which lead to carcinogenesis, neurotoxicity, gastrointestinal complications, infertility, lung and kidney toxicity [136].
Animal studies have shown that subacute and chronic administration of vanadate leads to different toxic effects. A six-week administration of sodium metavanadate (Figure 3F; 1.25 mg/mL concentration) led to hematologic toxicity manifested as a reduction in body weight, fluid, and food intake, and a decrease in hematologic parameters such as red blood cell (RBC) count, hemoglobin (Hb), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) [137].
Dose-dependent toxicity of sodium metavanadate was reported in a rat model following its supplementation in drinking water for 12 weeks. These experiments showed neurobehavioral changes, demonstrated by an impairment in learning and memory, the decline in striatal acetylcholine (ACh) and 5-hydroxytryptamine (5-HT), an increase in gamma-aminobutyric acid (GABA) levels, and degenerative changes in the histopathology of the striatum [138].
The oral administration of vanadyl sulfate (Figure 3G) to pregnant mice provoked a range of toxic effects including maternal toxicity, embryotoxicity, fetal toxicity, and teratogenic potential. Maternal toxicity was evidenced by a decrease in body weight by the 18th day of gestation, a significant reduction in absolute weight of the liver and kidney, and a significant decrease in early resorption per litter. Moreover, fetal toxicity was evidenced by reduced fetal weight and fetal length [139].
Recent studies in murine models showed that 1 h per week inhalation of vanadium pentoxide for 4 weeks (Figure 7A; 0.02 M in saline) produced a negligible reduction in corporal weight, but a significant increase in uterine thickness and reduction in estrous cycling and serum progesterone levels in CD-1 mice [140]. Contrary to these findings, it has been reported that there are no significant hematological, biochemical, or histopathologic changes following the administration of vanadium for 5–12 months [141]. However, there is a significant reduction in systemic blood pressure and an increase in serum insulin in experimentally induced hypertensive rats [142].
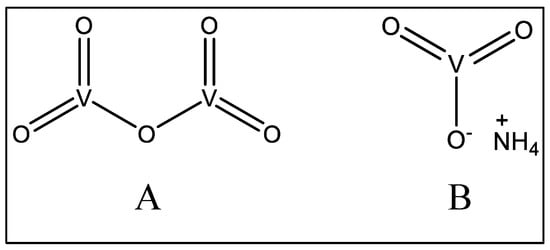
Figure 7.
Structures of some vanadate compounds.
The brains of experimental animals exposed to vanadium showed morphological changes in neurons and astroglia cells [143]. The brain morphological alterations were attributed to increased oxidative stress in the presence of vanadium complexes in the cerebellum and hippocampus [144]. Moreover, vanadium compounds induced the proteolytic activation of caspase-3-dependent protein kinase C gamma (PKC-δ) and apoptosis via neurotoxicity in dopaminergic neuronal cells [145]. Spatial memory impairment in the experimental animals was attributed to changes in the hippocampus neuropiles and loss of dendritic spines [144].
Vanadium inhalation in mouse models increased thrombogenesis and enhanced platelet production (attributed to megakaryocytic proliferation) [146]. Long-term vanadium treatment in normal and pre-diabetic STZ rats led to bradycardia and a smaller leukocyte count in the peripheral blood of STZ-induced diabetic animals [142]. Moreover, five-months’ administration of vanadyl sulfate to the streptozotocin-diabetic rat enhanced morphological alterations in kidneys [141,147].
The gastrointestinal (GI) system is most vulnerable to vanadium toxicity following inhalational or oral exposure. The ingestion of >14 mg of vanadium in humans causes GI symptoms [72]. The oral administration of vanadium salts often results in GI symptoms, such as abdominal cramps, diarrhea, vomiting, and weight reduction, in NIDDM patients with heart disease [69]. Oral administration of vanadium salts often results in gastrointestinal symptoms such as abdominal cramps, diarrhea, vomiting, and weight reduction in NIDDM patients with heart disease [69]. However, only slight GI distress was reported in diabetic patients with cardiovascular disorders following short-term treatment with vanadium derivatives [135]. Moreover, there was no hematologic alteration in blood cell count or biochemical markers for the liver and kidney after a three-month test period in athletes [148,149]. However, the continuous administration of vanadium may result in tissue accumulation accompanying the side effects in clinical settings. Experimental studies revealed that metal chelating agents, such as deferoxamine(N′-[5-(Acetyl-hydroxy-amino)pentyl]-N-[5-[3-(5-aminopentyl-hydroxy-carbamoyl) propanoylamino]pentyl]-N-hydroxy-butane diamide) and tiron (Disodium 4,5-dihydroxybenzene-1,3-disulfonate), significantly reduce vanadium accumulation in the kidney, bone, and liver [63].
The fatal intoxication of ammonium vanadate (Figure 7B) has been documented [150] in a 24-year-old woman admitted to the emergency department and presenting several GI tract complications, including pain, nausea, vomiting, and diarrhea, as well as hypoglycemia and severe acute renal failure with glomerular filtration rate.
Finally, people exposed to vanadium dust through work or other daily activities manifest respiratory system complications, such as cough, sputum, ENT (ear, nose, and throat) inflammation, exertional dyspnea, and wheezing [151]. Chinese workers exposed to vanadium showed increased negative emotions (e.g., excessive anger, depression, tiredness, and inertia) and decreased positive emotions (coordination, short-term memory, and interaction with others) [144].
9. Conclusions
Like other transition metals, such as copper, zinc, and iron, vanadium plays significant roles in many biological processes. More information is available on those other transition metals regarding their biological effects, and less attention has been given to vanadium for the synthesis of new complexes as candidate therapeutics. In this review, we highlighted the unique chemical structures and chemical properties of vanadium complexes as well as reviewed their significant biological effects.
Vanadium is a toxic metal ion, and its toxicity in humans mostly depends on the nature of its metal complexes (organic or inorganic), oxidation state, exposure route, time, and dose. However, vanadium compounds show beneficial effects as insulin-mimetic formulas in type 2 diabetes and enhance osteogenic processes in bone formation and mineralization. Moreover, selected vanadium compounds inhibit cell proliferation and thus could have anticancer potential. The distinct vanadium biochemistries of viruses, microorganisms, and parasites could be exploited to selectively target human pathogens with certain vanadium compounds. More study of vanadium biochemistry could lead to a new era in vanadium biomedicine.
Author Contributions
Conceptualization, A.A.S. and A.-H.E.; methodology, A.A.S. and A.A.S.; software, F.M.; writing—original draft preparation, I.M.A.-Y., H.A.M., M.D., F.M., H.A.A., M.J.A., K.A.Q., M.A.H., M.A., M.J., N.A.; writing—review and editing, J.I.L., A.-H.E., M.J., H.A.M., K.A.Q. and A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Peter Karagiannis, King Abdullah University of Science and Technology (KAUST), Core Labs, for his valuable comments and edits. M.J. would like to thank KAUST for financial support (baseline no. BAS/1/1085-01-01).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fontani, M.; Costa, M.; Orna, M.V. The Lost Elements: The Periodic Table’s Shadow Side; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Barceloux, D.G.; Barceloux, D. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Caswell, L.R. Andrés del Río, Alexander Von Humboldt, and the twice-discovered element. Bull. Hist. Chem. 2003, 28, 35–41. [Google Scholar]
- Pessoa, J.C. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J. Snowball Earth to Global Warming: Coupled vanadium-carbonaceous deposits in the Cryogenian-Cambrian. Ore Geol. Rev. 2022, 145, 104876. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Santos, M.F.; Correia, I.; Sanna, D.; Sciortino, G.; Garribba, E. Binding of vanadium ions and complexes to proteins and enzymes in aqueous solution. Coord. Chem. Rev. 2021, 449, 214192. [Google Scholar] [CrossRef]
- Sanna, D.; Micera, G.; Garribba, E. On the transport of vanadium in blood serum. Inorg. Chem. 2009, 48, 5747–5757. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Sakurai, H.; Crans, D.C.; Micera, G.; Garribba, E. Structural and redox requirements for the action of anti-diabetic vanadium compounds. Dalton Trans. 2014, 43, 6965–6972. [Google Scholar] [CrossRef]
- Maurya, M.R.; Kumar, A.; Pessoa, J.C. Vanadium complexes immobilized on solid supports and their use as catalysts for oxidation and functionalization of alkanes and alkenes. Coord. Chem. Rev. 2011, 255, 2315–2344. [Google Scholar] [CrossRef]
- Maurya, M.R.; Uprety, B.; Avecilla, F.; Adão, P.; Pessoa, J.C. Vanadium (V) complexes of a tripodal ligand, their characterisation and biological implications. Dalton Trans. 2015, 44, 17736–17755. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef]
- Jakusch, T.; Pessoa, J.C.; Kiss, T. The speciation of vanadium in human serum. Coord. Chem. Rev. 2011, 255, 2218–2226. [Google Scholar] [CrossRef]
- Correia, I.; Pessoa, J.C.; Duarte, M.T.; Da Piedade, M.F.M.; Jackush, T.; Kiss, T.; Castro, M.M.C.; Geraldes, C.F.; Avecilla, F. Vanadium (IV and V) complexes of Schiff bases and reduced Schiff bases derived from the reaction of aromatic o-hydroxyaldehydes and diamines: Synthesis, characterisation and solution studies. Eur. J. Inorg. Chem. 2005, 732–744. [Google Scholar] [CrossRef]
- Buglyó, P.; Culeddu, N.; Kiss, T.; Micera, G.; Sanna, D. Vanadium (IV) and vanadium (V) complexes of deferoxamine B in aqueous solution. J. Inorg. Biochem. 1995, 60, 45–59. [Google Scholar] [CrossRef]
- Del Carpio, E.; Hernández, L.; Ciangherotti, C.; Coa, V.V.; Jiménez, L.; Lubes, V.; Lubes, G. Vanadium: History, chemistry, interactions with α-amino acids and potential therapeutic applications. Coord. Chem. Rev. 2018, 372, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, N.S.; Sharfalddin, A.A.; Domyati, D.; Basaleh, A.S.; Hussien, M.A. Experiment versus theory of copper (II) complexes based imidazole derivatives as anti-cancer agents. J. Indian Chem. Soc. 2022, 99, 100692. [Google Scholar] [CrossRef]
- Babgi, B.A.; Alsayari, J.; Alenezi, H.M.; Abdellatif, M.H.; Eltayeb, N.E.; Emwas, A.-H.M.; Jaremko, M.; Hussien, M.A. Alteration of anticancer and protein-binding properties of gold (I) Alkynyl by phenolic Schiff bases moieties. Pharmaceutics 2021, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, J.I.; Pichiri, G.; Piludu, M.; Fais, S.; Orrù, G.; Congiu, T.; Piras, M.; Faa, G.; Fanni, D.; Dalla Torre, G. Thymosin β4 Is an Endogenous Iron Chelator and Molecular Switcher of Ferroptosis. Int. J. Mol. Sci. 2022, 23, 551. [Google Scholar] [CrossRef]
- Basaleh, A.S.; Howsaui, H.B.; Sharfalddin, A.A.; Hussien, M.A. Substitution effect on new Schiff base ligand in complexation with some divalent Metal ion; Synthesis, Characterization, DFT and Cytotoxicity Studies. Results Chem. 2022, 4, 100445. [Google Scholar] [CrossRef]
- Barrio, A.D.; Etcheverry, B.S. Potential use of vanadium compounds in therapeutics. Curr. Med. Chem. 2010, 17, 3632–3642. [Google Scholar] [CrossRef]
- Semiz, S. Vanadium as potential therapeutic agent for COVID-19: A focus on its antiviral, antiinflamatory, and antihyperglycemic effects. J. Trace Elem. Med. Biol. 2022, 69, 126887. [Google Scholar] [CrossRef]
- Brownsey, R.W.; Dong, G.W. Evidence for selective effects of vanadium on adipose cell metabolism involving actions on cAMP-dependent protein kinase. In Vanadium Compounds: Biochemical and Therapeutic Applications; Springer: Berlin, Germany, 1995; pp. 131–137. [Google Scholar]
- Clark, A.S.; Fagan, J.; Mitch, W. Selectivity of the insulin-like actions of vanadate on glucose and protein metabolism in skeletal muscle. Biochem. J. 1985, 232, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A. Overview of Research on Vanadium-Quercetin Complexes with a Historical Outline. Antioxidants 2022, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-D.; Zhang, G.-H.; Xu, R.; Wang, Y.; Chou, K.-C. Fabrication of pure V2O3 powders by reducing V2O5 powders with CO-CO2 mixed gases. Ceram. Int. 2019, 45, 2117–2123. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Emwas, A.H.; Jaremko, M.; Hussien, M.A. Synthesis and Theoretical Calculations of Metal–Antibiotic Chelation with Thiamphenicol; In vitro DNA and HSA Binding, Molecular Docking, and Cytotoxic Studies. N. J. Chem. 2021, 45, 9598–9613. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Emwas, A.-H.; Jaremko, M.; Hussien, M.A. Complexation of uranyl (UO2) 2+ with bidentate ligands: XRD, spectroscopic, computational, and biological studies. PLoS ONE 2021, 16, e0256186. [Google Scholar] [CrossRef]
- García-García, A.; Noriega, L.; Meléndez-Bustamante, F.J.; Castro, M.E.; Sánchez-Gaytán, B.L.; Choquesillo-Lazarte, D.; González-Vergara, E.; Rodríguez-Diéguez, A. 2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity. Inorganics 2021, 9, 67. [Google Scholar] [CrossRef]
- Lima, L.M.; Belian, M.F.; Silva, W.E.; Postal, K.; Kostenkova, K.; Crans, D.C.; Rossiter, A.K.F.; da Silva Júnior, V.A. Vanadium (IV)-diamine complex with hypoglycemic activity and a reduction in testicular atrophy. J. Inorg. Biochem. 2021, 216, 111312. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Garribba, E.; Santos, M.F.; Santos-Silva, T. Vanadium and proteins: Uptake, transport, structure, activity and function. Coord. Chem. Rev. 2015, 301, 49–86. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Biochemical and medical importance of vanadium compounds. Acta Biochim. Pol. 2012, 59, 195–200. [Google Scholar] [CrossRef]
- Kiersztan, A.; Modzelewska, A.; Jarzyna, R.; Jagielska, E.; Bryła, J. Inhibition of gluconeogenesis by vanadium and metformin in kidney-cortex tubules isolated from control and diabetic rabbits. Biochem. Pharmacol. 2002, 63, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Fantus, I.G.; Kadota, S.; Deragon, G.; Foster, B.; Posner, B.I. Pervanadate [peroxide (s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry 1989, 28, 8864–8871. [Google Scholar] [CrossRef] [PubMed]
- Schieven, G.L.; Kirihara, J.M.; Myers, D.E.; Ledbetter, J.A.; Uckun, F.M. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood 1993, 5, 1212–1220. [Google Scholar] [CrossRef]
- Boruah, J.J.; Kalita, D.; Das, S.P.; Paul, S.; Islam, N.S. Polymer-anchored peroxo compounds of vanadium (V) and molybdenum (VI): Synthesis, stability, and their activities with alkaline phosphatase and catalase. Inorg. Chem. 2011, 50, 8046–8062. [Google Scholar] [CrossRef] [PubMed]
- Parente, J.E.; Naso, L.G.; Jori, K.; Franca, C.A.; da Costa Ferreira, A.M.; Williams, P.A.; Ferrer, E.G. In vitro experiments and infrared spectroscopy analysis of acid and alkaline phosphatase inhibition by vanadium complexes. N. J. Chem. 2019, 43, 17603–17619. [Google Scholar] [CrossRef]
- Shehzad, S. The potential effect of vanadium compounds on glucose-6-phosphatase. Biosci. Horiz. Int. J. Stud. Res. 2013, 6, hzt002. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, S.M. Synthesis, characterization, antioxidant and anti-diabetic activities of a novel protein–vanadium complex. Appl. Organomet. Chem. 2019, 33, e5102. [Google Scholar] [CrossRef]
- Ashiq, U.; Jamal, R.A.; Mahroof-Tahir, M.; Maqsood, Z.T.; Khan, K.M.; Omer, I.; Choudhary, M.I. Enzyme inhibition, radical scavenging, and spectroscopic studies of vanadium (IV)–hydrazide complexes. J. Enzym. Inhib. Med. Chem. 2009, 24, 1336–1343. [Google Scholar] [CrossRef]
- Platt, D.C.; Rink, J.; Braich, K.; McLauchlan, C.C.; Jones, M.A. 2′-3′-Cyclic Nucleotide 3′-Phosphodiesterase Inhibition by Organometallic Vanadium Complexes: A Potential New Paradigm for Studying CNS Degeneration. Brain Sci. 2021, 11, 588. [Google Scholar] [CrossRef]
- Percival, M.D.; Doherty, K.; Gresser, M.J. Inhibition of phosphoglucomutase by vanadate. Biochemistry 1990, 29, 2764–2769. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Buglyó, P.; Crans, D.C.; Nagy, E.M.; Lindo, R.L.; Yang, L.; Smee, J.J.; Jin, W.; Chi, L.-H.; Godzala, M.E.; Willsky, G.R. Aqueous chemistry of the vanadiumIII (VIII) and the VIII− dipicolinate systems and a comparison of the effect of three oxidation states of vanadium compounds on diabetic hyperglycemia in rats. Inorg. Chem. 2005, 44, 5416–5427. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.G.; Davis, M.G.; Howard, B.W.; Pokross, M.; Rastogi, V.; Diven, C.; Greis, K.D.; Eby-Wilkens, E.; Maier, M.; Evdokimov, A. Mechanism of insulin sensitization by BMOV (bis maltolato oxo vanadium); unliganded vanadium (VO4) as the active component. J. Inorg. Biochem. 2003, 96, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. Bioinorganic Vanadium Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 30. [Google Scholar]
- Rehder, D. Vanadium in health issues. Chem.Texts 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Mehdi, M.Z.; Pandey, S.K.; Théberge, J.-F.; Srivastava, A.K. Insulin signal mimicry as a mechanism for the insulin-like effects of vanadium. Cell Biochem. Biophys. 2006, 44, 73–81. [Google Scholar] [CrossRef]
- Mohammad, A.; Sharma, V.; McNeill, J.H. Vanadium increases GLUT4 in diabetic rat skeletal muscle. Mol. Cell. Biochem. 2002, 233, 139–143. [Google Scholar] [CrossRef]
- Kenner, K.A.; Hill, D.E.; Olefsky, J.M.; Kusari, J. Regulation of protein tyrosine phosphatases by insulin and insulin-like growth factor I. J. Biol. Chem. 1993, 268, 25455–25462. [Google Scholar] [CrossRef]
- Yang, X.; Wang, K.; Lu, J.; Crans, D.C. Membrane transport of vanadium compounds and the interaction with the erythrocyte membrane. Coord. Chem. Rev. 2003, 237, 103–111. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Cantley, J.; Ashcroft, F.M. Q&A: Insulin secretion and type 2 diabetes: Why do β-cells fail? BMC Biol. 2015, 13, 1–7. [Google Scholar]
- Lyonnet, B. L’emploi therapeutique des derives du vanadium. Presse Med. 1899, 1, 191–192. [Google Scholar]
- Thompson, K.H.; Orvig, C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Jakusch, T.; Kiss, T. In vitro study of the antidiabetic behavior of vanadium compounds. Coord. Chem. Rev. 2017, 351, 118–126. [Google Scholar] [CrossRef]
- Mehtab, S.; Gonçalves, G.; Roy, S.; Tomaz, A.I.; Santos-Silva, T.; Santos, M.F.; Romão, M.J.; Jakusch, T.; Kiss, T.; Pessoa, J.C. Interaction of vanadium (IV) with human serum apo-transferrin. J. Inorg. Biochem. 2013, 121, 187–195. [Google Scholar] [CrossRef]
- Correia, I.; Jakusch, T.; Cobbinna, E.; Mehtab, S.; Tomaz, I.; Nagy, N.V.; Rockenbauer, A.; Pessoa, J.C.; Kiss, T. Evaluation of the binding of oxovanadium (IV) to human serum albumin. Dalton Trans. 2012, 41, 6477–6487. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Yoshida, J.; Kodera, Y.; Katoh, A.; Takada, J.; Sakurai, H. Bis (allixinato) oxovanadium (IV) complex is a potent antidiabetic agent: Studies on structure−activity relationship for a series of hydroxypyrone−vanadium complexes. J. Med. Chem. 2006, 49, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-B.; Yang, X.-D. Synthesis, characterization and anti-diabetic therapeutic potential of a new benzyl acid-derivatized kojic acid vanadyl complex. Biometals 2012, 25, 1261–1268. [Google Scholar] [CrossRef]
- Reul, B.A.; Amin, S.S.; Buchet, J.P.; Ongemba, L.N.; Crans, D.C.; Brichard, S.M. Effects of vanadium complexes with organic ligands on glucose metabolism: A comparison study in diabetic rats. Br. J. Pharmacol. 1999, 126, 467–477. [Google Scholar] [CrossRef]
- McNeill, J.H.; Yuen, V.; Hoveyda, H.; Orvig, C. Bis (maltolato) oxovanadium (IV) is a potent insulin mimic. J. Med. Chem. 1992, 35, 1489–1491. [Google Scholar] [CrossRef]
- Thompson, K.H.; Liboiron, B.D.; Sun, Y.; Bellman, K.D.; Setyawati, I.A.; Patrick, B.O.; Karunaratne, V.; Rawji, G.; Wheeler, J.; Sutton, K. Preparation and characterization of vanadyl complexes with bidentate maltol-type ligands; in vivo comparisons of anti-diabetic therapeutic potential. JBIC J. Biol. Inorg. Chem. 2003, 8, 66–74. [Google Scholar] [CrossRef]
- Costa Pessoa, J.; Tomaz, I. Transport of therapeutic vanadium and ruthenium complexes by blood plasma components. Curr. Med. Chem. 2010, 17, 3701–3738. [Google Scholar] [CrossRef] [PubMed]
- Goldfine, A.B.; Simonson, D.C.; Folli, F.; Patti, M.-E.; Kahn, C.R. Metabolic effects of sodium metavanadate in humans with insulin-dependent and noninsulin-dependent diabetes mellitus in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 1995, 80, 3311–3320. [Google Scholar] [PubMed]
- Rehder, D.; Costa Pessoa, J.; Geraldes, C.F.; Castro, M.M.; Kabanos, T.; Kiss, T.; Meier, B.; Micera, G.; Pettersson, L.; Rangel, M. In vitro study of the insulin-mimetic behaviour of vanadium (IV, V) coordination compounds. JBIC J. Biol. Inorg. Chem. 2002, 7, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Shteinman, A.A.; Degerman, E.; Enyedy, E.A.; Kiss, T.; Behrens, U.; Rehder, D.; Nordlander, E. Salicylamide and salicylglycine oxidovanadium complexes with insulin-mimetic properties. J. Inorg. Biochem. 2011, 105, 1795–1800. [Google Scholar] [CrossRef]
- Afkhami-Arekani, M.; Karimi, M.; Mohammadi Mohammad, S.; Nourani, F. Effect of sodium metavanadate supplementation on lipid and glucose metabolism biomarkers in type e diabetic patients. Malays J. Nutr 2008, 14, 113–119. [Google Scholar]
- Goldfine, A.B.; Patti, M.-E.; Zuberi, L.; Goldstein, B.J.; LeBlanc, R.; Landaker, E.J.; Jiang, Z.Y.; Willsky, G.R.; Kahn, C.R. Metabolic effects of vanadyl sulfate in humans with non—Insulin-dependent diabetes mellitus: In vivo and in vitro studies. Metabolism 2000, 49, 400–410. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Ruiz, J.; van Rossum, G.D.; Turco, S. Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non—Insulin-dependent diabetes mellitus. Metabolism 1996, 45, 1130–1135. [Google Scholar] [CrossRef]
- Bariyanga, J.; Luyt, A. Synthesis, Fourier transform infrared, nuclear magnetic resonance and thermal analysis of sodium and platinum complexes of 6-mercaptopurine. J. Mol. Struct. 2001, 559, 49–54. [Google Scholar] [CrossRef]
- Halberstam, M.; Cohen, N.; Shlimovich, P.; Rossetti, L.; Shamoon, H. Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes 1996, 45, 659–666. [Google Scholar] [CrossRef]
- Fereshteh, B.; Ali-Reza, A.; Nastaran, M.; Mohsen, T.; Mehdi, M.S. Evaluating the effects of vanadyl sulfate on biomarkers of oxidative stress and inflammation in renal tissue of rats with diabetes type 2. Braz. J. Pharm. Sci. 2020, 56, 18–56. [Google Scholar] [CrossRef]
- Shah, S.Z.H.; Naveed, A.K.; Rashid, A. Effects of oral vanadium on glycaemic and lipid profile in rats. J. Pak. Med. Assoc. 2016, 66, 1592–1596. [Google Scholar]
- Jacques-Camarena, O.; González-Ortiz, M.; Martínez-Abundis, E.; López-Madrueño, J.P.; Medina-Santillán, R. Effect of vanadium on insulin sensitivity in patients with impaired glucose tolerance. Ann. Nutr. Metab. 2008, 53, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Jakusch, T.; Hollender, D.; Dörnyei, Á.; Enyedy, É.A.; Pessoa, J.C.; Sakurai, H.; Sanz-Medel, A. Biospeciation of antidiabetic VO (IV) complexes. Coord. Chem. Rev. 2008, 252, 1153–1162. [Google Scholar] [CrossRef]
- Barrio, D.; Etcheverry, S. Vanadium and bone development: Putative signaling pathways. Can. J. Physiol. Pharmacol. 2006, 84, 677–686. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Cortizo, A.M.; Etcheverry, S.B. Vanadium derivatives act as growth factor—Mimetic compounds upon differentiation and proliferation of osteoblast-like UMR106 cells. Mol. Cell. Biochem. 1995, 145, 97–102. [Google Scholar] [CrossRef]
- Tiago, D.M. Role of Insulin and Insulin-like Peptides in Bone Formation: Identification of Bone-Specific Target Genes and Regulatory Mechanisms, and Characterization of the Insulin-Mimetic Effect of Vanadium; Universidade do Algarve (Portugal): Faro, Portugal, 2008. [Google Scholar]
- Cortizo, A.M.; Molinuevo, M.S.; Barrio, D.A.; Bruzzone, L. Osteogenic activity of vanadyl (IV)–ascorbate complex: Evaluation of its mechanism of action. Int. J. Biochem. Cell Biol. 2006, 38, 1171–1180. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, H.; Wang, J.; Liu, W.; Cheng, M.; Peng, X.; Qin, H.; Wei, J.; Jin, P.; Li, J. Nano vanadium dioxide films deposited on biomedical titanium: A novel approach for simultaneously enhanced osteogenic and antibacterial effects. Artif. Cells Nanomed. Biotechnol. 2018, 46, 58–74. [Google Scholar] [CrossRef]
- Lohberger, B.; Eck, N.; Glaenzer, D.; Kaltenegger, H.; Leithner, A. Surface Modifications of Titanium Aluminium Vanadium Improve Biocompatibility and Osteogenic Differentiation Potential. Materials 2021, 14, 1574. [Google Scholar] [CrossRef]
- Ferrer, E.G.; Salinas, M.V.; Correa, M.J.; Naso, L.; Barrio, D.A.; Etcheverry, S.B.; Lezama, L.; Rojo, T.; Williams, P.A. Synthesis, characterization, antitumoral and osteogenic activities of quercetin vanadyl (IV) complexes. JBIC J. Biol. Inorg. Chem. 2006, 11, 791–801. [Google Scholar] [CrossRef]
- Cortizo, A.M.; Ruderman, G.; Mazzini, F.N.; Molinuevo, M.S.; Mogilner, I.G. Novel vanadium-loaded ordered collagen scaffold promotes osteochondral differentiation of bone marrow progenitor cells. Int. J. Biomater. 2016, 2016, 9191ec. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, V.A.; León, I.E. An Overview of Vanadium and Cell Signaling in Potential Cancer Treatments. Inorganics 2022, 10, 47. [Google Scholar] [CrossRef]
- Evangelou, A.M. Vanadium in cancer treatment. Crit. Rev. Oncol./Hematol. 2002, 42, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Koepf-Maier, P.; Koepf, H. Non-platinum group metal antitumor agents. History, current status, and perspectives. Chem. Rev. 1987, 87, 1137–1152. [Google Scholar] [CrossRef]
- Narla, R.K.; Dong, Y.; Klis, D.; Uckun, F.M. Bis (4, 7-dimethyl-1, 10-phenanthroline) sulfatooxovanadium (IV) as a novel antileukemic agent with matrix metalloproteinase inhibitory activity. Clin. Cancer Res. 2001, 7, 1094–1101. [Google Scholar]
- Banerjee, A.; Dash, S.P.; Mohanty, M.; Sahu, G.; Sciortino, G.; Garribba, E.; Carvalho, M.F.N.; Marques, F.; Costa Pessoa, J.O.; Kaminsky, W. New VIV, VIVO, VVO, and VVO2 systems: Exploring their interconversion in solution, protein interactions, and cytotoxicity. Inorg. Chem. 2020, 59, 14042–14057. [Google Scholar] [CrossRef] [PubMed]
- Rozzo, C.; Sanna, D.; Garribba, E.; Serra, M.; Cantara, A.; Palmieri, G.; Pisano, M. Antitumoral effect of vanadium compounds in malignant melanoma cell lines. J. Inorg. Biochem. 2017, 174, 14–24. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Bawazeer, A.M.; Shaaban, S.; Adam, M.S.S. Targeting ctDNA binding and elaborated in-vitro assessments concerning novel Schiff base complexes: Synthesis, characterization, DFT and detailed in-silico confirmation. J. Mol. Liq. 2021, 322, 114977. [Google Scholar] [CrossRef]
- Biswal, D.; Pramanik, N.R.; Chakrabarti, S.; Drew, M.G.; Acharya, K.; Chandra, S. Syntheses, crystal structures, DFT calculations, protein interaction and anticancer activities of water soluble dipicolinic acid-imidazole based oxidovanadium (iv) complexes. Dalton Trans. 2017, 46, 16682–16702. [Google Scholar] [CrossRef]
- Nunes, P.; Yildizhan, Y.; Adiguzel, Z.; Marques, F.; Costa Pessoa, J.; Acilan, C.; Correia, I. Copper (II) and oxidovanadium (IV) complexes of chromone Schiff bases as potential anticancer agents. JBIC J. Biol. Inorg. Chem. 2022, 27, 89–109. [Google Scholar] [CrossRef]
- Ni, L.; Zhao, H.; Tao, L.; Li, X.; Zhou, Z.; Sun, Y.; Chen, C.; Wei, D.; Liu, Y.; Diao, G. Synthesis, in vitro cytotoxicity, and structure–activity relationships (SAR) of multidentate oxidovanadium (iv) complexes as anticancer agents. Dalton Trans. 2018, 47, 10035–10045. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Hussien, M.A. Bivalence Metal Complexes of Antithyroid Drug Carbimazole; Synthesis, Characterization, Computational simulation, and Biological Studies. J. Mol. Struct. 2020, 1228, 129725. [Google Scholar] [CrossRef]
- Alomari, F.Y.; Sharfalddin, A.A.; Abdellattif, M.H.; Domyati, D.; Basaleh, A.S.; Hussien, M.A. QSAR Modeling, Molecular Docking and Cytotoxic Evaluation for Novel Oxidovanadium(IV) Complexes as Colon Anticancer Agents. Molecules 2022, 27, 649. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Gong, S.; Fu, D. A spectroscopic study on the DNA binding behavior of the anticancer drug dacarbazine. Spectrosc. Lett. 2002, 35, 751–756. [Google Scholar] [CrossRef]
- Basaleh, A.S.; Alomari, F.Y.; Sharfalddin, A.A.; Al-Radadi, N.S.; Domyati, D.; Hussien, M.A. Theoretical Investigation by DFT and Molecular Docking of Synthesized Oxidovanadium (IV)-Based Imidazole Drug Complexes as Promising Anticancer Agents. Molecules 2022, 27, 2796. [Google Scholar] [CrossRef]
- Inamdar, P.; Angappan, S. DNA binding behaviour of mixed ligand vanadium (V) complex based on novel tridentate hydrazone and benzhydroxamic acid ligand systems. Appl. Organomet. Chem. 2017, 31, e3573. [Google Scholar] [CrossRef]
- Maia, P.I.d.S.; Pavan, F.R.; Leite, C.Q.; Lemos, S.S.; de Sousa, G.F.; Batista, A.A.; Nascimento, O.R.; Ellena, J.; Castellano, E.E.; Niquet, E. Vanadium complexes with thiosemicarbazones: Synthesis, characterization, crystal structures and anti-Mycobacterium tuberculosis activity. Polyhedron 2009, 28, 398–406. [Google Scholar] [CrossRef]
- Collins, F.M.; Klayman, D.L.; Morrison, N.E. Correlations between structure and antimycobacterial activity in a series of 2-acetylpyridine thiosemicarbazones. Microbiology 1982, 128, 1349–1356. [Google Scholar] [CrossRef]
- Cohen, M.D. Vanadium. In Handbook on the Toxicology of Metals; Academic Press: Cambridge, MA, USA, 2022; Volume 11, pp. 937–961. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Guo, G.; Cheng, T.; Peng, X.; Mao, X.; Li, J.; Zhang, X. A functionalized surface modification with vanadium nanoparticles of various valences against implant-associated bloodstream infection. Int. J. Nanomed. 2017, 12, 3121. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, C.; Gunvanthrao Yernale, N.; Prasad, M. Synthesis, spectral characterization, and antibacterial and antifungal studies of PANI/V2O5 nanocomposites. Int. J. Chem. Eng. 2016, 2016, 3479248. [Google Scholar] [CrossRef]
- He, L.-Y.; Qiu, X.-Y.; Cheng, J.-Y.; Liu, S.-J.; Wu, S.-M. Synthesis, characterization and crystal structures of vanadium (V) complexes derived from halido-substituted tridentate hydrazone compounds with antimicrobial activity. Polyhedron 2018, 156, 105–110. [Google Scholar] [CrossRef]
- Sheikhshoaie, I.; Ebrahimipour, S.Y.; Lotfi, N.; Mague, J.T.; Khaleghi, M. Synthesis, spectral characterization, X-ray crystal structure and antimicrobial activities of two cis dioxido-vanadium (V) complexes incorporating unsymmetrical dimalonitrile-based (NNO) Schiff base ligands. Inorg. Chim. Acta 2016, 442, 151–157. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Dong, Y.; Uckun, F.M. Potent dual anti-HIV and spermicidal activities of novel oxovanadium (V) complexes with thiourea non-nucleoside inhibitors of HIV-1 reverse transcriptase. Biochem. Biophys. Res. Commun. 2003, 302, 253–264. [Google Scholar] [CrossRef]
- Sun, R.W.-Y.; Ma, D.-L.; Wong, E.L.-M.; Che, C.-M. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Trans. 2007, 43, 4884–4892. [Google Scholar]
- Shigeta, S.; Mori, S.; Kodama, E.; Kodama, J.; Takahashi, K.; Yamase, T. Broad spectrum anti-RNA virus activities of titanium and vanadium substituted polyoxotungstates. Antivir. Res. 2003, 58, 265–271. [Google Scholar] [CrossRef]
- Scior, T.; Abdallah, H.H.; Mustafa, S.F.Z.; Guevara-García, J.A.; Rehder, D. Are vanadium complexes druggable against the main protease mpro of sars-cov-2?–a computational approach. Inorg. Chim. Acta 2021, 519, 120287. [Google Scholar] [CrossRef]
- Vlasiou, M.C.; Pafti, K.S. Screening possible drug molecules for Covid-19. The example of vanadium (III/IV/V) complex molecules with computational chemistry and molecular docking. Comput. Toxicol. 2021, 18, 100157. [Google Scholar] [CrossRef] [PubMed]
- Miloud, M.; El-ajaily, M.; Al-noor, T.; Al-barki, N. Antifungal activity of some mixed ligand complexes incorporating Schiff bases. J. Bacteriol Mycol 2020, 7, 1122. [Google Scholar]
- Maurya, M.R.; Bharti, N. Synthesis, thermal and spectral studies of oxoperoxo and dioxo complexes of vanadium (V), molybdenum (VI) and tungsten (VI) with 2-(α-hydroxyalkyl/aryl) benzimidazole. Transit. Met. Chem. 1999, 24, 389–393. [Google Scholar] [CrossRef]
- Maurya, M.R.; Haldar, C.; Khan, A.A.; Azam, A.; Salahuddin, A.; Kumar, A.; Costa Pessoa, J. Synthesis, characterization, catalytic and antiamoebic activity of vanadium complexes of binucleating bis (dibasic tridentate ONS donor) ligand systems. Eur. J. Inorg. Chem. 2012, 2012, 2560–2577. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Chidawanyika, W.; Antunes, E.; Fernandes, M.A.; Nyokong, T.; Torto, N.; Tshentu, Z.R. Oxovanadium (IV)-catalysed oxidation of dibenzothiophene and 4, 6-dimethyldibenzothiophene. Dalton Trans. 2012, 41, 13908–13918. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Machado, P.; Mota, V.Z.; de Lima Cavalli, A.C.; de Carvalho, G.S.G.; Da Silva, A.D.; Gameiro, J.; Cuin, A.; Coimbra, E.S. High selective antileishmanial activity of vanadium complex with stilbene derivative. Acta Trop. 2015, 148, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Varela, J.; Correia, I.; Birriel, E.; Castiglioni, J.; Moreno, V.; Pessoa, J.C.; Cerecetto, H.; González, M.; Gambino, D. A new series of heteroleptic oxidovanadium (IV) compounds with phenanthroline-derived co-ligands: Selective Trypanosoma cruzi growth inhibitors. Dalton Trans. 2013, 42, 11900–11911. [Google Scholar] [CrossRef] [PubMed]
- Benítez, J.; Correia, I.; Becco, L.; Fernández, M.; Garat, B.; Gallardo, H.; Conte, G.; Kuznetsov, M.L.; Neves, A.; Moreno, V. Searching for Vanadium-Based Prospective Agents against Trypanosoma cruzi: Oxidovanadium (IV) Compounds with Phenanthroline Derivatives as Ligands. Z. Anorg. Allg. Chem. 2013, 639, 1417–1425. [Google Scholar] [CrossRef]
- Tiago, T.; Martel, P.; Gutiérrez-Merino, C.; Aureliano, M. Binding modes of decavanadate to myosin and inhibition of the actomyosin ATPase activity. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2007, 1774, 474–480. [Google Scholar] [CrossRef]
- Aureliano, M. Decavanadate toxicology and pharmacological activities: V10 or V1, both or none? Oxidative Med. Cell. Longev. 2016, 2016, 22–45. [Google Scholar] [CrossRef]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286-) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1837. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Stabilities and biological activities of vanadium drugs: What is the nature of the active species? Chem. Asian J. 2017, 12, 1692–1699. [Google Scholar] [CrossRef]
- Mbatha, B.; Khathi, A.; Sibiya, N.; Booysen, I.; Mangundu, P.; Ngubane, P. Cardio-protective effects of a dioxidovanadium (V) complex in male sprague–dawley rats with streptozotocin-induced diabetes. BioMetals 2021, 34, 161–173. [Google Scholar] [CrossRef]
- Bhanot, S.; McNeill, J.H. Vanadyl sulfate lowers plasma insulin and blood pressure in spontaneously hypertensive rats. Hypertension 1994, 24, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.; Takada, Y.; Shioda, N.; Moriguchi, S.; Kasahara, J.; Fukunaga, K. Cardioprotective effect of vanadyl sulfate on ischemia/reperfusion-induced injury in rat heart in vivo is mediated by activation of protein kinase B and induction of FLICE-inhibitory protein. Cardiovasc. Drug Rev. 2008, 26, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Hashimoto, M.; Kasahara, J.; Aihara, K.; Fukunaga, K. Cytoprotective effect of sodium orthovanadate on ischemia/reperfusion-induced injury in the rat heart involves Akt activation and inhibition of fodrin breakdown and apoptosis. J. Pharmacol. Exp. Ther. 2004, 311, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, S.; Bryer-Ash, M.; Cheung, A.; McNeill, J.H. Bis (maltolato) oxovanadium (IV) attenuates hyperinsulinemia and hypertension in spontaneously hypertensive rats. Diabetes 1994, 43, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Liem, D.A.; Gho, C.C.; Gho, B.C.; Kazim, S.; Manintveld, O.C.; Verdouw, P.D.; Duncker, D. The tyrosine phosphatase inhibitor bis (maltolato) oxovanadium attenuates myocardial reperfusion injury by opening ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 2004, 309, 1256–1262. [Google Scholar] [CrossRef]
- Yuen, V.G.; Orvig, C.; Thompson, K.H.; McNeill, J.H. Improvement in cardiac dysfunction in streptozotocin-induced diabetic rats following chronic oral administration of bis (maltolato) oxovanadium (IV). Can. J. Physiol. Pharmacol. 1993, 71, 270–276. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Shioda, N.; Shibuya, M.; Iwabuchi, Y.; Fukunaga, K. Activation of endothelial nitric oxide synthase by a vanadium compound ameliorates pressure overload-induced cardiac injury in ovariectomized rats. Hypertension 2009, 53, 57–63. [Google Scholar] [CrossRef]
- Terada, Y.; Higashi, N.; Hidaka, Y.; Isomoto, Y.; Yayama, K. Protein tyrosine phosphatase inhibitor, orthovanadate, induces contraction via Rho kinase activation in mouse thoracic aortas. Biol. Pharm. Bull. 2019, 42, 877–885. [Google Scholar] [CrossRef]
- Goc, A. Biological activity of vanadium compounds. Cent. Eur. J. Biol. 2006, 1, 314–332. [Google Scholar] [CrossRef]
- Domingo, J.L. Vanadium and tungsten derivatives as antidiabetic agents. Biol. Trace Elem. Res. 2002, 88, 97–112. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; Yang, F.; Guo, X.; Peng, J.; Wang, X.; Fang, Y.; Chen, J.; Yi, X.; Cao, H. New sight into interaction between endoplasmic reticulum stress and autophagy induced by vanadium in duck renal tubule epithelial cells. Chem.-Biol. Interact. 2022, 1, 109981. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A.; Zaporowska, H.; Ostrowski, J. Selected haematological and biochemical parameters of blood in rats after subchronic administration of vanadium and/or magnesium in drinking water. Arch. Environ. Contam. Toxicol. 2006, 51, 287–295. [Google Scholar] [CrossRef]
- Sun, L.; Wang, K.; Li, Y.; Fan, Q.; Zheng, W.; Li, H. Vanadium exposure-induced striatal learning and memory alterations in rats. Neurotoxicology 2017, 62, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Paternain, J.; Domingo, J.; Gomez, M.; Ortega, A.; Corbella, J. Developmental toxicity of vanadium in mice after oral administration. J. Appl. Toxicol. 1990, 10, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Garcia, N.; Garcia-Ibarra, F.; Bizarro-Nevares, P.; Rojas-Lemus, M.; Lopez-Valdez, N.; González-Villalva, A.; Ayala-Escobar, M.E.; García-Vázquez, F.; Fortoul, T.I. Changes in Ovarian and Uterine Morphology and Estrous Cycle in CD-1 Mice After Vanadium Inhalation. Int. J. Toxicol. 2020, 39, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.; Pederson, R.; Brownsey, R.; McNeill, J. Long-term effectiveness of oral vanadyl sulphate in streptozotocin-diabetic rats. Diabetologia 1993, 36, 218–224. [Google Scholar] [CrossRef]
- Dai, S.; McNeill, J. One-year treatment of non-diabetic and streptozotocin-diabetic rats with vanadyl sulphate did not alter blood pressure or haematological indices. Pharmacol. Toxicol. 1994, 74, 110–115. [Google Scholar] [CrossRef]
- Soazo, M.; Garcia, G.B. Vanadium exposure through lactation produces behavioral alterations and CNS myelin deficit in neonatal rats. Neurotoxicology Teratol. 2007, 29, 503–510. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Zhang, Q.; Feng, C.; Zheng, W.; He, K.; Lan, Y. Vanadium exposure-induced neurobehavioral alterations among Chinese workers. Neurotoxicology 2013, 36, 49–54. [Google Scholar] [CrossRef]
- Carvour, M.; Song, C.; Kaul, S.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A. Chronic low dose oxidative stress induces caspase-3 dependent PKCδ proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1139, 197. [Google Scholar] [CrossRef]
- Fortoul, T.I.; Piñón-Zarate, G.; Diaz-Bech, M.E.; González-Villalva, A.; Mussali-Galante, P.; Rodriguez-Lara, V.; Colin-Barenque, L.; Martinez Pedraza, M.; Montaño, L.F. Spleen and bone marrow megakaryocytes as targets for inhaled vanadium. Histol. Histopathol. 2008, 1, 1vvr. [Google Scholar]
- Chen, L.-C.; Maciejczyk, P.; Thurston, G.D. Metals and air pollution. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–182. [Google Scholar]
- Zenz, C.; Berg, B.A. Human responses to controlled vanadium pentoxide exposure. Arch. Environ. Health Int. J. 1967, 14, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.; Farquhar, S.; Thou, T.; Shand, B. Oral vanadyl sulphate does not affect blood cells, viscosity or biochemistry in humans. Pharmacol. Toxicol. 1997, 80, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Boulassel, B.; Sadeg, N.; Roussel, O.; Perrin, M.; Belhadj-Tahar, H. Fatal poisoning by vanadium. Forensic Sci. Int. 2011, 206, e79–e81. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, B.; Sudha, S. Vanadium toxicity. Asian J. Exp. Sci. 2005, 19, 127–134. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).