Layered Gadolinium-Europium-Terbium Hydroxides Sensitised with 4-Sulfobenzoate as All Solid-State Luminescent Thermometers
Abstract
1. Introduction
2. Results and Discussion
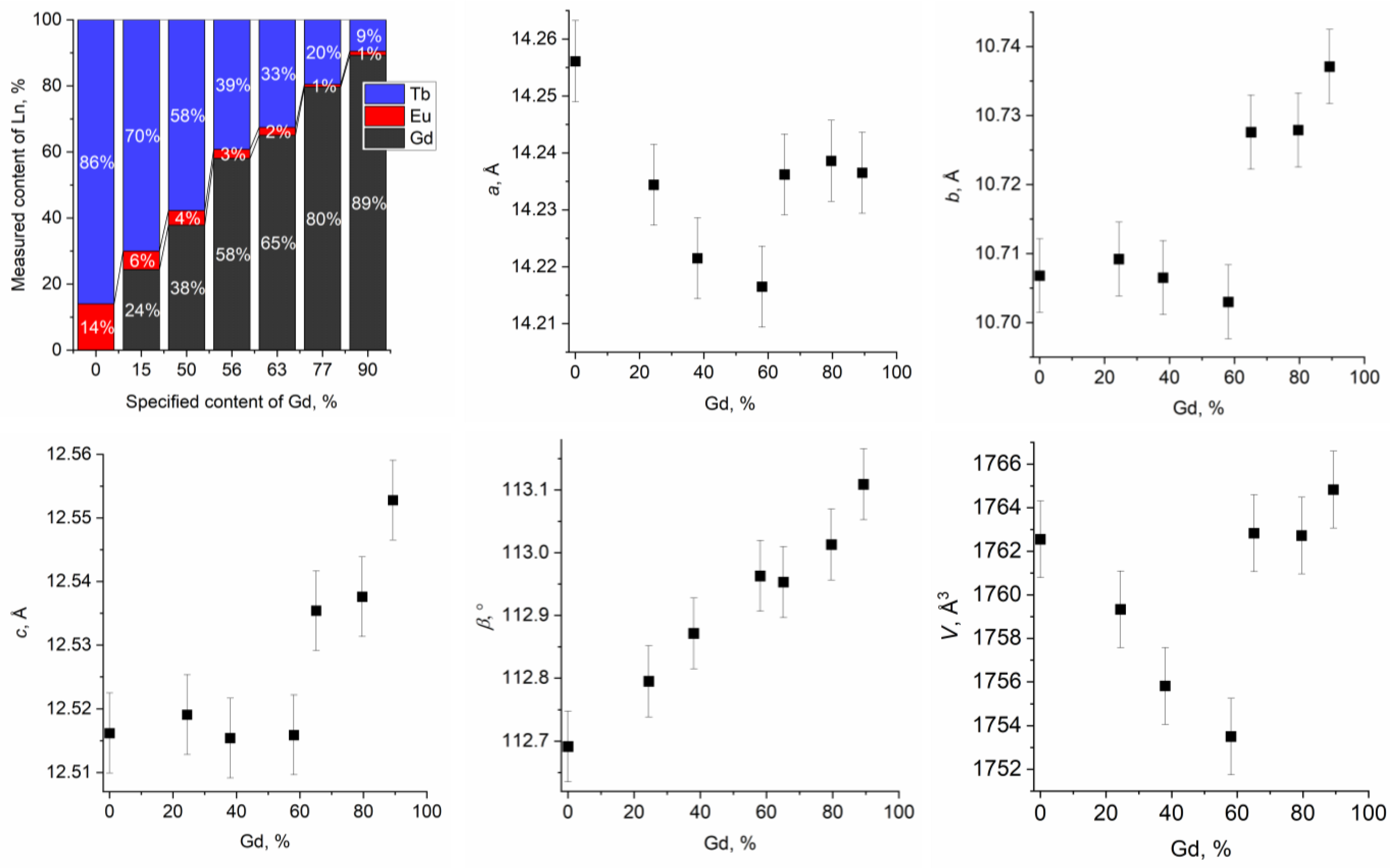
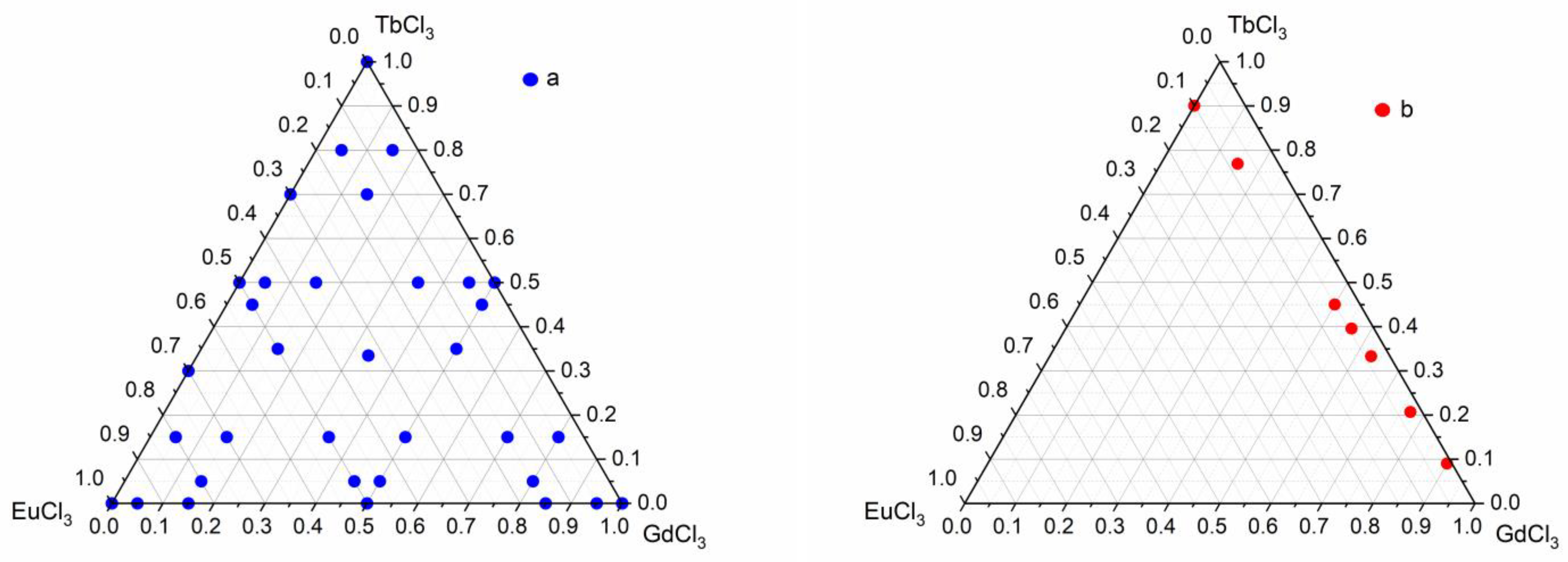
2.1. Synthesis of Ternary Layered Rare Earth Basic Chlorides
2.2. Anion Exchange with Potassium 4-Sulfobenzoate
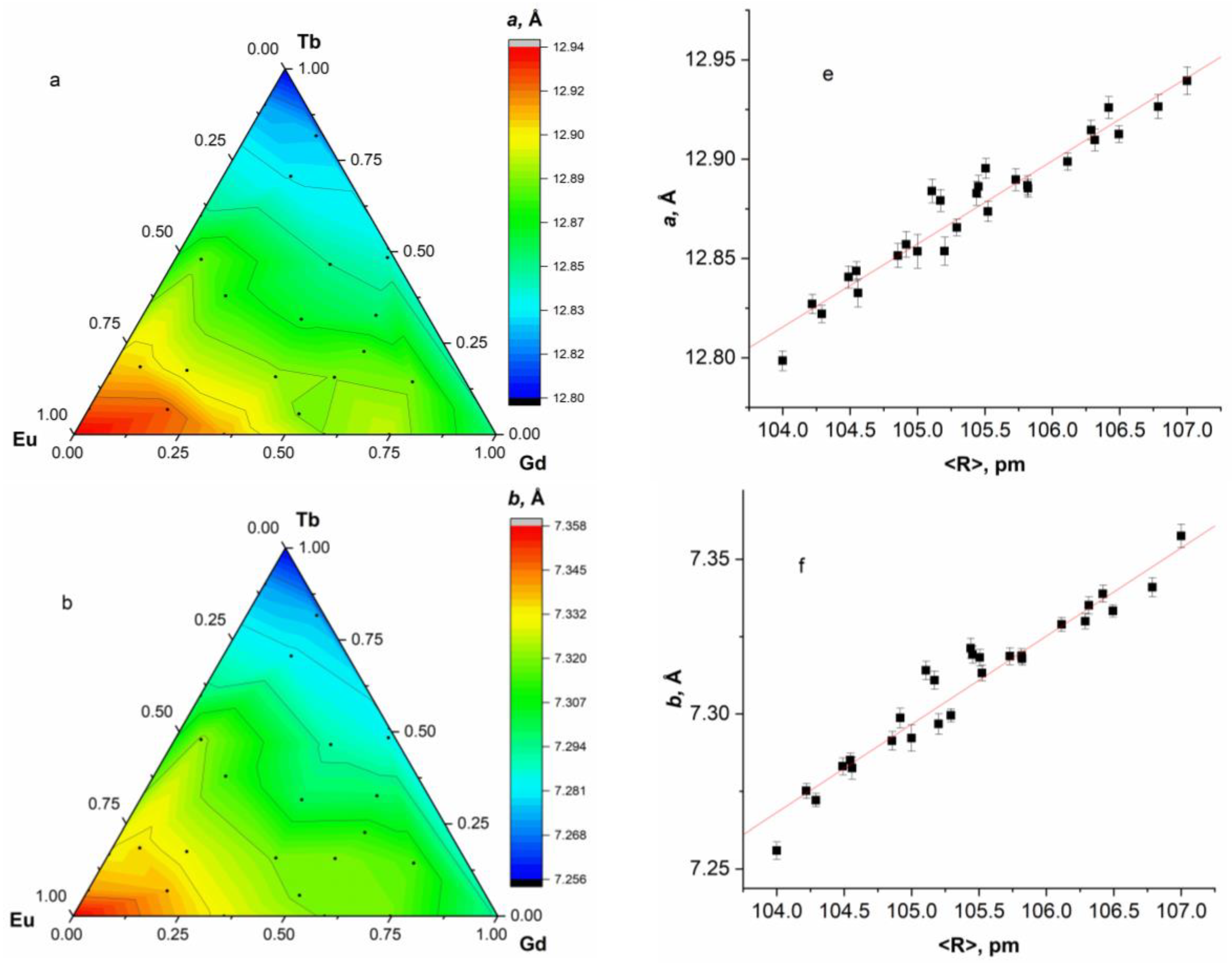
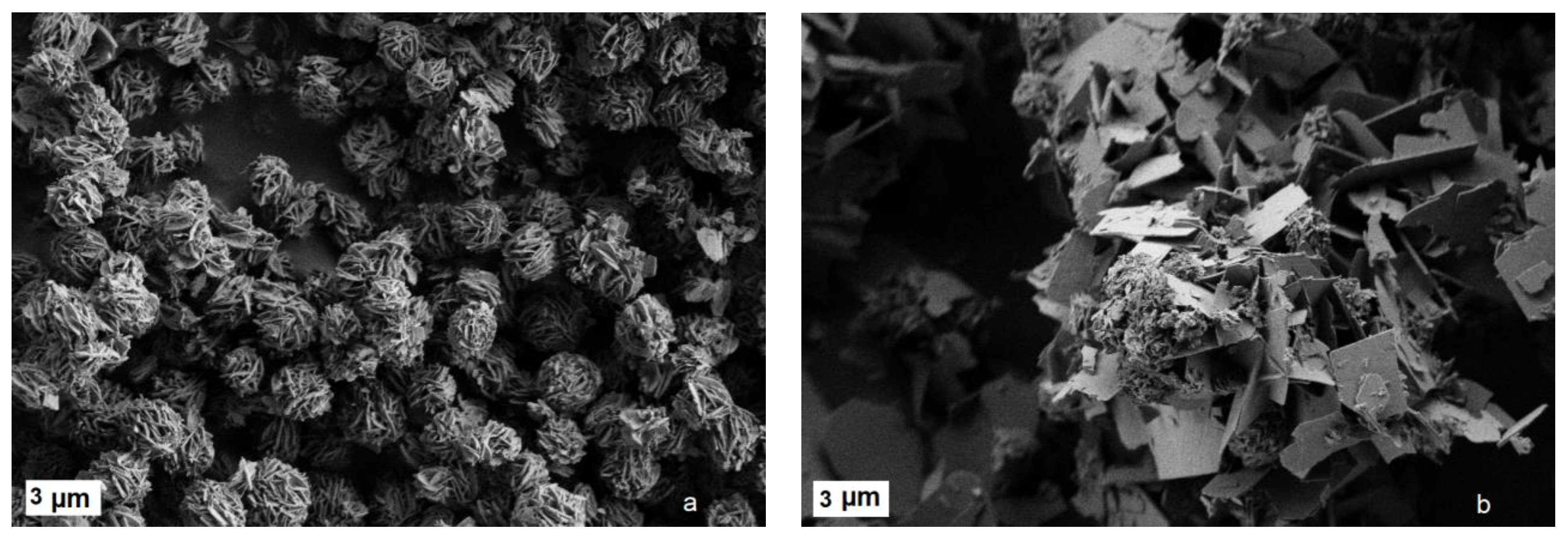
2.3. Single-Stage Synthesis of Ternary Layered Rare Earth Gd-Eu-Tb Basic Sulfobenzoates
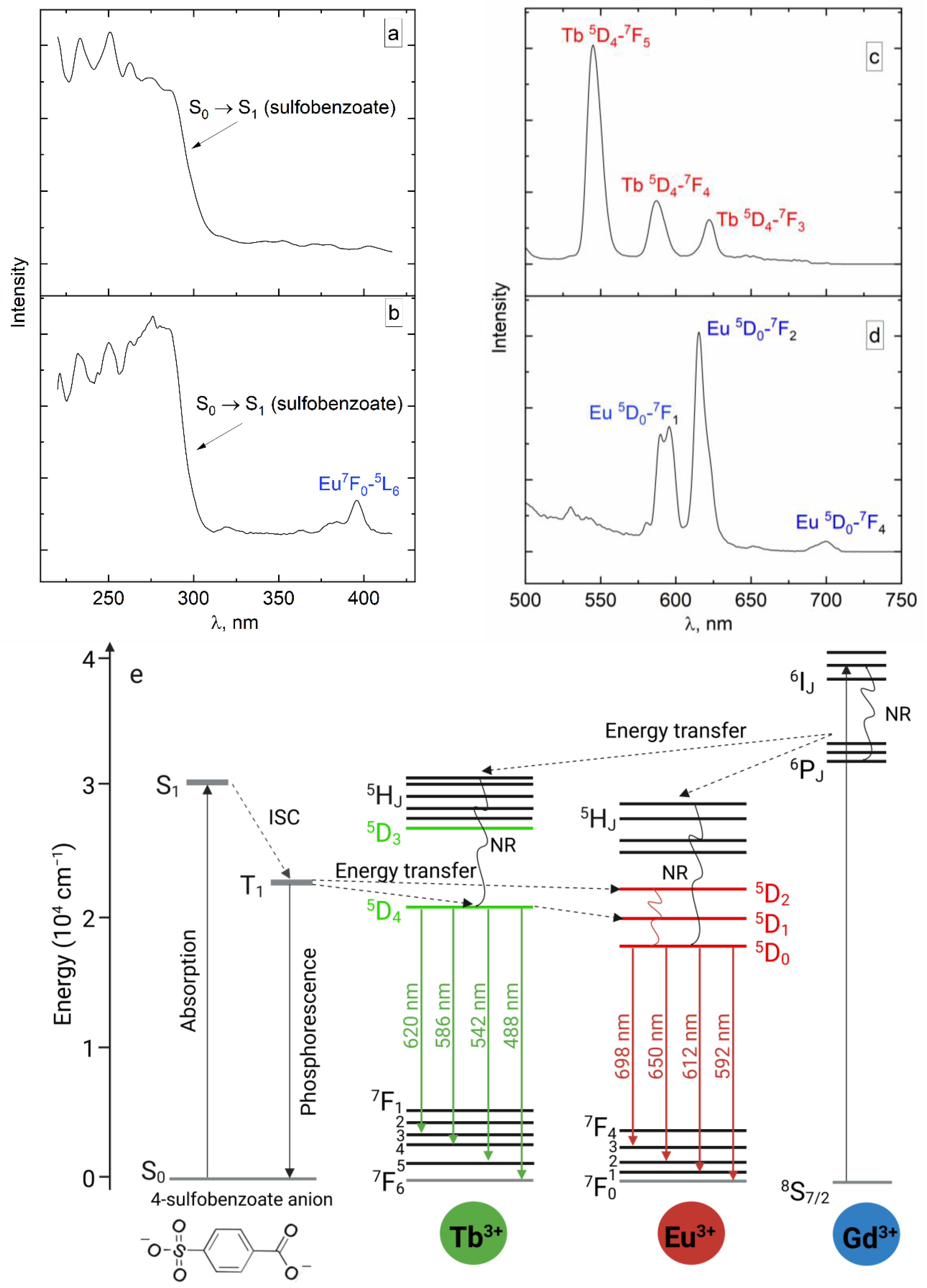
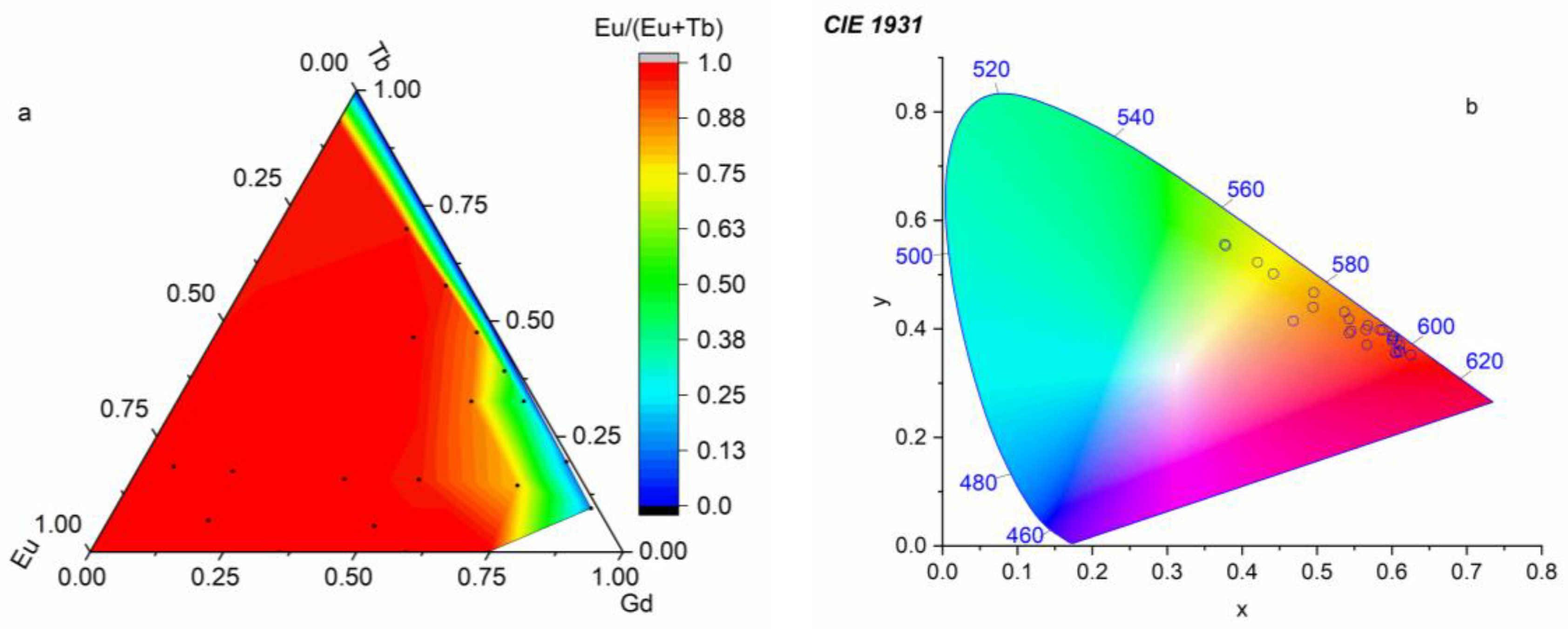
2.4. Luminescent Properties
2.5. Temperature Dependence of Luminescence
3. Materials and Methods
3.1. Materials
3.2. Anion Exchange Reactions
3.3. Single-Stage Synthesis of (Gd1−xTb0.9xEu0.1x)3(OH)7(C7H4O5S)·nH2O
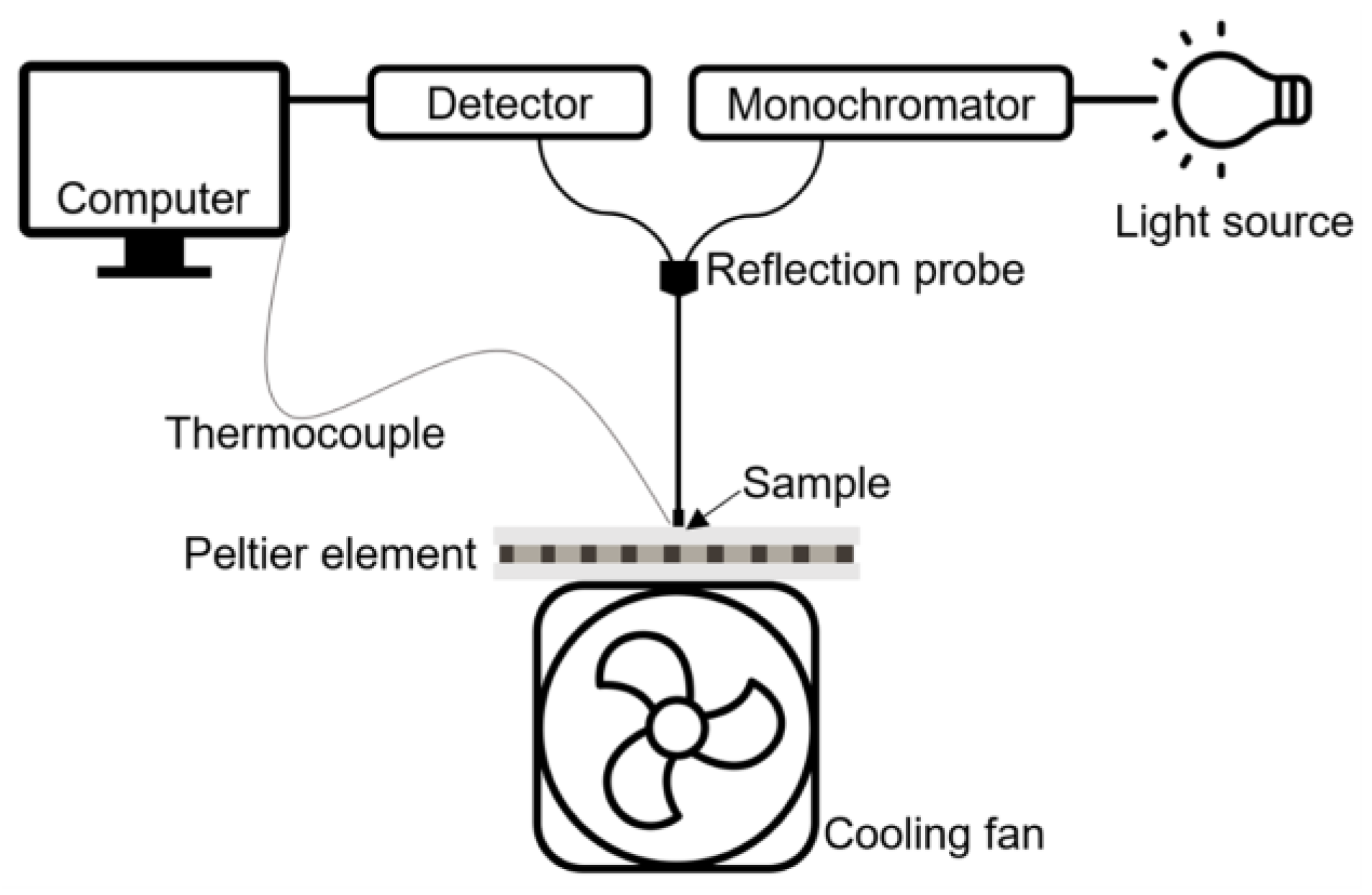
3.4. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haschke, J.M. Preparation, phase equilibriums, crystal chemistry, and some properties of lanthanide hydroxide nitrates. Inorg. Chem. 1974, 13, 1812–1818. [Google Scholar] [CrossRef]
- Serre, C.; Millange, F.; Marrot, J.; Férey, G. Hydrothermal synthesis, structure determination, and thermal behavior of new three-dimensional europium terephthalates. Chem. Mater. 2002, 14, 2409–2415. [Google Scholar] [CrossRef]
- Snejko, N.; Gándara, F.; Perles, J.; Monge, M.Á.; Gutiérrez-Puebla, E.; Gómez-Lor, B.; Iglesias, M. Layered Rare-Earth Hydroxides: A Class of Pillared Crystalline Compounds for Intercalation Chemistry. Angew. Chem. Int. Ed. 2006, 45, 7998–8001. [Google Scholar] [CrossRef]
- Rodina, A.A.; Yapryntsev, A.D.; Churakov, A.V.; Baranchikov, A.E. Layered Rare Earth Hydroxides React with Formamide to Give [Ln(HCOO)3 2(HCONH2)]. Russ. J. Inorg. Chem. 2021, 66, 125–132. [Google Scholar] [CrossRef]
- Bai, M.; Liu, X.; Sasaki, T.; Ma, R. Superlattice films of semiconducting oxide and rare-earth hydroxide nanosheets for tunable and efficient photoluminescent energy transfer. Nanoscale 2021, 13, 4551–4561. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, H.; Su, F.; Liang, Z.; Ma, S. Layered yttrium hydroxide composite as supersensitive fluorescent sensor on Fe(III) ions. Mater. Res. Bull. 2021, 135, 111135. [Google Scholar] [CrossRef]
- Ren, Y.; Feng, J. Skin-Inspired Multifunctional Luminescent Hydrogel Containing Layered Rare-Earth Hydroxide with 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces 2020, 12, 6797–6805. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.G.; Liu, W.; Cui, H.; Liu, M.; Chen, J.; Zhu, H.; Li, X.; Sun, X. A novel method for improving particle growth and photoluminescence through F− substituting for gallery NO3− in layered Y/Eu hydroxides. Chem. Eng. J. 2020, 380, 122618. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, F.; Ren, J.; Guan, Q.; Wei, B.; Bai, C.; Sheng, L. Preparation of polyacrylamide/nanosheets LYH:Eu nanocomposite film and enhanced photoluminescence. Opt. Mater. 2020, 108, 110437. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Sun, M.; Du, P.; Liu, W.; Huang, S.; Li, J.G. Phase-conversion synthesis of LaF3:Yb/RE (RE = Ho, Er) nanocrystals with Ln2(OH)4SO4·2H2O type layered compound as a new template, phase/morphology evolution, and upconversion luminescence. J. Mater. Res. Technol. 2020, 9, 10659–10668. [Google Scholar] [CrossRef]
- Yang, W.; Li, Q.; Zheng, X.; Li, X.; Li, X. Luminescent sensing film based on sulfosalicylic acid modified Tb(III)-doped yttrium hydroxide nanosheets. J. Adv. Ceram. 2018, 7, 352–361. [Google Scholar] [CrossRef]
- Li, X.; Xue, Z.; Xia, J.; Zhou, G.; Jiang, D.; Dai, M.; Wang, W.; Miu, J.; Heng, Y.; Yu, C.; et al. Gd/Y hydroxide nanosheets as highly efficient T1/T2 MRI contrast agents. Nanomaterials 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tang, G.; Meng, W.; Feng, H.; Zhang, Z.; Zhao, J. Controlled synthesis of hydrophilic yttrium-based fluorids by transformation from layered rare-earth hydroxides. Opt. Mater. 2020, 108, 110220. [Google Scholar] [CrossRef]
- Shao, B.; Zhang, X.; Wang, X.; Cui, F.; Yang, X. The optical sensitive detection of molybdate ions by layered europium hydroxides. Opt. Mater. 2020, 100, 109597. [Google Scholar] [CrossRef]
- Omwoma, S.; Stephen Odongo, A.; Otieno, Z.; Lagat, S.; Lalah, J.O. Layered Rare-Earth Hydroxide Unilameller Nanosheets: Synthesis, Characterization, and Adsorption. J. Chem. 2020, 2020, 8923871. [Google Scholar] [CrossRef]
- Omwoma, S. Trace Metal Detection in Aqueous Reservoirs Using Stilbene Intercalated Layered Rare-Earth Hydroxide Tablets. J. Anal. Methods Chem. 2020, 2020, 9712872. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Li, J.; Qi, X.; Guo, Z.; Wei, H.; Chu, H. Gold Nanoparticles on Nanosheets Derived from Layered Rare-Earth Hydroxides for Catalytic Glycerol-to-Lactic Acid Conversion. ACS Appl. Mater. Interfaces 2021, 13, 522–530. [Google Scholar] [CrossRef]
- Gu, Q.; Li, J.; Ji, L.; Ju, R.; Jin, H.; Zhang, R. Fabrication of novel bifunctional nanohybrid based on layered rare-earth hydroxide with magnetic and fluorescent properties. Front. Mater. Sci. 2020, 14, 488–496. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Abo-zeid, Y.; Bear, J.C.; Davies, G.L.; Lei, X.; Williams, G.R. SiO2-coated layered gadolinium hydroxides for simultaneous drug delivery and magnetic resonance imaging. J. Solid State Chem. 2020, 286, 121291. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B.-I.; Jeong, H.; Byeon, S.-H. Relationship between interlayer anions and photoluminescence of layered rare earth hydroxides. J. Mater. Chem. C 2015, 3, 7437–7445. [Google Scholar] [CrossRef]
- Liu, L.; Yu, M.; Zhang, J.; Wang, B.; Liu, W.; Tang, Y.; Wang, B.; Tang, Y.; Liu, L.; Zhang, J.; et al. Facile fabrication of color-tunable and white light emitting nano-composite films based on layered rare-earth hydroxides. J. Mater. Chem. C 2015, 3, 2326–2333. [Google Scholar] [CrossRef]
- Yapryntsev, A.D.; Baranchikov, A.E.; Ivanov, V.K. Layered rare-earth hydroxides: A new family of anion-exchangeable layered inorganic materials. Russ. Chem. Rev. 2020, 89, 629–666. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.V. Photophysics of Lanthanoid Coordination Compounds. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 339–398. [Google Scholar]
- Liu, L.; Wang, Q.; Gao, C.; Chen, H.; Liu, W.; Tang, Y. Dramatically enhanced luminescence of layered terbium hydroxides as induced by the synergistic effect of Gd3+ and organic sensitizers. J. Phys. Chem. C 2014, 118, 14511–14520. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B.I.; Byeon, S.H. The inner filter effect of Cr(VI) on Tb-doped layered rare earth hydroxychlorides: New fluorescent adsorbents for the simple detection of Cr(VI). Chem. Commun. 2015, 51, 725–728. [Google Scholar] [CrossRef]
- Gu, Q.; Sun, Y.; Chu, N.; Ma, S.; Jia, Z.; Yang, X. Intercalation of amino acids into Eu3+-doped layered gadolinium hydroxide and quenching of Eu3+ luminescence. Eur. J. Inorg. Chem. 2012, 2012, 4407–4412. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, B.-I.; Byeon, S.-H. Antenna Effect on the Organic Spacer-Modified Eu-Doped Layered Gadolinium Hydroxide for the Detection of Vanadate Ions over a Wide pH Range. ACS Appl. Mater. Interfaces 2016, 8, 10946–10953. [Google Scholar] [CrossRef]
- Xie, L.; Liu, C.; Ma, L.; Xiao, C.; Ma, S.; Sun, G.; Li, H.; Yang, X. A unique delaminated MoS4/OS-LEuH composite exhibiting turn-on luminescence sensing for detection of water in formamide. Dalt. Trans. 2017, 46, 3110–3114. [Google Scholar] [CrossRef]
- Al-Enezi, E.; Vakurov, A.; Eades, A.; Ding, M.; Jose, G.; Saha, S.; Millner, P. Affimer-Based Europium Chelates Allow Sensitive Optical Biosensing in a Range of Human Disease Biomarkers. Sensors 2021, 21, 831. [Google Scholar] [CrossRef]
- Selivanova, N.; Galyametdinov, Y. Terbium(III) as a Fluorescent Probe for Molecular Detection of Ascorbic Acid. Chemosensors 2021, 9, 134. [Google Scholar] [CrossRef]
- Vialtsev, M.B.; Tcelykh, L.O.; Bobrovsky, A.Y.; Utochnikova, V.V. Lanthanide complexes for elevated temperature luminescence thermometry: Mixture vs. bimetallic compound. J. Alloys Compd. 2022, 924, 166421. [Google Scholar] [CrossRef]
- Tcelykh, L.O.; Kozhevnikova, V.Y.; Goloveshkin, A.S.; Latipov, E.V.; Gordeeva, E.O.; Utochnikova, V.V. Sensing temperature with Tb-Eu-based luminescent thermometer: A novel approach to increase the sensitivity. Sens. Actuators A Phys. 2022, 345, 113787. [Google Scholar] [CrossRef]
- Popelensky, T.Y.; Utochnikova, V.V. How does the ligand affect the sensitivity of the luminescent thermometers based on Tb–Eu complexes. Dalt. Trans. 2020, 49, 12156–12160. [Google Scholar] [CrossRef] [PubMed]
- Vialtsev, M.B.; Dalinger, A.I.; Latipov, E.V.; Lepnev, L.S.; Kushnir, S.E.; Vatsadze, S.Z.; Utochnikova, V.V. New approach to increase the sensitivity of Tb–Eu-based luminescent thermometer. Phys. Chem. Chem. Phys. 2020, 22, 25450–25454. [Google Scholar] [CrossRef] [PubMed]
- Quici, S.; Casoni, A.; Foschi, F.; Armelao, L.; Bottaro, G.; Seraglia, R.; Bolzati, C.; Salvarese, N.; Carpanese, D.; Rosato, A. Folic Acid-Conjugated Europium Complexes as Luminescent Probes for Selective Targeting of Cancer Cells. J. Med. Chem. 2015, 58, 2003–2014. [Google Scholar] [CrossRef]
- Galyametdinov, Y.G.; Krupin, A.S.; Knyazev, A.A. Temperature-Sensitive Chameleon Luminescent Films Based on PMMA Doped with Europium(III) and Terbium(III) Anisometric Complexes. Inorganics 2022, 10, 94. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Mamonova, D.V.; Kurochkin, M.A.; Medvedev, V.A.; Kolesnikov, E.Y. Ratiometric dual-center Gd2O3:Tb3+/Eu3+ nanothermometers with enhanced thermometric performances. J. Alloys Compd. 2022, 922, 166182. [Google Scholar] [CrossRef]
- Khudoleeva, V.; Tcelykh, L.; Kovalenko, A.; Kalyakina, A.; Goloveshkin, A.; Lepnev, L.; Utochnikova, V. Terbium-europium fluorides surface modified with benzoate and terephthalate anions for temperature sensing: Does sensitivity depend on the ligand? J. Lumin. 2018, 201, 500–508. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, S.; Jin, J.; Xu, Z.; Li, X.; Sun, X.; Li, J.-G.G. Luminescent Thermometry by a Y/Eu Binary Layered Rare-Earth Hydroxide (LRH) via In Situ Intercalation with Neutral Terbium(III) Complexes. Chem.-Asian J. 2018, 13, 3664–3669. [Google Scholar] [CrossRef]
- Sakuma, K.; Fujihara, S. Synthesis of carboxylate-intercalated layered yttrium hydroxides by anion exchange reactions and their application to Ln3+-activated luminescent materials. J. Ceram. Process. Res. 2013, 14, s26–s29. [Google Scholar]
- Chu, N.; Sun, Y.; Zhao, Y.; Li, X.; Sun, G.; Ma, S.; Yang, X. Intercalation of organic sensitisers into layered europium hydroxide and enhanced luminescence property. Dalt. Trans. 2012, 41, 7409–7414. [Google Scholar] [CrossRef]
- Yapryntsev, A.; Abdusatorov, B.; Yakushev, I.; Svetogorov, R.; Gavrikov, A.; Rodina, A.; Fatyushina, Y.; Baranchikov, A.; Zubavichus, Y.; Ivanov, V. Eu-Doped layered yttrium hydroxides sensitized by a series of benzenedicarboxylate and sulphobenzoate anions. Dalt. Trans. 2019, 48, 6111–6122. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Guo, R.; Yu, Z.; Li, J.; Liang, Z.; Shi, K.; Ma, S.; Sun, G.; Li, H. Layered rare-earth hydroxide (LRH, R = Tb, Y) composites with fluorescein: Delamination, tunable luminescence and application in chemosensoring for detecting Fe(III) ions. Dalt. Trans. 2018, 47, 5380–5389. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wu, X.; Zhang, H.; Li, J.G. Ultrathin (Y0.98Re0.02)2(OH)5NO3·nH2O (Re = Pr, Sm, Eu, Tb, Dy, Ho, Er, Tm) nanosheets and well-dispersed oxide nanoparticles: Facile co-precipitation synthesis and multi-color luminescent properties. Opt. Mater. 2020, 105, 109884. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Ren, K.; Zhang, R.; Wu, X.; Li, J. guang Fabrication of oriented oxide films from exfoliated yttrium hydroxide layers: Enhanced photoluminescence and unexplored behavior of energy transfer. J. Alloys Compd. 2018, 763, 815–821. [Google Scholar] [CrossRef]
- Wu, L.; Gao, C.; Li, Z.; Chen, G. Tunable photoluminescence from layered rare-earth hydroxide/polymer nanocomposite hydrogels by a cascaded energy transfer effect. J. Mater. Chem. C 2017, 5, 5207–5213. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.-G.G.; Guo, M.; Yang, X. Structural and photoluminescent investigation of LTbH/LEuH nanosheets and their color-tunable colloidal hybrids. J. Mater. Chem. C 2013, 1, 3584–3592. [Google Scholar] [CrossRef]
- Jeon, H.G.; Kim, H.; Byeon, S.H. Flexibly transparent luminescent organic-inorganic-polymer composite films: Intense full-color emissions at a single excitation wavelength. Chem. Eng. J. 2021, 405, 126675. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Q.; Li, D.; Li, Z.; Zhang, Y. Effects of A-site cationic radius and cationic disorder on the electromagnetic properties of La0.7Ca0.3MnO3 ceramic with added Sr, Pb, and Ba. Ceram. Int. 2018, 44, 5378–5384. [Google Scholar] [CrossRef]
- Giri, R.; Singh, H.K.; Tiwari, R.S.; Srivastava, O.N. Effect of cationic size in Hg(Tl/Bi)Ba2Ca2Cu3O8+δ on superconducting and microstructural characteristics. Bull. Mater. Sci. 2001, 24, 523–528. [Google Scholar] [CrossRef]
- Muralidhar, M.; Chauhan, H.S.; Saitoh, T.; Kamada, K.; Segawa, K.; Murakami, M. Effect of mixing three rare-earth elements on the superconducting properties of REBa2Cu3Oy. Supercond. Sci. Technol. 1997, 10, 663–670. [Google Scholar] [CrossRef]
- Phor, L.; Kumar, V. Structural, thermomagnetic, and dielectric properties of Mn0.5Zn0.5GdxFe2−xO4 (x = 0, 0.025, 0.050, 0.075, and 0.1). J. Adv. Ceram. 2020, 9, 243–254. [Google Scholar] [CrossRef]
- Fattakhova, Z.A.; Vovkotrub, E.G.; Zakharova, G.S. Microwave-Assisted Hydrothermal Synthesis of α-MoO3. Russ. J. Inorg. Chem. 2021, 66, 35–41. [Google Scholar] [CrossRef]
- Dumrongrojthanath, P.; Phuruangrat, A.; Sakhon, T.; Thongtem, T.; Thongtem, S. Effect of Gd Dopant on Visible-Light-Driven Photocatalytic Properties of CeO2 Nanowires Synthesized Microwave-Assisted Hydrothermal Method. Russ. J. Inorg. Chem. 2022, 67, 1880–1887. [Google Scholar] [CrossRef]
- Lomakin, M.S.; Proskurina, O.V.; Levin, A.A.; Sergeev, A.A.; Leonov, A.A.; Nevedomsky, V.N.; Voznesenskiy, S.S. Pyrochlore Phase in the Bi2O3–Fe2O3–WO3–(H2O) System: Its Formation by Hydrothermal-Microwave Synthesis and Optical Properties. Russ. J. Inorg. Chem. 2022, 67, 820–829. [Google Scholar] [CrossRef]
- Feng, P.; Wang, X.; Zhao, Y.; Fang, D.-C.C.; Yang, X. Energy transfer between rare earths in layered rare-earth hydroxides. RSC Adv. 2018, 8, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Grishko, A.Y.; Utochnikova, V.V.; Averin, A.A.; Mironov, A.V.; Kuzmina, N.P. Unusual Luminescence Properties of Heterometallic REE Terephthalates. Eur. J. Inorg. Chem. 2015, 2015, 1660–1664. [Google Scholar] [CrossRef]
- Sun, Y.; Chu, N.; Gu, Q.; Pan, G.; Sun, G.; Ma, S.; Yang, X. Hybrid of europium-doped layered yttrium hydroxide and organic sensitizer-effect of solvent on structure and luminescence behavior. Eur. J. Inorg. Chem. 2013, 2013, 32–38. [Google Scholar] [CrossRef]
- Li, J.; Chen, H. Theory prediction of band structures, densities of states, mechanical and thermodynamic properties of solid solutions BaxK1-xBi0.92Mg0.08O3. Phys. B Condens. Matter 2021, 618, 413163. [Google Scholar] [CrossRef]
- Glinskaya, A.; Petrov, G.; Romanovski, V. Crystal structure, physicochemical, and sensory properties of solid solutions Bi1−xLaxFe1−xCoxO3 (x = 0, 0.05, 0.1). J. Mater. Sci. Mater. Electron. 2021, 32, 22579–22587. [Google Scholar] [CrossRef]
- Baidya, T.; Bera, P.; Kröcher, O.; Safonova, O.; Abdala, P.M.; Gerke, B.; Pöttgen, R.; Priolkar, K.R.; Mandal, T.K. Understanding the anomalous behavior of Vegard’s law in Ce1-xMxO2 (M = Sn and Ti; 0 < x ≤ 0.5) solid solutions. Phys. Chem. Chem. Phys. 2016, 18, 13974–13983. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Liu, B.; Duan, G.; Liu, Z. Enhanced Dy3+ white emission via energy transfer in spherical (Lu,Gd)3Al5O12 garnet phosphors. Sci. Rep. 2020, 10, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Li, X.; Sun, X.; Ishigaki, T. Monodispersed colloidal spheres for uniform Y2O3:Eu3+ red-phosphor particles and greatly enhanced luminescence by simultaneous Gd3+ doping. J. Phys. Chem. C 2008, 112, 11707–11716. [Google Scholar] [CrossRef]
- Steblevskaya, N.I.; Belobeletskaya, M.V.; Medkov, M.A. Luminescent Properties of Lanthanum Borates LaBO3:Eu and La(BO2)3:Eu Obtained by the Extraction-Pyrolytic Method. Russ. J. Inorg. Chem. 2021, 66, 468–476. [Google Scholar] [CrossRef]
- Steblevskaya, N.I.; Belobeletskaya, M.V.; Yarovaya, T.P.; Nedozorov, P.M. Luminescent Composites Based on Europium(III) and Europium(II) Tungstate, Phosphate, and Titanate. Russ. J. Inorg. Chem. 2022, 67, 245–251. [Google Scholar] [CrossRef]
- Steblevskaya, N.I.; Belobeletskaya, M.V.; Medkov, M.A.; Shlyk, D.K. Luminescent Properties of La0.95Eu0.05BO3:M and La0.95Eu0.05(BO2)3:M Borates (M = Tb, Bi) Synthesized by the Extraction-Pyrolytic Method. Russ. J. Inorg. Chem. 2022, 67, 1228–1238. [Google Scholar] [CrossRef]
- Wu, L.; Chen, G.; Li, Z. Layered Rare-Earth Hydroxide/Polyacrylamide Nanocomposite Hydrogels with Highly Tunable Photoluminescence. Small 2017, 13, 1604070. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Kuzmina, N.P. Photoluminescence of lanthanide aromatic carboxylates. Russ. J. Coord. Chem. 2016, 42, 679–694. [Google Scholar] [CrossRef]
- Nakazawa, E.; Shionoya, S. Energy Transfer between Trivalent Rare-Earth Ions in Inorganic Solids. J. Chem. Phys. 1967, 47, 3211–3219. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; pp. 339–427. [Google Scholar]
- Bao, G.; Wong, K.-L.; Jin, D.; Tanner, P.A. A stoichiometric terbium-europium dyad molecular thermometer: Energy transfer properties. Light Sci. Appl. 2018, 7, 96. [Google Scholar] [CrossRef]
- Wu, M.; Li, L.; Yu, X.; Zhang, D.; Sun, T.; Li, X.; Sun, L.; Lui, S.; Huang, X.; Bi, F.; et al. Multifunctional layered gadolinium hydroxide nanoplates for ultrahigh field magnetic resonance imaging, Computed tomography and fluorescence bioimaging. J. Biomed. Nanotechnol. 2014, 10, 3620–3630. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Yapryntsev, A.D.; Baranchikov, A.E.; Skogareva, L.S.; Goldt, A.E.; Stolyarov, I.P.; Ivanova, O.S.; Kozik, V.V.; Ivanov, V.K. High-yield microwave synthesis of layered Y2(OH)5NO3 xH2O materials. CrystEngComm 2015, 17, 2667–2674. [Google Scholar] [CrossRef]
- Yapryntsev, A.D.D.; Baranchikov, A.E.E.; Zabolotskaya, A.V.V.; Borilo, L.P.P.; Ivanov, V.K.K. Synthesis of gadolinium hydroxo nitrate under microwave-hydrothermal treatment conditions. Russ. J. Inorg. Chem. 2014, 59, 1383–1391. [Google Scholar] [CrossRef]
- Yapryntsev, A.; Baranchikov, A.; Goldt, A.; Ivanov, V. Microwave-Assisted Hydrothermal Synthesis of Layered Europium Hydroxynynitrate, Eu2(OH)5NO3∙xH2O. Curr. Microw. Chem. 2016, 3, 3–8. [Google Scholar] [CrossRef]
- Yapryntsev, A.D.; Bykov, A.Y.; Baranchikov, A.E.; Zhizhin, K.Y.; Ivanov, V.K.; Kuznetsov, N.T. closo-Dodecaborate Intercalated Yttrium Hydroxide as a First Example of Boron Cluster Anion-Containing Layered Inorganic Substances. Inorg. Chem. 2017, 56, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Teplonogova, M.A.; Yapryntsev, A.D.; Baranchikov, A.E.; Ivanov, V.K. Selective hydrothermal synthesis of ammonium vanadates(V) and (IV,V). Transit. Met. Chem. 2019, 44, 25–30. [Google Scholar] [CrossRef]
- Egorova, A.A.; Bushkova, T.M.; Kolesnik, I.V.; Yapryntsev, A.D.; Kottsov, S.Y.; Baranchikov, A.E. Selective Synthesis of Manganese Dioxide Polymorphs by the Hydrothermal Treatment of Aqueous KMnO4 Solutions. Russ. J. Inorg. Chem. 2021, 66, 146–152. [Google Scholar] [CrossRef]
- Meskin, P.E.; Gavrilov, A.I.; Maksimov, V.D.; Ivanov, V.K.; Churagulov, B.P. Hydrothermal/microwave and hydrothermal/ultrasonic synthesis of nanocrystalline titania, zirconia, and hafnia. Russ. J. Inorg. Chem. 2007, 52, 1648–1656. [Google Scholar] [CrossRef]
- Sadovnikov, A.A.; Baranchikov, A.E.; Zubavichus, Y.V.; Ivanova, O.S.; Murzin, V.Y.; Kozik, V.V.; Ivanov, V.K.; Baranchikov, A.E.; Ivanov, V.K.; Sadovnikov, A.A.; et al. Photocatalytically active fluorinated nano-titania synthesized by microwave-assisted hydrothermal treatment. J. Photochem. Photobiol. A Chem. 2015, 303–304, 36–43. [Google Scholar] [CrossRef]
- Geng, F.; Xin, H.; Matsushita, Y.; Ma, R.; Tanaka, M.; Izumi, F.; Iyi, N.; Sasaki, T.; Xin, H.; Geng, F.; et al. New layered rare-earth hydroxides with anion-exchange properties. Chem.-Eur. J. 2008, 14, 9255–9260. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodina, A.A.; Yapryntsev, A.D.; Abdusatorov, B.A.; Belova, E.V.; Baranchikov, A.E.; Ivanov, V.K. Layered Gadolinium-Europium-Terbium Hydroxides Sensitised with 4-Sulfobenzoate as All Solid-State Luminescent Thermometers. Inorganics 2022, 10, 233. https://doi.org/10.3390/inorganics10120233
Rodina AA, Yapryntsev AD, Abdusatorov BA, Belova EV, Baranchikov AE, Ivanov VK. Layered Gadolinium-Europium-Terbium Hydroxides Sensitised with 4-Sulfobenzoate as All Solid-State Luminescent Thermometers. Inorganics. 2022; 10(12):233. https://doi.org/10.3390/inorganics10120233
Chicago/Turabian StyleRodina, Anfisa A., Alexey D. Yapryntsev, Bakhodur A. Abdusatorov, Ekaterina V. Belova, Alexander E. Baranchikov, and Vladimir K. Ivanov. 2022. "Layered Gadolinium-Europium-Terbium Hydroxides Sensitised with 4-Sulfobenzoate as All Solid-State Luminescent Thermometers" Inorganics 10, no. 12: 233. https://doi.org/10.3390/inorganics10120233
APA StyleRodina, A. A., Yapryntsev, A. D., Abdusatorov, B. A., Belova, E. V., Baranchikov, A. E., & Ivanov, V. K. (2022). Layered Gadolinium-Europium-Terbium Hydroxides Sensitised with 4-Sulfobenzoate as All Solid-State Luminescent Thermometers. Inorganics, 10(12), 233. https://doi.org/10.3390/inorganics10120233







