Synthesis of a New Dinuclear Ag(I) Complex with Asymmetric Azine Type Ligand: X-ray Structure and Biological Studies
Abstract
1. Introduction
2. Results and Discussion
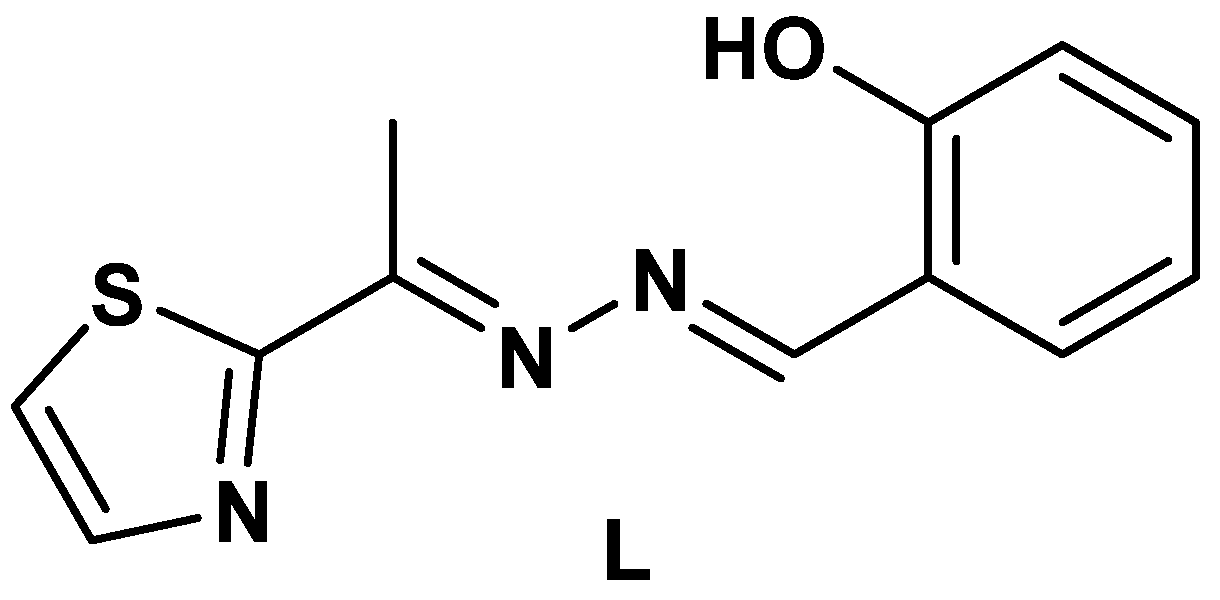
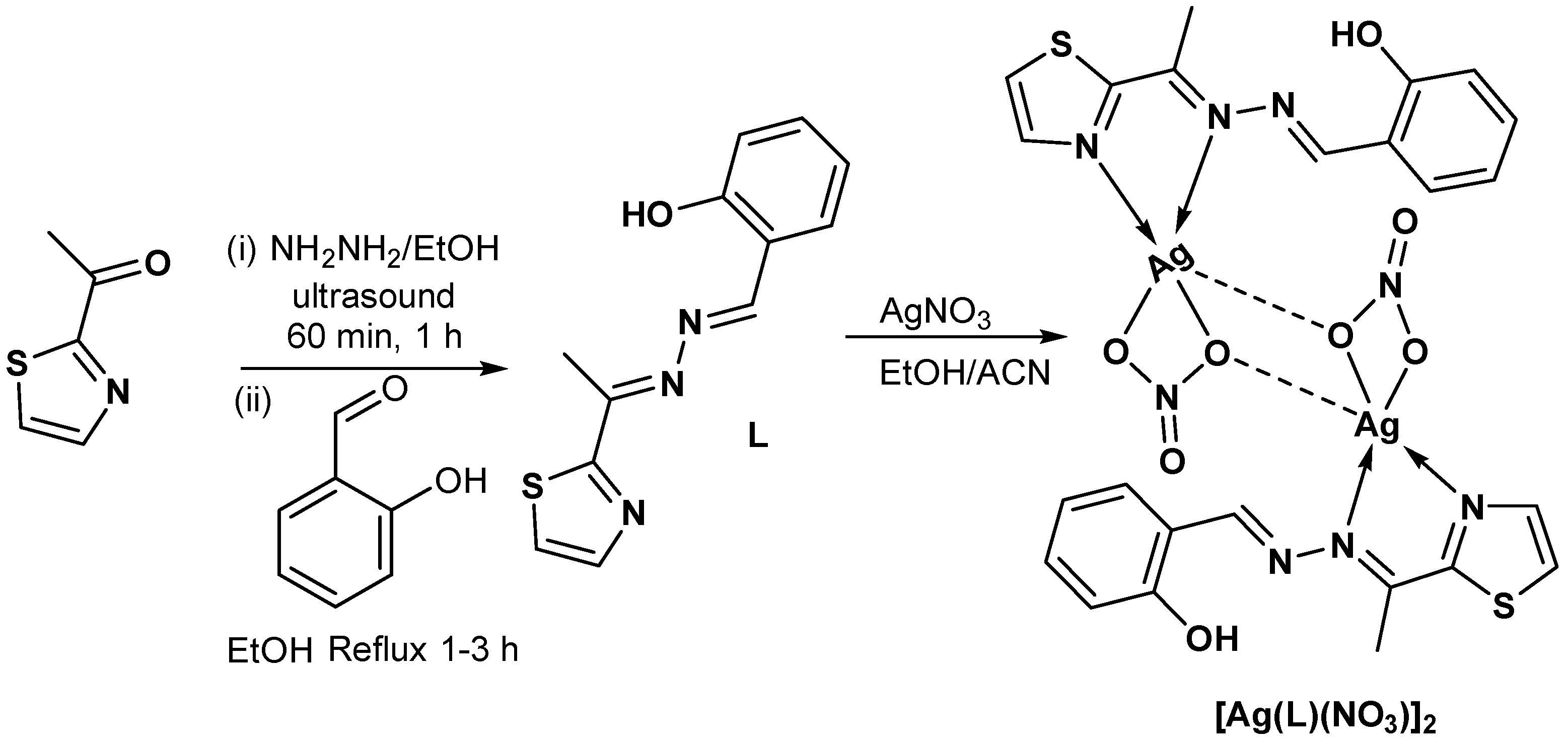
2.1. Synthesis and Characterizations
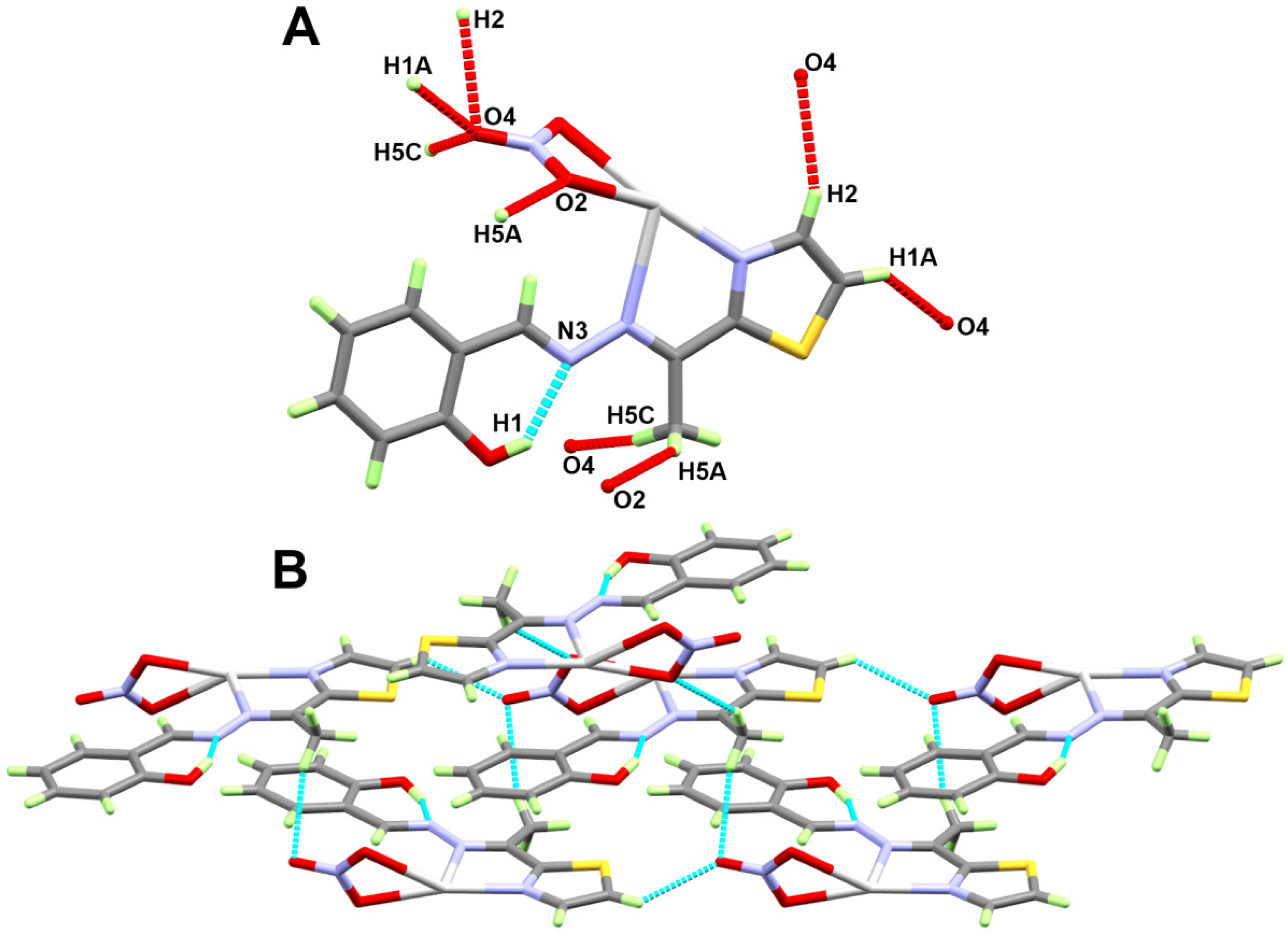
2.2. X-ray Structure Description of [Ag(L)(NO3)]2 Complex
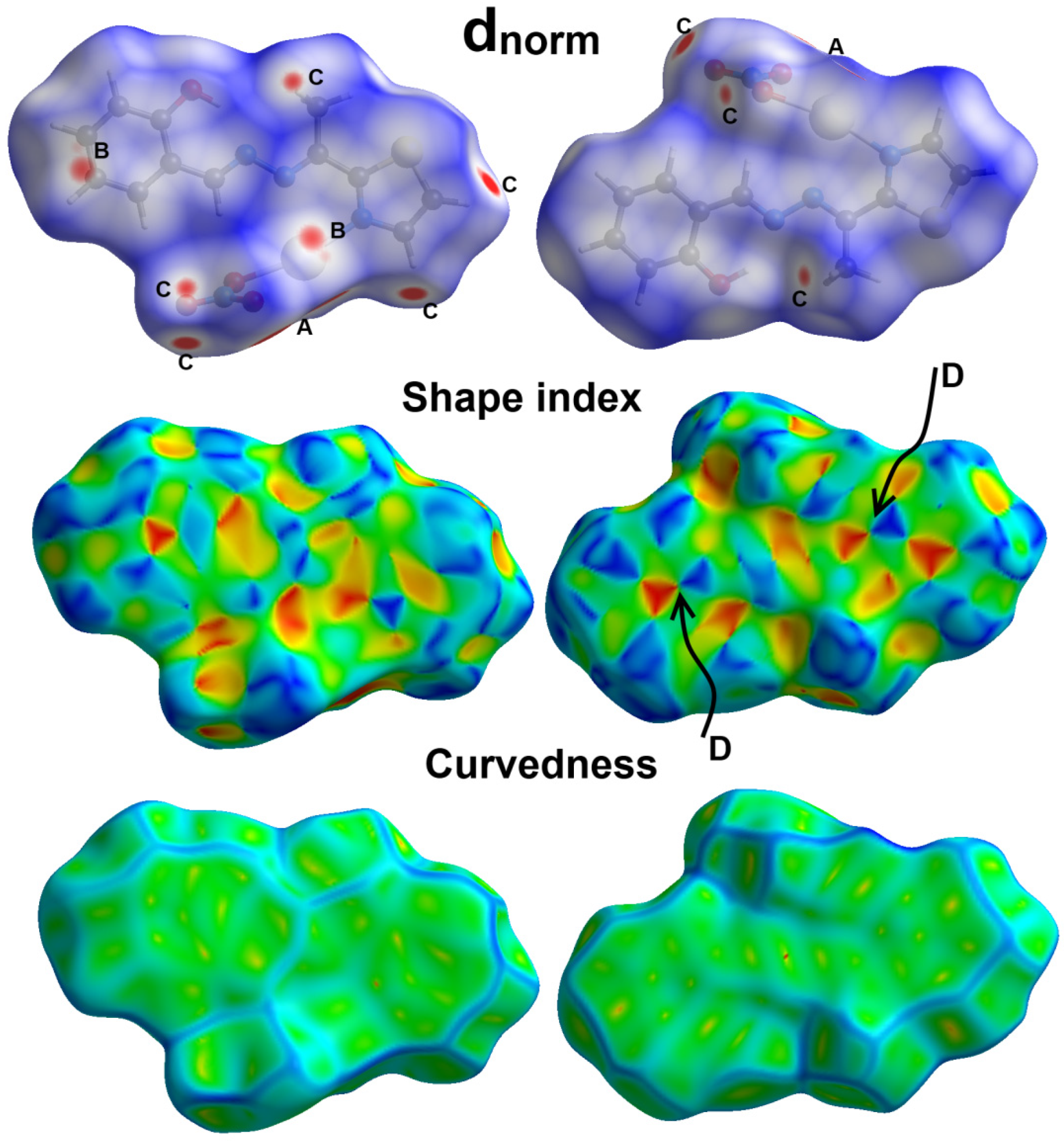
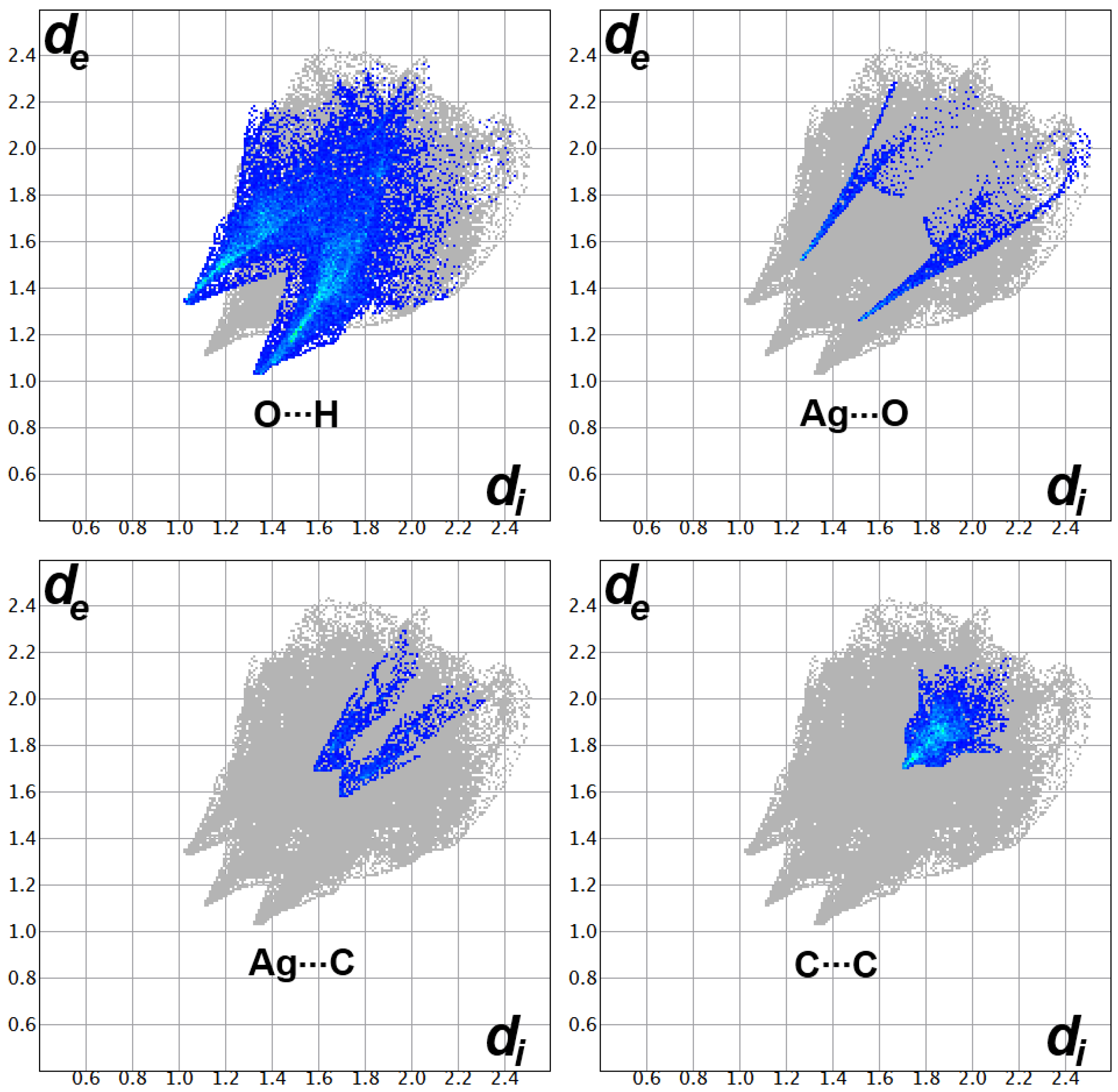
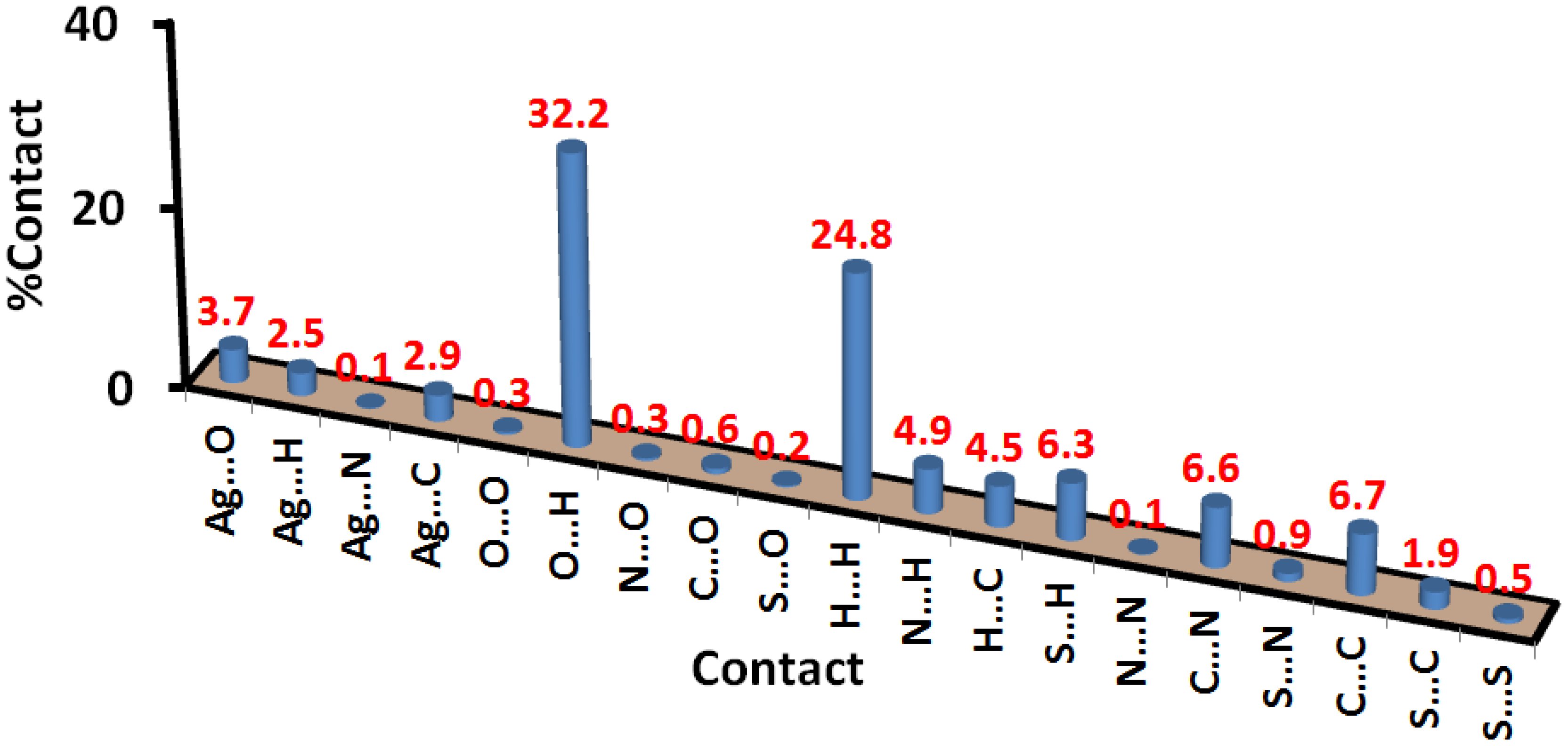
2.3. Hirshfeld Analysis
2.4. Biological Studies
2.4.1. Antimicrobial Activity
2.4.2. Anticancer and Antioxidant Activities
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis of [Ag(L)(NO3)]2 Complex
3.4. Hirshfeld Calculations
3.5. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adsule, S.; Barve, V.; Chen, D.; Ahmed, F.; Dou, Q.P.; Padhye, S.; Sarkar, F.H. Novel Schiff base copper complexes of quinoline-2 carboxaldehyde as proteasome inhibitors in human prostate cancer cells. J. Med. Chem. 2006, 49, 7242–7246. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Serafin, A.; Michie, J.; Van Rensburg, C.; Swarts, J.; Bohm, L. Cytotoxicity and cell death pathways invoked by two new rhodium-ferrocene complexes in benign and malignant prostatic cell lines. Anticancer Res. 2004, 24, 763–770. [Google Scholar] [PubMed]
- Shago, R.F.; Swarts, J.C.; Kreft, E.; Van Rensburg, C.E. Antineoplastic activity of a series of ferrocene-containing alcohols. Anticancer Res. 2007, 27, 3431–3433. [Google Scholar] [PubMed]
- Basu Baul, T.S.; Basu, S.; de Vos, D.; Linden, A. Amino acetate functionalized Schiff base organotin (IV) complexes as anticancer drugs: Synthesis, structural characterization, and in vitro cytotoxicity studies. Investig. New Drugs 2009, 27, 419–431. [Google Scholar] [CrossRef]
- Chan, M.-H.E.; Crouse, K.A.; Tahir, M.I.M.; Rosli, R.; Umar-Tsafe, N.; Cowley, A.R. Synthesis and characterization of cobalt (II), nickel (II), copper (II), zinc (II) and cadmium (II) complexes of benzyl N-[1-(thiophen-2-yl) ethylidene] hydrazine carbodithioate and benzyl N-[1-(thiophen-3-yl) ethylidene] hydrazine carbodithioate and the X-ray crystal structure of bis nickel (II). Polyhedron 2008, 27, 1141–1149. [Google Scholar]
- Zhang, C.X.; Lippard, S.J. New metal complexes as potential therapeutics. Curr. Opin. Chem. Biol. 2003, 7, 481–489. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Johnson, R.K.; Giovenella, A.J.; Faucette, L.F.; Mirabelli, C.K.; Sadler, P.J. Antimicrobial and anticancer activity of tetrahedral, chelated, diphosphine silver (I) complexes: Comparison with copper and gold. J. Inorg. Biochem. 1988, 33, 285–295. [Google Scholar] [CrossRef]
- Zartilas, S.; Hadjikakou, S.K.; Hadjiliadis, N.; Kourkoumelis, N.; Kyros, L.; Kubicki, M.; Baril, M.; Butler, I.S.; Karkabounas, S.; Balzarini, J. Tetrameric 1: 1 and monomeric 1: 3 complexes of silver (I) halides with tri (p-tolyl)-phosphine: A structural and biological study. Inorg. Chim. Acta 2009, 362, 1003–1010. [Google Scholar] [CrossRef]
- Liu, J.J.; Galettis, P.; Farr, A.; Maharaj, L.; Samarasinha, H.; McGechan, A.C.; Baguley, B.C.; Bowen, R.J.; Berners-Price, S.J.; McKeage, M.J. In vitro antitumour and hepatotoxicity profiles of Au (I) and Ag (I) bidentate pyridyl phosphine complexes and relationships to cellular uptake. J. Inorg. Biochem. 2008, 102, 303–310. [Google Scholar] [CrossRef]
- Teyssot, M.-L.; Jarrousse, A.-S.; Manin, M.; Chevry, A.; Roche, S.; Norre, F.; Beaudoin, C.; Morel, L.; Boyer, D.; Mahiou, R. Metal-NHC complexes: A survey of anti-cancer properties. Dalton Trans. 2009, 35, 6894–6902. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-L.; Zhang, X.-M.; Liu, X.-Y.; Wang, X.-J.; Liu, G.-F.; Usman, A.; Fun, H.-K. Clear Ag–Ag bonds in three silver (I) carboxylate complexes with high cytotoxicity properties. Inorg. Chem. Commun. 2003, 6, 1113–1116. [Google Scholar] [CrossRef]
- Patil, S.; Claffey, J.; Deally, A.; Hogan, M.; Gleeson, B.; Menéndez Méndez, L.M.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Synthesis, Cytotoxicity and Antibacterial Studies of p-Methoxybenzyl-Substituted and Benzyl-Substituted N-Heterocyclic Carbene–Silver Complexes; Wiley Online Library: New York, NJ, USA, 2010. [Google Scholar]
- Siciliano, T.J.; Deblock, M.C.; Hindi, K.M.; Durmus, S.; Panzner, M.J.; Tessier, C.A.; Youngs, W.J. Synthesis and anticancer properties of gold (I) and silver (I) N-heterocyclic carbene complexes. J. Organomet. Chem. 2011, 696, 1066–1071. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. Coordination ability of silver (I) with antimycins and actinomycins–Properties of the T-shaped chromophores. Polyhedron 2012, 38, 235–244. [Google Scholar] [CrossRef]
- Pandeya, S.; Sriram, D.; Nath, G.; DeClercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl) thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Chen, H.; Rhodes, J. Schiff base forming drugs: Mechanisms of immune potentiation and therapeutic potential. J. Mol. Med. 1996, 74, 497–504. [Google Scholar] [CrossRef]
- Sakıyan, I.; Logoglu, E.; Arslan, S.; Sari, N.; Şakiyan, N. Antimicrobial activities of N-(2-hydroxy-1-naphthalidene)-amino acid (glycine, alanine, phenylalanine, histidine, tryptophane) Schiff bases and their manganese (III) complexes. Biometals 2004, 17, 115–120. [Google Scholar] [CrossRef]
- Holla, B.S.; Rao, B.S.; Shridhara, K.; Akberali, P. Studies on arylfuran derivatives: Part XI. Synthesis, characterisation and biological studies on some Mannich bases carrying 2, 4-dichlorophenylfurfural moiety. Il Farmaco 2000, 55, 338–344. [Google Scholar] [CrossRef]
- Hearn, M.J.; Cynamon, M.H.; Chen, M.F.; Coppins, R.; Davis, J.; Kang, H.J.-O.; Noble, A.; Tu-Sekine, B.; Terrot, M.S.; Trombino, D. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur. J. Med. Chem. 2009, 44, 4169–4178. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, I.M. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef]
- Ali, M.; Jesmin, M.; Salahuddin, M.; Habib, M.; Khanam, J. Antineoplastic activity of N–salicylideneglycinato–di–aquanickel (II) complex against Ehrlich Ascites Carcinoma (EAC) cells in mice. Int. J. Biol. Chem. Sci. 2008, 2, 292–298. [Google Scholar] [CrossRef][Green Version]
- Ali, M.A.; Haroon, C.M.; Nazimuddin, M.; Majumder, S.; Tarafder, M.T.; Khair, M.A. Synthesis, characterization and biological activities of some new nickel (II), copper (II), zinc (II) and cadmium (II) complexes of quadridentate SNNS ligands. Transit. Met. Chem. 1992, 17, 133–136. [Google Scholar] [CrossRef]
- Hossain, M.E.; Alam, M.N.; Ali, M.A.; Nazimuddin, M.; Smith, F.E.; Hynes, R.C. The synthesis, characterization and bioactivities of some copper (II) complexes of the 2-acetylpyridine Schiff bases of s-methyl-and s-benzyldithiocarbazate, and the x-ray crystal structure of the nitrato (s-benzyl-β-n-(2-acetylpyridyl) methylenedithiocarbazato) copper (II) complex. Polyhedron 1996, 15, 973–980. [Google Scholar]
- Hossain, M.E.; Alam, M.; Begum, J.; Ali, M.A.; Nazimuddin, M.; Smith, F.; Hynes, R. The preparation, characterization, crystal structure and biological activities of some copper (II) complexes of the 2-benzoylpyridine Schiff bases of S-methyl-and S-benzyldithiocarbazate. Inorg. Chim. Acta 1996, 249, 207–213. [Google Scholar] [CrossRef]
- Wang, Q.; Yuen, M.C.W.; Lu, G.L.; Ho, C.L.; Zhou, G.J.; Keung, O.M.; Lam, K.H.; Gambari, R.; Tao, X.M.; Wong, R.S.M. Synthesis of 9, 9-Dialkyl-4, 5-diazafluorene Derivatives and Their Structure–Activity Relationships Toward Human Carcinoma Cell Lines. ChemMedChem Chem. Enabling Drug Discov. 2010, 5, 559–566. [Google Scholar] [CrossRef]
- Constable, E.C.; Steel, P.J. N, N′-Chelating biheteroaromatic ligands; a survey. Coord. Chem. Rev. 1989, 93, 205–223. [Google Scholar] [CrossRef]
- Yamamoto, T.; Zhou, Z.-h.; Kanbara, T.; Shimura, M.; Kizu, K.; Maruyama, T.; Nakamura, Y.; Fukuda, T.; Lee, B.-L.; Ooba, N. π-conjugated donor− acceptor copolymers constituted of π-excessive and π-deficient arylene units. Optical and electrochemical properties in relation to CT structure of the polymer. J. Am. Chem. Soc. 1996, 118, 10389–10399. [Google Scholar] [CrossRef]
- Prasad, K.T.; Gupta, G.; Rao, A.V.; Das, B.; Rao, K.M. New series of platinum group metal complexes bearing η5-and η6-cyclichydrocarbons and Schiff base derived from 2-acetylthiazole: Syntheses and structural studies. Polyhedron 2009, 28, 2649–2654. [Google Scholar] [CrossRef]
- Tan, X.-J.; Liu, H.-Z.; Ye, C.-Z.; Lou, J.-F.; Liu, Y.; Xing, D.-X.; Li, S.-P.; Liu, S.-L.; Song, L.-Z. Synthesis, characterization and in vitro cytotoxic properties of new silver (I) complexes of two novel Schiff bases derived from thiazole and pyrazine. Polyhedron 2014, 71, 119–132. [Google Scholar] [CrossRef]
- Rogovoy, M.I.; Tomilenko, A.V.; Samsonenko, D.G.; Nedolya, N.A.; Rakhmanova, M.I.; Artem’ev, A.V. New silver (I) thiazole-based coordination polymers: Structural and photophysical investigation. Mendeleev Commun. 2020, 30, 728–730. [Google Scholar] [CrossRef]
- Soliman, S.M.; Albering, J.H.; Barakat, A. Unexpected formation of polymeric silver (I) complexes of azine-type ligand via self-assembly of Ag-salts with isatin oxamohydrazide. R. Soc. Open Sci. 2018, 5, 180434. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; Barakat, A. Self-assembly of azine-based hydrolysis of pyridine and isatin oxamohydrazides with AgNO3; synthesis and structural studies of a novel four coordinated Ag (I)-azine 2D coordination polymer. Inorg. Chim. Acta 2019, 490, 227–234. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Khalil, S.M.; Al-Wahaib, D.; Barakat, A.; Soliman, S.M.; Eldissouky, A. Ag (I)-mediated hydrolysis of hydrazone to azine: Synthesis, X-ray structure, and biological investigations of two new Ag (I)-azine complexes. Appl. Organomet. Chem. 2022, 36, e6757. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; do Rego, A.M.B. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Volkov, I.L.; Smirnova, A.; Makarova, A.A.; Reveguk, Z.V.; Ramazanov, R.R.; Usachov, D.Y.; Adamchuk, V.K.; Kononov, A.I. DNA with ionic, atomic, and clustered silver: An XPS study. J. Phys. Chem. B 2017, 121, 2400–2406. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- El-Faham, A.; Soliman, S.M.; Ghabbour, H.A.; Elnakady, Y.A.; Mohaya, T.A.; Siddiqui, M.R.; Albericio, F. Ultrasonic promoted synthesis of novel s-triazine-Schiff base derivatives; molecular structure, spectroscopic studies and their preliminary anti-proliferative activities. J. Mol. Struct. 2016, 1125, 121–135. [Google Scholar] [CrossRef]
- Shang, Y.-F.; Wang, Q.-M.; Zhu, M.-L.; Zhang, Y.-H. 2-(Hydrazonomethyl) phenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o3023. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement; CLSI supplement M100–M121; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
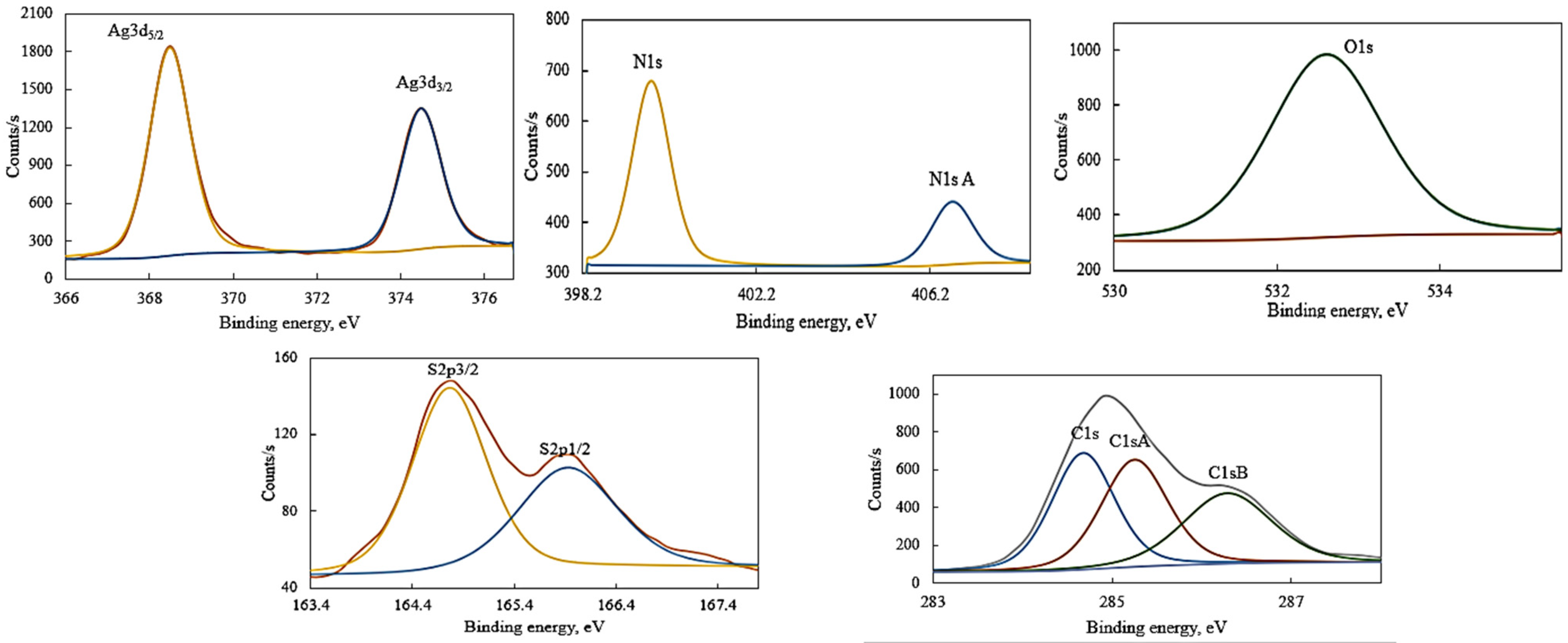


| Name | Peak BE (eV) | Atomic % |
|---|---|---|
| C1s | 284.68 | 21.45 |
| C1s A | 285.25 | 21.08 |
| C1s B | 286.29 | 18.25 |
| O1s | 532.60 | 18.34 |
| N1s | 399.78 | 7.91 |
| N1s A | 406.71 | 2.96 |
| Ag3d, 5/2 | 368.49 | 3.95 |
| Ag3d, 3/2 | 374.50 | 2.66 |
| S2p, 3/2 | 164.76 | 1.97 |
| S2p, 1/2 | 165.92 | 1.42 |
| Bond | Distance (Å) | Bond(s) | Angle (°) |
|---|---|---|---|
| Ag1-N1 | 2.218(5) | N1-Ag1-O2 | 156.3(2) |
| Ag1-O2 | 2.347(6) | N1-Ag1-N2 | 69.37(16) |
| Ag1-N2 | 2.603(4) | O2-Ag1-N2 | 95.65(17) |
| Ag1-O3 | 2.631(6) | O2-Ag1-O3 | 50.4(2) |
| Ag1-O3 # | 2.781(7) | N2-Ag1-O3 # | 164.8(2) |
| O2-Ag1-O3 # | 99.0(2) | ||
| O3-Ag1-O3 # | 79.2(2) |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) | Symm. Code |
|---|---|---|---|---|---|
| O1-H1...N3 | 0.91(11) | 1.82(11) | 2.652(8) | 152(9) | |
| C1-H1A...O4 | 0.93 | 2.46 | 3.149(8) | 131 | x,1+y,z |
| C5-H5A...O2 | 0.96 | 2.58 | 3.435(10) | 149 | 1−x,1−y,1−z |
| C5-H5C...O4 | 0.96 | 2.59 | 3.487(10) | 155 | 3/2−x,1/2+y,3/2−z |
| Microbe | [Ag(L)(NO3)]2 | Free L | Control |
|---|---|---|---|
| A. fumigatus | 31 (20) | - | 17(156) b |
| C. albicans | 18 (625) | - | 20(312) b |
| S. aureus | 8 (5000) | 11(1250) | 24(10) c |
| B. subtilis | 9 (5000) | - | 26(5) c |
| E. coli | 12 (1250) | - | 30(5) c |
| P. vulgaris | 15 (625) | - | 25(5) c |
| Sample Conc. (µg/mL) | Viability % | Inhibitory % | S.D. (±) a |
|---|---|---|---|
| 500 | 1.28 | 98.72 | 0.46 |
| 250 | 3.96 | 96.04 | 0.32 |
| 125 | 8.48 | 91.52 | 0.74 |
| 62.5 | 15.27 | 84.73 | 1.35 |
| 31.25 | 29.96 | 70.04 | 1.82 |
| 15.6 | 42.81 | 57.19 | 1.65 |
| 7.8 | 61.29 | 38.71 | 2.73 |
| 3.9 | 75.46 | 24.54 | 2.08 |
| 2 | 86.13 | 13.87 | 1.95 |
| 1 | 90.47 | 9.53 | 1.21 |
| 0 | 100 | 0 | 0 |
| Sample Conc. (µg/mL) | DPPH Scavenging % | S.D. (±) |
|---|---|---|
| 1280 | 75.18 | 1.68 |
| 640 | 50.73 | 2.09 |
| 320 | 32.91 | 2.43 |
| 160 | 15.29 | 1.17 |
| 80 | 9.73 | 0.65 |
| 40 | 6.28 | 0.46 |
| 20 | 4.09 | 0.35 |
| 10 | 1.85 | 0.29 |
| 5 | 0.91 | 0.17 |
| 2.5 | 0.23 | 0.09 |
| 0 | 0 | 0 |
| CCDC | 2207716 |
|---|---|
| Empirical formula | C24H22Ag2N8O8S2 |
| Fw | 830.35 g/mol |
| Temp | 296(2) K |
| λ | 1.54178 Å |
| cryst syst | Monoclinic |
| Space group | P21/n |
| a/Å | 10.3274(2) Å |
| b/Å | 11.4504(3) Å |
| c/Å | 12.7137(3) Å |
| α/° | 90° |
| β/° | 108.2560(10)° |
| γ/° | 90° |
| V | 1427.76(6) Å3 |
| Z | 2 |
| ρcalc | 1.931 g/cm3 |
| μ(Cu Kα) | 12.933 mm−1 |
| Reflections collected | 15004 |
| Independent reflections | 2487 [R(int) = 0.0628] |
| Completeness to theta = 66.67° | 98.6% |
| Data/restraints/parameters | 2487/0/204 |
| GOOF (F2) | 1.094 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0595, wR2 = 0.1687 |
| R indices (all data) | R1 = 0.0662, wR2 = 0.1793 |
| Largest diff. peak and hole | 1.574 and −1.154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Soliman, S.M.; Al-Wahaib, D.; Barakat, A.; Ali, A.E.; Elbadawy, H.A. Synthesis of a New Dinuclear Ag(I) Complex with Asymmetric Azine Type Ligand: X-ray Structure and Biological Studies. Inorganics 2022, 10, 209. https://doi.org/10.3390/inorganics10110209
Altowyan MS, Soliman SM, Al-Wahaib D, Barakat A, Ali AE, Elbadawy HA. Synthesis of a New Dinuclear Ag(I) Complex with Asymmetric Azine Type Ligand: X-ray Structure and Biological Studies. Inorganics. 2022; 10(11):209. https://doi.org/10.3390/inorganics10110209
Chicago/Turabian StyleAltowyan, Mezna Saleh, Saied M. Soliman, Dhuha Al-Wahaib, Assem Barakat, Ali Eldissouky Ali, and Hemmat A. Elbadawy. 2022. "Synthesis of a New Dinuclear Ag(I) Complex with Asymmetric Azine Type Ligand: X-ray Structure and Biological Studies" Inorganics 10, no. 11: 209. https://doi.org/10.3390/inorganics10110209
APA StyleAltowyan, M. S., Soliman, S. M., Al-Wahaib, D., Barakat, A., Ali, A. E., & Elbadawy, H. A. (2022). Synthesis of a New Dinuclear Ag(I) Complex with Asymmetric Azine Type Ligand: X-ray Structure and Biological Studies. Inorganics, 10(11), 209. https://doi.org/10.3390/inorganics10110209









