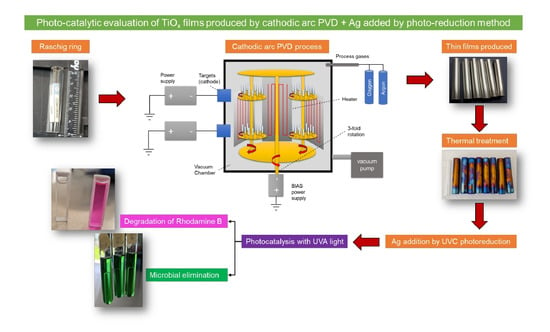
Photocatalytic Evaluation of TiOx Films Produced by Cathodic Arc-PVD with Silver Addition by UVC Photo-Reduction Method
Abstract
1. Introduction
2. Results
2.1. Characterization of the TiOx Arc-PVD Coatings in the As-Coated State
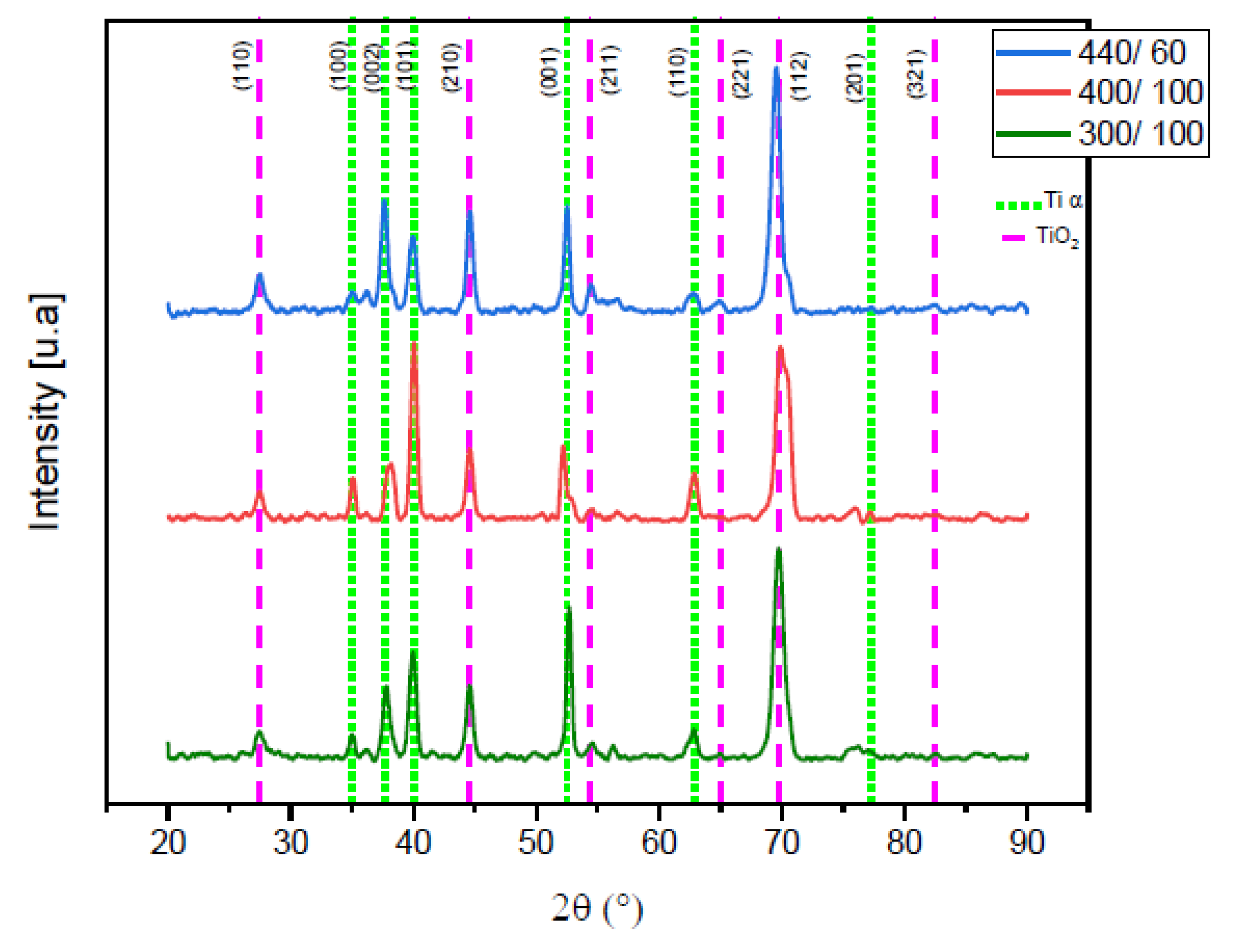
2.1.1. X-ray Diffraction (XRD)
2.1.2. Scanning Electron Microscopy (SEM)
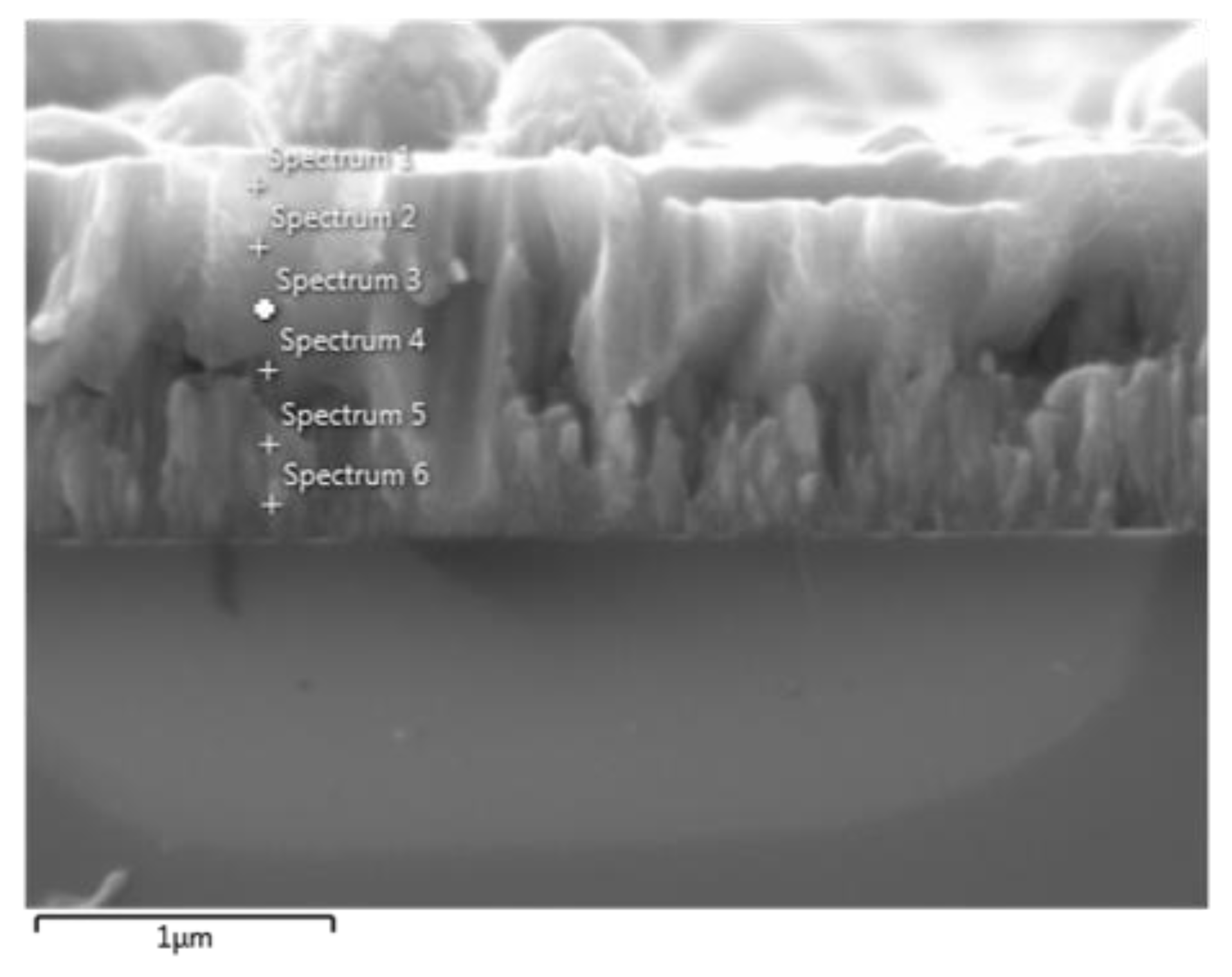
2.1.3. X-ray Energy Dispersive Spectroscopy (EDS)
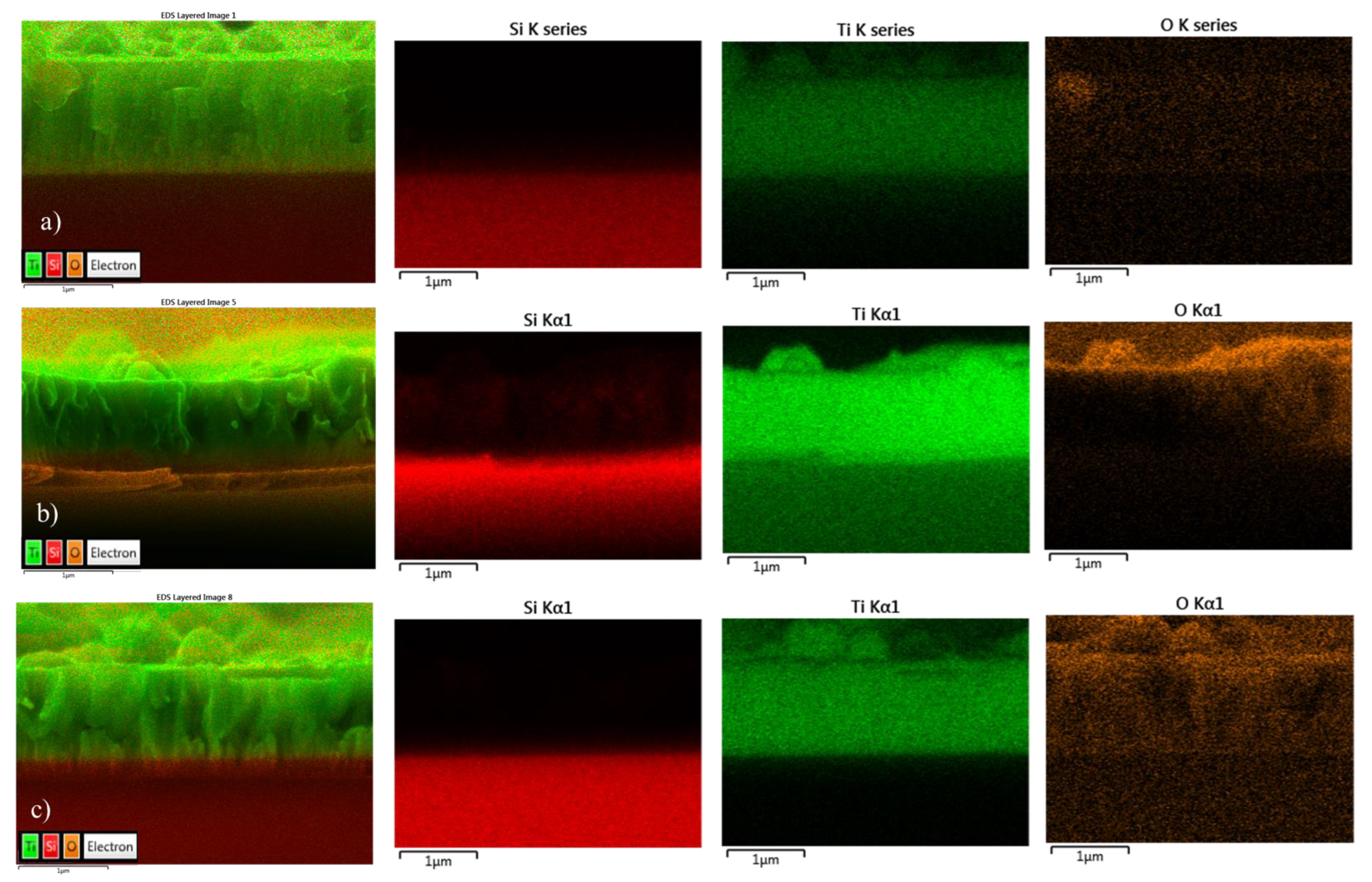
2.1.4. EDS Mapping
2.1.5. EDS Spectra of the Coating with Fluxes of 300 Ar /100 O2
2.2. Characterization of Thin Film by PVD and Heat Treatment
2.2.1. X-ray Diffraction (XRD)
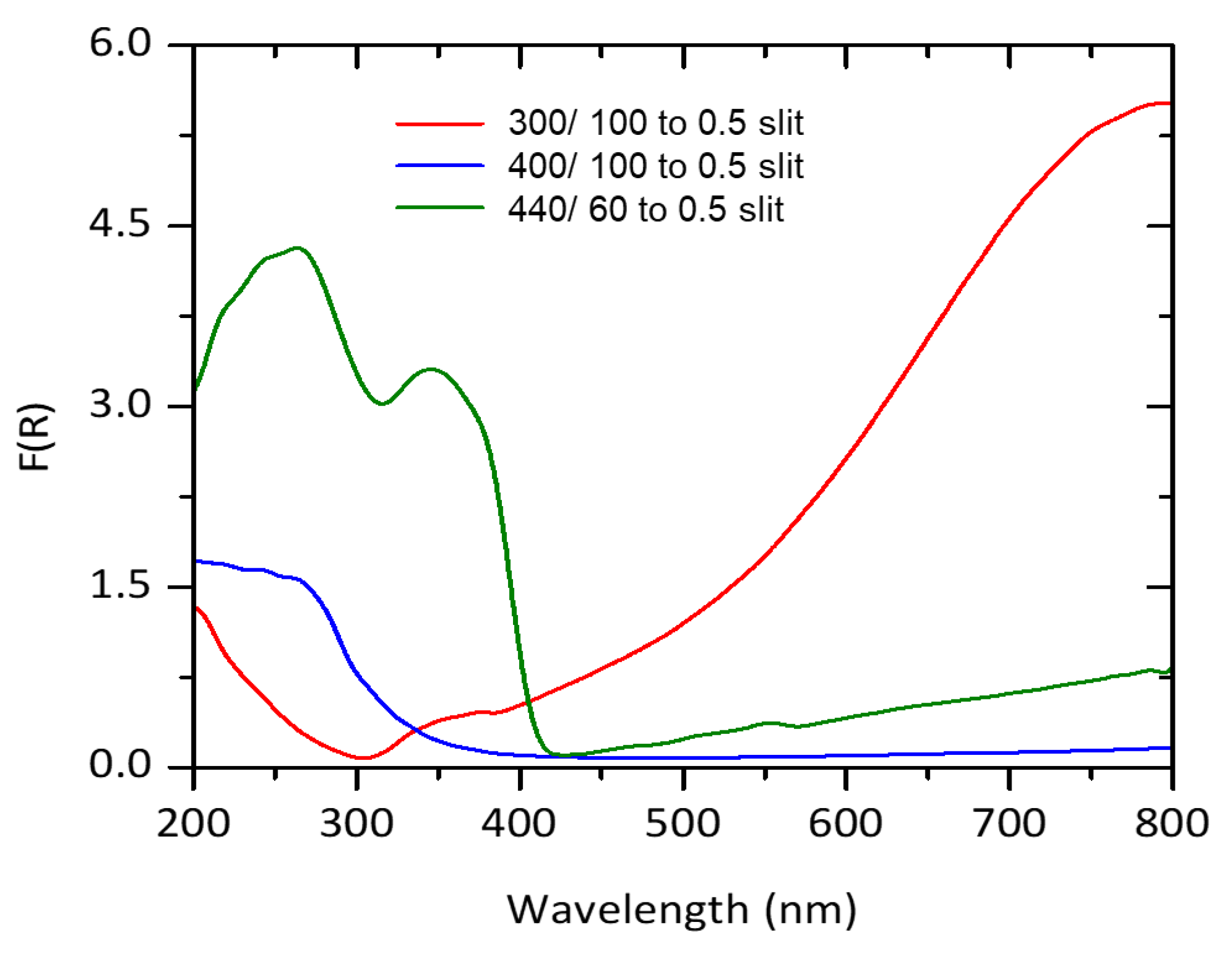
2.2.2. Diffuse Reflectance
2.3. The Effect of Ag Deposited by Photo-Reduction on the TiOx Arc-PVD Coatings
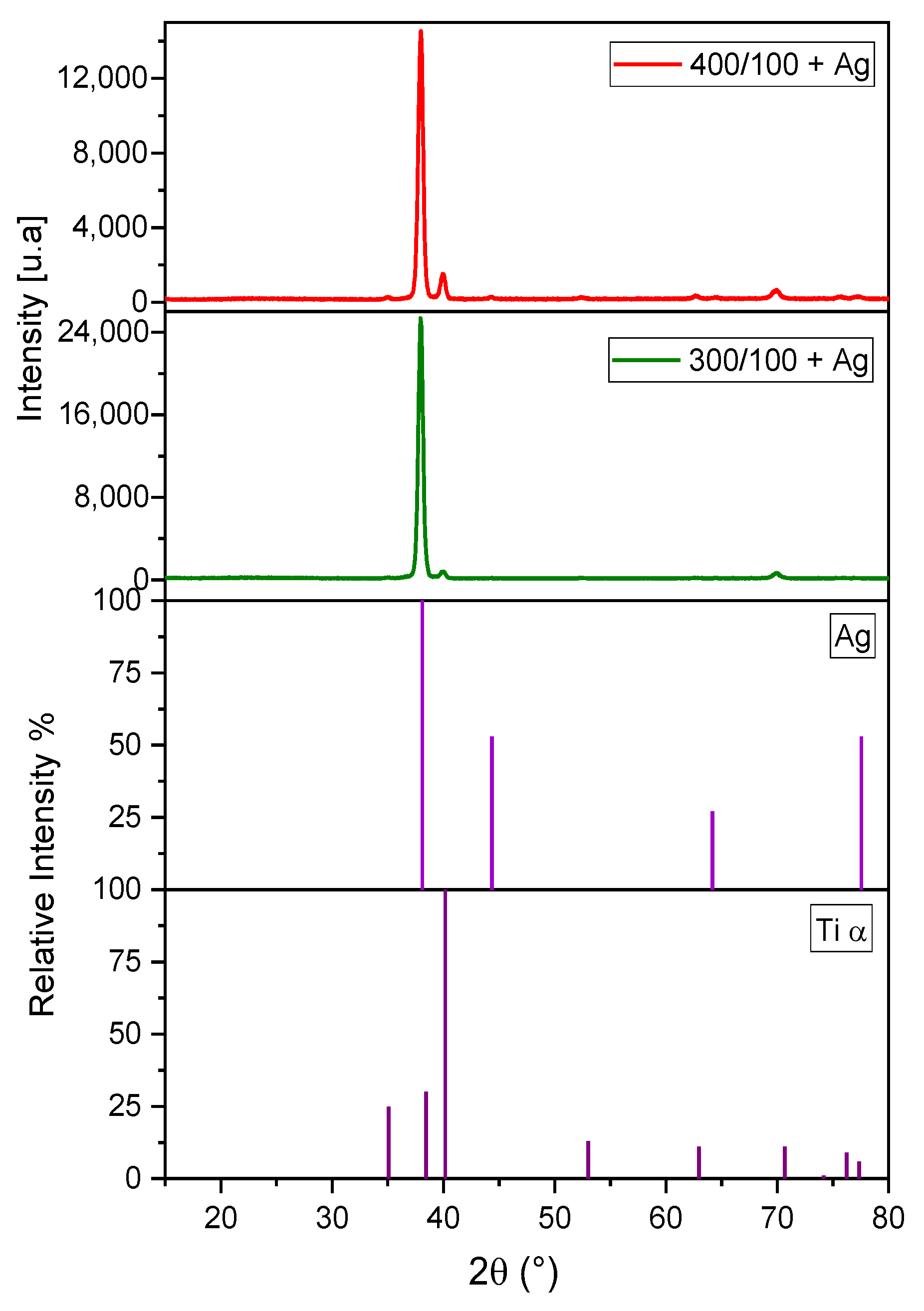
2.3.1. X-ray Diffraction (XRD)
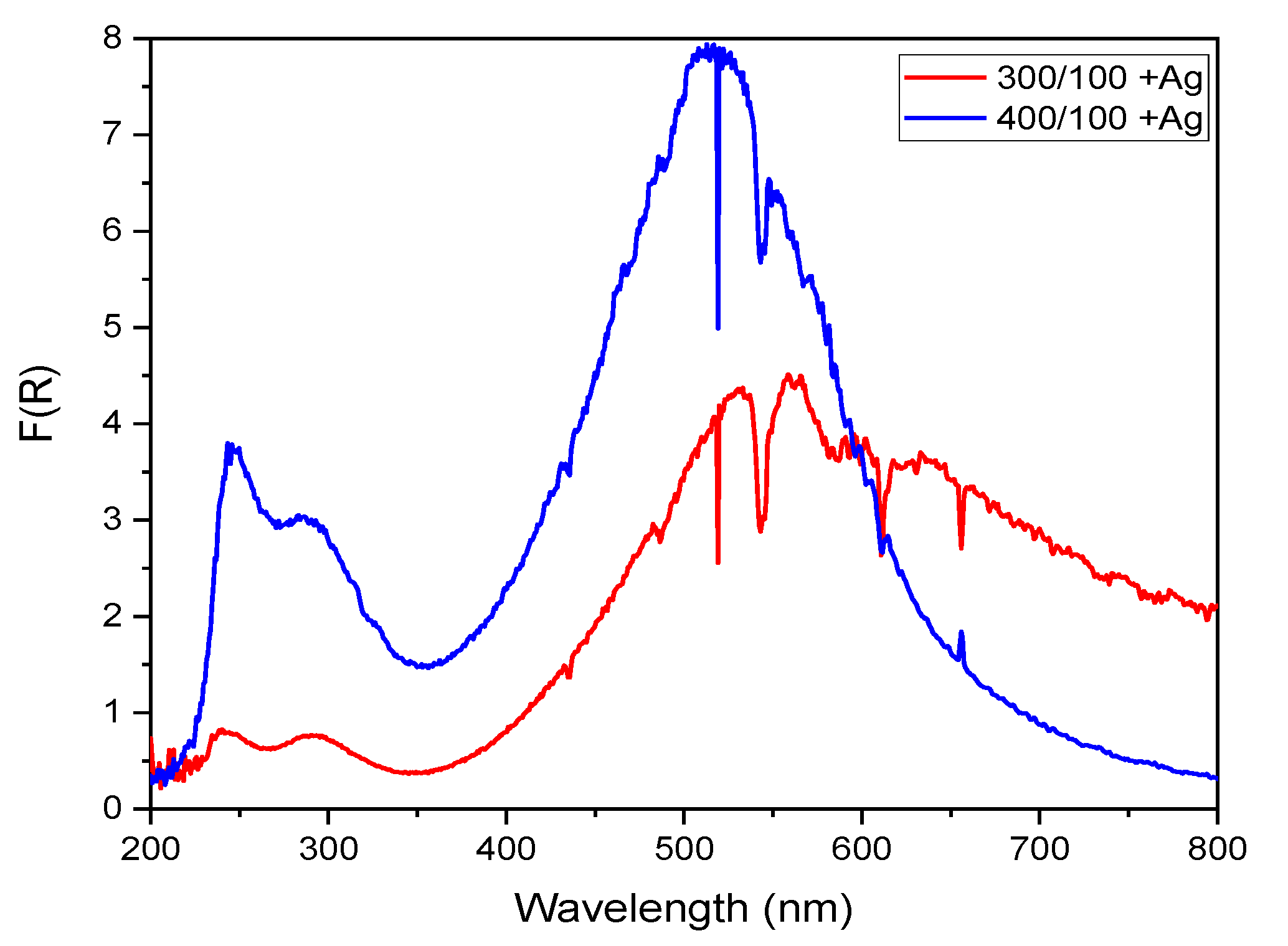
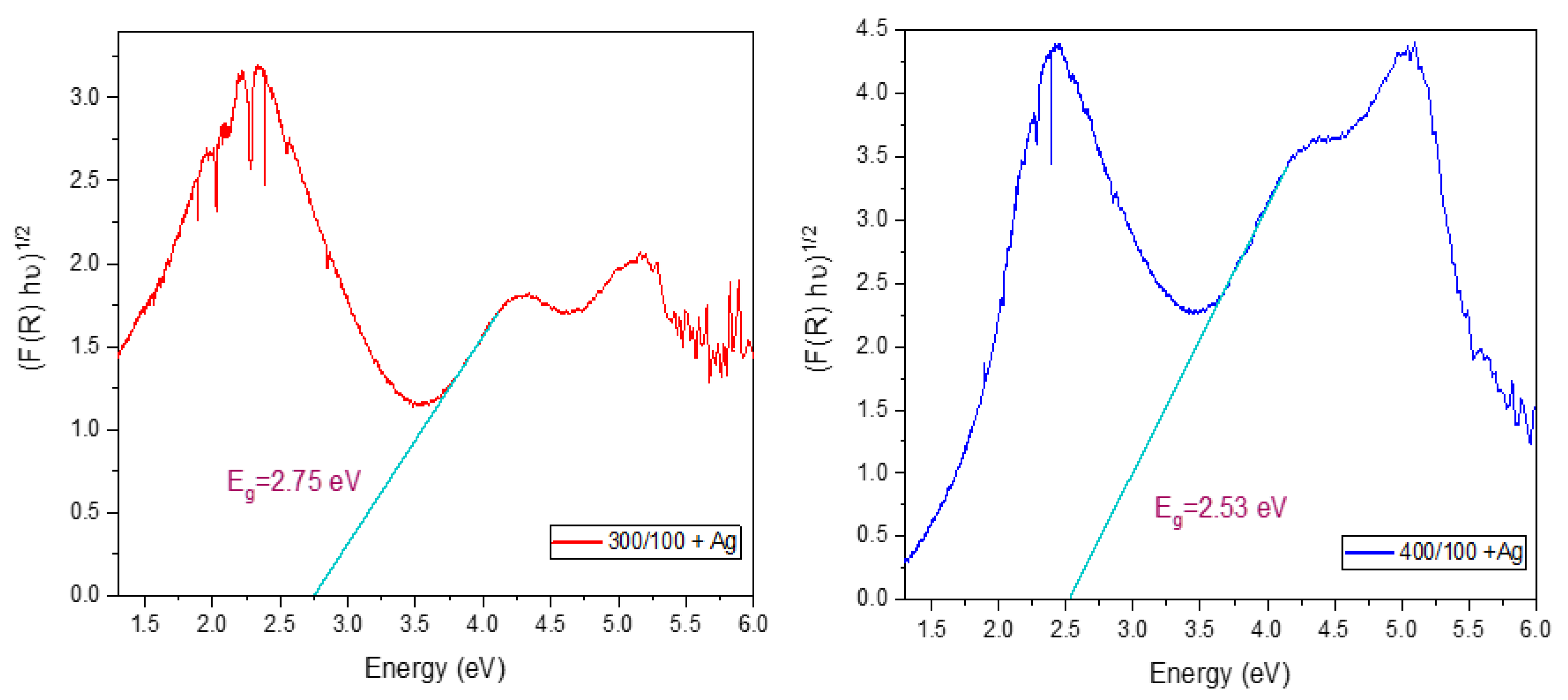
2.3.2. Diffuse Reflectance
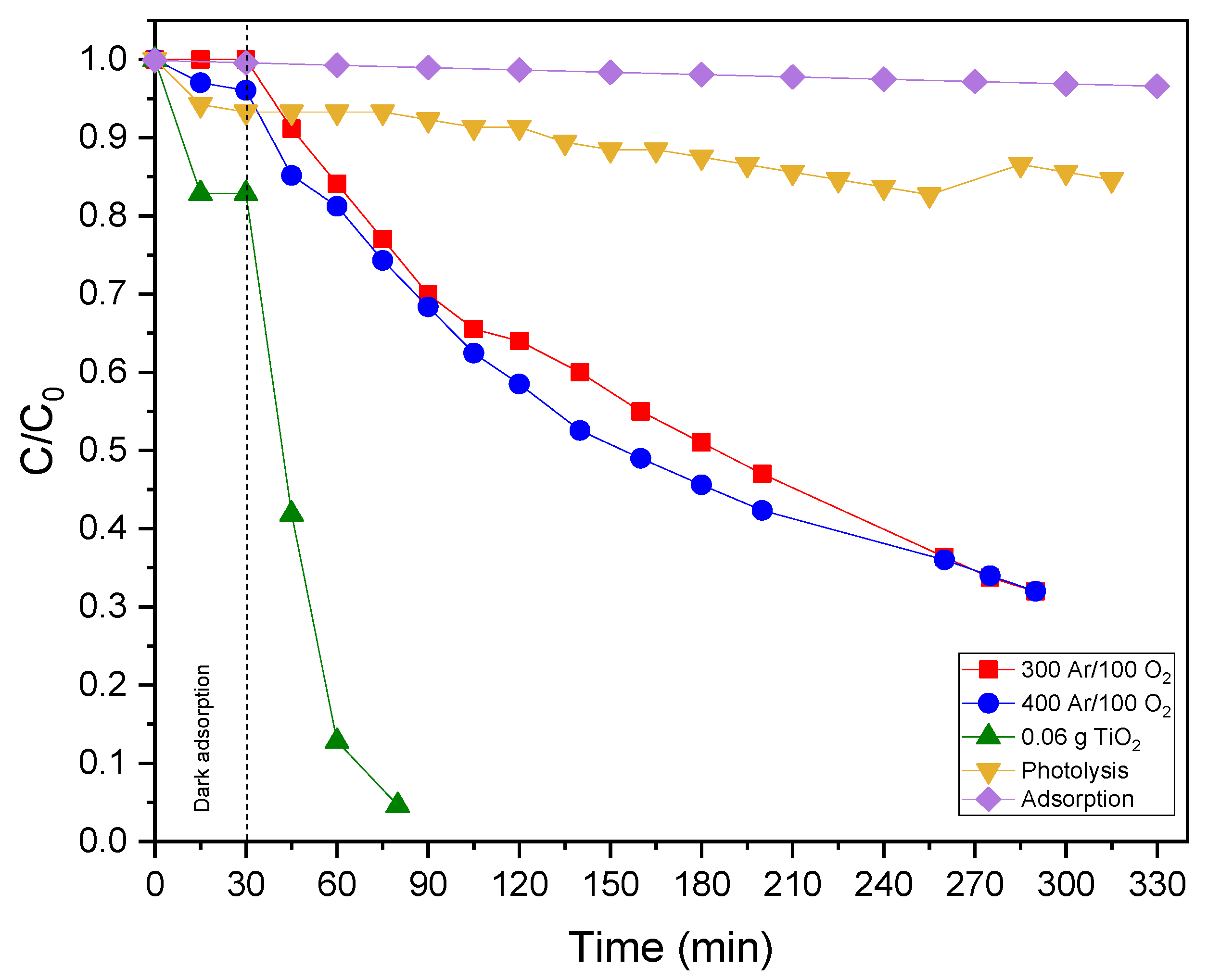
2.4. Degradation of Rhodamine B Dye by the Ag-TiOx Photo-Catalysts
2.5. Determination of Reaction Kinetic Constants
2.6. Disinfection of Secondary Treatment Effluent by the Fixed Ag-TiOx Photo-Catalysts
2.7. Disinfection of Synthetic Water by the Fixed Ag-TiOx Photo-Catalysts
3. Materials and Methods
3.1. Synthesis of Thin Films by PVD on Borosilicate Raschig Rings
3.2. Heat Treatment after PVD Coating
3.3. Doping of the TiOx with Silver
3.4. Dye Degradation Experiments
3.5. Disinfection Experiments
4. Conclusions
- Targeting the application of fixed photo-catalysts, TiOx coatings were deposited by cathodic arc physical vapor deposition on borosilicate Raschig rings. The deposit was designed for O grading using flow ratios of 440 Ar/60 O2, 400 Ar/100 O2, and 300 Ar/100 O2 in a semi-industrial coater unit. The arc-PVD plasma was stable in all Ar/O2 mixtures.
- In the as-coated state, the TiOx catalysts are composed of pure Ti in the α phases. The EDS analysis showed that O and Ti varies in the depth of the coating, suggesting the formation of a system with a low amount of O producing a nonstoichiometric oxide (TiOx). A heat treatment was needed in order to increase the amount of O in the coating and build the crystalline TiO2 rutile phase.
- After the thermal treatment, the analysis by UV vis diffuse reflectance spectroscopy indicated that the TiOx catalytic coatings using 400 Ar/100 O2 and 300 Ar/100 O2 are activated under UVA radiation, while the coating 440 Ar/60 O2 absorbs in the visible spectrum.
- Silver, confirmed by XRD, was added by the UVC photo-reduction method in the TiOx photo-catalysts deposited using 400 Ar/100 O2 and 300 Ar/100 O2 sccm sets. In this Ag-TiOx photo-catalyst, a decrease in the bandgap energy was observed, thus showing moderate photo-catalytic activity in the degradation of rhodamine B at 0.5 ppm and the removal of fecal coliforms from a secondary treatment effluent and synthetic water.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naffouti, W.; Nasr, T.B.; Mehdi, A.; Kamoun-Turki, N. Effect of sprayed solution flow rate on the physical properties of anatase TiO2 thin films. J. Electron. Mater. 2014, 43, 4033–4040. [Google Scholar] [CrossRef]
- Giordano, F.; Abate, A.; Correa, J.P.; Saliba, M.; Matsui, T.; Im, S.H.; Graetzel, M. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 10379. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jin, T.; Liu, C.; Li, G.; Yan, M. Three-Dimensional Graphene-TiO2 Nanocomposite Photocatalyst Synthesized by Covalent Attachment. ACS Omega 2016, 1, 351–356. [Google Scholar] [CrossRef]
- Rane, S.S.; Kajale, D.A.; Arbuj, S.S.; Rane, S.B.; Gosavi, S.W. Hydrogen, ethanol and ammonia gas sensing properties of nano-structured titanium dioxide thick films. J. Mater. Sci. Mater. Electron. 2017, 28, 9011–9016. [Google Scholar] [CrossRef]
- Lanin, S.N.; Vlasenko, E.V.; Kovaleva, N.V.; Zung, F.T. The adsorption properties of titanium dioxide. Russ. J. Phys. Chem. 2008, 82, 2152–2155. [Google Scholar] [CrossRef]
- Nunes, D.; Fortunato, E.; Martins, R. Flexible nanostructured TiO2-based gas and UV sensors: A review. Discov. Mater. 2022, 2, 2. [Google Scholar] [CrossRef]
- Ding, S.N.; Gao, B.H.; Shan, D.; Sun, Y.M.; Cosnier, S. TiO2 nanocrystals electrochemiluminescence quenching by biological enlarged nanogold particles and its application for biosensing. Biosens. Bioelectron. 2013, 39, 342–345. [Google Scholar] [CrossRef]
- Mun, K.S.; Alvarez, S.D.; Choi, W.Y.; Sailor, M.J. A stable, label-free optical interferometric biosensor based on TiO2 Nanotube Arrays. ACS Nano 2010, 4, 2070–2076. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Y.; Deng, K.; Chen, Y.; Li, X.; Deng, X.; Cheng, Z.; Lian, H.; Li, C.; Lin, J. UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: Near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano 2015, 9, 2584–2599. [Google Scholar] [CrossRef]
- Deepagan, V.G.; You, D.G.; Um, W.; Ko, H.; Kwon, S.; Choi, K.Y.; Yi, G.R.; Lee, J.Y.; Lee, D.S.; Kim, K.; et al. Long-Circulating Au-TiO2 Nanocomposite as a Sonosensitizer for ROS-Mediated Eradication of Cancer. Nano Lett. 2016, 16, 6257–6264. [Google Scholar] [CrossRef]
- Chinnamuthu, P.; Dhar, J.C.; Mondal, A.; Bhattacharyya, A.; Singh, N.K. Ultraviolet detection using TiO2 nanowire array with Ag Schottky contact. J. Phys. D Appl. Phys. 2012, 45, 135102–135105. [Google Scholar] [CrossRef]
- Zhang, H.; Ruan, S.; Li, H.; Zhang, M.; Lv, K.; Feng, C.; Chen, W. Schottky diode ultraviolet detector based on TiO2 nanowire array. IEEE Electron. Device Lett. 2012, 33, 83–85. [Google Scholar] [CrossRef]
- Yu, H.; Chen, J.; Wang, W.; Dai, Q. Optical waveguide lightmode spectroscopy (OWLS) as a sensor for thin film and quantum dot corrosion. Sensors 2012, 12, 17330–17342. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Chen, J.; Tao, X. Enhanced photoelectrocatalytic activity of reduced graphene oxide/TiO2 composite films for dye degradation. Chem. Eng. J. 2012, 198, 547–554. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Morozova, M.; Kluson, P.; Krysa, J.; Zlamal, M.; Solcova, O.; Kment, S.; Steck, T. Role of the template molecular structure on the photo-electrochemical functionality of the sol-gel titania thin films. J. Sol-Gel Sci. Technol. 2009, 52, 398–407. [Google Scholar] [CrossRef]
- Kment, S.; Hubicka, Z.; Kmentova, H.; Kluson, P.; Krysa, J.; Gregora, I.; Morozova, M.; Cada, M.; Petras, D.; Dytrych, P.; et al. Photoelectrochemical properties of hierarchical nanocomposite structure: Carbon nanofibers/TiO2/ZnO thin films. Catal. Today 2011, 161, 8–14. [Google Scholar] [CrossRef]
- Kment, S.; Kluson, P.; Hubicka, Z.; Krysa, J.; Cada, M.; Gregora, I.; Deyneka, A.; Remes, Z.; Zabova, H.; Jastrabik, L. Double hollow cathode plasma jet-low temperature method for the TiO2-xNx photoresponding films. Electrochim. Acta 2010, 55, 1548–1556. [Google Scholar] [CrossRef]
- Kment, S.; Kluson, P.; Bartkova, H.; Krysa, J.; Churpita, O.; Cada, M.; Virostko, P.; Kohout, M.; Hubicka, Z. Advanced methods for titanium (IV) oxide thin functional coatings. Surf. Coat. Technol. 2008, 202, 2379–2383. [Google Scholar] [CrossRef]
- Karuppuchamy, S.; Nonomura, K.; Yoshida, T.; Sugiura, T.; Minoura, H. Cathodic electrodeposition of oxide semiconductor thin films and their application to dye-sensitized solar cells. Solid State Ion. 2002, 151, 19–27. [Google Scholar] [CrossRef]
- Djošić, M.; Mišković-Stanković, V.; Janaćković, D.T.; Kačarević-Popović, Z.; Petrović, R. Electrophoretic deposition and characterization of boehmite coatings on titanium substrate. Colloids Surf. 2006, 274, 185–191. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X. Preparation and characterization of a novel activated carbon-supported N-doped visible light response photocatalyst (TiO2 − xNy/AC). J. Chem. Technol. Biotechnol. 2007, 82, 453–459. [Google Scholar] [CrossRef]
- Okada, K.; Takamatsu, Y.; Tokudome, Y.; Nakahira, A.; Takahashi, M. Highly oriented growth of titanate nanotubes (TNTs) in micro and confinement spaces on sol-gel derived amorphous TiO2 thin films under moderate hydrothermal condition. J. Sol-Gel Sci. Technol. 2013, 65, 36–40. [Google Scholar] [CrossRef]
- Kment, S.; Kmentova, H.; Hubicka, Z.; Olejnicek, J.; Cada, M.; Krysa, J. Enhanced photocatalytic activity of silver-doped nanoparticulate TiO2 thin films with respect to the method of doping. Res. Chem. Intermed. 2015, 41, 9343–9355. [Google Scholar] [CrossRef]
- Muaz, A.K.M.; Hashim, U.; Ibrahim, F.; Thong, K.L.; Mohktar, M.S.; Liu, W.W. Effect of annealing temperatures on the morphology, optical and electrical properties of TiO2 thin films synthesized by the sol–gel method and deposited on Al/TiO2/SiO2/p-Si. Microsyst. Technol. 2016, 22, 871–881. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Lou, L. Photodegradation of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J. Photochem. Photobiol. 2004, 163, 281–287. [Google Scholar] [CrossRef]
- Tonejc, A.M.; Turković, A.; Gotić, M.; Musić, S.; Vuković, M.; Trojko, R.; Tonejc, A. HREM, TEM and XRD observation of nanocrystalline phases in TiO2 obtained by the sol-gel method. Mater. Lett. 1997, 31, 127–131. [Google Scholar] [CrossRef]
- Kavaliunas, V.; Sestakauskaite, A.; Sriubas, M.; Laukaitis, G. Influence of Deposition Parameters on the Structure of TiO Thin Films Prepared by Reactive Magnetron Sputtering Technique. Lect. Notes Netw. Syst. 2019, 53, 49–57. [Google Scholar] [CrossRef]
- Sobrinho, V.S.S.; Neto, J.Q.M.; Lima, L.L.F.; Souza, I.A.; Libório, M.S.; Queiroz, J.C.A.; Sousa, R.R.M.; Almeida, E.O.; Feitor, M.C.; Costa, T.H.C. Optical-Electrical Properties and Thickness Analysis of TiO2 Thin Films Obtained by Magnetron Sputtering. Braz. J. Phys. 2020, 50, 771–779. [Google Scholar] [CrossRef]
- Horprathum, M.; Eiamchai, P.; Chindaudom, P.; Pokaipisit, A.; Limsuwan, P. Oxygen partial pressure dependence of the properties of TiO2 thin films deposited by DC reactive magnetron sputtering. Procedia Eng. 2012, 32, 676–682. [Google Scholar] [CrossRef]
- Guo, D.; Ito, A.; Goto, T.; Tu, R.; Wang, C.; Shen, Q.; Zhang, L. Preparation of rutile TiO2 thin films by laser chemical vapor deposition method. J. Adv. Ceram. 2013, 2, 162–166. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Jehng, J.; Liu, L. Preparation of TiO2/MCM-41 by plasma enhanced chemical vapor deposition method and its photocatalytic activity. Front. Environ. Sci. Eng. 2011, 6, 304–312. [Google Scholar] [CrossRef]
- Naffouti, W.; Jrad, A.; Nasr, T.B.; Ammar, S.; Turki-Kamoun, N. Structural, morphological and optical properties of TiO2: Mn thin films prepared by spray pyrolysis technique. J. Mater. Sci. Mater. Electron. 2016, 27, 4622–4630. [Google Scholar] [CrossRef]
- Doubi, Y.; Hartiti, B.; Labrim, H.; Fadili, S.; Tahri, M.; Belafhaili, A.; Siadat, M.; Thevenin, P. Experimental study of properties of TiO2 thin films deposited by spray pyrolysis for future sensory applications. Appl. Phys. A 2021, 127, 475. [Google Scholar] [CrossRef]
- Lahiri, R.; Ghosh, A.; Dwivedi, S.M.M.D.; Chakrabartty, S.; Chinnamuthu, P.; Mondal, A. Performance of Erbium-doped TiO2 thin film grown by physical vapor deposition technique. Appl. Phys. A Mater. Sci. Processing 2017, 123, 573. [Google Scholar] [CrossRef]
- Shubham, K.; Chakrabarti, P. Fabrication and characterization of metal/insulator/semiconductor structures based on TiO2 and TiO2/SiO2 thin films prepared by low-temperature arc vapor deposition. Electron. Mater. Lett. 2014, 10, 579–584. [Google Scholar] [CrossRef]
- Yap, Y.K.; Zhang, D. Physical Vapor Deposition. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrech, The Netherlands, 2015; pp. 1–8. [Google Scholar] [CrossRef]
- György, G.; Socol, E.; Axente, I.N.; Mihailescu, C.; Ducu, C.; Ciuca, S. Anatase phase TiO2 thin films obtained by pulsed laser deposition for gas sensing applications. Appl. Surf. Sci. 2005, 247, 429–433. [Google Scholar] [CrossRef]
- Brunella, M.; Diamanti, M.; Pedeferri, M.; Fonzo, F.; Casari, C.; Bassi, A. Photocatalytic behavior of different titanium dioxide layers. Thin Solid Film. 2007, 515, 6309–6313. [Google Scholar] [CrossRef]
- Sahm, H.; Zywitzki, O.; Glöß, D.; Modes, T.; Frach, P.; Goedicke, K. Structure and properties of crystalline titanium oxide layers deposited by reactive pulse magnetron sputtering. Surf. Coat. Technol. 2004, 180, 538–543. [Google Scholar]
- Heo, C.; Lee, S.; Boo, J. Deposition of TiO2 thin films using RF magnetron sputtering method and study of their surface characteristics. Thin Solid Film. 2005, 475, 183–188. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Li, C.; Wang, H.; Chen, L.; Liu, X. Rutile-type titanium oxide films synthesized by filtered arc deposition. Surf. Coat. Technol. 1998, 110, 136–139. [Google Scholar] [CrossRef]
- Takikawa, H.; Matsui, T.; Sakakibara, A.; Bendavid, A.; Martin, P. Properties of titanium oxide film prepared by reactive cathodic vacuum arc deposition. Thin Solid Film. 1999, 348, 145–151. [Google Scholar] [CrossRef]
- Attenberger, W.; Thorwarth, G.; Manova, D.; Mändl, S.; Stritzker, B.; Rauschenbach, B. Interface properties of TiO2 on Si formed by simultaneous implantation and deposition of titanium and oxygen ions. Surf. Coat. Technol. 2001, 142, 412–417. [Google Scholar] [CrossRef]
- Leng, Y.X.; Chen, J.Y.; Sun, H.; Yang, P.; Wan, G.J.; Wang, J.; Huang, N. Properties of titanium oxide synthesized by pulsed metal vacuum arc deposition. Surf. Coat. Technol. 2004, 176, 141–147. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, H.; Guo, J.; Dong, J.; Jia, S.; Zhu, Z. P–N co-doping induced structural recovery of TiO2 for overall water splitting under visible light irradiation. J. Alloy. Compd. 2014, 615, 79–83. [Google Scholar] [CrossRef]
- Noguez, C. Surface Plasmons on Metal Nanoparticles: The Influence of Shape and Physical Environment. J. Phys. Chem. C 2007, 111, 3606–3619. [Google Scholar] [CrossRef]
- Trabelsi, K.; Hajjaji, A.; Ka, I.; Gaidi, M.; Bessais, B.; el Khakani, M.A. Optoelectronic and photocatalytic properties of in situ platinum-doped TiO2 films deposited by means of pulsed laser ablation technique. J. Mater. Sci. Mater. Electron. 2017, 28, 3317–3324. [Google Scholar] [CrossRef]
- Hassan, A.; Kayani, Z.N.; Anwar, M. Effect of Au ions on structural, optical, magnetic, dielectric, and antibacterial properties of TiO2 dip-coated thin films. J. Mater. Sci. Mater. Electron. 2021, 32, 14398–14419. [Google Scholar] [CrossRef]
- Khan, S.; ul Haq, M.; Ma, Y.; Nisar, M.; Li, Y.; Khan, R.; Han, G.; Liu, Y. Structural and optical properties of macroporous Ag@TiO2 thin films prepared by a facile one-step sol–gel method. J. Sol-Gel Sci. Technol. 2019, 93, 273–280. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Badawi, A. Structural and Optical Characteristic of Cu-Doped TiO2 Thin Film. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2853–2862. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Chinthala, M.; Vo, D.V.N. Biopolymer-supported TiO2 as a sustainable photocatalyst for wastewater treatment: A review. Environ. Chem. Lett. 2022, 3, 1–28. [Google Scholar] [CrossRef]
- Obregón, S.; Rodríguez-González, V. Photocatalytic TiO2 thin films and coatings prepared by sol–gel processing: A brief review. J. Sol-Gel Sci. Technol. 2021, 102, 125–141. [Google Scholar] [CrossRef]
- ACLAC. Economic Commission for Latin America and the Caribbean. 2004. Available online: https://observatoriop10.cepal.org/es/tratados/convenio-estocolmo-contaminantes-organicos-persistentes (accessed on 3 March 2022).
- Anjaneyulu, R.B.; Mohan, B.S.; Naidu, G.P.; Muralikrishna, R. Visible light enhanced photocatalytic degradation of methylene blue by ternary nanocomposite, MoO3/Fe2O3/rGO. J. Asian Ceram. Soc. 2018, 6, 183–195. [Google Scholar] [CrossRef]
- Klut, M.E.; Bisalputra, T.; Antia, N.J. The use of fluorochromes in the cytochemical characterization of some phytoflagellates. Histochem. J. 1988, 20, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.A.; Pitts, B.; Stewart, P.S. Rapid Diffusion of Fluorescent Tracers into Staphylococcus epidermidis Biofilms Visualized by Time Lapse Microscopy. Antimicrob. Agents Chemother. 2005, 49, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Rinne, W.E.; Deacon, J.E. Fluorescent Pigment and Immersion Stain Marking Techniques for Lepidomeda mollispinis and Cyprinodon nevadensis. Trans. Am. Fish. Soc. 1973, 102, 459462. [Google Scholar] [CrossRef]
- Sagoo, S.K.; Jockusch, R.A. The fluorescence properties of cationic rhodamine B in the gas phase. J. Photochem. Photobiol. A Chem. 2011, 220, 173–178. [Google Scholar] [CrossRef]
- Wilhelm, P.; Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J. Photochem. Photobiol. A Chem. 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, Y. Synergetic Degradation of Rhodamine B at a Porous ZnWO 4 Film Electrode by Combined Electro-Oxidation and Photocatalysis. Environ. Sci. Technol. 2006, 40, 3367–3372. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Baruah, S.; Anal, A.K.; Dutta, J. Microbial Pathogen Inactivation Using Heterogeneous Photocatalysis. In Environmental Chemistry for a Sustainable World; Springer: Dordrech, The Netherlands, 2012; pp. 511–541. [Google Scholar]
- Hossain, M.A.; Elias, M.; Sarker, D.R.; Diba, Z.R.; Mithun, J.M.; Azad, M.A.K.; Siddiquey, I.A.; Rahman, M.M.; Uddin, J.; Uddin, M. Synthesis of Fe- or Ag-doped TiO2–MWCNT nanocomposite thin films and their visible-light-induced catalysis of dye degradation and antibacterial activity. Res. Chem. Intermed. 2018, 44, 2667–2683. [Google Scholar] [CrossRef]
- Hou, X.; Wu, X.; Liu, A. Studies on photocatalytic activity of Ag/TiO2 films. Front. Chem. China 2006, 1, 402–407. [Google Scholar] [CrossRef]
- Kader, S.; Al-Mamun, M.R.; Suhan, M.B.K.; Shuchi, S.B.; Islam, M.S. Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3 and Ag doped TiO2 photocatalysts. Environ. Technol. Innov. 2022, 27, 102476. [Google Scholar] [CrossRef]
- Wang, X.; Hou, X.; Luan, W.; Li, D.; Yao, K. The antibacterial and hydrophilic properties of silver-doped TiO2 thin films using sol–gel method. Appl. Surf. Sci. 2012, 258, 8241–8246. [Google Scholar] [CrossRef]
- Molina-Reyes, J.; Romero-Morán, A.; Sánchez-Salas, J.L. Enhanced photocatalytic bacterial inactivation of atomic-layer deposited anatase-TiO2 thin films on rutile-TiO2 nanotubes. Photochem. Photobiol. Sci. 2020, 19, 399–405. [Google Scholar] [CrossRef]
- Araujo-Scharnber, A. Porous ceramic supported TiO2 nanoparticles: Enhanced photocatalytic activity for rhodamine B degradation. Bol. Soc. Esp. Cerám. Vidr. 2020, 59, 230–238. [Google Scholar] [CrossRef]
- Polo-Lopez, M.I.; Fernandez-Ibañez, P.; Garcia-Fernandez, I.; Oller, I.; Salgado-Trnsito, I.; Sichel, C. Resistance of Fusarium sp spores to tolar TiO2 photocatalysis: Influence of spore type and water. Scaling-Up Results 2010, 85, 1038–1048. [Google Scholar]
- Sichel, C.; Blanco, J.; Malato, S.; Fernández-Ibáñez, P. Effects of experimental conditions on E. coli survival during solar photocatalytic water disinfection. J. Photochem. Photobiol. A Chem. 2007, 189, 239–246. [Google Scholar] [CrossRef]
- Christensen, P.; Curtis, T.; Egerton, T.; Kosa, S.; Tinlin, J. Photoelectrocatalytic and photocatalytic disinfection of E. coli suspensions by titanium dioxide. Apllied Catal. B Environ. 2003, 41, 371–386. [Google Scholar] [CrossRef]
- Lopes, P.; Montagnoli, R.; Bidoia, E. Photocatalytic Degradation of phenol by thermal titanium dioxide thin layer electrodes. Water Air Soil Pollut. 2012, 223, 3673–3688. [Google Scholar] [CrossRef]
- Santos, A. Water Disinfection by Photocatalytic Process Using Titanium Dioxide Thermal Electrodes for Inactivation of Escherichia Coli and Staphylococcus aureus; Bioscience Institute; UNESP—Univ Estadual Paulista: Rio Claro, Brazil, 2008. [Google Scholar]
- Zhao, G.; Kozuka, H.; Yoko, T. Sol—gel preparation and photoelectrochemical properties of TiO2 films containing Au and Ag metal particles. Thin Solid Films 1996, 277, 147–154. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; Gonzalez-Elipe, A.R.; Fernandez, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Zarei, M.; Jamnejad, A.; Khajehali, E. Antibacterial effect of silver nanoparticles against four foodborme pathogens. Jundishapur J. Microbiol. 2014, 7, e8720. [Google Scholar] [CrossRef] [PubMed]






| Photocatalyst | Pollutant | Pollutant Concentration | Reaction Time and Type of Irradiation | Results |
|---|---|---|---|---|
| Ag-doped TiO2 thin films using a modified sol-gel method and photo-chemical deposition [24] | Rhodamine B (RhB) | 10 mL of RhB solution with initial concentration of 10−4 mol−1 | 90 min, UV lamp (400 W) | Degradation efficiency from 38% to 90%. |
| Fe- or Ag-doped TiO2 –MWCNT nanocomposite thin films by sol–gel drop coating method [63] | Methylene blue (MB) | 200 mL of MB solution with initial concentration of 5 × 10−6 M | 240 min, visible-light (200 W) | Degradation efficiency from 40.39% to 58.27%. kap = 0.002 and 0.003 min−1 |
| Ag/TiO2 films by hybrid sol-gel method [64] | Methyl orange (MO) | 100 mL of MO solution with initial concentration of 5 × 10−5 mol/L | 60 min, UV lamp (1000 W) | Kap = 0.0014 min−1 |
| Synthesis of Ag/TiO2 nanocomposites immobilized by Dip-coating on borosilicate glass [65] | Methyl orange (MO) | 125 mL of MO solution with initial concentration of 10 ppm at a fixed pH (7.0) | 5.5 h, UV lamp (100 W) | Degradation efficiency from 59.5% to 75.8%. kap = 0.003 min−1 |
| Ag–TiO2 composite thin films deposited on glass slides by sol–gel spin coating technique [66] | Escherichia coli | 10 mL E. coli solution with initial concentration of 4.46 × 108 CFU/mL | 80 s, UV-C lamp (15 W) | Bacterial elimination 94% |
| Atomic layer deposited anatase-TiO2 thin films on rutile-TiO2 nanotubes [67] | Escherichia coli | ∼106 CFU mL−1 | 120 min, UV-A lamp | Bacterial elimination 40% |
| Ti α | ||
|---|---|---|
| 2ϴ Angle | hkl Plane | Intensity [a.u.] |
| 35.094 | (100) | 315.3 |
| 38.422 | (002) | 36,126.04 |
| 40.171 | (101) | 1690.58 |
| 53.005 | (102) | 20.89 |
| 77.37 | (201) | 20.24 |
| 82.292 | (004) | 1033.52 |
| Spectrum | % Weight | % Atomic | ||
|---|---|---|---|---|
| O | Ti | O | Ti | |
| 1 | 12.17 | 87.83 | 29.32 | 70.68 |
| 2 | 6.86 | 93.14 | 18.07 | 81.93 |
| 3 | 4.58 | 95.42 | 12.56 | 87.44 |
| 4 | 3.66 | 96.34 | 10.21 | 89.79 |
| 5 | 4.89 | 95.11 | 13.34 | 86.66 |
| 6 | 5.99 | 94.01 | 16.01 | 83.99 |
| 7 | 9.28 | 90.72 | 23.44 | 76.56 |
| Reaction Order | Reaction Speed (kap) | Integration of kap | Line Graph to Determine kap | Half Life Time (t1/2) |
|---|---|---|---|---|
| First order |
| Photocatalyst | C/C0 | kap (min−1) | Half Life Time (t1/2) (min) | R2 |
|---|---|---|---|---|
| TiOx coating 400 Ar /100 O2 | 0.318 | 0.0041 | 169.06 | 0.9937 |
| TiOx coating 300 Ar/100 O2 | 0.319 | 0.0038 | 182.4 | 0.9951 |
| Powder-TiO2 | 0.04 | 0.0595 | 11.64 | 0.9904 |
| NMP/100 mL | ||||
|---|---|---|---|---|
| Ag-TiOx Coatings | ||||
| Time | 300 Ar/100 O2 | 400 Ar/100 O2 | Photolysis | Rings in the dark |
| 0 | 46,000 | 9300 | 400 | 400 |
| 4 h | 7500 | 900 | 400 | 400 |
| % removal | 83.69 | 90.32 | 0 | 0 |
| logarithmic units | ||||
| 0 | 4.66 | 3.96 | 2.60 | 2.60 |
| 4 h | 3.87 | 2.95 | 2.60 | 2.60 |
| Ulog reduction | 0.79 | 1.01 | 0 | 0 |
| NMP/100 mL | ||||
|---|---|---|---|---|
| Ag-TiOx Coatings | ||||
| Time | 300 Ar/100 O2 | 400 Ar/100 O2 | Photolysis | Rings in the dark |
| 0 | 150,000 | 11,000,000 | 24,000,000 | 11,000,000 |
| 4 h | 21,000 | 2,400,000 | 24,000,000 | 11,000,000 |
| % removal | 86 | 78.18 | 0 | 0 |
| logarithmic units | ||||
| 0 | 5.17 | 7.04 | 7.38 | 7.04 |
| 4 h | 4.32 | 6.38 | 7.38 | 7.04 |
| Ulog Reduction | 0.85 | 0.66 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raya-Tapia, A.Y.; Ung-Medina, F.; Mondragón-Rodríguez, G.C.; Rivera-Muñoz, E.M.; Apolinar-Cortés, J.; Méndez, F.J.; Huirache-Acuña, R. Photocatalytic Evaluation of TiOx Films Produced by Cathodic Arc-PVD with Silver Addition by UVC Photo-Reduction Method. Inorganics 2022, 10, 148. https://doi.org/10.3390/inorganics10100148
Raya-Tapia AY, Ung-Medina F, Mondragón-Rodríguez GC, Rivera-Muñoz EM, Apolinar-Cortés J, Méndez FJ, Huirache-Acuña R. Photocatalytic Evaluation of TiOx Films Produced by Cathodic Arc-PVD with Silver Addition by UVC Photo-Reduction Method. Inorganics. 2022; 10(10):148. https://doi.org/10.3390/inorganics10100148
Chicago/Turabian StyleRaya-Tapia, Alma Yunuen, Francisco Ung-Medina, Guillermo César Mondragón-Rodríguez, Eric Mauricio Rivera-Muñoz, José Apolinar-Cortés, Franklin J. Méndez, and Rafael Huirache-Acuña. 2022. "Photocatalytic Evaluation of TiOx Films Produced by Cathodic Arc-PVD with Silver Addition by UVC Photo-Reduction Method" Inorganics 10, no. 10: 148. https://doi.org/10.3390/inorganics10100148
APA StyleRaya-Tapia, A. Y., Ung-Medina, F., Mondragón-Rodríguez, G. C., Rivera-Muñoz, E. M., Apolinar-Cortés, J., Méndez, F. J., & Huirache-Acuña, R. (2022). Photocatalytic Evaluation of TiOx Films Produced by Cathodic Arc-PVD with Silver Addition by UVC Photo-Reduction Method. Inorganics, 10(10), 148. https://doi.org/10.3390/inorganics10100148