Anisometric Ln(III) Complexes with Efficient Near-IR Luminescence
Abstract
1. Introduction
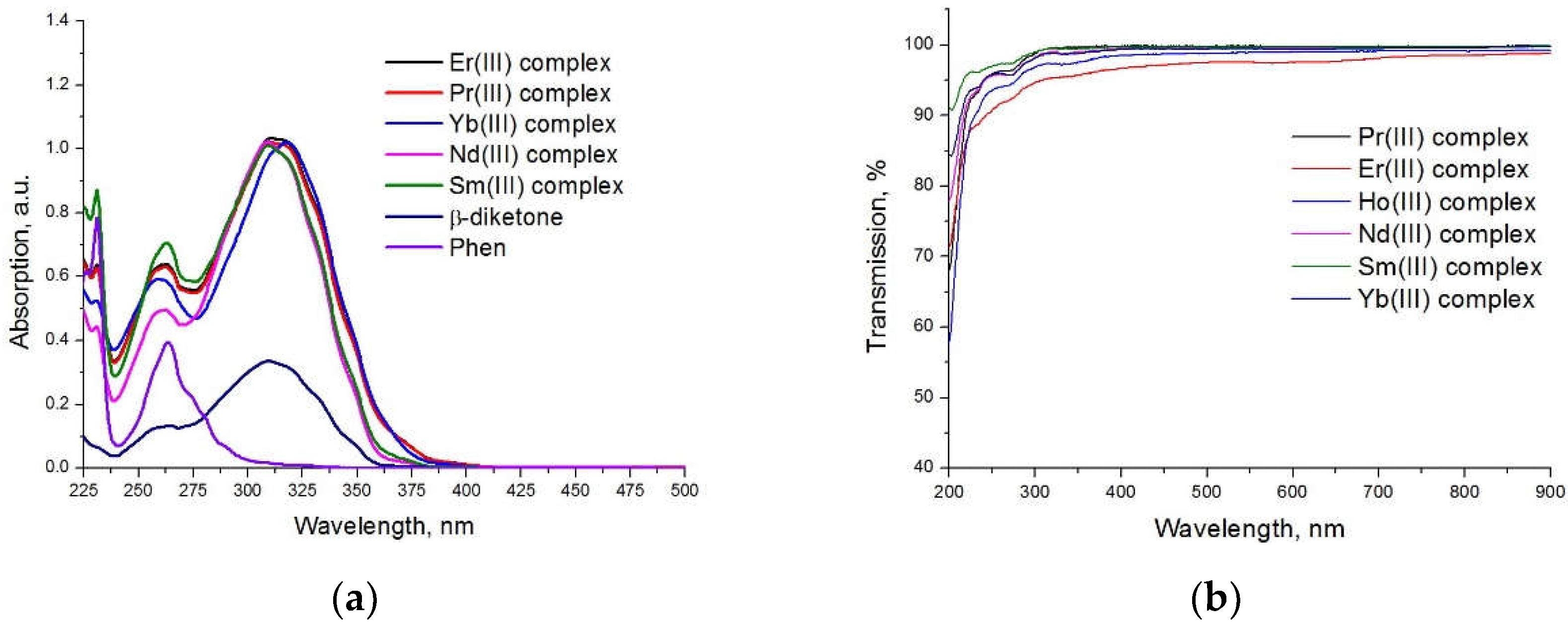
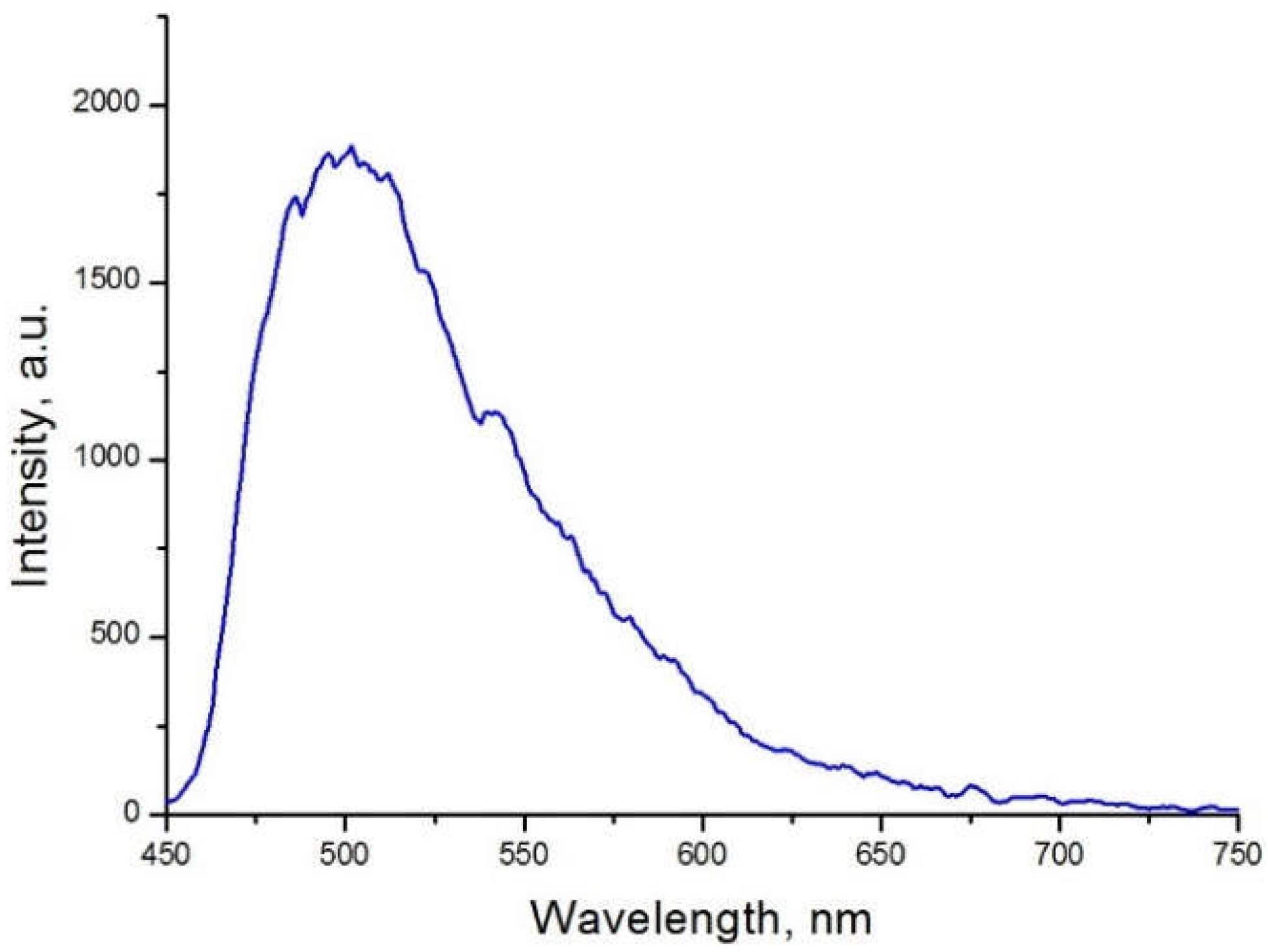
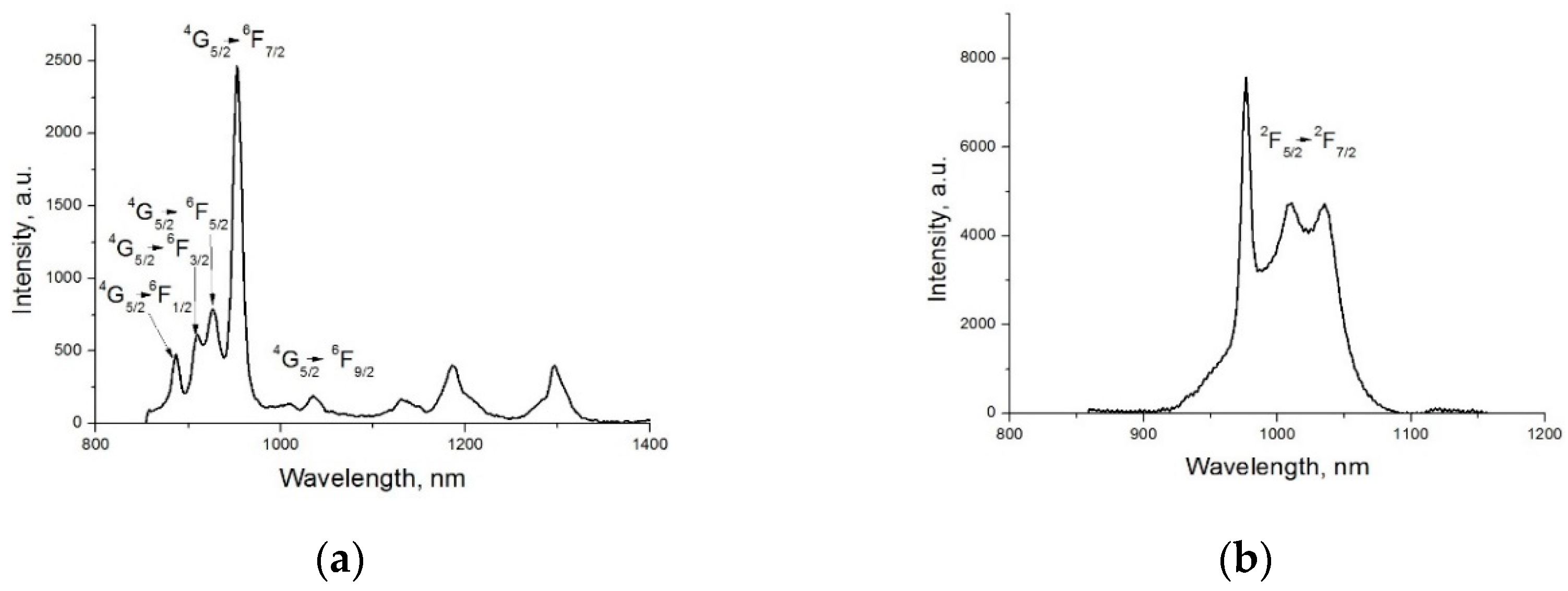
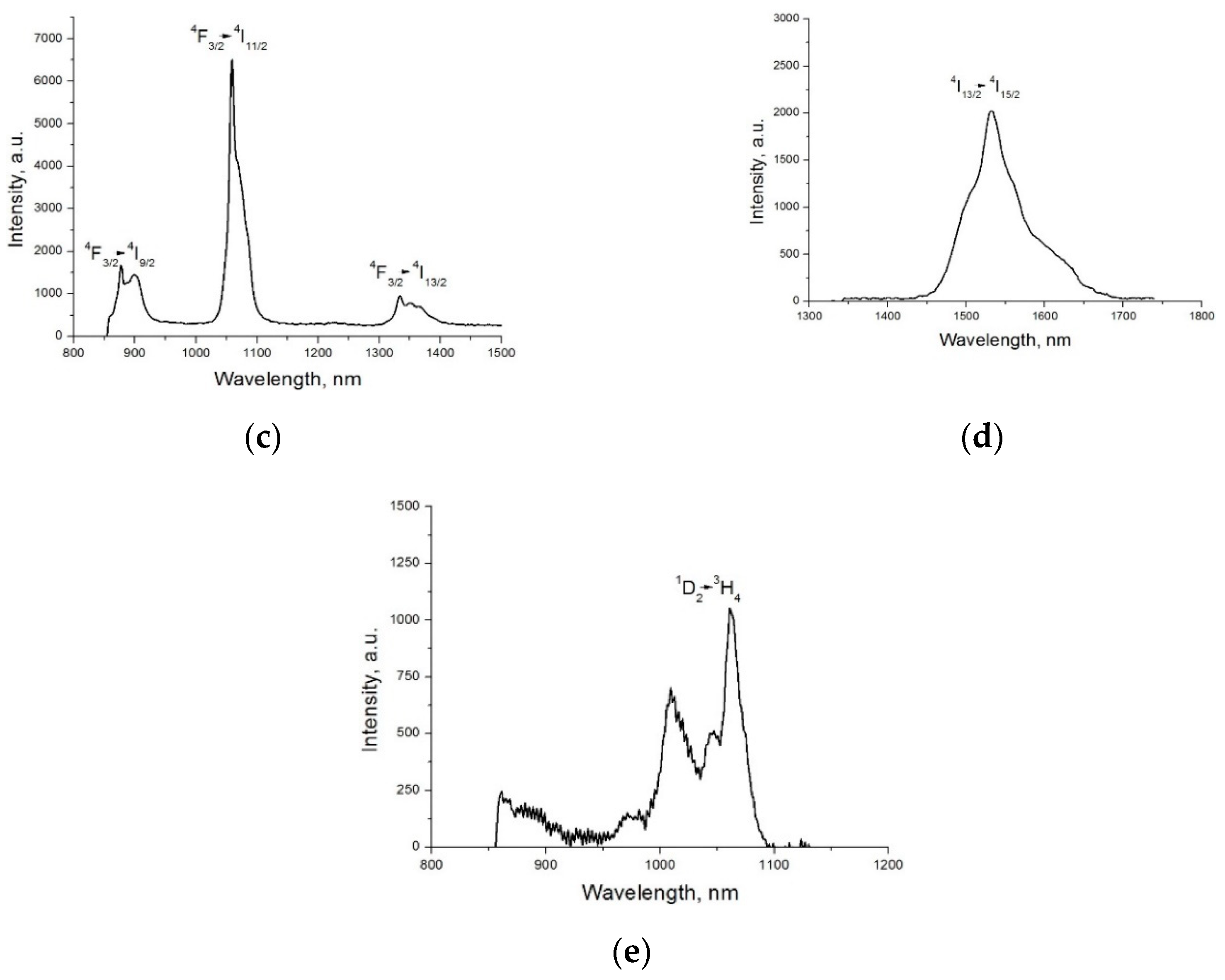
2. Results and Discussion
| # | Structure of β-Diketone | 3T1, cm−1 | Reference |
|---|---|---|---|
| 1 | 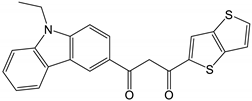 | 19,230 | [48] |
| 2 | 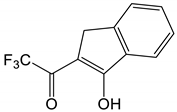 | 19,607 | [49] |
| 3 | 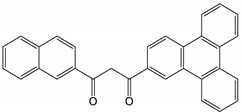 | 19,300 | [50] |
| 4 |  | 19,300 | [50] |
| 5 |  | 18,975 | [31] |
| 6 |  | 20,200 | In this paper |
| 7 | 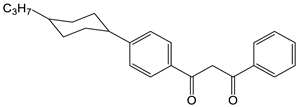 | 19,200 | [51] |
| 8 |  | 18,200 | [52] |
| 9 | 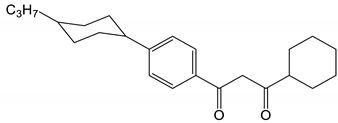 | 19,160 | [53] |
| 10 | 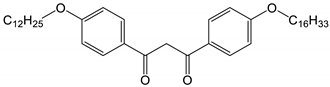 | 19,200 | [54] |
3. Materials and Methods
3.1. Materials
3.2. Characterization Techniques
3.3. Synthesis of Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. Near-Infrared Spectroscopy: Principles, Instruments, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 352761267X. [Google Scholar]
- Ozaki, Y.; Huck, C.; Tsuchikawa, S.; Engelsen, S.B. Near-Infrared Spectroscopy: Theory, Spectral Analysis, Instrumentation, and Applications; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 9811586489. [Google Scholar]
- Wang, Z.Y. Near-Infrared Organic Materials and Emerging Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Svoboda, K.; Block, S.M. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 247–285. [Google Scholar] [CrossRef]
- Ferrari, M.; Wolf, M.; Quaresima, V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J. Biomed. Opt. 2007, 12, 062104. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Bremer, C.; Weissleder, R. Fluorescence imaging with near-infrared light: New technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003, 13, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.; Chang, H.; Liu, R.-S. (INVITED) Recent progress on broadband near-infrared phosphors-converted light emitting diodes for future miniature spectrometers. Opt. Mater. X 2019, 1, 100011. [Google Scholar] [CrossRef]
- Rajendran, V.; Fang, M.-H.; De Guzman, G.N.; Lesniewski, T.; Mahlik, S.; Grinberg, M.; Leniec, G.; Kaczmarek, S.M.; Lin, Y.-S.; Lu, K.-M.; et al. Super Broadband Near-Infrared Phosphors with High Radiant Flux as Future Light Sources for Spectroscopy Applications. ACS Energy Lett. 2018, 3, 2679–2684. [Google Scholar] [CrossRef]
- Shao, Q.; Ding, H.; Yao, L.; Xu, J.; Liang, C.; Jiang, J. Photoluminescence properties of a ScBO3:Cr3+ phosphor and its applications for broadband near-infrared LEDs. RSC Adv. 2018, 8, 12035–12042. [Google Scholar] [CrossRef]
- Shang, M.; Li, C.; Lin, J. How to produce white light in a single-phase host? Chem. Soc. Rev. 2014, 43, 1372–1386. [Google Scholar] [CrossRef]
- Shao, Q.; Ding, H.; Yao, L.; Xu, J.; Liang, C.; Li, Z.; Dong, Y.; Jiang, J. Broadband near-infrared light source derived from Cr3+-doped phosphors and a blue LED chip. Opt. Lett. 2018, 43, 5251–5254. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lu, Y.-Y.; Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 2011, 11, 58–63. [Google Scholar] [CrossRef]
- Herbst, J.; Croat, J. Neodymium-iron-boron permanent magnets. J. Magn. Magn. Mater. 1991, 100, 57–78. [Google Scholar] [CrossRef]
- Kränkel, C.; Marzahl, D.-T.; Moglia, F.; Huber, G.; Metz, P.W. Out of the blue: Semiconductor laser pumped visible rare-earth doped lasers. Laser Photon-Rev. 2016, 10, 548–568. [Google Scholar] [CrossRef]
- Nakazawa, M. Evolution of EDFA from single-core to multi-core and related recent progress in optical communication. Opt. Rev. 2014, 21, 862–874. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal–organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176. [Google Scholar] [CrossRef]
- Zeng, H.; Zhou, T.; Wang, L.; Xie, R.-J. Two-Site Occupation for Exploring Ultra-Broadband Near-Infrared Phosphor—Double-Perovskite La2MgZrO6:Cr3+. Chem. Mater. 2019, 31, 5245–5253. [Google Scholar] [CrossRef]
- Gao, T.; Zhuang, W.; Liu, R.; Liu, Y.; Yan, C.; Tian, J.; Chen, G.; Chen, X.; Zheng, Y.; Wang, L. Site occupancy and enhanced luminescence of broadband NIR gallogermanate phosphors by energy transfer. J. Am. Ceram. Soc. 2020, 103, 202–213. [Google Scholar] [CrossRef]
- Zhong, Y.; Gai, S.; Xia, M.; Gu, S.; Zhang, Y.; Wu, X.; Wang, J.; Zhou, N.; Zhou, Z. Enhancing quantum efficiency and tuning photoluminescence properties in far-red-emitting phosphor Ca14Ga10Zn6O35:Mn4+ based on chemical unit engineering. Chem. Eng. J. 2019, 374, 381–391. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Hao, Z.; Zhang, X.; Pan, G.; Wu, H.; Zhang, J. Cr3+-Doped Broadband NIR Garnet Phosphor with Enhanced Luminescence and its Application in NIR Spectroscopy. Adv. Opt. Mater. 2019, 7, 1900185. [Google Scholar] [CrossRef]
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the biological windows: Current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 2016, 1, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Comby, S.; Bünzli, J.-C.G. Chapter 235 Lanthanide Near-Infrared Luminescence in Molecular Probes and Devices. Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Sabbatini, N.; Guardigli, M.; Lehn, J.-M. Luminescent lanthanide complexes as photochemical supramolecular devices. Co-Ord. Chem. Rev. 1993, 123, 201–228. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Yan, P.; Chen, P.; Hou, G.; Li, G. Synthesis, Crystal Structure, and Luminescent Properties of 2-(2,2,2-Trifluoroethyl)-1-indone Lanthanide Complexes. Inorg. Chem. 2012, 51, 5050–5057. [Google Scholar] [CrossRef] [PubMed]
- Quirino, W.; Legnani, C.; dos Santos, R.; Teixeira, K.; Cremona, M.; Guedes, M.; Brito, H. Electroluminescent devices based on rare-earth tetrakis β-diketonate complexes. Thin Solid Films 2008, 517, 1096–1100. [Google Scholar] [CrossRef]
- Divya, V.; Sankar, V.; Raghu, K.G.; Reddy, M.L.P. A mitochondria-specific visible-light sensitized europium β-diketonate complex with red emission. Dalton Trans. 2013, 42, 12317–12323. [Google Scholar] [CrossRef]
- Biju, S.; Reddy, M.; Freire, R. 3-Phenyl-4-aroyl-5-isoxazolonate complexes of Tb3+ as promising light-conversion molecular devices. Inorg. Chem. Commun. 2007, 10, 393–396. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, C.; Liu, L.; Li, H.; He, Y.; Lü, X.; Wong, W.-K.; Jones, R.A. Efficient near-infrared (NIR) luminescent PMMA-supported hybrid materials doped with tris-β-diketonate Ln3+ complex (Ln=Nd or Yb). J. Photochem. Photobiol. A Chem. 2016, 314, 104–113. [Google Scholar] [CrossRef]
- Werts, M.H. Making sense of Lanthanide Luminescence. Sci. Prog. 2005, 88, 101–131. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Wada, Y.; Yanagida, S. Strategies for the design of luminescent lanthanide(III) complexes and their photonic applications. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 183–202. [Google Scholar] [CrossRef]
- Bunzli, J.-C.; Comby, S.; Chauvin, A.-S.; Vandevyver, C.D. New Opportunities for Lanthanide Luminescence. J. Rare Earths 2007, 25, 257–274. [Google Scholar] [CrossRef]
- Kuriki, K.; Koike, Y.; Okamoto, Y. Plastic Optical Fiber Lasers and Amplifiers Containing Lanthanide Complexes. Chem. Rev. 2002, 102, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, G.; Gryczynski, I.; Maliwal, B.P.; Lakowicz, J.R. Multi-Photon Sensitized Excitation of Near Infrared Emitting Lanthanides. J. Fluoresc. 2002, 12, 15–17. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Cheng, C.C.W.; Kwok, W.-M.; Tam, H.-L.; Hao, J.; Kwong, D.W.J.; Wong, W.-K. Water-Soluble Mitochondria-Specific Ytterbium Complex with Impressive NIR Emission. J. Am. Chem. Soc. 2011, 133, 20120–20122. [Google Scholar] [CrossRef] [PubMed]
- D’Aléo, A.; Bourdolle, A.; Brustlein, S.; Fauquier, T.; Grichine, A.; Duperray, A.; Baldeck, P.L.; Andraud, C.; Brasselet, S.; Maury, O. Ytterbium-Based Bioprobes for Near-Infrared Two-Photon Scanning Laser Microscopy Imaging. Angew. Chem. Int. Ed. 2012, 51, 6622–6625. [Google Scholar] [CrossRef]
- Bui, A.T.; Grichine, A.; Brasselet, S.; Duperray, A.; Andraud, C.; Maury, O. Unexpected Efficiency of a Luminescent Samarium(III) Complex for Combined Visible and Near-Infrared Biphotonic Microscopy. Chem.-A Eur. J. 2015, 21, 17757–17761. [Google Scholar] [CrossRef]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. The evolution of β-diketone or β-diketophenol ligands and related complexes. Coord. Chem. Rev. 2009, 253, 1099–1201. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Z.; Liu, L.; Feng, W.; Lü, X.; Wong, W.-K.; Jones, R.A. Near-infrared (NIR) luminescent PMMA-based hybrid materials doped with Ln-β-diketonate (Ln=Nd or Yb) complexes. Inorg. Chem. Commun. 2014, 49, 30–33. [Google Scholar] [CrossRef]
- Dang, S.; Yu, J.-B.; Wang, X.-F.; Guo, Z.-Y.; Sun, L.-N.; Deng, R.-P.; Feng, J.; Fan, W.-Q.; Zhang, H.-J. A study on the NIR-luminescence emitted from ternary lanthanide [Er(III), Nd(III) and Yb(III)] complexes containing fluorinated-ligand and 4,5-diazafluoren-9-one. J. Photochem. Photobiol. A Chem. 2010, 214, 152–160. [Google Scholar] [CrossRef]
- Ahmed, Z.; Iftikhar, K. Sensitization of Visible and NIR Emitting Lanthanide(III) Ions in Noncentrosymmetric Complexes of Hexafluoroacetylacetone and Unsubstituted Monodentate Pyrazole. J. Phys. Chem. A 2013, 117, 11183–11201. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ramos, P.; da Silva, P.S.P.; Lavín, V.; Martín, I.R.; Lahoz, F.; Chamorro-Posada, P.; Silva, M.R.; Martín-Gil, J. Structure and NIR-luminescence of ytterbium(III) beta-diketonate complexes with 5-nitro-1,10-phenanthroline ancillary ligand: Assessment of chain length and fluorination impact. Dalton Trans. 2013, 42, 13516–13526. [Google Scholar] [CrossRef]
- Kang, T.-S.; Harrison, B.; Bouguettaya, M.; Foley, T.; Boncella, J.; Schanze, K.; Reynolds, J. Near-Infrared Light-Emitting Diodes (LEDs) Based on Poly(phenylene)/Yb-tris(β-Diketonate) Complexes. Adv. Funct. Mater. 2003, 13, 205–210. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. Luminescence behavior of PMMA films doped with Tb(III) and Eu(III) complexes. J. Lumin. 2021, 242, 118609. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.; Heinrich, B.; Donnio, B.; Galyametdinov, Y. Controlled polarized luminescence of smectic lanthanide complexes. Dye. Pigment. 2018, 148, 492–500. [Google Scholar] [CrossRef]
- Baek, N.S.; Kim, Y.H.; Eom, Y.K.; Oh, J.H.; Kim, H.K.; Aebischer, A.; Gumy, F.; Chauvin, A.-S.; Bünzli, J.-C.G. Sensitized near-IR luminescence of lanthanide complexes based on push-pull diketone derivatives. Dalton Trans. 2010, 39, 1532–1538. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Li, H.; Yan, P.; Hou, G.; Li, G. NIR luminescence of 2-(2,2,2-trifluoroethyl)-1-indone (TFI) neodymium and ytterbium complexes. J. Lumin. 2014, 146, 205–210. [Google Scholar] [CrossRef]
- Baek, N.; Kwak, B.; Kim, Y.; Kim, H. Sensitized Near IR Luminescence of Er(III) Ion in Lanthanide Complexes Based on Diketone Derivatives: Synthesis and Photophysical Behaviors. Bull. Korean Chem. Soc. 2007, 28, 1256–1260. [Google Scholar] [CrossRef][Green Version]
- Knyazev, A.A.; Karyakin, M.E.; Romanova, K.A.; Heinrich, B.; Donnio, B.; Galyametdinov, Y.G. Influence of Lewis Bases on the Mesogenic and Luminescent Properties of Homogeneous Films of Europium(III) Tris(β-diketonate) Adducts. Eur. J. Inorg. Chem. 2017, 2017, 639–645. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.; Romanova, K.A.; Galyametdinov, Y. Luminescence and energy transfer in poly(N-vinylcarbazole) blends doped by a highly anisometric Eu(III) complex. J. Co-Ord. Chem. 2016, 69, 1473–1483. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Dzhabarov, V.I.; Lapaev, D.V.; Lobkov, V.S.; Haase, W.; Galyametdinov, Y.G. New nematogenic β-diketones for synthesis of lanthanidomesogens. Russ. J. Gen. Chem. 2010, 80, 756–760. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Galyametdinov, Y.G.; Goderis, B.; Driesen, K.; Goossens, K.; Görller-Walrand, C.; Binnemans, K.; Cardinaels, T. Liquid-Crystalline Ternary Rare-Earth Complexes. Eur. J. Inorg. Chem. 2008, 2008, 756–761. [Google Scholar] [CrossRef]
- Knyazev, A.; Karyakin, M.; Galyametdinov, Y. Photostable Anisometric Lanthanide Complexes as Promising Materials for Optical Applications. Photonics 2019, 6, 110. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.E.; Heinrich, B.; Donnio, B.; Galyametdinov, Y.G. A facile approach for the creation of heteroionic lanthanidomesogens-containing uniform films with enhanced luminescence efficiency. Dye. Pigment. 2020, 187, 109050. [Google Scholar] [CrossRef]
- Lapaev, D.V.; Nikiforov, V.G.; Lobkov, V.S.; Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. New insights into UV laser irradiation effect on luminescent behavior of vitrified films based on mesogenic lanthanide(III) β-diketonate complexes. J. Photochem. Photobiol. A Chem. 2019, 382, 111962. [Google Scholar] [CrossRef]
- Caspers, H.H.; Miller, S.A.; Rast, H.E.; Fry, J.L. Electronic Spectrum and Energy Levels of Gd3+ in LaF3. Phys. Rev. 1969, 180, 329–333. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From Antenna to Assay: Lessons Learned in Lanthanide Luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef]
- Li, S.; Zhu, W.; Xu, Z.; Pan, J.; Tian, H. Antenna-functionalized dendritic β-diketonates and europium complexes: Synthetic approaches to generation growth. Tetrahedron 2006, 62, 5035–5048. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.; Krupin, A.; Galyametdinov, Y. Synthesis and luminescence properties of hybrid systems based on liquid crystal terbium(III) and europium(III) complexes. Russ. J. Gen. Chem. 2015, 85, 2806–2812. [Google Scholar] [CrossRef]
- Knyazev, A.; Krupin, A.; Gubaidullin, A.; Galyametdinov, Y. Optical and structural characteristics of PMMA films doped with a new anisometric EuIII complex. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 570–577. [Google Scholar] [CrossRef]


| Lanthanide Ion | Yield, % | Elemental | ESI-MS (m/z) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C, % | H, % | N, % | O, % | Ln, % | ||||||||
| Calcd. | Found | Calcd. | Found | Calcd. | Found | Calcd. | Found | Calcd. | Found | |||
| Pr | 75 | 72.30 | 72.01 | 8.01 | 8.17 | 2.08 | 2.05 | 7.13 | 7.22 | 10.47 | 10.45 | 1368 (M + Na)+ |
| Nd | 73 | 72.12 | 71.98 | 7.99 | 8.06 | 2.08 | 2.05 | 7.12 | 7.26 | 10.69 | 10.81 | 1370 (M + Na)+ |
| Sm | 78 | 71.79 | 71.59 | 7.96 | 8.12 | 2.07 | 2.04 | 7.08 | 7.23 | 11.10 | 11.47 | 1377 (M + Na)+ |
| Gd | 74 | 71.43 | 71.25 | 7.92 | 8.15 | 2.06 | 2.04 | 7.05 | 7.27 | 11.55 | 11.63 | 1385 (M + Na)+ |
| Ho | 71 | 71.03 | 70.87 | 7.87 | 8.11 | 2.05 | 2.02 | 7.01 | 7.42 | 12.04 | 12.16 | 1391 (M + Na)+ |
| Er | 72 | 70.91 | 70.78 | 7.86 | 8.05 | 2.04 | 2.02 | 7.00 | 7.22 | 12.19 | 12.03 | 1394 (M + Na)+ |
| Yb | 73 | 70.61 | 70.48 | 7.83 | 8.02 | 2.03 | 2.01 | 6.97 | 7.16 | 12.56 | 12.41 | 1399 (M + Na)+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. Anisometric Ln(III) Complexes with Efficient Near-IR Luminescence. Inorganics 2022, 10, 9. https://doi.org/10.3390/inorganics10010009
Knyazev AA, Krupin AS, Galyametdinov YG. Anisometric Ln(III) Complexes with Efficient Near-IR Luminescence. Inorganics. 2022; 10(1):9. https://doi.org/10.3390/inorganics10010009
Chicago/Turabian StyleKnyazev, Andrey A., Aleksandr S. Krupin, and Yuriy G. Galyametdinov. 2022. "Anisometric Ln(III) Complexes with Efficient Near-IR Luminescence" Inorganics 10, no. 1: 9. https://doi.org/10.3390/inorganics10010009
APA StyleKnyazev, A. A., Krupin, A. S., & Galyametdinov, Y. G. (2022). Anisometric Ln(III) Complexes with Efficient Near-IR Luminescence. Inorganics, 10(1), 9. https://doi.org/10.3390/inorganics10010009






