Facile Preparation of Flexible Lateral 2D MoS2 Nanosheets for Photoelectrochemical Hydrogen Generation and Optoelectronic Applications
Abstract
:1. Introduction
2. Experiments and Measurements
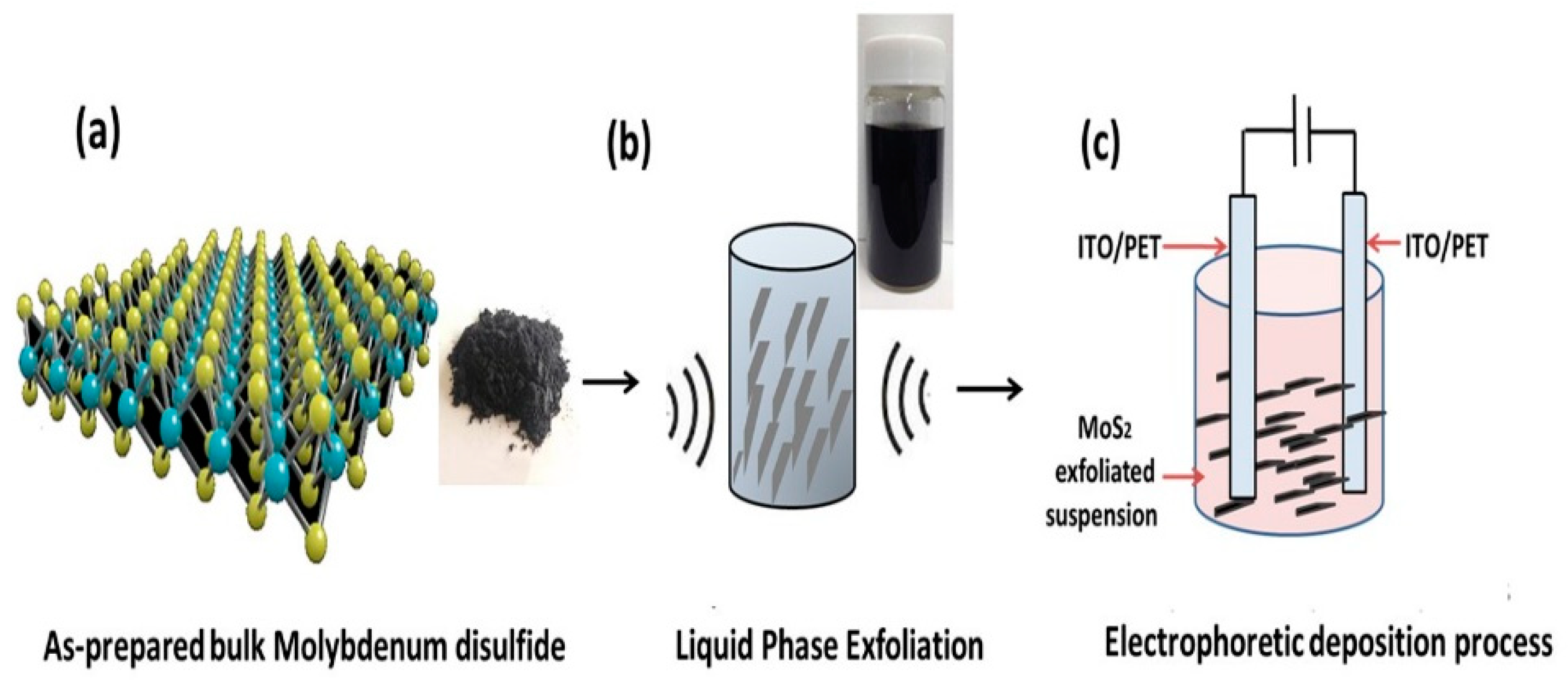
2.1. Synthesis of Bulk MoS2
2.2. Synthesis of 2D MoS2 Nanosheets Suspensions
2.3. Preparation of 2D MoS2 Nanosheets Film
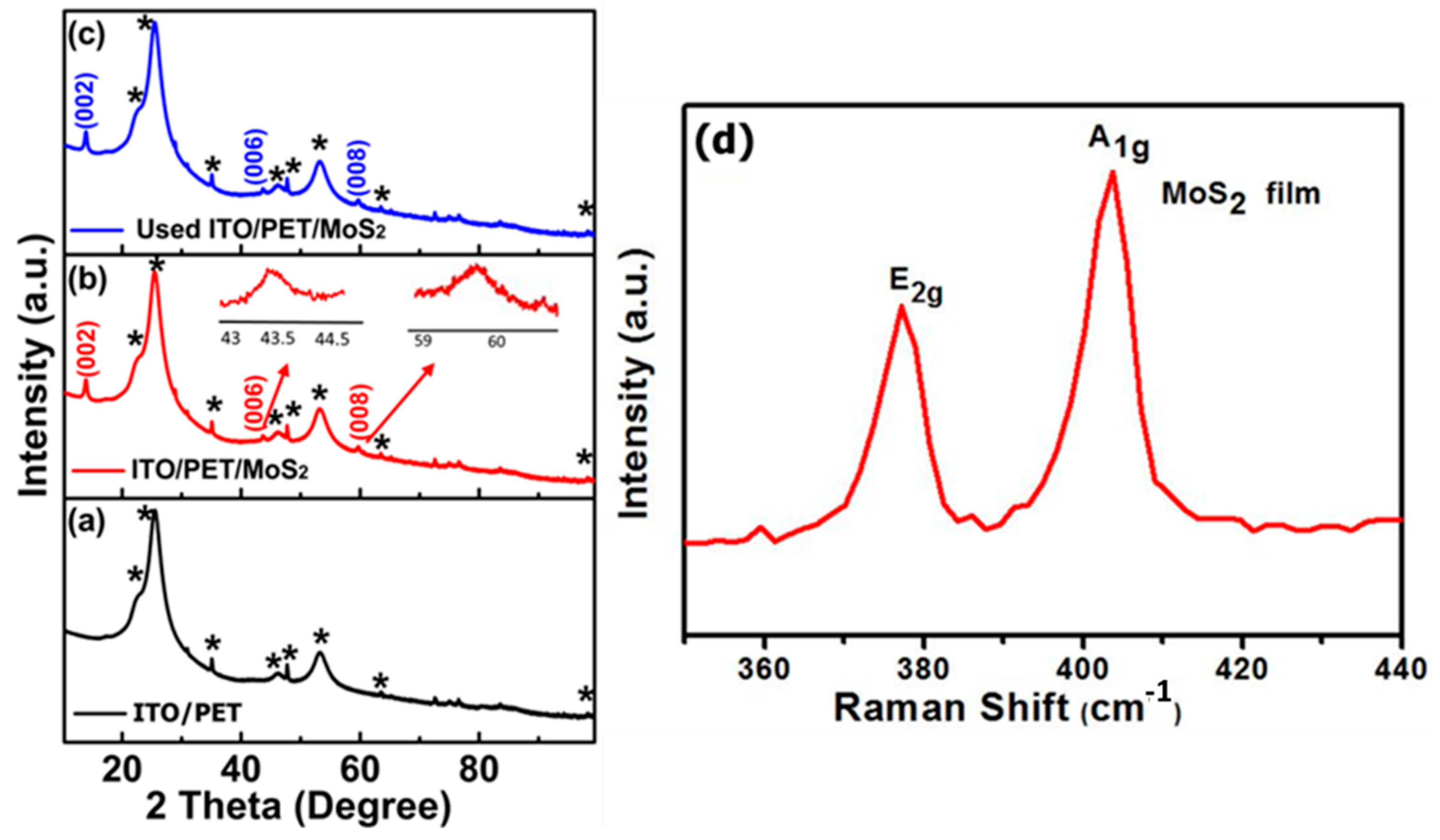
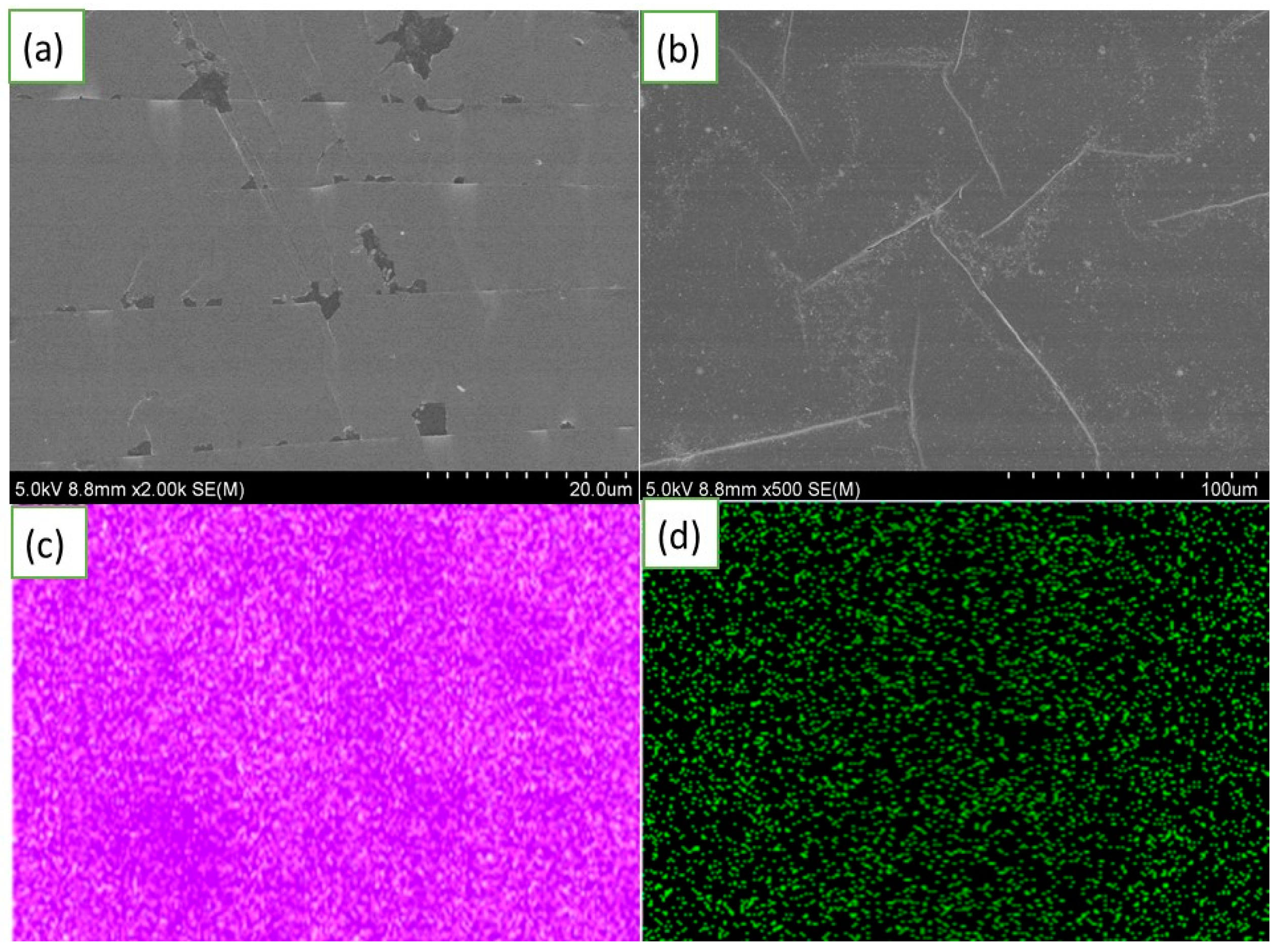
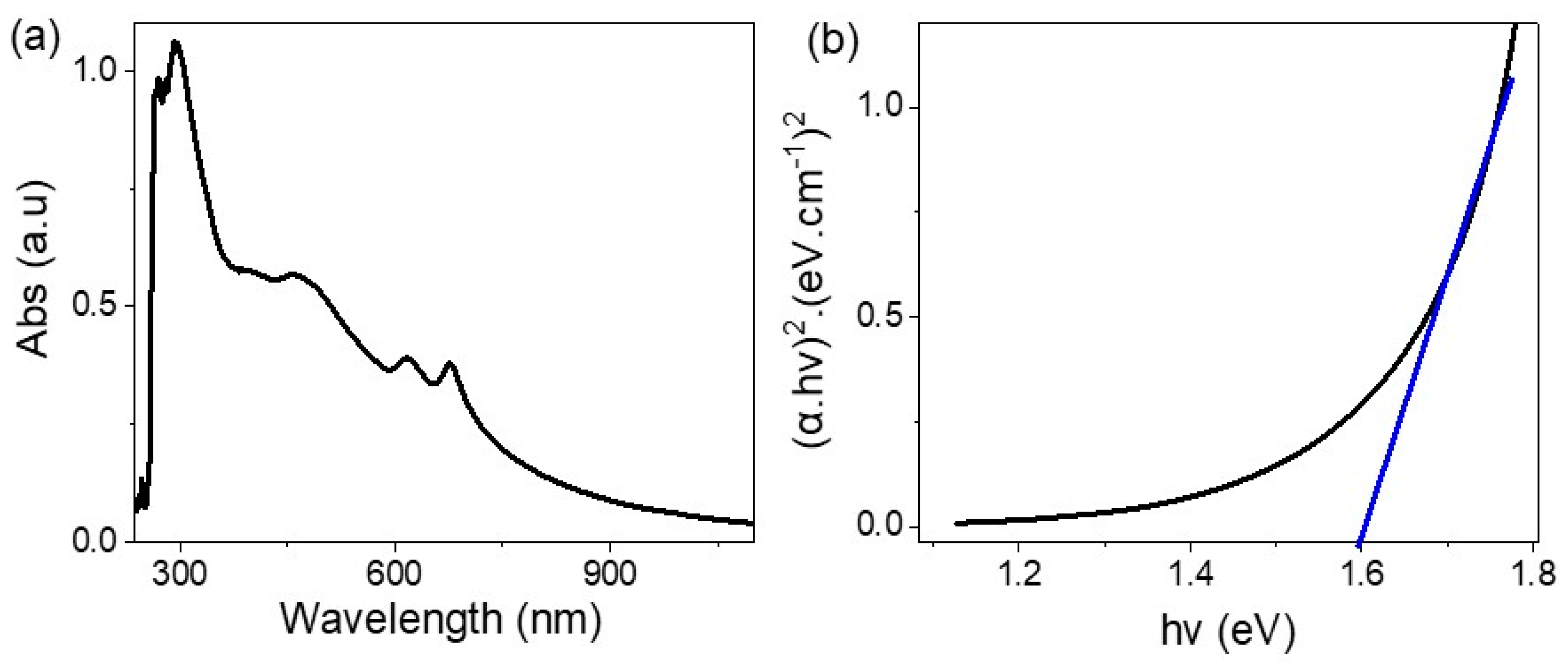
2.4. Characterization and Measurement
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Avcu, E.; Bastan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Ounaies, Z.; Watson, K.A.; Crooks, R.E.; Smith, J., Jr.; Lowther, S.E.; Connell, J.W.; Siochi, E.J.; Harrison, J.S.; St Clair, T.L. Dispersion of single wall carbon nanotubes by in situ Dispersion of single wall carbon nanotubes by in situ polymerization under sonication. Chem. Phys. Lett. 2002, 364, 303–308. [Google Scholar] [CrossRef]
- Yu, Y.; Li, C.; Liu, Y.; Su, L.; Zhang, Y.; Cao, L. Controlled Scalable Synthesis of Uniform, High-Quality Monolayer and Few-layer MoS2 Films. Sci. Rep. 2013, 3, srep01866. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Tilley, S.D. Recent Advances and Emerging Trends in Photo-Electrochemical Solar Energy Conversion. Adv. Energy Mater. 2019, 9, 1802877. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Eugene, Y.J.; Khiavi, N.D.; Katal, R.; Gong, X. Aluminum-incorporated p-CuO/n-ZnO photocathode coated with nanocrystal-engineered TiO2 protective layer for photoelectrochemical water splitting and hydrogen generation. J. Mater. Chem. A 2018, 6, 11951–11965. [Google Scholar] [CrossRef]
- Diez-Perez, I.; Li, Z.; Guo, S.; Madden, C.; Huang, H.; Che, Y.; Yang, X.; Zang, L.; Tao, N. Ambipolar transport in an electrochemically gated single-molecule field-effect transistor. ACS Nano 2012, 6, 7044–7052. [Google Scholar] [CrossRef] [PubMed]
- Akama, T.; Okita, W.; Nagai, R.; Li, C.; Kaneko, T.; Kato, T. Schottky solar cell using few-layered transition metal dichalcogenides toward large-scale fabrication of semitransparent and flexible power generator. Sci. Rep. 2017, 7, 11967. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, A.-P.; Wang, F.-Z.; Tang, R.; Liu, M.; Luo, Z.-C.; Xu, W.-C.; Zhao, C.-J.; Zhang, H. Femtosecond pulse erbium-doped fiber laser by a few-layer MoS2 saturable absorber. Opt. Lett. 2014, 39, 4591–4594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, S.B.; Zheng, J.; Du, J.; Wen, S.C.; Tang, D.Y.; Loh, K. Molybdenum disulfide (MoS2) as a broadband saturable absorber for ultra-fast photonics. Opt. Express 2014, 22, 7249–7260. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Lee, Y.T.; Lee, H.S.; Lee, Y.J.; Shokouh, S.H.H.; Kyhm, J.; Lee, J.; Kim, H.H.; Yoo, T.-H.; Nam, S.H.; et al. Ultrasensitive PbS quantum-dot-sensitized InGaZnO hybrid photoinverter for near-infrared detection and imaging with high photogain. NPG Asia Mater. 2016, 8, e233. [Google Scholar] [CrossRef]
- Wang, S.; Yu, H.; Zhang, H.; Wang, A.; Zhao, M.; Chen, Y.; Mei, L.; Wang, J. Broadband few-layer MoS2 saturable absorbers. Adv. Mater. 2014, 26, 3538–3544. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2-based photocatalysis: A review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ Blue titania via band shape engineering for exceptional solar H2 production in rutile TiOAppl. Catal. B Environ. 2021, 297, 120380. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Choi, H.C. Synthesis and properties of molybdenum disulphide: From bulk to atomic layers. RSC Adv. 2015, 5, 7495–7514. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Nikonovas, T.; Spessa, A.; Doerr, S.H.; Clay, G.D.; Mezbahuddin, S. Near-complete loss of fire-resistant primary tropical forest cover in Sumatra and Kalimantan. Commun. Earth Environ. 2020, 1, 65. [Google Scholar] [CrossRef]
- Pagliaro, M. Preparing for the future: Solar energy and bioeconomy in the United Arab Emirates. Energy Sci. Eng. 2019, 7, 1451–1457. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Peng, X.; Wang, Z.; Zhou, L.; Yin, Q. A Novel Route to Manufacture 2D Layer MoS2 and g-C3N4 by Atmospheric Plasma with Enhanced Visible-Light-Driven Photocatalysis. Nanomaterials 2019, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, Q.; Zhang, G.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Exfoliated MoS2 supported Au–Pd bimetallic nanoparticles with core–shell structures and superior peroxidase-like activities. RSC Adv. 2015, 5, 10352–10357. [Google Scholar] [CrossRef]
- De-Mello, G.B.; Smith, L.; Rowley-Neale, S.J.; Gruber, J.; Hutton, S.J.; Banks, C.E. Surfactant-exfoliated 2D molybdenum disulphide (2D-MoS2): The role of surfactant upon the hydrogen evolution reaction. RSC Adv. 2017, 7, 36208–36213. [Google Scholar] [CrossRef]
- Yue, X.; Yang, J.; Li, W.; Jing, Y.; Dong, L.; Zhang, Y.; Li, X. Electron Irradiation Induces the Conversion from 2H-WSe2 to 1T-WSe2 and Promotes the Performance of Electrocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2022, 10, 2420–2428. [Google Scholar] [CrossRef]
- Sharma, U.; Karazhanov, S.; Jose, R.; Das, S. Plasmonic hot-electron assisted phase transformation in 2D-MoS2 for the hydrogen evolution reaction: Current status and future prospects. J. Mater. Chem. A 2022, 10, 8626–8655. [Google Scholar] [CrossRef]
- Saha, D.; Kruse, P. Editors’ choice—Review—Conductive forms of MoS2 and their applications in energy storage and conversion. J. Electrochem. Soc. 2020, 167, 126517. [Google Scholar] [CrossRef]
- Xia, Z.; Tao, Y.; Pan, Z.; Shen, X. Enhanced photocatalytic performance and stability of 1T MoS2 transformed from 2H MoS2 via Li intercalation. Results Phys. 2019, 12, 2218–2224. [Google Scholar] [CrossRef]
- Ho, Y.T.; Ma, C.H.; Luong, T.T.; Wei, L.L.; Yen, T.C.; Hsu, W.T.; Chang, W.H.; Chu, Y.C.; Tu, Y.Y.; Pande, K.P.; et al. Layered MoS2 grown on c-sapphire by pulsed laser deposition. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2015, 9, 187–191. [Google Scholar] [CrossRef]
- Lewandowska, R. Number of layers of MoS2 determined using Raman Spectroscopy. Horiba Sci. 2014, 104, 109933. [Google Scholar]
- Baishya, K.; Ray, J.S.; Dutta, P.; Das, P.P.; Das, S.K. Graphene-mediated band gap engineering of WO3 nanoparticle and a relook at Tauc equation for band gap evaluation. Appl. Phys. A 2018, 124, 704. [Google Scholar] [CrossRef]
- Ingle, N.N.; Shirsat, S.; Sayyad, P.; Bodkhe, G.; Patil, H.; Deshmukh, M.; Mahadik, M.; Singh, F.; Shirsat, M. Influence of swift heavy ion irradiation on sensing properties of nickel-(NRs-Ni3HHTP2) metal-organic framework. J. Mater. Sci. Mater. Electron. 2021, 32, 18657–18668. [Google Scholar] [CrossRef]
- Abd-Elmageed, A.; Al-Hossainy, A.; Fawzy, E.; Almutlaq, N.; Eid, M.; Bourezgui, A.; Abdel-Hamid, S.; Elsharkawy, N.; Zwawi, M.; Abdel-Aziz, M.; et al. Synthesis, characterization and DFT molecular modeling of doped poly (para-nitroaniline-co-para-toluidine) thin film for optoelectronic devices applications. Opt. Mater. 2020, 99, 109593. [Google Scholar] [CrossRef]
- Abdelazeez, A.A.A.; El-Fatah, G.A.; Shaban, M.; Ahmed, A.M.; Rabia, M. ITO/Poly-3-Methylaniline/Au Electrode for Electrochemical Water Splitting and Dye Removal. ECS J. Solid State Sci. Technol. 2021, 10, 123009. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, H.S.H.; Shaban, M.; Taha, S. Preparation of polyaniline/PbS core-shell nano/microcomposite and its application for photocatalytic H2 electrogeneration from H2O. Sci. Rep. 2018, 8, 1107. [Google Scholar] [CrossRef]
- Mohamed, S.; Zhao, H.; Romanus, H.; El-Hossary, F.; El-Kassem, M.A.; Awad, M.; Rabia, M.; Lei, Y. Optical, water splitting and wettability of titanium nitride/titanium oxynitride bilayer films for hydrogen generation and solar cells applications. Mater. Sci. Semicond. Process. 2020, 105, 104704. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Rabia, M.; Elkader, Y.A.; El-Halim, M.R.A. Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr(VI) contaminants from aqueous solutions; kinetic and equilibrium studies. Rendiconti Lince Sci. Fis. E Nat. 2018, 29, 141–154. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Eldakrory, M.G.; Maree, R.M.; Ahmed, A.M. Efficient photoselectrochemical hydrogen production utilizing of APbI3 (A = Na, Cs, and Li) perovskites nanorods. Int. J. Energy Res. 2021, 45, 7436–7446. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Shaban, M.; Aly, A.H.; Ahmed, A.M.; Rabia, M. Preparation and characterization of a high-efficiency photoelectric detector composed of hexagonal Al2O3/TiO2/TiN/Au nanoporous array. Mater. Sci. Semicond. Process. 2022, 139, 106348. [Google Scholar] [CrossRef]
- Yao, H.; Luo, S.; Duesberg, G.S.; Qi, X.; Lu, D.; Yue, C.; Zhong, J. Enhanced photoresponse of graphene oxide functionalised SnSe films. AIP Adv. 2018, 8, 075123. [Google Scholar] [CrossRef]
- Arulanantham, A.; Valanarasu, S.; Jeyadheepan, K.; Ganesh, V.; Shkir, M. Development of SnS (FTO/CdS/SnS) thin films by nebulizer spray pyrolysis (NSP) for solar cell applications. J. Mol. Struct. 2018, 1152, 137–144. [Google Scholar] [CrossRef]
- Tyona, M. Doped Zinc Oxide Nanostructures for Photovoltaic Solar Cells application. In Zinc Oxide Based Nano Materials and Devices; IntechOpen: London, UK, 2019. [Google Scholar]
- Patel, A.B.; Machhi, H.K.; Chauhan, P.; Narayan, S.; Dixit, V.; Soni, S.S.; Jha, P.K.; Solanki, G.K.; Patel, K.D.; Pathak, V.M. Electrophoretically deposited MoSe2/WSe2 heterojunction from ultrasonically exfoliated nanocrystals for enhanced electrochemical photoresponse. ACS Appl. Mater. Interfaces 2019, 11, 4093–4102. [Google Scholar] [CrossRef]
- Pesci, F.M.; Sokolikova, M.S.; Grotta, C.; Sherrell, P.C.; Reale, F.; Sharda, K.; Ni, N.; Palczynski, P.; Mattevi, C. MoS2/WS2 heterojunction for photoelectrochemical water oxidation. ACS Catal. 2017, 7, 4990–4998. [Google Scholar] [CrossRef]
- Norton, K.; Alam, F.; Lewis, D. A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS). Appl. Sci. 2021, 11, 2062. [Google Scholar] [CrossRef]


| Materials | Electrolyte | Light Intensity (mW/cm2) | Synthesis Method | Grain Size (nm) | JSC (mA/cm2) |
|---|---|---|---|---|---|
| MoS2 (this work) | 0.5 M Na2SO4 | 100 | Liquid exfoliation/EPD | 80 | 0.98 |
| ZnSe/SnSe [45] | tetracycline hydrochloride | N/A | Hydrothermal process | Microscale | 0.01 |
| 1D–0D CdS–SnS2 [46] | Na2S/Na2S2O3 | 100 | Hydrothermal process | 1D–0D ~(125*30)−14 | 0.02 |
| Cu-doped zinc oxide [47] | 0.1 M Na2SO4 | 80 | Chemical bath deposition/annealing | Microscale | 0.09 |
| MoSe2/WSe2 [48] | 0.5 M H2SO4 | 100 | Liquid exfoliation/EPD | ~45 | 0.04 |
| MoS2/WS2 [49] | 3 M Potassium Chloride (KCl) solution | 100 | Redox reaction of Li-intercalated powders in water | 4 nm–2 μm | 0.0122 |
| SnSe2/RGO [50] | N/A | 0.075 | Hydrothermal process | Microspikes/RGO sheets | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelazeez, A.A.A.; Ben Gouider Trabelsi, A.; Alkallas, F.H.; Elaissi, S.; Rabia, M. Facile Preparation of Flexible Lateral 2D MoS2 Nanosheets for Photoelectrochemical Hydrogen Generation and Optoelectronic Applications. Photonics 2022, 9, 638. https://doi.org/10.3390/photonics9090638
Abdelazeez AAA, Ben Gouider Trabelsi A, Alkallas FH, Elaissi S, Rabia M. Facile Preparation of Flexible Lateral 2D MoS2 Nanosheets for Photoelectrochemical Hydrogen Generation and Optoelectronic Applications. Photonics. 2022; 9(9):638. https://doi.org/10.3390/photonics9090638
Chicago/Turabian StyleAbdelazeez, Ahmed Adel A., Amira Ben Gouider Trabelsi, Fatemah. H. Alkallas, Samira Elaissi, and Mohamed Rabia. 2022. "Facile Preparation of Flexible Lateral 2D MoS2 Nanosheets for Photoelectrochemical Hydrogen Generation and Optoelectronic Applications" Photonics 9, no. 9: 638. https://doi.org/10.3390/photonics9090638
APA StyleAbdelazeez, A. A. A., Ben Gouider Trabelsi, A., Alkallas, F. H., Elaissi, S., & Rabia, M. (2022). Facile Preparation of Flexible Lateral 2D MoS2 Nanosheets for Photoelectrochemical Hydrogen Generation and Optoelectronic Applications. Photonics, 9(9), 638. https://doi.org/10.3390/photonics9090638






