Orbital and Spin Dynamics of Electron’s States Transition in Hydrogen Atom Driven by Electric Field
Abstract
:1. Introduction
2. Quantum Hamilton Mechanics
2.1. Quantum Hamilton-Jacobi Equation
2.2. Quantum Equation of Motion in Central Force Field
2.3. Orbital Dynamics of Electron in Hydrogen Atom
2.4. Electronic Quantum Motion in Eigenstates
3. Spin Dynamics of Electron in Hydrogen Atom
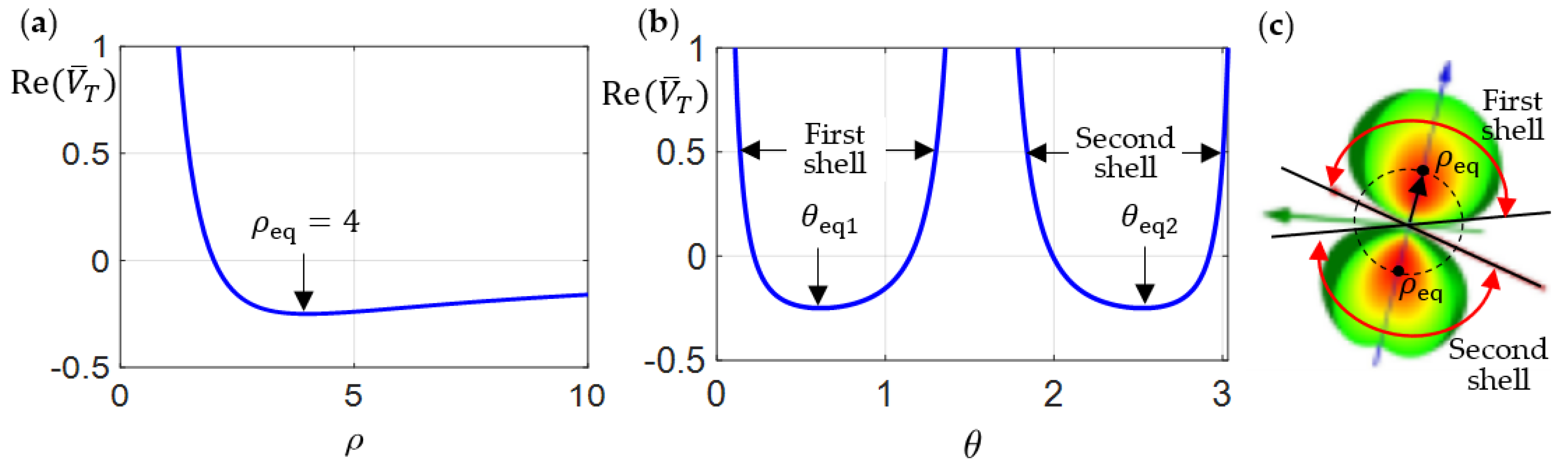
3.1. Spinless Dynamics
3.2. Spin-Up and Spin-Own Dynamics
- Spin-down region
- Spinless region
- Spin-up region
3.3. Deriving Spin Dynamics from Schrödinger Equation
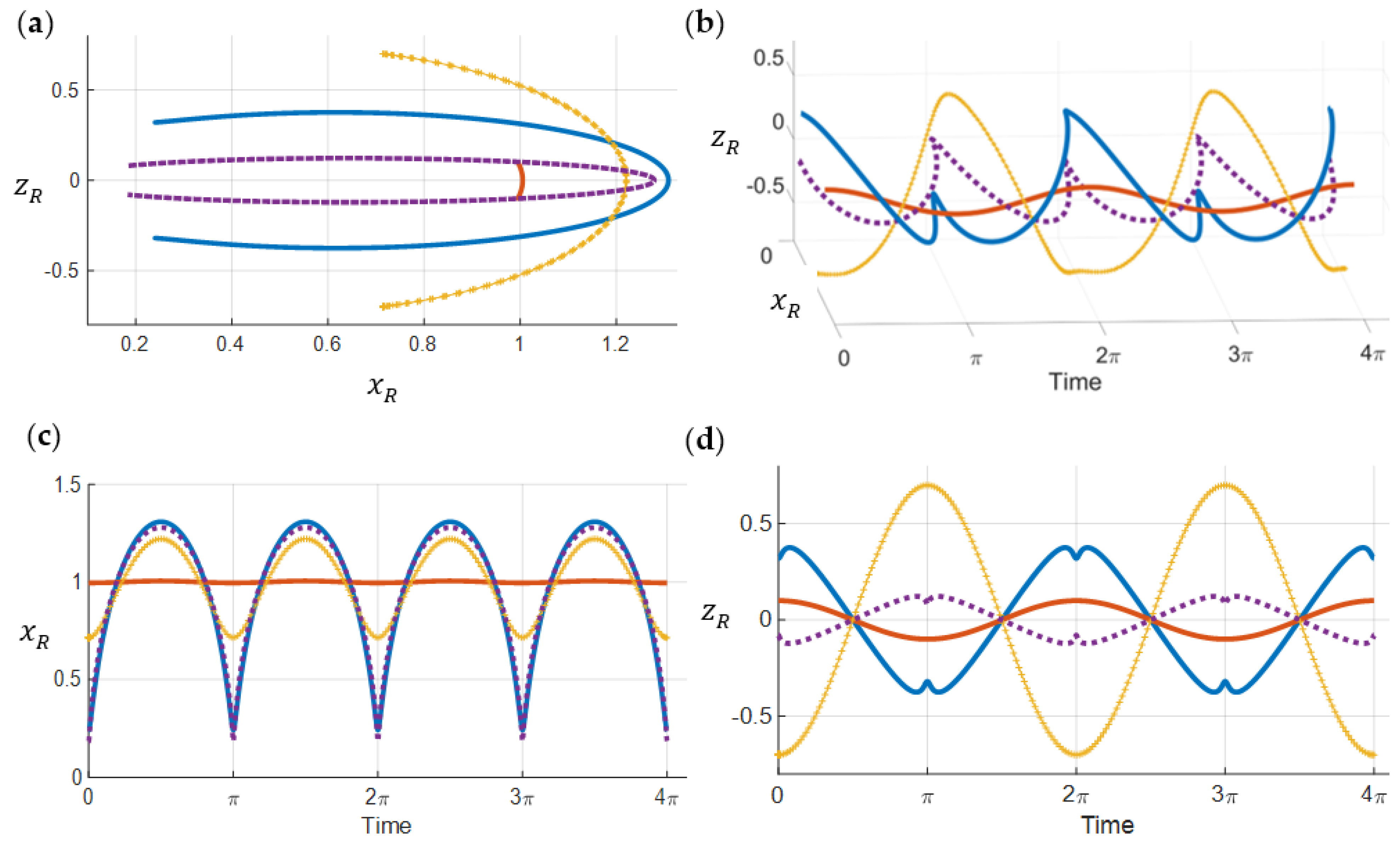
4. Dynamics of Electron’s States Transitions
4.1. Energy Level Differences and Electric Field
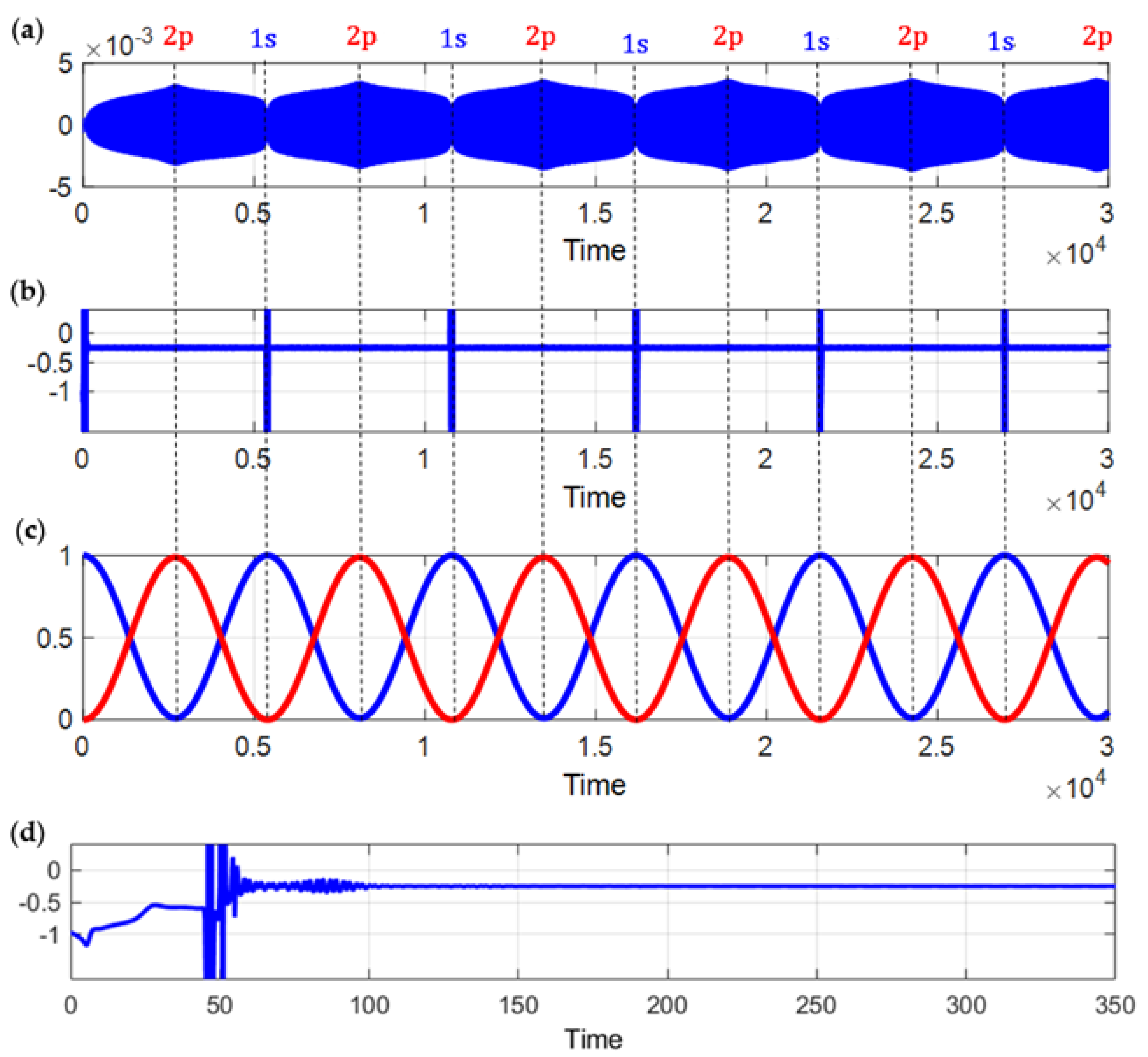
4.2. 1s–2p State Transition
5. Discussions
- Through the equivalence of the Schrödinger equation and the Hamilton-Jacobi equation, the electron transition behavior, which was originally described by the quantum probability, can now be described by the dynamic trajectories of the combined orbital and spin motions.
- By extending motion domain from the real space to the complex space, the physical observables, which were originally represented by abstract quantum operators, can now be represented by complex variables or complex functions.
- Spin motion, which was originally described by relativistic quantum mechanics, can now be described by the Schrödinger equation defined in the complex domain.
- Quantum phenomena, which were inexplicable by classical mechanics and can only be predicted by the quantum probability, can now be understood intuitively by the action of the quantum potential .
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brion, E.; Molmer, K. Quantum computing with collective ensembles of multilevel systems. Phys. Rev. Lett. 2007, 99, 260501. [Google Scholar] [CrossRef] [PubMed]
- Li, W.P.; Yin, J.W.; Yu, Y.F.; Xiao, J.L.; Wang, Z.W. Quantum transition of two-level system in a parabolic quantum dot. Int. J. Theor. Phys. 2009, 48, 3339. [Google Scholar] [CrossRef]
- Yang, C.D.; Han, S.Y. Tunneling quantum dynamics in ammonia. Int. J. Mol. Sci. 2021, 22, 8282. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Lee, A.; Sinha, K.; Fatemi, F.K.; Rolston, S.L. Observation of vacuum-induced collective quantum beats. Phys. Rev. Lett. 2021, 127, 073604. [Google Scholar] [CrossRef]
- Lei, X.; Ma, L.; Yan, J.; Zhou, X.; Yan, Z.; Jia, X. Electromagnetically induced transparency quantum memory for non-classical states of light. Adv. Phys. X 2022, 7, 2060133. [Google Scholar] [CrossRef]
- Rao, J.; Li, B. Resonances of the hydrogen atom in strong parallel magnetic and electric fields. Phys. Rev. A 1995, 51, 4526–4530. [Google Scholar] [CrossRef]
- Bartsch, T.; Main, J.; Wunner, G. The hydrogen atom in an electric field: Closed-orbit theory with bifurcating orbits. J. Phys. B At. Mol. Opt. Phys. 2003, 36, 1231–1254. [Google Scholar] [CrossRef]
- Tian, M.; Barber, Z.W.; Fischer, J.A.; Babbitt, W.R. Geometric manipulation of the quantum states of two-level atoms. Phys. Rev. A 2004, 69, 050301. [Google Scholar] [CrossRef]
- Gao, J.; Daal, M.; Vayonakis, A.; Kumar, S.; Zmuidzinas, J.; Sadoulet, B.; Mazin, B.A.; Day, P.K.; Leduc, H.G. Experimental evidence for a surface distribution of two-level systems in superconducting lithographed microwave resonators. Appl. Phys. Lett. 2008, 92, 152505. [Google Scholar] [CrossRef]
- Bowman, P.J. A “local observables” method for wave mechanics applied to atomic hydrogen. Am. J. Phys. 2008, 76, 1120–1129. [Google Scholar] [CrossRef]
- Messina, A.; Nakazato, H. Analytically solvable Hamiltonians for quantum two-level systems and their dynamics. J. Phys. A Math. Theor. 2014, 47, 445302. [Google Scholar] [CrossRef]
- Lopez Carreno, J.C.; Sanchez Munoz, C.; del Valle, E.; Laussy, F.P. Excitation with quantum light. II. Exciting a two-level system. Phys. Rev. A 2016, 94, 063826. [Google Scholar] [CrossRef]
- Oberreiter, L.; Burkhardt, J.; Main, J.; Wunner, G. Population transfer at exceptional points in the spectra of the hydrogen atom in parallel electric and magnetic fields. Phys. Rev. A 2018, 98, 013417. [Google Scholar] [CrossRef]
- Stavrou, V.N. Spin qubits: Spin relaxation in coupled quantum dots. J. Phys. Condens. Matter. 2018, 30, 455301. [Google Scholar] [CrossRef] [PubMed]
- Sumner, I.; Anthony, H. Electron trajectories in molecular orbitals. Int. J. Quantum. Chem. 2020, 120, e26371. [Google Scholar] [CrossRef]
- Stipsic, P.; Milivojevic, M. Control of a spin qubit in a lateral GaAs quantum dot based on symmetry of gating potential. Phys. Rev. B 2020, 101, 165302. [Google Scholar] [CrossRef]
- Zheng, C. Quantum simulation of Pseudo-Hermitian-φ-symmetric two-level systems. Entropy 2022, 24, 867. [Google Scholar] [CrossRef]
- Holland, P. Uniqueness of paths in quantum mechanics. Phys. Rev. A 1999, 60, 4326. [Google Scholar] [CrossRef]
- Colijn, C.; Vrscay, E.R. Spin-dependent Bohm trajectories for hydrogen eigenstates. Phys. Lett. A. 2002, 300, 334–340. [Google Scholar] [CrossRef]
- Colijn, C.; Vrscay, E.R. Spin-dependent Bohm trajectories associated with an electronic transition in hydrogen. J. Phys. A Math. Gen. 2003, 36, 4689–4702. [Google Scholar] [CrossRef] [Green Version]
- Colijn, C.; Vrscay, E.R. Spin-dependent Bohm trajectories for Pauli and Dirac eigenstates of hydrogen. Found. Phys. Lett. 2003, 36, 303–323. [Google Scholar] [CrossRef]
- Colijn, C.; Vrscay, E.R. Quantum relaxation in hydrogen eigenstates and two-state transitions. Phys. Lett. A 2004, 327, 113–122. [Google Scholar] [CrossRef]
- Shakov, K.; McGuire, J.H. Population control of 2s–2p transitions in hydrogen. Phys. Rev. A 2003, 67, 033405. [Google Scholar] [CrossRef]
- Scheffler, T.B.; Malherbe, J.B. Complex energy eigenvalues of a zero-range atom in a uniform electric field. J. Phys. A Math. Gen. 1979, 12, 1011–1023. [Google Scholar] [CrossRef]
- Farrelly, D.; Reinhardt, W.P. Uniform semiclassical and accurate quantum calculations of complex energy eigenvalues for the hydrogen atom in a uniform electric field. J. Phys. B Atom. Mol. Phys. 1983, 16, 2103–2117. [Google Scholar] [CrossRef]
- Hertzberg, M.P.; Yamada, M. Vacuum decay in real time and imaginary time formalisms. Phys. Rev. D 2019, 100, 016011. [Google Scholar] [CrossRef]
- Liu, C.S. The quantum phase transitions of dimer chain driven by an imaginary alternating field. Phys. E Low-Dimens. Syst. Nanostructures 2021, 134, 114871. [Google Scholar] [CrossRef]
- Turro, F.; Roggero, A.; Amitrano, V.; Luchi, P.; Wendt, K.A.; Dubois, J.L.; Quaglioni, S.; Pederiva, F. Imaginary-time propagation on a quantum chip. Phys. Rev. A 2022, 105, 022440. [Google Scholar] [CrossRef]
- Wang, M.S. Stochastic interpretation of quantum mechanics in complex space. Phys. Rev. Lett. 1997, 79, 3319–3322. [Google Scholar] [CrossRef]
- Kanatchikov, I.V. De Donder-Weyl theory and a hypercomplex extension of quantum mechanics to field theory. Rep. Math. Phys. 1998, 42, 157–170. [Google Scholar] [CrossRef] [Green Version]
- John, M.V. Modified de Broglie-Bohm approach to quantum mechanics. Found. Phys. Lett. 2002, 15, 329–343. [Google Scholar] [CrossRef]
- Schomerus, H. Topologically protected midgap states in complex photonic lattices. Opt. Lett. 2013, 38, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Aharonov, Y.; Albert, D.Z.; Vaidman, L. How the result of a measurement of a component of a spin-1/2 particle can turn to be 100. Phys. Rev. Lett. 1988, 60, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Aharonov, Y.; Botero, A. Quantum averages of weak values. Phys. Rev. A 2005, 72, 052111. [Google Scholar] [CrossRef]
- Jozsa, R. Complex weak values in quantum measurement. Phys. Rev. A 2007, 76, 044103. [Google Scholar] [CrossRef]
- Matzkin, A. Weak measurements of trajectories in quantum systems: Classical, Bohmian and sum over paths. J. Phys. A Math. Theor. 2015, 48, 305301. [Google Scholar] [CrossRef]
- Kocsis, S.; Braverman, B.; Ravets, S.; Stevens, M.J.; Mirin, R.P.; Shalm, L.K.; Steinberg, A.M. Observing the Average Trajectories of Single Photons in a Two-Slit Interferometer. Science 2011, 332, 1170–1173. [Google Scholar] [CrossRef]
- Murch, K.W.; Weber, S.J.; Macklin, C.; Siddiqi, I. Observing Single Quantum Trajectories of a Superconducting Quantum Bit. Nature 2013, 502, 211–214. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Liu, X.; Kedem, Y.; Cui, J.M.; Li, Z.F.; Hua, Y.L.; Li, C.F.; Guo, G.C. Experimental observation of anomalous trajectories of single photons. Phys. Rev. A 2017, 95, 042121. [Google Scholar] [CrossRef]
- Procopio, L.M.; Rozema, L.A.; Wong, Z.J.; Hamel, D.R.; O’Brien, K.; Zhang, X.; Dakić, B.; Walther, P. Single-Photon Test of Hyper-Complex Quantum Theories Using a Metamaterial. Nat. Commun. 2017, 8, 15044. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Mason, D.; Chen, J.; Schliesser, A. Observing and verifying the quantum trajectory of a mechanical resonator. Phys. Rev. Lett. 2019, 123, 163601. [Google Scholar] [CrossRef]
- Ghafari, F.; Tischler, N.; Di Franco, C.; Thompson, J.; Gu, M.; Pryde, G.J. Interfering trajectories in experimental quantum-enhanced stochastic simulation. Nat. Commun. 2019, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Naghiloo, M.; Tan, D.; Harrington, O.M.; Alonso, J.J.; Lutz, E.; Romito, A.; Murch, K.W. Heat and work along individual trajectories of a quantum bit. Phys. Rev. Lett. 2020, 124, 110604. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Pekola, J.P. Quantum trajectory analysis of single microwave photon detection by nanocalorimetry. Phys. Rev. Lett. 2020, 124, 170601. [Google Scholar] [CrossRef] [PubMed]
- Steinfurth, A.; Krešić, I.; Weidemann, S.; Kremer, M.; Makris, K.G.; Heinrich, M.; Rotter, S.; Szameit, A. Observation of photonic constant-intensity waves and induced transparency in tailored non-Hermitian lattices. Sci. Adv. 2022, 8, eabl7412. [Google Scholar] [CrossRef]
- Lee, T.E.; Chan, C.K. Heralded magnetism in non-Hermitian atomic systems. Phys. Rev. X 2014, 4, 041001. [Google Scholar] [CrossRef]
- Helbig, T.; Hofmann, T.; Imhof, S.; Abdelghany, M.; Kiessling, T.; Molenkamp, L.W.; Lee, C.H.; Szameit, A.; Greiter, M.; Thomale, R. Generalized bulk-boundary correspondence in non-Hermitian topolectrical circuits. Nat. Phys. 2020, 16, 747–750. [Google Scholar] [CrossRef]
- Li, J.; Harter, A.K.; Liu, J.; de Melo, L.; Joglekar, Y.N.; Luo, L. Observation of parity time symmetry breaking transitions in a dissipative floquent system of ultracold atoms. Nat. Commun. 2019, 10, 855. [Google Scholar] [CrossRef]
- Renou, M.O.; Trillo, D.; Weilenmann, M.; Le, T.P.; Tavakoli, A.; Gisin, N.; Acín, A.; Navascués, M. Quantum theory based on real number can be experimentally falsified. Nature 2021, 600, 625–629. [Google Scholar] [CrossRef]
- Wu, K.D.; Kondra, T.V.; Rana, S.; Scandolo, C.M.; Xiang, G.Y.; Li, C.F.; Guo, G.C.; Streltsov, A. Resource theory of imaginarity: Quantification and state conversion. Phys. Rev. A 2021, 103, 032401. [Google Scholar] [CrossRef]
- Wu, K.D.; Kondra, T.V.; Rana, S.; Scandolo, C.M.; Xiang, G.Y.; Li, C.F.; Guo, G.C.; Streltsov, A. Operational resource theory of imaginarity. Phys. Rev. Lett. 2021, 126, 090401. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Wang, C.; Liu, F.M.; Wang, J.W.; Ying, C.; Shang, Z.X.; Wu, Y.; Gong, M.; Deng, H.; Liang, F.T.; et al. Ruling out real-valued standard formalism of quantum theory. Phys. Rev. Lett. 2022, 128, 040403. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.D. Wave-particle duality in complex space. Ann. Phys. 2005, 319, 444–470. [Google Scholar] [CrossRef]
- Yang, C.D. Quantum Hamilton mechanics: Hamilton equations of quantum motion, origin of quantum operators, and proof of quantization axiom. Ann. Phys. 2006, 321, 2876–2926. [Google Scholar] [CrossRef]
- Yang, C.D. Trajectory interpretation of the uncertainty principle in 1D systems using complex Bohmian mechanics. Phys. Lett. A 2008, 372, 6240–6253. [Google Scholar] [CrossRef]
- Yang, C.D.; Han, S.Y. Trajectory interpretation of correspondence principle: Solution of nodal issue. Found. Phys. 2020, 50, 960–976. [Google Scholar] [CrossRef]
- Yang, C.D.; Han, S.Y. Extending quantum probability from real axis to complex plane. Entropy 2021, 23, 210. [Google Scholar] [CrossRef]
- Yang, C.D. Quantum dynamics of hydrogen atom. Ann. Phys. 2005, 319, 399–443. [Google Scholar] [CrossRef]
- Yang, C.D. Complex spin and anti-spin dynamics: A generalization of de Broglie-Bohm theory to complex space. Chaos Soli Frac. 2009, 41, 317–333. [Google Scholar] [CrossRef]
- Yang, C.D. Spin: Nonlinear zero dynamics of orbital motion. Chaos Soli Frac. 2008, 37, 1158–1171. [Google Scholar] [CrossRef]
- Yang, C.D. Complex tunneling dynamics. Chaos Soli Frac. 2007, 32, 312–345. [Google Scholar] [CrossRef]
- Colangelo, G.; Ciurana, F.M.; Bianchet, L.C.; Sewell, R.J.; Mitchell, M.W. Simultaneous tracking of spin angle and amplitude beyond classical limits. Nature 2017, 543, 525. [Google Scholar] [CrossRef] [PubMed]
- Cujia, K.S.; Boss, J.M.; Herb, K.; Zopes, J.; Degen, C.L. Tracking the precession of single nuclear spins by weak measurements. Nature 2019, 571, 230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Chen, S.; Wen, S.; Luo, H. Multiple-weak-value quantum measurement for precision estimation of time delay. Phys. Rev. A 2022, 105, 033521. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-D.; Han, S.-Y. Orbital and Spin Dynamics of Electron’s States Transition in Hydrogen Atom Driven by Electric Field. Photonics 2022, 9, 634. https://doi.org/10.3390/photonics9090634
Yang C-D, Han S-Y. Orbital and Spin Dynamics of Electron’s States Transition in Hydrogen Atom Driven by Electric Field. Photonics. 2022; 9(9):634. https://doi.org/10.3390/photonics9090634
Chicago/Turabian StyleYang, Ciann-Dong, and Shiang-Yi Han. 2022. "Orbital and Spin Dynamics of Electron’s States Transition in Hydrogen Atom Driven by Electric Field" Photonics 9, no. 9: 634. https://doi.org/10.3390/photonics9090634
APA StyleYang, C.-D., & Han, S.-Y. (2022). Orbital and Spin Dynamics of Electron’s States Transition in Hydrogen Atom Driven by Electric Field. Photonics, 9(9), 634. https://doi.org/10.3390/photonics9090634





