Identifying Collector Channel Orifices In Vivo with Phase-Sensitive Optical Coherence Tomography: A Preliminary Study
Abstract
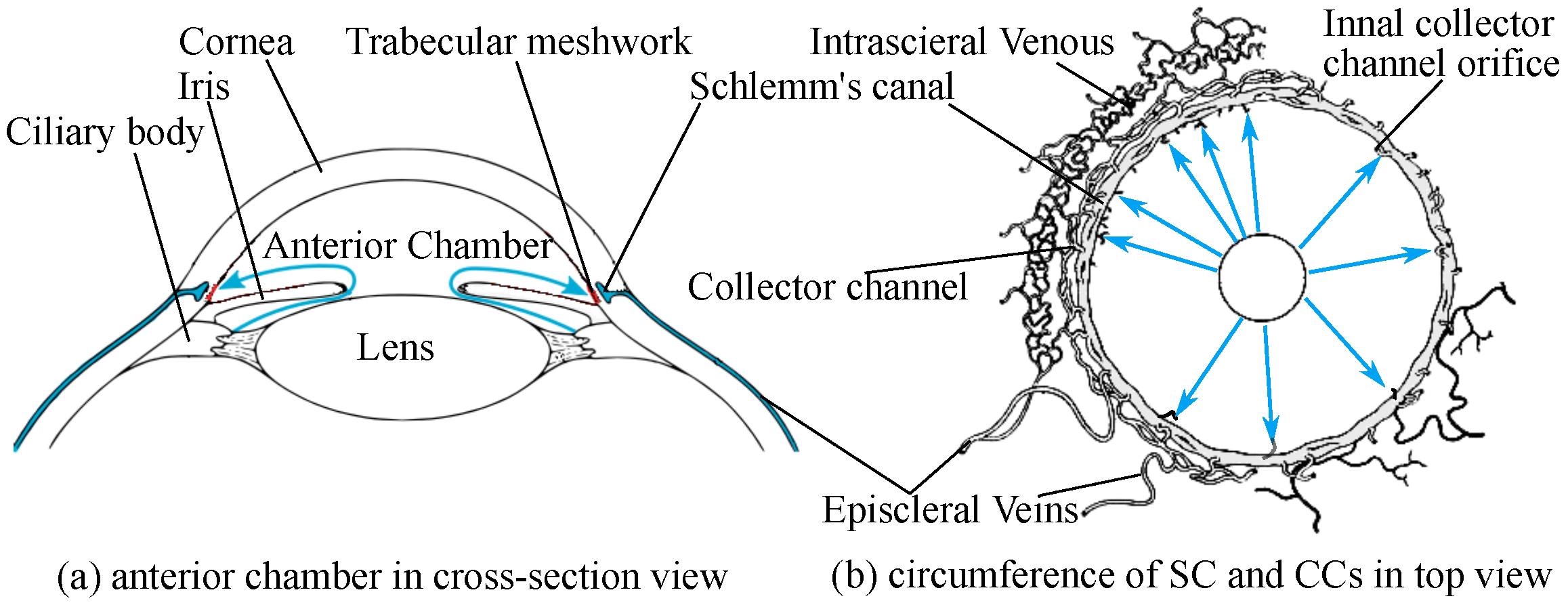
1. Introduction
- Imaging AHO pathways in vivo
- OCT for imaging AHO pathways
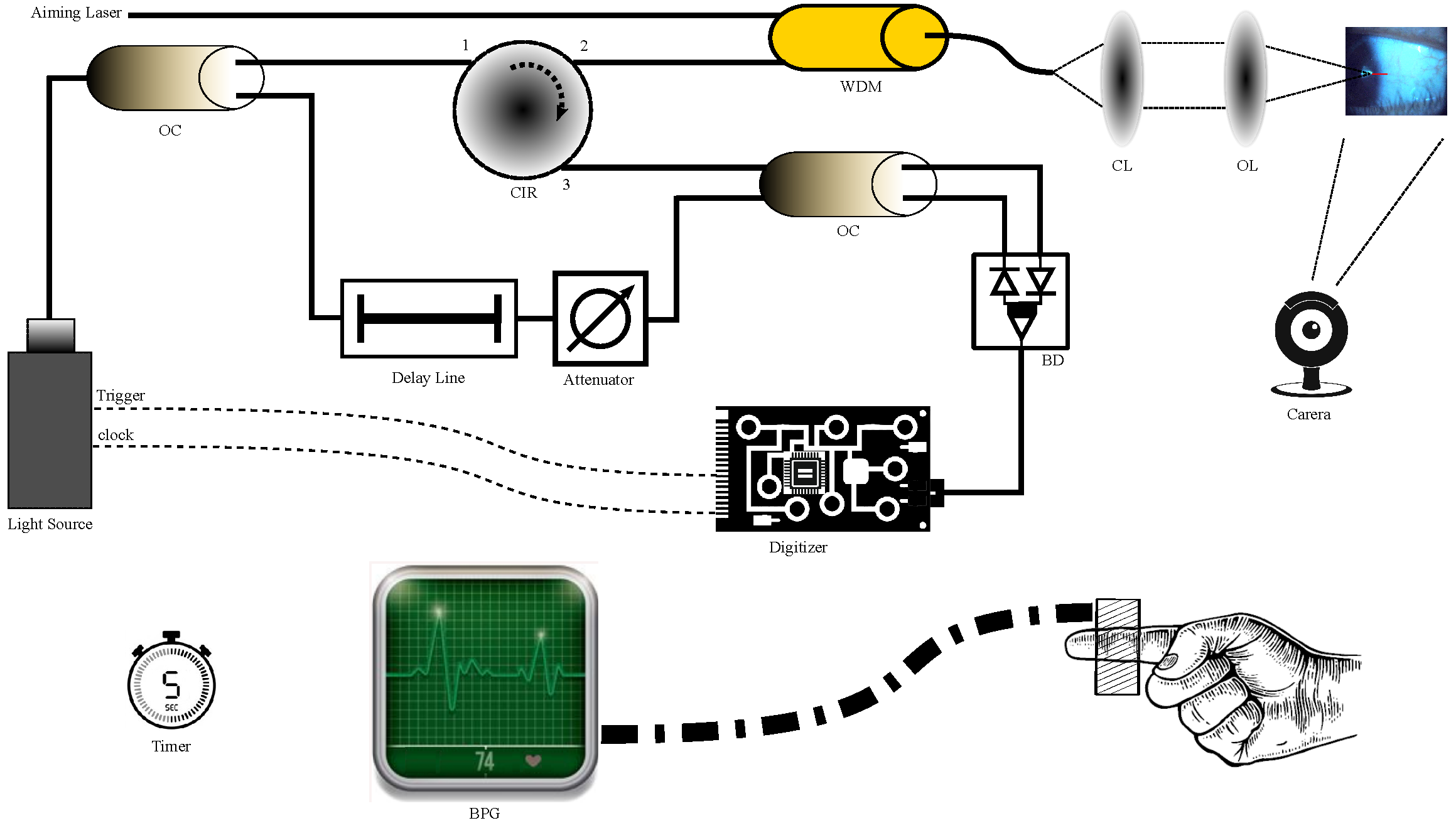
2. Phs-OCT Displacement Methods
2.1. System Setup
2.2. Principle of PhS-OCT
2.3. Phase Stabilization
- (1)
- Phase unwrapping. Since the length of the OCT axial scan was short in this study (less than 12 mm), it can be assumed that phase-wrapping occurred only once along the A scan. Consequently, when phase wrapping occurred, the phase signals were divided into two discontinued sets, and the wrapped phase was recognized as a negative value. The average value of the squared phase signal was used as a criterion to define the origin coordinate. Then, an offset of was given to the negative set to reverse the wrapping effect. Consequently, the phase ramp trend was recovered.
- (2)
- Phase disambiguation and jitter compensation. PhDiff can be expressed as , which is depth-dependent. The value of is a random radian value within a specific range, depending on the amplitude of the synchronization jitter. If the gradient term is depth-z-dependent, the jitter-induced phase change is calculable. One way to exclude the phase noise is median filtering. According to the phase variation statistics, after compensation processing, the phase stability was reduced from 1.8 radians of standard deviation to [46].
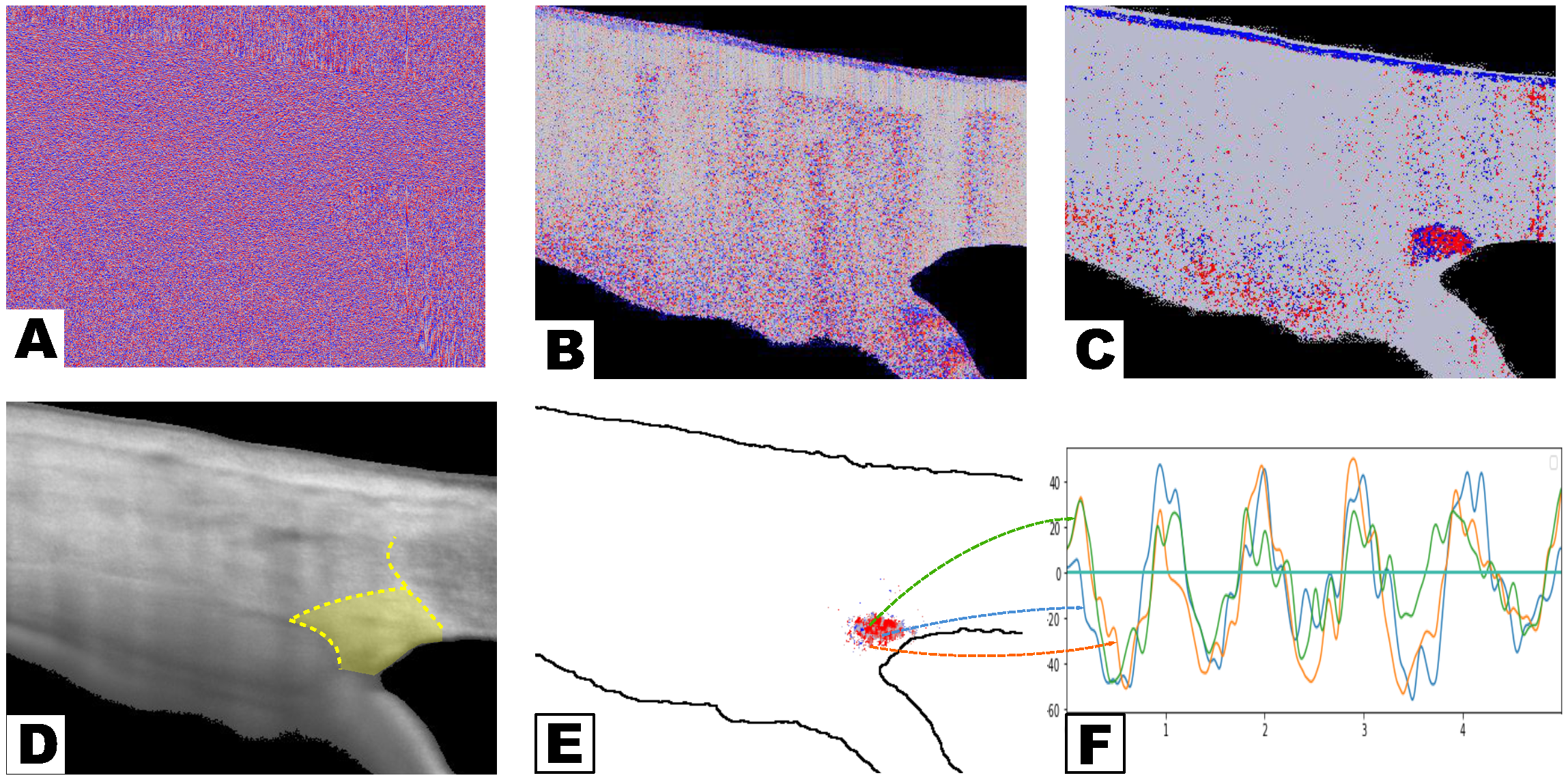
3. Signal Processing
3.1. Data Acquisition and Preprocessing
3.2. TM Motion Identification and Reconstruction
- Signal screening by waveform quality
- Signal reconstruction
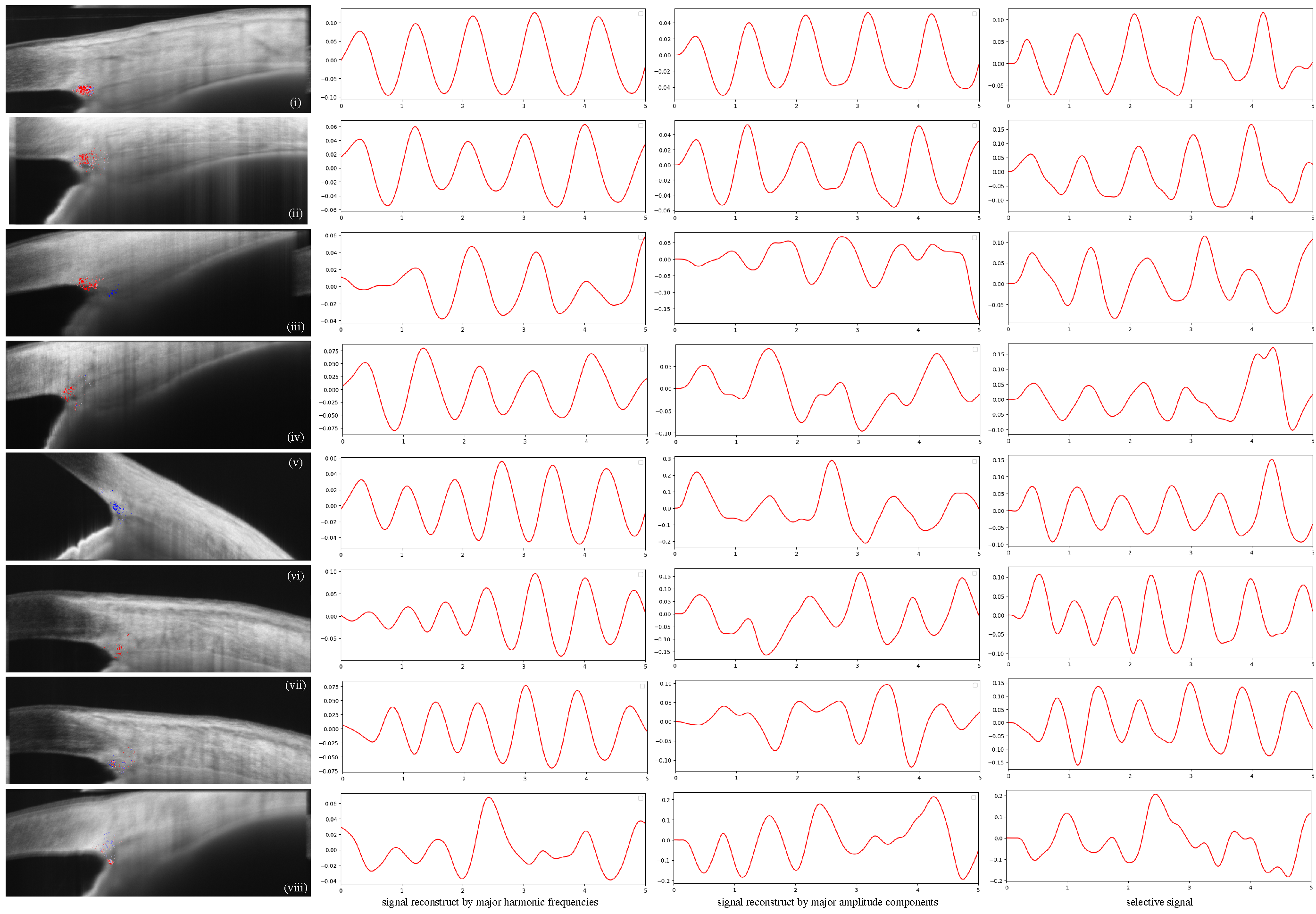
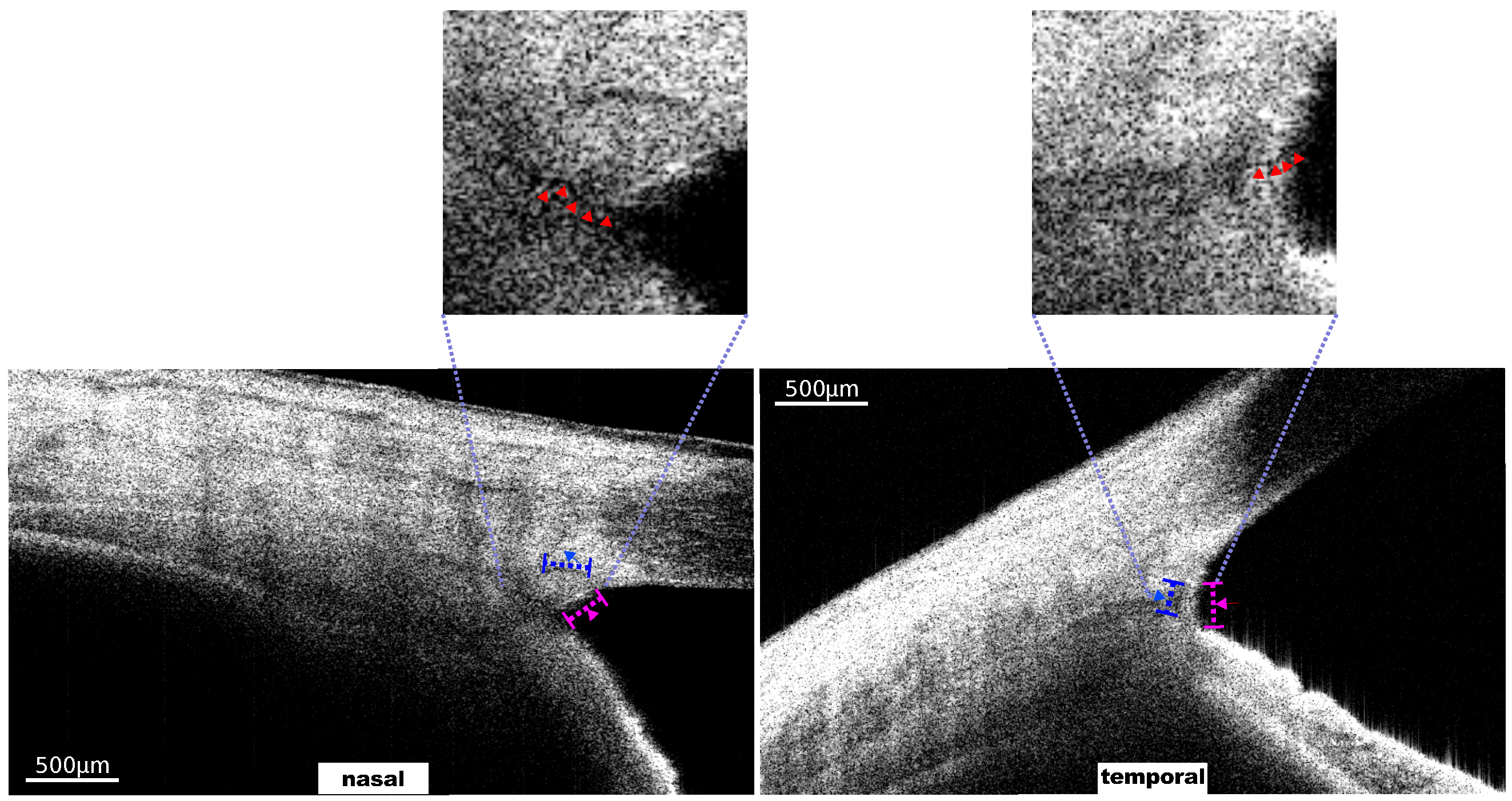
4. Results and Discussion
Results of TM Motion Signal Reconstruction
5. Conclusions
Funding
Conflicts of Interest
References
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lütjen-Drecoll, E.; Shimizu, T.; Rohrbach, M.; Rohen, J.W. Quantitative analysis of ‘plaque material’ in the inner- and outer wall of Schlemm’s canal in normal- and glaucomatous eyes. Exp. Eye Res. 1986, 42, 443–455. [Google Scholar] [CrossRef]
- Johnstone, M.A. The aqueous outflow system as a mechanical pump: Evidence from examination of tissue and aqueous movement in human and non-human primates. J. Glaucoma 2004, 13, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.; Alvarado, J.; Weddell, J. Histology of the Human Eye: An Atlas and Textbook; Saunders: Philadelphia, PA, USA, 1971; pp. 136–153, 260–319. [Google Scholar]
- Richter, G.M.; Coleman, A.L. Minimally invasive glaucoma surgery: Current status and future prospects. Clin. Ophthalmol. 2016, 10, 189–206. [Google Scholar]
- Yuan, F.; Schieber, A.; Camras, L.; Harasymowycz, P.; Herndon, L.; RR, A. Mathematical Modeling of Outflow Facility Increase with Trabecular Meshwork Bypass and Schlemm Canal Dilation. J. Glaucoma 2016, 25, 355–364. [Google Scholar] [CrossRef]
- Xin, C.; Wang, R.K.; Song, S.; Shen, T.; Wen, J.; Martin, E.; Jiang, Y.; Padilla, S.; Johnstone, M. Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Exp. Eye Res. 2017, 158, 171–186. [Google Scholar] [CrossRef]
- Ahuja, Y.; Pyi, S.M.K.; Malihi, M.; Hodge, D.O.; Sit, A.J. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: The Mayo Clinic series in Rochester, Minnesota. Am. J. Ophthalmol. 2013, 156, 927–935. [Google Scholar] [CrossRef]
- Hariri, S.; Johnstone, M.; Jiang, Y.; Padilla, S.; Zhou, Z.; Reif, R.; Wang, R.K. Platform to investigate aqueous outflow system structure and pressure-dependent motion using high-resolution spectral domain optical coherence tomography. J. Biomed. Opt. 2014, 19, 1–11. [Google Scholar] [CrossRef]
- Huang, A.S.; Mohindroo, C.; Weinreb, R.N. Aqueous Humor Outflow Structure and Function Imaging At the Bench and Bedside: A Review. J. Clin. Exp. Ophthalmol. 2016, 7. [Google Scholar] [CrossRef]
- Hann, C.R.; Fautsch, M.P. Preferential fluid flow in the human trabecular meshwork near collector channels. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1692–1697. [Google Scholar] [CrossRef]
- Rohen, J.W.; Rentsch, F.J. Morphology of Schlemm’s canal and related vessels in the human eye. Albrecht Von Graefe’S Arch. Clin. Exp. Ophthalmol. 1968, 176, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Hann, C.R.; Bentley, M.D.; Vercnocke, A.; Ritman, E.L.; Fautsch, M.P. Imaging the aqueous humor outflow pathway in human eyes by three-dimensional micro-computed tomography (3D micro-CT). Exp. Eye Res. 2011, 92, 104–111. [Google Scholar] [CrossRef]
- Johnstone, M.A. A new model describes an aqueous outflow pump and explores causes of pump failure in glaucoma. In Essentials in Ophthalmology; Grehn, F., Stamper, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Chapter Glaucoma; pp. 3–34. [Google Scholar] [CrossRef]
- Johnstone, M.; Xin, C.; Tan, J.; Martin, E.; Wen, J.; Wang, R.K. Aqueous outflow regulation—21st century concepts. Prog. Retin. Eye Res. 2021, 83, 100917. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.D.; Hann, C.R.; Fautsch, M.P. Anatomical Variation of Human Collector Channel Orifices. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1153–1159. [Google Scholar] [CrossRef]
- Fellman, R.L.; Feuer, W.J.; Grover, D.S. Episcleral Venous Fluid Wave Correlates with Trabectome Outcomes: Intraoperative Evaluation of the Trabecular Outflow Pathway. Ophthalmology 2015, 122, 2385–2391.e1. [Google Scholar] [CrossRef]
- Grieshaber, M.C.; Pienaar, A.; Olivier, J.; Stegmann, R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1498–1504. [Google Scholar] [CrossRef]
- Jhanji, V.; Chan, E.; Das, S.; Zhang, H.; Vajpayee, R.B. Trypan blue dye for anterior segment surgeries. Eye 2011, 25, 1113–1120. [Google Scholar] [CrossRef]
- Simpson, T.; Fonn, D. Optical coherence tomography of the anterior segment. Ocul. Surf. 2008, 6, 117–127. [Google Scholar] [CrossRef]
- Leung, C.K.s.; Cheung, C.Y.l.; Lin, D.; Pang, C.P.; Lam, D.S.C.; Weinreb, R.N. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4886–4892. [Google Scholar] [CrossRef]
- Grewal, D.S.; Tanna, A.P. Diagnosis of glaucoma and detection of glaucoma progression using spectral domain optical coherence tomography. Curr. Opin. Ophthalmol. 2013, 24, 150–161. [Google Scholar] [CrossRef]
- Tatham, A.J.; Medeiros, F.A. Detecting Structural Progression in Glaucoma with Optical Coherence Tomography. Ophthalmology 2017, 124, S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha, D.S.V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Kagemann, L.; Wang, B.; Wollstein, G.; Ishikawa, H.; Nevins, J.E.; Nadler, Z.; Sigal, I.A.; Bilonick, R.A.; Schuman, J.S. IOP elevation reduces Schlemm’s canal cross-sectional area. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1805–1809. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Milner, T.E.; Srinivas, S.; Wang, X.; Malekafzali, A.; van Gemert, M.J.C.; Nelson, J.S. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Opt. Lett. 1997, 22, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, Y.; Saxer, C.; Xiang, S.; de Boer, J.F.; Nelson, J.S. Phase-resolved OCT/ODT for imaging tissue microcirculation. In Proceedings of the Conference on Lasers and Electro-Optics, San Francisco, CA, USA, 7–11 May 2000; Optical Society of America: Washington, DC, USA, 2000; p. CThF1. [Google Scholar]
- Vakoc, B.; Yun, S.; de Boer, J.; Tearney, G.; Bouma, B. Phase-resolved optical frequency domain imaging. Opt. Express 2005, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Kirkpatrick, S.; Hinds, M. Phase-sensitive optical coherence elastography for mapping tissue microstrains in real time. Appl. Phys. Lett. 2007, 90, 164105. [Google Scholar] [CrossRef]
- Wang, R.K.; Nuttall, A.L. Phase-sensitive optical coherence tomography imaging of the tissue motion within the organ of Corti at a subnanometer scale: A preliminary study. J. Biomed. Opt. 2010, 15, 056005. [Google Scholar] [CrossRef]
- Szkulmowski, M.; Szkulmowska, A.; Bajraszewski, T.; Kowalczyk, A.; Wojtkowski, M. Flow velocity estimation using joint Spectral and Time domain Optical Coherence Tomography. Opt. Express 2008, 16, 6008–6025. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Fingler, J.; Kim, D.Y.; Zawadzki, R.; Morse, L.S.; Park, S.S.C.; Fraser, S.E.; Werner, J.S. Phase-variance optical coherence tomography: A technique for noninvasive angiography. Ophthalmology 2014, 121, 180–187. [Google Scholar] [CrossRef]
- Li, P.; Reif, R.; Zhi, Z.; Shen, T.; Wang, R.K.; Martin, E.; Johnstone, M. Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo nonhuman primate eyes. J. Biomed. Opt. 2012, 17, 076026. [Google Scholar] [CrossRef]
- Li, P.; Reif, R.; Zhi, Z.; An, L.; Martin, E.; Shen, T.T.; Johnstone, M.; Wang, R.K. Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo non-human primate eyes. In Proceedings of the Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XVII. International Society for Optics and Photonics, San Francisco, CA, USA, 20 March 2013; Volume 8571, p. 85711S. [Google Scholar] [CrossRef]
- Gao, K.; Song, S.; Johnstone, M.A.; Zhang, Q.; Xu, J.; Zhang, X.; Wang, R.K.; Wen, J.C. Reduced Pulsatile Trabecular Meshwork Motion in Eyes With Primary Open Angle Glaucoma Using Phase-Sensitive Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 21. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Wang, X.; Wang, N.; Wang, R.; Johnstone, M. Trabecular Meshwork Motion Profile from Pulsatile Pressure Transients: A New Platform to Simulate Transitory Responses in Humans and Nonhuman Primates. Appl. Sci. 2022, 12, 11. [Google Scholar] [CrossRef]
- Wu, C.; Aglyamov, S.R.; Han, Z.; Singh, M.; Liu, C.H.; Larin, K.V. Assessing the biomechanical properties of the porcine crystalline lens as a function of intraocular pressure with optical coherence elastography. Biomed. Opt. Express 2018, 9, 6455–6466. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Gille, H.K.; Wu, J.; Yang, C. Ex vivo optical coherence tomography imaging of collector channels with a scanning endoscopic probe. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3921–3925. [Google Scholar] [CrossRef]
- Xin, C.; Chen, X.; Li, M.; Shi, Y.; Wang, H.; Wang, R.; Wang, N. Imaging collector channel entrance with a new intraocular micro-probe swept-source optical coherence tomography. Acta Ophthalmol. 2017, 95, 602–607. [Google Scholar] [CrossRef]
- Akagi, T.; Uji, A.; Huang, A.S.; Weinreb, R.N.; Yamada, T.; Miyata, M.; Kameda, T.; Ikeda, H.O.; Tsujikawa, A. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. Am. J. Ophthalmol. 2018, 196, 1–9. [Google Scholar] [CrossRef]
- Parc, C.E.; Johnson, D.H.; Brilakis, H.S. Giant vacuoles are found preferentially near collector channels. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2984–2990. [Google Scholar]
- Xu, J.; Song, S.; Men, S.; Wang, R.K. Long ranging swept-source optical coherence tomography-based angiography outperforms its spectral-domain counterpart in imaging human skin microcirculations. J. Biomed. Opt. 2017, 22, 1–11. [Google Scholar] [CrossRef]
- Song, S.; Zhou, K.; Xu, J.J.; Zhang, Q.; Lyu, S.; Wang, R. Development of a clinical prototype of a miniature hand-held optical coherence tomography probe for prematurity and pediatric ophthalmic imaging. Biomed. Opt. Express 2019, 10, 2383–2398. [Google Scholar] [CrossRef]
- Kirkpatrick, S.J.; Wang, R.K.; Duncan, D.D. OCT-based elastography for large and small deformations. Opt. Express 2006, 14, 11585–11597. [Google Scholar] [CrossRef]
- Duncan, D.D.; Kirkpatrick, S.J.; Mark, F.F.; Hunter, L.W. Transform method of processing for speckle strain-rate measurements. Appl. Opt. 1994, 33, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xu, J.; Men, S.; Shen, T.T.; Wang, R.K. Robust numerical phase stabilization for long-range swept-source optical coherence tomography. J. Biophotonics 2017, 10, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. Optimal signal quality index for photoplethysmogram signals. Bioengineering 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Crowell, E.L.; Baker, L.; Chuang, A.Z.; Feldman, R.M.; Bell, N.P.; Chévez-Barrios, P.; Blieden, L.S. Characterizing anterior segment OCT angle landmarks of the trabecular meshwork complex. Ophthalmology 2018, 125, 994–1002. [Google Scholar] [CrossRef]
- Elgendi, M.; Norton, I.; Abbott, D.; Schuurmans, D. Systolic peak detection in acceleration photoplethysmograms measured from emergency responders in tropical conditions. PLoS ONE 2013, 8, e76585. [Google Scholar] [CrossRef]
- Reddy, K.A.; Kumar, V.J. Motion artifact reduction in photoplethysmographic signals using singular value decomposition. In Proceedings of the 2007 IEEE Instrumentation & Measurement Technology Conference IMTC 2007, Warsaw, Poland, 1–3 May 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 1–4. [Google Scholar]
- Gavish, M.; Donoho, D.L. The optimal hard threshold for singular values is 4/. IEEE Trans. Inf. Theory 2014, 60, 5040–5053. [Google Scholar] [CrossRef]
- Ashton, N. Anatomical study of Schlemm’s canal and aqueous veins by means of neoprene casts: Part I. aqueous veins. Br. J. Ophthalmol. 1951, 35, 291. [Google Scholar] [CrossRef]
- Xin, C.; Song, S.; Johnstone, M.; Wang, N.; Wang, R.K. Quantification of pulse-dependent trabecular meshwork motion in normal humans using phase-sensitive OCT. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3675–3681. [Google Scholar] [CrossRef]



| OCT | Central Wavelength | Spectral Bandwidth | Incident Energy | Sweeping Rate |
|---|---|---|---|---|
| Protocol | 1300 nm | ≥170 nm | 5 mW | 20 kHz |
| image | axial resolution | lateral resolution | frame rate | |
| information | ∼9 m | 4.7 m | 200 fps |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G. Identifying Collector Channel Orifices In Vivo with Phase-Sensitive Optical Coherence Tomography: A Preliminary Study. Photonics 2022, 9, 593. https://doi.org/10.3390/photonics9080593
Li G. Identifying Collector Channel Orifices In Vivo with Phase-Sensitive Optical Coherence Tomography: A Preliminary Study. Photonics. 2022; 9(8):593. https://doi.org/10.3390/photonics9080593
Chicago/Turabian StyleLi, Guangxu. 2022. "Identifying Collector Channel Orifices In Vivo with Phase-Sensitive Optical Coherence Tomography: A Preliminary Study" Photonics 9, no. 8: 593. https://doi.org/10.3390/photonics9080593
APA StyleLi, G. (2022). Identifying Collector Channel Orifices In Vivo with Phase-Sensitive Optical Coherence Tomography: A Preliminary Study. Photonics, 9(8), 593. https://doi.org/10.3390/photonics9080593





