Abstract
A sodium nitrate sensor with graphene/gold coating is presented in this paper. A Kretschmann setup with angle interrogation was used to detect sodium nitrate in the range of 0–15%. Using a graphene coating on top of the 50 nm gold layer showed an improvement in the sensitivity of the sensor. The gold-coated setups had a sensitivity of 0.198°/%. In contrast, the graphene/gold-coated samples showed a sensitivity of 0.244°/% due to the charge transfer between the graphene and the gold and the resulting excited solid electric field. The graphene/gold-coated sensor showed good stability with time in the temperature range of 19–34 °C. This shows that this setup may be beneficial in detecting sodium nitrate.
1. Introduction
Sodium nitrate is a common food additive used in preservation and pickling processes. However, some health concerns were raised, leading to the establishment of a daily intake limit for food and beverages reported as 3.7 mg/kg body weight (bw) per day [1,2,3]. As a result, detecting sodium nitrate levels in food and beverages is critical. Traditional nitrate sensors, which rely on resistive and capacitive elements, face frequency- and temperature-dependence issues, so photonics sensing may be a solution to these issues [4,5,6]. Although tapered and D-shaped fiber sensors have been used to detect sodium nitrate, they were found to be fragile and difficult to handle [7,8]. On the other hand, surface plasmon resonance (SPR) has demonstrated high sensitivity and resolution; further development is needed to ensure cost-effectiveness that may be required.
SPR sensors are also used to detect toxic gases [9] and pollutants [10] and in a variety of bio-sensing applications [11]. When incident light energy couples with electrons in a dielectric–metal interface, they move parallel to the metal surface, causing surface plasmon resonance. These plasmons produce an electric field with a range of approximately 300 nm from the metal–dielectric boundary. This energy coupling is visible as a dip in the SPR sensor’s reflectivity curve at the resonance angle. The incident light must be at the resonance angle where the photons’ propagation constant matches the plasmons [12]. The refractive index of the metal–dielectric interface influences the propagation constant, causing the resonance angle to change. By tracking the changes in resonance angles, valuable information about the refractive index of the sensor environment can be obtained [13,14]. In this study, a Kretschmann setup was used to build the sensor. Kretschmann setups can be a challenge to miniaturize, but they have shown good sensitivity and stability and are widely used.
The surface plasmons are associated with an electric field that is at its peak at the interface, which exponentially decays in the metal and the dielectric material. Surface plasmons need TM transverse magnetic light to be excited because the plasmons are transverse magnetic. When boundary conditions are appropriately applied for the metal–dielectric interface, the propagation constant of plasmons becomes the following:
In the equation, is the surface plasmons’ wave propagation constant, is the incident light propagation constant at wavelength , and and are, respectively, the dielectric constant of the metal and dielectric interfaces, which depend on wavelength [12].
At a given wavelength, the signs of and are opposite. Consequently, the surface plasmons’ propagation constant is larger than the propagation constant of the incident light propagating in air. Thus, the excitation of surface plasmons cannot happen in direct light. Additional momentum and energy are needed to achieve plasmon resonance. The incident light must have additional momentum to satisfy phase matching and excite the surface plasmons. To achieve this prism, grating, waveguide, or fiber-optic coupling methods have been used before [15].
Graphene can be used to improve SPR sensing performance. Graphene is a two-dimensional material with super-speed electron transport [16,17]. Graphene has proven to enhance the sensitivity of bio SPR sensors. Charge transfer from a graphene coating to the thin gold coating of an SPR sensor makes the sensor more sensitive to changes in the refractive index of the sensing environment. This is caused by the stronger excited electric field on the thin gold coating [18,19].
Graphene-based SPR sensors to detect immunoglobulin G (IgG) antibodies had a resolution 5–10 ng/mL and a maximum standard deviation of 4.1 × 10−4 [20]. However, using polarization measurements can be a challenge setup for compact sensing. A graphene boron-doped (BG) nano disk array was structured on a ZnSe prism for biomolecule detection in aqueous solutions. It showed a 0.5 nM limit of detection. However, homogeneous doping is still a challenge for further development [21]. This shows that using graphene for SPR sensing is a promising direction but that developing feasible and cost-effective methods need to be addressed as well.
In this paper, a Kretschmann SPR sensor is presented for sodium nitrate in liquid solutions in which the concentration varied between 0 and 15%. The sensor used a glass prism with a gold and graphene coating. Angle interrogations were used to track the changes in the resonance angle when the sodium nitrate concentration changed.
2. Experiment Setup and Materials Preparation
The experiment setup for the proposed sensor is shown in Figure 1. It consists of a prism coated first with 50 nm gold using sputter coating. The sputter coating machine controls the thickness of the gold coating. The prism is positioned on a rotational stage to vary the incident light angle. A He-Ne light source with a wavelength 632.8 nm was used with a polarizer to ensure proper operations. The photodetector (Thorlabs OPM PM100D) used in this experiment was positioned on a linear translation stage to follow the movement of the prism. The light reflected from the coated surface of the prism was collected and saved using a computer. The sodium nitrate liquid solution was applied to a flow cell secured on the coated surface of the prism.

Figure 1.
The experimental setup for the surface plasmon resonance sensor.
The reflected light was collected for every angle of interest to identify the angle at which the least light was reflected, known as the resonance angle. This process was repeated for the different concentrations of sodium nitrate. The resonance angles recorded for the sodium nitrate solutions were in the range of 0–15% weight percent at room temperature, 25 °C.
The current refractive index measurements available from previous works were recorded [7,8,22]. Sodium nitrate exists in food and drinks in low concentrations and needs to be monitored in those low ranges. Thus, low concentrations between 0 and 15% were reported.
After identifying the resonance angles for different sodium nitrate concentrations, stability was tested. At the resonance angle, the light output level was recorded continuously for several minutes to gauge the stability of the sensor over time. The temperature effect on the sensor was investigated by measuring the light output level at the resonance angle while the temperature varied from 19 to 34 °C degrees.
Angle interrogation was conducted by varying the incident angle of the input light. A graphene coating was carefully applied to the gold-coated surface of the prism. A graphene suspension in acetone was sonicated for 72 h. Then, the sonicated solution was applied to the prism and placed on a spin-coating platform for an hour. This was to ensure even coating on the prism surface. Next, the prism was placed in an oven for the coating to adhere to the surface and at the same time evaporate the acetone. The prism was left in the oven at the temperature of 60 °C for an hour. The prism was stored in a 30% humidity and 25 °C temperature-controlled chamber for 24 h before the experiment.
After each iteration of the solution, distilled water was carefully applied to the sensor surface and then removed from the flow cell. The DI water was carefully used to rinse all the deposit off the sensor and to ensure that the sodium nitrate solution was removed. A microscope was used to check if there was any coating damage. Even though the sensor was exposed to several rounds of sodium nitrate solution, the image under the microscope shows that the graphene coating was not affected.
In future work, a compact and cost-effective sodium food safety sensor can be proposed to align with a compact light source and waveguide coupling to overcome bulkiness. Another reason for using waveguides is to be able to characterize the sensor coatings. The current setup uses a prism that cannot be attached to machinery for surface inspection. Instead, small glass slides can help in conducting characterization testing successfully. However, the sensing waveguide still has the potential to be damaged during the characterization process.
Currently, the scope of this work is to successfully coat the sensor and test it with various concentrations of sodium nitrate solution using angle interrogation method. Furthermore, the proposed work investigates the sensitivity of the sensor towards the changes in the concentrations of the sodium nitrate solutions. The sensing performance was measured based on the changes in the resonance angles at the different concentrations. Testing for selectivity will be addressed in a future work that will combine several coating layers in addition to graphene. Additional coating with different materials may improve the selectivity. In future work, we will focus on intensity-based SPR sensing to calculate limit of detection.
The current Kretschmann setup is appropriate for testing liquid samples safely. Sodium nitrate is a common food additive for pickling and preserving food. A method for examining sodium nitrate presence in food samples needs to be developed. The proposed sensor will be adapted in the future to test real samples from pickled foods.
3. Results and Discussion
This study’s sensor was proposed using a Kretschmann setup with angle interrogation. The changes in the refractive index were observed with changes in the concentrations of sodium nitrate solution. The change in concentration changes the refractive index of the sensing environment at the metal–dielectric interface. Consequently, changes in the wave vector of the plasmon affect the angle at which light couples with the electrons on the metal layers’ field. By observing the different resonance angles for the solutions, the presence and concentration of sodium nitrate can be detected.
Initially, a gold-coated sensor was studied to ensure that it could achieve surface plasmon resonance before applying the graphene coating. Angle interrogation was used to verify that the sensor could achieve SPR with distilled water (sodium nitrate 0%) applied on the surface. Figure 2 shows the resonance dip that occurred when distilled water was applied to the sensor surface. Upon applying the graphene coating, the same process was repeated, and the resonance dip was studied. As shown in Figure 2, the full width at half maximum (FWHM) of the resonance dip widens with graphene coating due to the slight increase in the thickness of the coating layers. The minimum reflectivity also increases due to the coating, which slightly affects the coupling of energy.
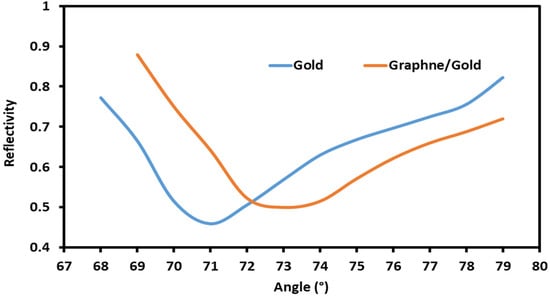
Figure 2.
The reflectivity of the SPR sensor with distilled water solution.
The resonance angle depends on the refractive index of the metal–dielectric interface and surrounding medium. Distilled water with sodium nitrate at 0% concentration is applied to the coated surface of sensor and the resonance angle is observed. The graphene/gold-coated surface of the sensor shows a shift in resonance angle due to the graphene layer’s presence, which changes the conditions of surface plasmon resonance. Furthermore, the application of the graphene coating layer increases the thickness of the coating surface, thus, limiting the energy coupling of the incident light and causing the minimum reflectivity to increase for the graphene/gold-coated sensor, in contrast with the one coated with gold only.
The effects of the sodium nitrate solutions on the gold-coated SPR setup are shown in Figure 3. Solutions of different concentrations of sodium nitrate in water (0%, 1%, 7% and 15%) were injected independently into the flow cell on the surface of sensor with the gold-coated prism. The angle interrogation showed that the resonance angle shift with the changing concentrations of sodium nitrate. This is due to the changes in the refractive indexes of the solutions in contact with the metal–dielectric surface and the subsequent change in the resonance conditions. The total angle shift when gold coating was used is smaller than when graphene/gold coating is used, as can be seen in Figure 3 and Figure 4.
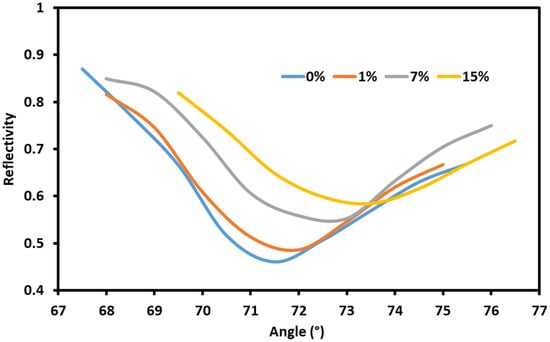
Figure 3.
The response of the gold-coated sensor to sodium nitrate solutions.
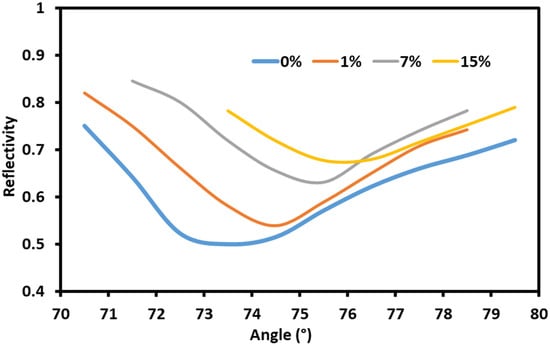
Figure 4.
The response of the sensor with the graphene/gold coating.
Next, the effect of graphene coating on the gold-coated prism was studied by repeating the same process. The angle interrogation showed that the resonance angle shifts with changing concentrations of the sodium nitrate. The wave vector of the plasmon depends on the refractive index of the metal–dielectric interface and the surrounding medium. Thus, sodium nitrate is able to affect the coupling condition and cause an angle shift as shown in Figure 4. Using graphene with gold had a pronounced effect on the response of the sensor to the sodium nitrate concentration changes. The charge transfer between the graphene and gold enhanced the sensor’s sensitivity to changes in the refractive index due to the strengthening of the excited electric field in the metal–dielectric interface.
The sensing performance of the setup shows the effect of using the graphene coatings on gold-coated prism samples as shown in Figure 5. The dashed lines show the best-fit straight line of the sensor response to changes in sodium nitrate concentrations. The sensor responded to the changes in sodium nitrate concentration and improved with graphene coating during the testing of several prisms coated with graphene. An average sensitivity of 0.244°/% was recorded with the graphene/gold samples. The average sensitivity of the prisms with only gold coating was recorded as 0.198°/%, which shows that coating graphene on top of gold can improve the performance of prism setups. This is because graphene is a two-dimensional material with super-speed electron transport. The sensing surface becomes more sensitive to changes in the surrounding due to the charge transfer from the surface of the graphene layer to the surface of the thin gold coating. The graphene also enhances the excited electric field at the gold layer surface. Thus, the use of graphene is beneficial for the sensor.

Figure 5.
The sensing performance of the angle interrogation for the Kretschmann setup sodium nitrate concentration changes in the range 0–15%.
Table 1 shows the performance of the graphene/gold-coated sensor. The experiment was performed three times. The standard deviation was also recorded and showed a maximum of 0.041 mW. The minimum light output reflectivity increased with changes in sodium nitrate concentration. This is an effect of the interaction between the graphene and gold layers with the changing concentrations in the solution and the resulting changes in the coupling conditions for the resonance.

Table 1.
The Kretschmann setup sensing parameters for the sodium nitrate–water solutions.
Several methods are reported for studying the performance of the sensor with the differing concentrations of sodium nitrate solutions. The summary is presented in Table 2. To show the comparison between the reported and proposed sensors for different concentrations of sodium nitrate solutions, most reported that fiber sensors use light output variations and peak wavelength shifts for sensing analytes, but this work uses resonance angle variations to detect the presence of analyte. Tapered fibers and D-shaped fibers are fragile and difficult to handle. Therefore, the proposed sensor uses a Kretschmann setup with prisms for the same purpose. The Kretschmann setup used in this work showed an average sensing performance of 0.244°/%.

Table 2.
The sensing parameters for sodium nitrate sensors in previous works.
Figure 6 shows the results of stability testing the graphene/gold-coated sensor over time. The sensor was tested at the resonance angle for different concentrations, and the light output level was continuously recorded for 300 s. The maximum fluctuation in light output power was 0.020 mw. The stability testing was conducted at room temperature of 25 °C and 30% relative humidity.
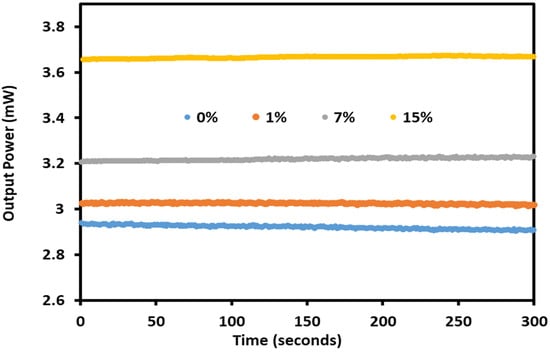
Figure 6.
The Kretschmann setup stability for sodium nitrate detection.
Investigating a graphene/gold-coated sensor with temperature effect is vital. The effects of temperature variation needs to be investigated to ensure that the light output of the sensor is stable. The sensor will be further developed into a compact food safety sensor in the future. Thus, room temperatures in the range of 19–34 °C were used for these investigations. This range of temperature provided a reliable and stable output for the current setup. In Figure 7, the light output level at the resonance angle was studied with temperature ranging between 19 and 34 °C. The sensor shows a maximum output power of 0.020 mW variation in the temperature range, which can be considered in the future development of the sensor. Gold coating shows better stability in SPR sensors than other plasmonic coatings such as silver due to oxidation.
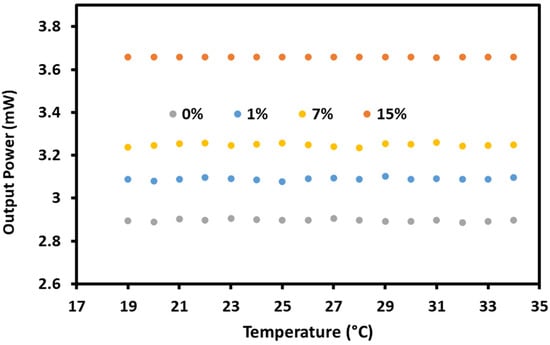
Figure 7.
The temperature effects on the Kretschmann setup for detecting sodium nitrate.
4. Conclusions
Surface plasmon resonance sensors are garnering attention for their good sensitivity and detection performance. Sodium nitrate is a chemical used for preserving food. However, it is recommended that its consumption be limited for a healthier life. A graphene/gold-coated Kretschmann SPR sensor was developed for sodium nitrate detection in the range of 0–15%. A He-Ne light source with wavelength of 632.8 nm was used as a light source with a prism coated with a 50 nm layer of gold and graphene. Angle interrogation was used to investigate the performance of the sensor. The graphene/gold-coated samples showed a sensitivity of 0.244°/%, while the gold-coated setups showed a sensitivity of 0.198°/%. The charge transfer between the graphene and gold occurred due to the strong excited electric field, which enhanced the sensor’s response to refractive index changes. The sensor showed good stability over time. Temperature’s effect on the sensor output was minimal at the temperature range between 19 and 34 °C. This shows that the proposed sensor is effective for detecting sodium nitrate. This sensor will be further developed into a compact intensity-based surface plasmon resonance sensor for food and drinking water safety.
Author Contributions
Conceptualization, S.W.H.; Data curation, H.A.Z.; Formal analysis, H.A.Z. and S.W.H.; Funding acquisition, M.B. and Z.H.; Investigation, H.A.Z.; Methodology, H.A.Z.; Project administration, M.B. and S.W.H.; Resources, S.W.H.; Supervision, M.B. and S.W.H.; Writing—original draft, H.A.Z. and M.B.; Writing—review & editing, Z.H., H.R.A.R. and S.W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by INTI IU SEEDING GRANT 2022, grant number INTI-FEQS-01-11-2022.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would also like to thank the research facility at Photonics Engineering Laboratory, University of Malaya (UM).
Conflicts of Interest
The authors declare no conflict of interest.
References
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of sodium nitrate (E 251) and potassium nitrate (E 252) as food additives. EFSA J. 2017, 15, e04787. [Google Scholar] [CrossRef]
- Aly, H.A.; Mansour, A.M.; Abo-Salem, O.M.; Abd-Ellah, H.F.; Abdel-Naim, A.B. Potential testicular toxicity of sodium nitrate in adult rats. Food Chem. Toxicol. 2010, 48, 572–578. [Google Scholar] [CrossRef]
- Katan, M.B. Nitrate in foods: Harmful or healthy? Am. J. Clin. Nutr. 2009, 90, 11–12. [Google Scholar] [CrossRef]
- Chen, X.; Pu, H.; Fu, Z.; Sui, X.; Chang, J.; Chen, J.; Mao, S. Real-time and selective detection of nitrates in water using graphene-based field-effect transistor sensors. Environ. Sci. Nano 2018, 5, 1990–1999. [Google Scholar] [CrossRef]
- Dean, R.N.; Guertal, E.A.; Newby, A.F. A low-cost environmental nitrate sensor. In Proceedings of the 2020 IEEE Green Technologies Conference (GreenTech), Oklahoma City, OK, USA, 1–3 April 2020. [Google Scholar]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water—A Review. Sensors 2019, 20, 54. [Google Scholar] [CrossRef]
- Kadir, N.A.A.; Irawati, N.; Jafry, A.A.A.; Razali, N.M.; Hamzah, A.; Harun, S.W. Sodium nitrate sensor based on D-shaped fiber structure. Measurement 2020, 163, 107927. [Google Scholar] [CrossRef]
- Yasin, M.; Irawati, N.; Harun, S.; Ahmad, F.; Khasanah, M. Sodium nitrate (NaNO3) sensor based on graphene coated microfiber. Measurement 2019, 146, 208–214. [Google Scholar] [CrossRef]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Surface plasmon resonance study on the optical sensing properties of tin oxide (SnO2) films to NH3gas. J. Appl. Phys. 2016, 119, 164502. [Google Scholar] [CrossRef]
- Yao, Q.; Ren, G.; Xu, K.; Zhu, L.; Khan, H.; Mohiuddin, M.; Khan, M.W.; Zhang, B.Y.; Jannat, A.; Haque, F.; et al. 2D Plasmonic Tungsten Oxide Enabled Ultrasensitive Fiber Optics Gas Sensor. Adv. Opt. Mater. 2019, 7, 1901383. [Google Scholar] [CrossRef]
- Yanik, A.A.; Huang, M.; Kamohara, O.; Artar, A.; Geisbert, T.W.; Connor, J.H.; Altug, H. An Optofluidic Nanoplasmonic Biosensor for Direct Detection of Live Viruses from Biological Media. Nano Lett. 2010, 10, 4962–4969. [Google Scholar] [CrossRef]
- Roh, S.; Chung, T.; Lee, B. Overview of the Characteristics of Micro- and Nano-Structured Surface Plasmon Resonance Sensors. Sensors 2011, 11, 1565–1588. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Homola, J. Electromagnetic Theory of Surface Plasmons; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–44. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef]
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M.; et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photon. 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef]
- Yang, Z.-W.; Pham, T.-T.; Hsu, C.-C.; Lien, C.-H.; Phan, Q.-H. Single-Layer-Graphene-Coated and Gold-Film-Based Surface Plasmon Resonance Prism Coupler Sensor for Immunoglobulin G Detection. Sensors 2022, 22, 1362. [Google Scholar] [CrossRef]
- Zheng, B.; Yang, X.; Li, J.; Shi, C.-F.; Wang, Z.-L.; Xia, X.-H. Graphene Plasmon-Enhanced IR Biosensing for in Situ Detection of Aqueous-Phase Molecules with an Attenuated Total Reflection Mode. Anal. Chem. 2018, 90, 10786–10794. [Google Scholar] [CrossRef]
- Zain, H.A.; Batumalay, M.; Harun, S.W.; Harith, Z.; Yasin, M.; Rahim, H.R.A. Graphene/PVA coated D-shaped fiber for sodium nitrate sensing. Sens. Actuators A Phys. 2021, 332, 113163. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).