Dose Response Effect of Photobiomodulation on Hemodynamic Responses and Glucose Levels in Men with Type 2 Diabetes: A Randomized, Crossover, Double-Blind, Sham-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
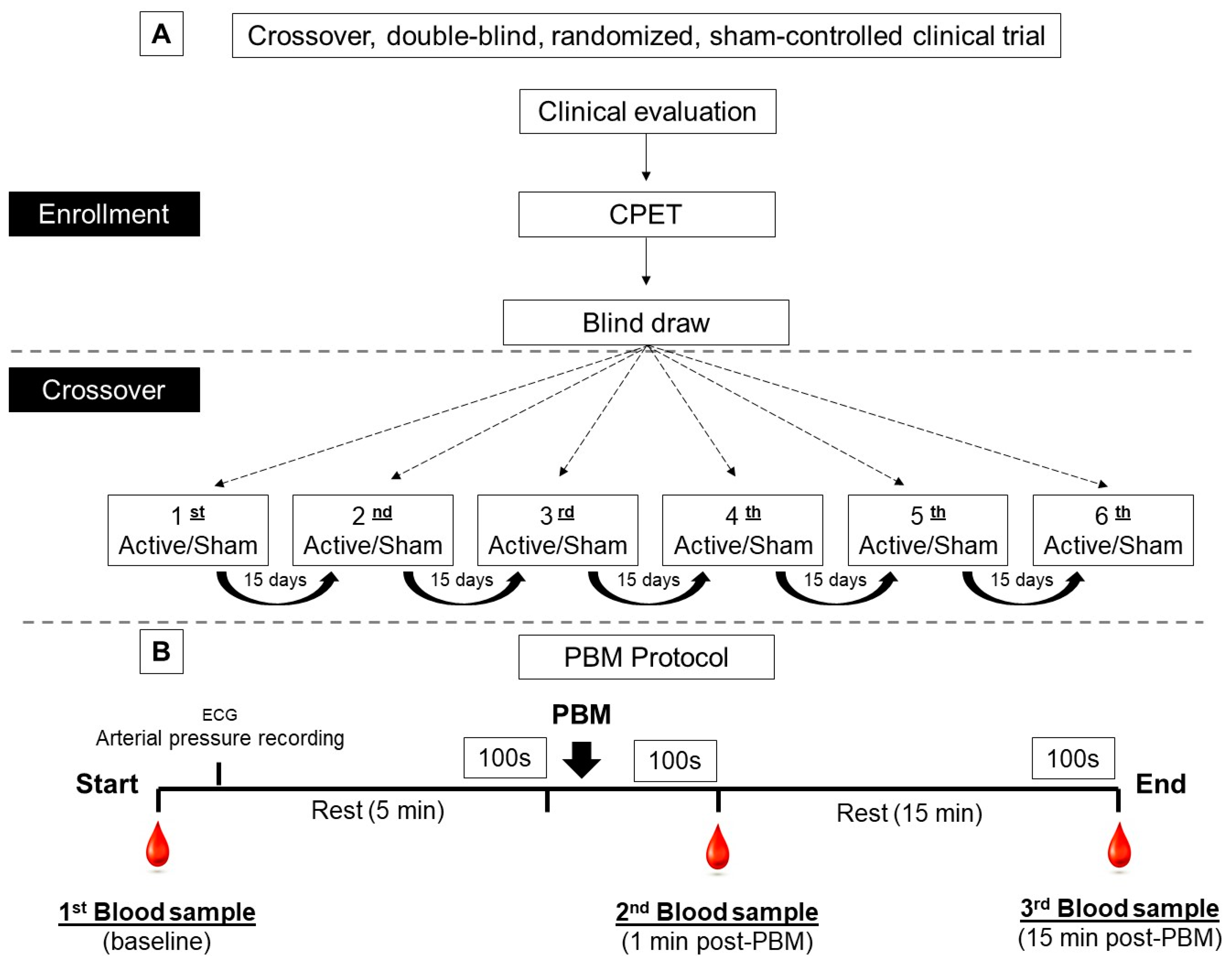
2.1. Study Design
2.2. Randomization and Blinding Procedures
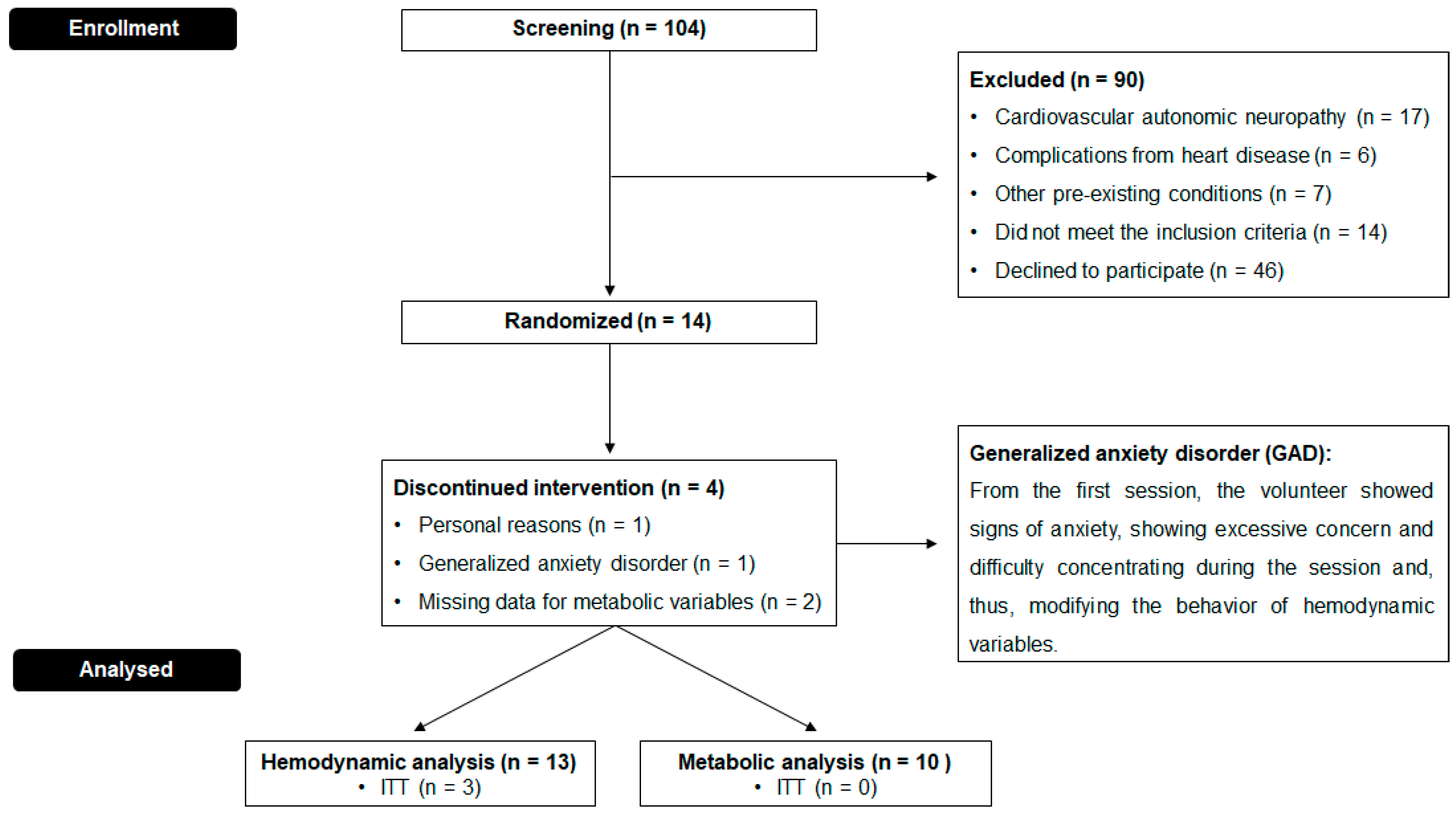
2.3. Study Population
2.4. Experimental Protocol
2.5. Photobiomodulation by Light-Emitting Diodes (LEDs)
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Hemodynamics Effects
4.2. Metabolic Responses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- ADA. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Poitras, V.J.; Hudson, R.W.; Tschakovsky, M.E. Exercise intolerance in Type 2 diabetes: Is there a cardiovascular contribution? J. Appl. Physiol. 2018, 124, 1117–1139. [Google Scholar] [CrossRef]
- De Francisco, C.O.; Beltrame, T.; Hughson, R.L.; Milan-Mattos, J.C.; Ferroli-Fabricio, A.M.; Galvão Benze, B.; Ferraresi, C.; Parizotto, N.A.; Bagnato, V.S.; Borghi-Silva, A.; et al. Effects of light-emitting diode therapy (LEDT) on cardiopulmonary and hemodynamic adjustments during aerobic exercise and glucose levels in patients with diabetes mellitus: A randomized, crossover, double-blind and placebo-controlled clinical trial. Complement. Ther. Med. 2019, 42, 178–183. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [Green Version]
- Linares, S.N.; Beltrame, T.; Ferraresi, C.; Galdino, G.A.M.; Catai, A.M. Photobiomodulation effect on local hemoglobin concentration assessed by near-infrared spectroscopy in humans. Lasers Med. Sci. 2020, 35, 641–649. [Google Scholar] [CrossRef]
- Nampo, F.K.; Cavalheri, V.; Dos Santos Soares, F.; de Paula Ramos, S.; Camargo, E.A. Low-level phototherapy to improve exercise capacity and muscle performance: A systematic review and meta-analysis. Lasers Med. Sci. 2016, 31, 1957–1970. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Soni, S.S.; Gonzalez-Lima, F.; Liu, H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016, 6, 30540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, G.; Ferraresi, C.; de Almeida, R.T.; Motta, M.L.; Paixão, T.; Ottone, V.O.; Fonseca, I.A.; Oliveira, M.X.; Rocha-Vieira, E.; Dias-Peixoto, M.F.; et al. Infrared photobiomodulation (PBM) therapy improves glucose metabolism and intracellular insulin pathway in adipose tissue of high-fat fed mice. Lasers Med. Sci. 2018, 33, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Gál, P.; Mokrý, M.; Vidinský, B.; Kilík, R.; Depta, F.; Harakalová, M.; Longauer, F.; Mozes, S.; Sabo, J. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and corticosteroid-treated rats. Lasers Med. Sci. 2009, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Du, Y.; Lee, C.A.; Talahalli, R.; Eells, J.T.; Kern, T.S. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: In vivo and in vitro. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3681–3690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, K.M.R.; de Paiva Carvalho, R.L.; Junior, G.M.R.; Tavares, B.A.; Simionato, L.H.; Bortoluci, C.H.F.; Soto, C.A.T.; Ferraresi, C. Can photobiomodulation therapy (PBMT) control blood glucose levels and alter muscle glycogen synthesis? J. Photochem. Photobiol. B Biol. 2020, 207, 111877. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, T.F.; Filho, J.C.C.; Oishi, J.C.; Almeida-Lopes, L.; Parizotto, N.A.; Rodrigues, G.J. Energy-dependent effect trial of photobiomodulation on blood pressure in hypertensive rats. Lasers Med. Sci. 2020, 35, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.M.; Sabino, C.P.; Ribeiro, M.S. Photobiomodulation reduces abdominal adipose tissue inflammatory infiltrate of diet-induced obese and hyperglycemic mice. J. Biophotonics 2016, 9, 1255–1262. [Google Scholar] [CrossRef]
- Beltrame, T.; Ferraresi, C.; Parizotto, N.A.; Bagnato, V.S.; Hughson, R.L. Light-emitting diode therapy (photobiomodulation) effects on oxygen uptake and cardiac output dynamics during moderate exercise transitions: A randomized, crossover, double-blind, and placebo-controlled study. Lasers Med. Sci. 2018, 33, 1065–1071. [Google Scholar] [CrossRef]
- Ferraresi, C.; Huang, Y.-Y.; Hamblin, M.R. Photobiomodulation in human muscle tissue: An advantage in sports performance? J. Biophotonics 2016, 9, 1273–1299. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, A.R.; Vanin, A.A.; Tomazoni, S.S.; Miranda, E.F.; Albuquerque-Pontes, G.M.; De Marchi, T.; Dos Santos Grandinetti, V.; de Paiva, P.R.V.; Imperatori, T.B.G.; de Carvalho, P.T.C.; et al. Pre-Exercise Infrared Photobiomodulation Therapy (810 nm) in Skeletal Muscle Performance and Postexercise Recovery in Humans: What Is the Optimal Power Output? Photomed. Laser Surg. 2017, 35, 595–603. [Google Scholar] [CrossRef]
- Milan-Mattos, J.C.; de Oliveira Francisco, C.; Ferroli-Fabrício, A.M.; Minatel, V.; Marcondes, A.C.A.; Porta, A.; Beltrame, T.; Parizotto, N.A.; Ferraresi, C.; Bagnato, V.S.; et al. Acute effect of photobiomodulation using light-emitting diodes (LEDs) on baroreflex sensitivity during and after constant loading exercise in patients with type 2 diabetes mellitus. Lasers Med. Sci. 2020, 35, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [CrossRef] [PubMed] [Green Version]
- Boulton, A.J.M.; Vinik, A.I.; Arezzo, J.C.; Bril, V.; Feldman, E.L.; Freeman, R.; Malik, R.A.; Maser, R.E.; Sosenko, J.M.; Ziegler, D. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care 2005, 28, 956–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Heart Association. Exercise Testing and Training of Apparently Healthy Individuals: A Handbook for Physicians; American Heart Association: Dallas, TX, USA, 1972; Available online: http://books.google.com/books?id=9o1qAAAAMAAJ (accessed on 1 June 2022).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.; Durland, J.; Makaryus, A.N. Physiology, Cardiac Index. In Treasure Island (FL); StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- de Waal, E.E.C.; Konings, M.K.; Kalkman, C.J.; Buhre, W.F. Assessment of stroke volume index with three different bioimpedance algorithms: Lack of agreement compared to thermodilution. Intensive Care Med. 2008, 34, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Hill, L.K.; Sollers Iii, J.J.; Thayer, J.F. Resistance reconstructed estimation of total peripheral resistance from computationally derived cardiac output-biomed 2013. Biomed. Sci. Instrum. 2013, 49, 216–223. [Google Scholar] [PubMed]
- da Cruz, J.P.; Messias, L.H.D.; Cetein, R.L.; Rasteiro, F.M.; Gobatto, C.A.; Manchado-Gobatto, F.B. Anaerobic and Agility Parameters of Salonists in Laboratory and Field Tests. Int. J. Sports Med. 2020, 41, 450–460. [Google Scholar] [CrossRef]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef] [Green Version]
- da Silva-Santos, J.E.; Assreuy, J. Long-lasting changes of rat blood pressure to vasoconstrictors and vasodilators induced by nitric oxide donor infusion: Involvement of potassium channels. J. Pharmacol. Exp. Ther. 1999, 290, 380–387. [Google Scholar]
- Paolillo, F.R.; Arena, R.; Dutra, D.B.; de Cassia Marqueti Durigan, R.; de Araujo, H.S.; de Souza, H.C.D.; Parizotto, N.A.; Cipriano, G.J.; Chiappa, G.; Borghi-Silva, A. Low-level laser therapy associated with high intensity resistance training on cardiac autonomic control of heart rate and skeletal muscle remodeling in wistar rats. Lasers Surg. Med. 2014, 46, 796–803. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose-Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Gavish, L.; Hoffer, O.; Rabin, N.; Halak, M.; Shkilevich, S.; Shayovitz, Y.; Weizman, G.; Haim, O.; Gavish, B.; Gertz, S.D.; et al. Microcirculatory Response to Photobiomodulation-Why Some Respond and Others Do Not: A Randomized Controlled Study. Lasers Surg. Med. 2020, 52, 863–872. [Google Scholar] [CrossRef]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.-Y.; de Sousa, M.V.P.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3-6 h. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Parizotto, N.A.; Pires de Sousa, M.V.; Kaippert, B.; Huang, Y.-Y.; Koiso, T.; Bagnato, V.S.; Hamblin, M.R. Light-emitting diode therapy in exercise-trained mice increases muscle performance, cytochrome c oxidase activity, ATP and cell proliferation. J. Biophotonics 2015, 8, 740–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraresi, C.; de Sousa, M.V.P.; Huang, Y.-Y.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med. Sci. 2015, 30, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Ferraresi, C.; de Brito Oliveira, T.; de Oliveira Zafalon, L.; de Menezes Reiff, R.B.; Baldissera, V.; de Andrade Perez, S.E.; Matheucci Júnior, E.; Parizotto, N.A. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med. Sci. 2011, 26, 349–358. [Google Scholar] [CrossRef]
- Azzone, G.F.; Carafoli, E. Biochemical properties of skeletal muscle mitochondria: I. Oxidative phosphorylation. Exp. Cell Res. 1960, 21, 447–455. [Google Scholar] [CrossRef]
- de Brito Vieira, W.H.; Ferraresi, C.; Schwantes, M.L.B.; de Andrade Perez, S.E.; Baldissera, V.; Cerqueira, M.S.; Parizotto, N.A. Photobiomodulation increases mitochondrial citrate synthase activity in rats submitted to aerobic training. Lasers Med. Sci. 2018, 33, 803–810. [Google Scholar] [CrossRef]
- Vanin, A.A.; Verhagen, E.; Barboza, S.D.; Costa, L.O.P.; Leal-Junior, E.C.P. Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: A systematic review and meta-analysis. Lasers Med. Sci. 2018, 33, 181–214. [Google Scholar] [CrossRef]
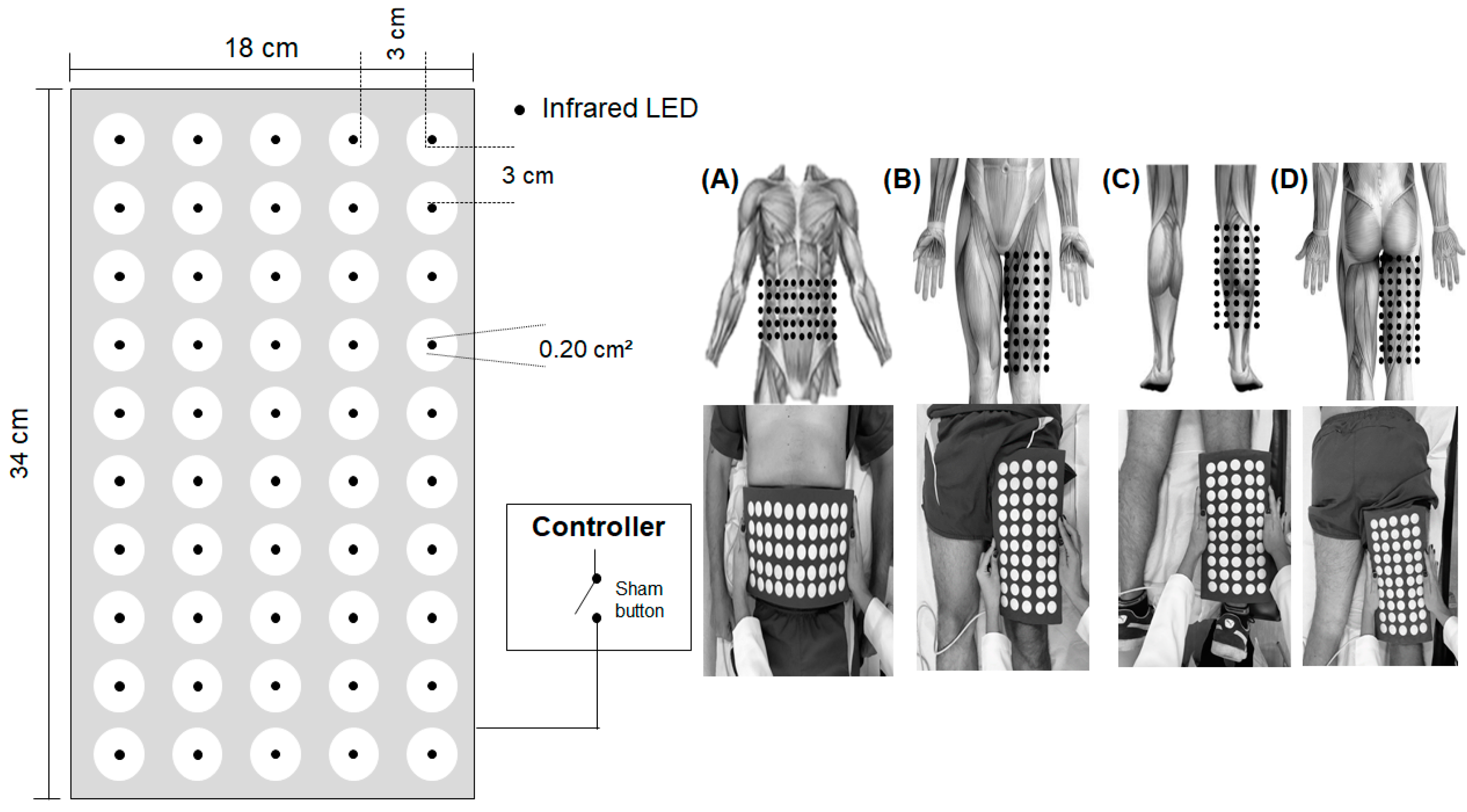

| PBM—Irradiation Parameters | ||||||
|---|---|---|---|---|---|---|
| Type of PBM | Ga-AI-As semiconductor diodes | |||||
| Number of points/LEDs | 50 | |||||
| Wavelength | 850 ± 20 nm | |||||
| Frequency | Continuous output | |||||
| Power output | 75 mW each diode | |||||
| Spot size (each LED) | 0.2 cm2 | |||||
| Energy per LED | 0 J, 1.5 J, 3 J, 6 J, 9 J, 12 J | |||||
| Power density per LED | 0 or 375 mW/cm2 | |||||
| Device area | 612 cm2 | |||||
| Application mode | Skin contact | |||||
| Sham | 75 J | 150 J | 300 J | 450 J | 600 J | |
| Treatment time over each muscle group (s) | 80 | 20 | 40 | 80 | 120 | 160 |
| Total energy delivered per muscle group | 0 J | 75 J | 150 J | 300 J | 450 J | 600 J |
| Total time (s) | 560 | 140 | 280 | 560 | 840 | 1.120 |
| Total energy delivered (7 regions) | 0 J | 525 J | 1.050 J | 2.100 J | 3.150 J | 4.200 J |
| Age (years) | 52 ± 7 |
| Anthropometric characteristics | |
| Weight (Kg) | 91.0 ± 10.8 |
| BMI (Kg/m2) | 29.3 ± 2.9 |
| BSA (m2) | 2.10 ± 0.14 |
| Clinical characteristics | |
| Duration of diabetes (years) | 13 ± 7 |
| HR at rest (bpm) | 75 ± 10 |
| SBP at rest (mmHg) | 126.7 ± 6.8 |
| DBP at rest (mmHg) | 72.2 ± 3.3 |
| Blood tests | |
| HbA1c (%) | 7.8 ± 1.8 |
| Fasting insulin (µU/mL) | 15.6 ± 14.1 |
| Fasting plasma glucose (mg/dL) | 180.1 ± 42.3 |
| Insulin sensitive (%, HOMA) | 4.0 ± 2.1 |
| Medications (n, (%)) | |
| Insulin | 3 (23.07) |
| Oral hypoglycemic | |
| Biguanides | 13 (100) |
| Sulphonylureas | 3 (23.07 |
| Glitazones | 3 (23.07) |
| SGLT2 inhibitors | 1 (7.69) |
| Antihypertensive | |
| Diuretics | 4 (30.76) |
| AT1-receiver blockers | 8 (61.53) |
| Hypercholesterolemia | |
| Fibrate | 1 (7.69) |
| Statin | 4 (30.76) |
| T4 replacement | 1 (7.69) |
| Sham | 75 J | 150 J | 300 J | 450 J | 600 J | |
|---|---|---|---|---|---|---|
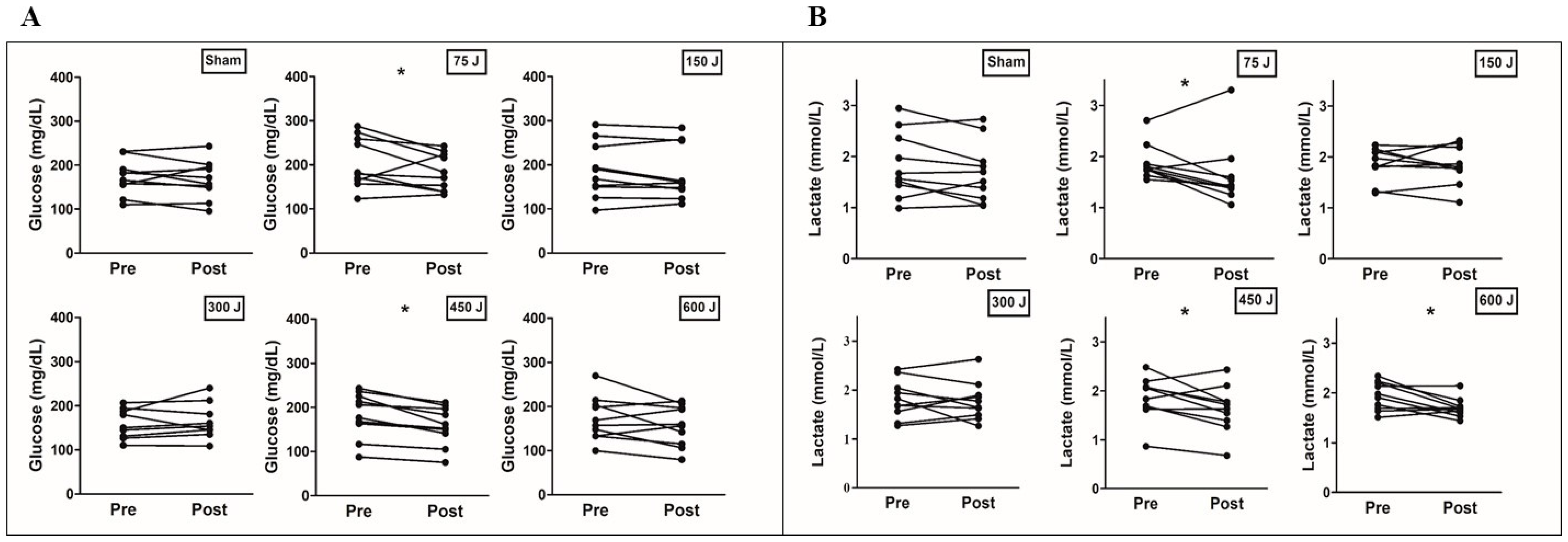
| Glucose (mg/dL) (n = 10) | ||||||
| Baseline | 172.7 ± 39.9 | 204.2 ± 56.8 | 187.3 ± 62.2 | 176.5 ± 63.6 | 183.4 ± 51.4 | 172.5 ± 49.5 |
| 1 min post-PBM | 172.4 ± 39.0 | 197.7 ± 50.8 | 190.6 ± 61.7 | 182.6 ± 42 | 168.4 ± 49.7 | 165.0 ± 49.5 |
| 15 min post-PBM | 167.1 ± 43.5 | 183.0 ± 42.1 * | 180.6 ± 61.2 | 176.9 ± 54.1 | 158.1 ± 43.8 * | 156.8 ± 45.9 |
| Lactate (mmol/L) (n = 10) | ||||||
| Baseline | 1.82 ± 0.63 | 1.87 ± 0.34 | 1.86 ± 0.32 | 1.81 ± 0.39 | 1.85 ± 0.44 | 1.93 ± 0.28 |
| 1 min post-PBM | 1.76 ± 0.64 | 1.75 ± 0.47 | 1.88 ± 0.35 | 1.90 ± 0.53 | 1.70 ± 0.44 | 1.78 ± 0.28 |
| 15 min post-PBM | 1.68 ± 0.58 | 1.63 ± 0.63 * | 1.83 ± 0.37 | 1.77 ± 0.39 | 1.63 ± 0.47 * | 1.69 ± 0.19 * |
| Sham | 75 J | 150 J | 300 J | 450 J | 600 J | |
|---|---|---|---|---|---|---|
| (L) (n = 13) | ||||||
| Baseline | 8.75 ± 2.4 | 8.13 ± 1.6 | 8.68 ± 2.0 | 8.56 ± 1.5 | 8.13 ± 1.3 | 8.83 ± 1.5 |
| 1 min post-PBM | 9.23 ± 2.6 | 8.97 ± 1.9 * | 8.84 ± 2.3 | 9.72 ± 1.7 * | 8.57 ± 1.5 | 8.69 ± 1.6 |
| 15 min post-PBM | 8.73 ± 2.5 | 8.78 ± 1.7 * | 8.85 ± 2.2 | 9.01 ± 1.5 # | 8.17 ± 1.7 | 8.54 ± 1.6 |
| CI (mL/m2) (n = 13) | ||||||
| Baseline | 4.20 ± 1.1 | 3.91 ± 0.8 | 4.16 ± 0.8 | 4.11 ± 0.6 | 3.91 ± 0.6 | 4.25 ± 0.7 |
| 1 min post-PBM | 4.43 ± 1.2 | 4.32 ± 0.9 * | 4.26 ± 1.0 | 4.66 ± 0.7 * | 4.12 ± 0.7 | 4.18 ± 0.7 |
| 15 min post-PBM | 4.18 ± 1.1 | 4.24 ± 0.9 * | 4.24 ± 0.9 | 4.33 ± 0.6 # | 3.93 ± 0.8 | 4.11 ± 0.7 |
| SV (mL) (n = 13) | ||||||
| Baseline | 114.5 ± 23.6 | 102.3 ± 20.7 | 115.7 ± 24.7 | 110.3 ± 21.3 | 110.9 ± 17.2 | 110.3 ± 20.2 |
| 1 min post-PBM | 119.4 ± 25.7 | 111.1 ± 21.6 * | 115.7 ± 27.0 | 120.9 ± 17.0 * | 113.6 ± 22.6 | 107.5 ± 16.5 |
| 15 min post-PBM | 116.0 ± 30.4 | 110.9 ± 23.0 * | 118.1 ± 27.3 | 116.8 ± 23.8 | 110.8 ± 25.0 | 108.4 ± 20.4 |
| SVI (mL/m2) (n = 13) | ||||||
| Baseline | 55.1 ± 11.1 | 49.2 ± 9.7 | 55.4 ± 10.8 | 53.0 ± 9.9 | 53.3 ± 7.8 | 53.0 ± 9.2 |
| 1 min post-PBM | 57.3 ± 11.9 | 53.5 ± 10.8 * | 55.5 ± 11.9 | 58.1 ± 7.5 * | 54.7 ± 10.7 | 51.6 ± 7.0 |
| 15 min post-PBM | 55.7 ± 14.6 | 53.5 ± 12.2 * | 56.6 ± 12.2 | 56.2 ± 11.2 | 53.3 ± 11.7 | 52.1 ± 9.2 |
| HR (bpm) (n = 13) | ||||||
| Baseline | 77 ± 12 | 78 ± 11 | 77 ± 11 | 79 ± 11 ¥ | 74 ± 8 | 79 ± 10 ¥ |
| 1 min post-PBM | 78 ± 11 | 79 ± 11 | 77 ± 10 | 81 ± 10 * § | 76 ± 7 * | 80 ± 8 |
| 15 min post-PBM | 77 ± 11 | 77 ± 11 | 76 ± 10 | 79 ± 11 # | 75 ± 9 | 78 ± 10 # |
| SBP (mmHg) (n =13) | ||||||
| Baseline | 138.3 ± 11.1 | 132.6 ± 11.6 | 132.4 ± 9.8 | 130.2 ± 8.6 | 133.5 ± 9.7 | 137.1 ± 12.1 |
| 1 min post-PBM | 138.0 ± 16.3 | 135.8 ± 15.8 | 130.2 ± 7.9 | 133.7 ± 12.4 | 135.0 ± 12.0 | 139.7 ± 17.3 |
| 15 min post-PBM | 137.3 ± 11.8 | 133.6 ± 13.6 | 133.2 ± 9.9 | 132.0 ± 8.1 | 134.7 ± 16.1 | 138.3 ± 16.0 |
| DBP (mmHg) (n = 13) | ||||||
| Baseline | 74.9 ± 7.7 | 73.1 ± 7.0 | 71.8 ± 4.9 | 71.6 ± 4.0 | 72.5 ± 5.0 | 74.1 ± 8.5 |
| 1 min post-PBM | 72.1 ± 10.6 | 71.4 ± 7.7 | 70.1 ± 5.6 | 70.1 ± 5.6 | 72.4 ± 6.3 | 75.3 ± 11.7 |
| 15 min post-PBM | 74.7 ± 7.8 | 71.6 ± 6.4 | 72.0 ± 4.3 | 71.0 ± 3.3 | 72.6 ± 6.2 | 74.1 ± 9.4 |
| MAP (mmHg) (n = 13) | ||||||
| Baseline | 96.0 ± 8.1 | 92.9 ± 7.8 | 92.0 ± 6.1 | 91.1 ± 4.2 | 92.8 ± 5.9 | 95.1 ± 8.8 |
| 1 min post-PBM | 94.1 ± 12.1 | 92.9 ± 9.9 | 90.8 ± 6.0 | 91.3 ± 7.3 | 93.3 ± 7.4 | 96.7 ± 13.1 |
| 15 min post-PBM | 95.7 ± 8.2 | 92.3 ± 7.7 | 92.4 ± 5.6 | 91.4 ± 3.3 | 93.3 ± 8.8 | 95.5 ± 10.5 |
| TPR (mmHg/L/min) (n = 13) | ||||||
| Baseline | 11.9 ± 3.0 | 12.3 ± 3.7 | 11.5 ± 2.4 | 11.1 ± 2.0 | 11.8 ± 2.1 | 11.1 ± 1.5 |
| 1 min post-PBM | 11.0 ± 2.5 | 11.0 ± 2.6 * | 11.2 ± 2.9 | 9.8 ± 1.4 * | 11.4 ± 2.2 | 11.5 ± 1.7 |
| 15 min post-PBM | 12.2 ± 4.4 # | 11.2 ± 2.9 * | 11.2 ± 2.4 | 10.5 ± 1.6 | 11.9 ± 1.9 | 11.7 ± 2.3 |
| TPRI (mmHg.min/L/m2) (n = 13) | ||||||
| Baseline | 5.7 ± 1.6 | 5.9 ± 1.9 | 5.6 ± 1.4 | 5.4 ± 1.2 | 5.7 ± 1.2 | 5.3 ± 0.8 |
| 1 min post-PBM | 5.3 ± 1.2 | 5.3 ± 1.3 * | 5.4 ± 1.6 | 4.7 ± 0.9 * | 5.5 ± 1.2 | 5.6 ± 0.9 |
| 15 min post-PBM | 5.9 ± 2.4 # | 5.4 ± 1.5 * | 5.4 ± 1.4 | 5.1 ± 1.0 | 5.8 ± 1.1 | 5.6 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares, S.N.; Beltrame, T.; Galdino, G.A.M.; Frade, M.C.M.; Milan-Mattos, J.C.; Gois, M.O.; Borghi-Silva, A.; de Biase, P.F.; Manchado-Gobatto, F.B.; Bagnato, V.S.; et al. Dose Response Effect of Photobiomodulation on Hemodynamic Responses and Glucose Levels in Men with Type 2 Diabetes: A Randomized, Crossover, Double-Blind, Sham-Controlled Trial. Photonics 2022, 9, 481. https://doi.org/10.3390/photonics9070481
Linares SN, Beltrame T, Galdino GAM, Frade MCM, Milan-Mattos JC, Gois MO, Borghi-Silva A, de Biase PF, Manchado-Gobatto FB, Bagnato VS, et al. Dose Response Effect of Photobiomodulation on Hemodynamic Responses and Glucose Levels in Men with Type 2 Diabetes: A Randomized, Crossover, Double-Blind, Sham-Controlled Trial. Photonics. 2022; 9(7):481. https://doi.org/10.3390/photonics9070481
Chicago/Turabian StyleLinares, Stephanie N., Thomas Beltrame, Gabriela A. M. Galdino, Maria Cecília M. Frade, Juliana C. Milan-Mattos, Mariana O. Gois, Audrey Borghi-Silva, Priscila F. de Biase, Fúlvia B. Manchado-Gobatto, Vanderlei S. Bagnato, and et al. 2022. "Dose Response Effect of Photobiomodulation on Hemodynamic Responses and Glucose Levels in Men with Type 2 Diabetes: A Randomized, Crossover, Double-Blind, Sham-Controlled Trial" Photonics 9, no. 7: 481. https://doi.org/10.3390/photonics9070481
APA StyleLinares, S. N., Beltrame, T., Galdino, G. A. M., Frade, M. C. M., Milan-Mattos, J. C., Gois, M. O., Borghi-Silva, A., de Biase, P. F., Manchado-Gobatto, F. B., Bagnato, V. S., Parizotto, N. A., Ferraresi, C., & Catai, A. M. (2022). Dose Response Effect of Photobiomodulation on Hemodynamic Responses and Glucose Levels in Men with Type 2 Diabetes: A Randomized, Crossover, Double-Blind, Sham-Controlled Trial. Photonics, 9(7), 481. https://doi.org/10.3390/photonics9070481





