The Effect of Photodynamic Therapy on the Early Outcome of Implants Placed on Patients with Periodontitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Patient Consent
2.2. Patient Selection
2.3. Groups
2.4. Treatment Protocol
2.5. Surgical Procedure
2.6. Statistical Analysis
3. Results
3.1. Age and Gender Distribution
3.2. Clinical Examination Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiba, A.; Sugimoto, S.; Sato, F.; Hori, S.; Mizunoe, Y. A refined technique for ex- traction of extracellular matrices from bacterial biofilms and its applicability. Microb. Biotechnol. 2015, 8, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Janam, P.; Narayanan, S.; Anil, S. Is Antimicrobial Photodynamic Therapy Effective as an Adjunct to Scaling and Root Planing in Patients with Chronic Periodontitis? A Systematic Review. Biomolecules 2017, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Haffajee, A.D.; Socransky, S.S.; Gunsolley, J.C. Systemic Anti-Infective Periodontal Therapy. A Systematic Review. Ann. Periodontol. 2003, 8, 115–181. [Google Scholar] [CrossRef] [PubMed]
- Drisko, C.L.; Cochran, D.L.; Blieden, T.; Bouwsma, O.J.; Cohen, R.E.; Damoulis, P.; Fine, J.B.; Greenstein, G.; Hinrichs, J.; Somerman, M.J.; et al. Research, Science and Therapy Committee of the American Accademy of Periodon-tology, Position paper: Sonic and ultrasonic scalers in periodontics. Research, Science and Therapy Committee of the American Academy of Periodotology. J. Periodontol. 2000, 71, 1792–1801. [Google Scholar] [PubMed] [Green Version]
- Laleman, I.; Cortellini, S.; de Winter, S.; Herrero, E.R.; Dekeyser, C.; Quirynen, M.; Teughels, W. Subgingival deb-ridement: End point, methods and how often? Periodontology 2000 2017, 75, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Rudney, J.D.; Chen, R.; Sedgewick, G.J. Intracellular Actinobacillus actinomyce- temcomitans and Porphyromonas gin-givalis in buccal epithelial cells collected from human subjects. Infect. Immun. 2001, 69, 2700–2707. [Google Scholar] [CrossRef] [Green Version]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, G.M.; Ash, M.M., Jr.; Caffesse, R.G. The Effectiveness of Subgingival Scaling and Root Planing in Calculus Removal. J. Periodontol. 1981, 52, 119–123. [Google Scholar] [CrossRef]
- Cobb, C.M. Non-Surgical Pocket Therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef]
- Gillies, M.; Ranakusuma, A.; Hoffmann, T.; Thorning, S.; McGuire, T.; Glasziou, P.; Del Mar, C. Common harms from amoxicillin: A systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Can. Med. Assoc. J. 2014, 187, E21–E31. [Google Scholar] [CrossRef] [Green Version]
- Grzech-Lesniak, K.; Matys, J.; Dominiak, M. Comparison of the clinical and micro- biological effects of antibiotic therapy in periodontal pockets following laser treatment: An in vivo study. Adv. Clin. Exp. Med. 2018, 2, 1263–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meimandi, M.; Ardakani, M.R.T.; Nejad, A.E.; Yousefnejad, P.; Saebi, K.; Tayeed, M.H. The effect of photodynamic therapy in the treatment of chronic periodontitis: A review of literature. J. Lasers Med. Sci. 2017, 8 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Van Winkelhoff, A.J.; Rams, T.E.; Slots, J. Systemic antibiotic therapy in periodontics. Periodontology 2000 1996, 10, 45–78. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, J.; Verma, N.; Nayan, K.; Saimbi, C.S.; Tripathi, A.K. Scope of photodynamic therapy in periodontics. Indian J. Dent. Res. 2015, 26, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, A.; Canakci, V.; Aydin, T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on out-comes of treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013, 84, 278–286. [Google Scholar] [CrossRef]
- Dobson, J.; Wilson, M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch. Oral Biol. 1992, 37, 883–887. [Google Scholar] [CrossRef]
- Komerik, N.; Nakanishi, H.; MacRobert, A.J.; Henderson, B.; Speight, P.; Wilson, M. In vivo killing of Porphyromonas gin-givalis by toluidine blue-mediated photo- sensitization in an animal model. Antimicrob. Agents Chemother. 2003, 47, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Petelin, M.; Perkic, K.; Seme, K.; Gaspirc, B. Effect of repeated adjunctive anti- microbial photodynamic therapy on sub-gingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med. Sci. 2015, 30, 1647–1656. [Google Scholar] [CrossRef]
- Akram, Z.; Al-Shareef, S.A.; Daood, U.; Asiri, F.Y.; Shah, A.H.; AlQahtani, M.A.; Vohra, F.; Javed, F. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: A systematic review. Photomed. Laser Surg. 2016, 34, 137–149. [Google Scholar] [CrossRef]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial photo- dynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Schwartz-Filho, H.O.; De Oliveira, R.R.; Feres, M.; Sato, S.; Figueiredo, L.C. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Microbiological profile. Lasers Med. Sci. 2011, 27, 389–395. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Silva, S.P.; Pires, J.R.; Soares, G.H.G.; Pontes, A.E.; Zuza, E.; Spolidorio, D.M.P.; De Toledo, B.E.C.; Garcia, V. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med. Sci. 2011, 27, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: A meta-analysis. Lasers Med. Sci. 2010, 25, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Balata, M.L.; Andrade, L.P.; Santos, D.B.; Cavalcanti, A.N.; Uda, R.T.; Edel, P.R.; Bittencourt, S. Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: A randomized controlled clinical trial. J. Appl. Oral Sci. 2013, 21, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Manocha, A.; Suresh, D.K. Photodynamic therapy—A strategic review. Indian J. Dent. Res. 2010, 21, 285–291. [Google Scholar] [CrossRef]
- Polansky, R.; Haas, M.; Heschl, A.; Wimmer, G. Clinical effectiveness of photo- dynamic therapy in the treatment of periodontitis. J. Clin. Periodontol. 2009, 36, 575–580. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Gatto, R.; Marzo, G.; Monaco, A. Photodynamic therapy in the treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2011, 28, 669–682. [Google Scholar] [CrossRef]
- Filho, G.A.N.; Casarin, R.C.; Casati, M.Z.; Giovani, E.M. PDT in non-surgical treatment of periodontitis in HIV patients: A split-mouth, randomized clinical trial. Lasers Surg. Med. 2012, 44, 296–302. [Google Scholar] [CrossRef]
- Birang, R.; Shahaboui, M.; Kiani, S.; Shadmehr, E.; Naghsh, N. Effect of Nonsurgical Periodontal Treatment Combined With Diode Laser or Photodynamic Therapy on Chronic Periodontitis: A Randomized Controlled Split-Mouth Clinical Trial. J. Lasers Med. Sci. 2015, 6, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Cappuyns, I.; Cionca, N.; Wick, P.; Giannopoulou, C.; Mombelli, A. Treatment of re- sidual pockets with photodynamic therapy, diode laser, or deep scaling. A rando- mized, split-mouth controlled clinical trial. Lasers Med. Sci. 2012, 27, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, V.F.; Andrade, P.V.; Rodrigues, M.F.; Hirata, M.H.; Hirata, R.D.; Pannuti, C.M.; de Micheli, G.; Conde, M.C. An-timicrobial photodynamic effect to treat residual pockets in periodontal patients: A randomized controlled clinical trial. J. Clin. Periodontol. 2015, 42, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Chitsazi, M.T.; Shirmohammadi, A.; Pourabbas, R.; Abolfazli, N.; Farhoudi, I.; Azar, B.D.; Farhadi, F. Clinical and Microbiological Effects of Photodynamic Therapy Associated with Non-surgical Treatment in Aggressive Periodontitis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 153–159. [Google Scholar] [CrossRef]
- Christodoulides, N.; Nikolidakis, D.; Chondros, P.; Becker, J.; Schwarz, F.; Rössler, R.; Sculean, A. Photodynamic Therapy as an Adjunct to Non-Surgical Periodontal Treatment: A Randomized, Controlled Clinical Trial. J. Periodontol. 2008, 79, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.-U.; Kim, J.-W.; Kim, S.-J.; Pang, E.-K. Effects of adjunctive daily phototherapy on chronic periodontitis: A randomized single-blind controlled trial. J. Periodontal Implant Sci. 2014, 44, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Kolbe, M.F.; Ribeiro, F.V.; Luchesi, V.H.; Casarin, R.C.; Sallum, E.A.; Nociti, F.; Ambrosano, G.M.; Cirano, F.; Pimentel, S.P.; Casati, M.Z. Photodynamic Therapy During Supportive Periodontal Care: Clinical, Microbiologic, Immunoinflammatory, and Patient-Centered Performance in a Split-Mouth Randomized Clinical Trial. J. Periodontol. 2014, 85, e277–e286. [Google Scholar] [CrossRef]
- Luchesi, V.H.; Pimentel, S.P.; Kolbe, M.F.; Ribeiro, F.V.; Casarin, R.C.; Nociti, E.A., Jr.; Sallum, F.H.; Casati, M.Z. Photodynamic therapy in the treatment of class II furca- tion: A randomized controlled clinical trial. J. Clin. Periodontol. 2013, 40, 781–788. [Google Scholar] [CrossRef]
- Rühling, A.; Fanghänel, J.; Houshmand, M.; Kuhr, A.; Meisel, P.; Schwahn, C.; Kocher, T. Photodynamic therapy of persistent pockets in maintenance patients—A clinical study. Clin. Oral Investig. 2009, 14, 637–644. [Google Scholar] [CrossRef]
- Sreedhar, A.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Kamath, V.; Barmappa, R. Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A split mouth clinical and microbiological study. J. Nat. Sci. Biol. Med. 2015, 6, S102–S109. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.; Lai, C.-H. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med. Sci. 2003, 18, 51–55. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Periodontitis, implant loss and peri-implantitis. A meta-analysis. Clin. Oral Implant. Res. 2015, 26, e8–e16. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Periodontally compromised vs. periodontally healthy patients and dental implants: A systematic review and meta-analysis. J. Dent. 2014, 42, 1509–1527. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Knight, E.T.; Al-Harthi, L.; Leichter, J.W. Chronic periodontitis and implant dentistry. Periodontology 2000 2017, 74, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Bonino, L.; Dalmasso, P.; Aglietta, M. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin. Oral Implant. Res. 2013, 25, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Buhara, O.; Pehlivan, S. Monte Carlo simulation of reasons for early failure of implants: Effects of two risk factors. Br. J. Oral Maxillofac. Surg. 2018, 57, 12–20. [Google Scholar] [CrossRef]
- Meyle, J.; Gersok, G.; Boedeker, R.-H.; Gonzales, J.R. Long-term analysis of osseointegrated implants in non-smoker patients with a previous history of periodontitis. J. Clin. Periodontol. 2014, 41, 504–512. [Google Scholar] [CrossRef]
- Graetz, C.; El-Sayed, K.F.; Geiken, A.; Plaumann, A.; Sälzer, S.; Behrens, E.; Wiltfang, J.; Dörfer, C.E. Effect of periodontitis history on implant success: A long-term evaluation during supportive periodontal therapy in a university setting. Clin. Oral Investig. 2017, 22, 235–244. [Google Scholar] [CrossRef]
- Li, S.; Di, P.; Zhang, Y.; Lin, Y. Immediate implant and rehabilitation based on All-on-4 concept in patients with generalized aggressive periodontitis: A medium-term prospective study. Clin. Implant Dent. Relat. Res. 2017, 19, 559–571. [Google Scholar] [CrossRef]
- Correia, F.; Gouveia, S.; Felino, A.C.; Costa, A.L.; Almeida, R.F. Survival rate of dental implants in patients with history of per-iodontal disease: A retrospective cohort study. Int. J. Oral Maxillofac. Implant. 2017, 32, 927–934. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, G.; Weigl, P.; Gu, X. Immediate placement of dental implants into infected versus noninfected sites in the esthetic zone: A systematic review and meta-analysis. J. Prosthet. Dent. 2018, 120, 658–667. [Google Scholar] [CrossRef]
- Amid, R.; Kadkhodazadeh, M.; Moscowchi, A. Immediate implant placement in compromised sockets: A systematic review and meta-analysis. J. Prosthet. Dent. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Janner, S.F.M.; Wittneben, J.-G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Carranza’s Clinical Periodontology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Shahabouee, M.; Rismanchian, M.; Yaghini, J.; Babashahi, A.; Badrian, H.; Goroohi, H. Microflora around teeth and dental implants. Dent. Res. J. 2012, 9, 215. [Google Scholar]
- Pazos, M.D.C.; Nader, H.B. Effect of photodynamic therapy on the extracellular matrix and associated components. Braz. J. Med. Biol. Res. 2007, 40, 1025–1035. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.-I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- De Almeida, J.M.; Theodoro, L.H.; Bosco, A.F.; Nagata, M.J.; Oshiiwa, M.; Garcia, V.G. Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J. Periodontol. 2007, 78, 566–575. [Google Scholar] [CrossRef]
- Kotsakis, G.; Salama, M.; Chrepa, V.; Hinrichs, J.E.; Gaillard, P. A Randomized, Blinded, Controlled Clinical Study of Particulate Anorganic Bovine Bone Mineral and Calcium Phosphosilicate Putty Bone Substitutes for Socket Preservation. Int. J. Oral Maxillofac. Implant. 2014, 29, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, K.; Chandra, R.V.; Reddy, A.A.; Reddy, B.H.; Reddy, C.; Naveen, A. Effect of a single session of antimicrobial photo-dynamic therapy using indocyanine green in the treatment of chronic periodontitis: A randomized controlled pilot trial. Quintessence Int. 2015, 46, 391–400. [Google Scholar]
- Karimi, M.R.; Hassani, A.; Khosroshahian, S. Efficacy of Antimicrobial Photodynamic Therapy as an Adjunctive to Mechanical Debridement in the Treatment of Peri-implant Diseases: A Randomized Controlled Clinical Trial. J. Lasers Med. Sci. 2016, 7, 139–145. [Google Scholar] [CrossRef] [Green Version]

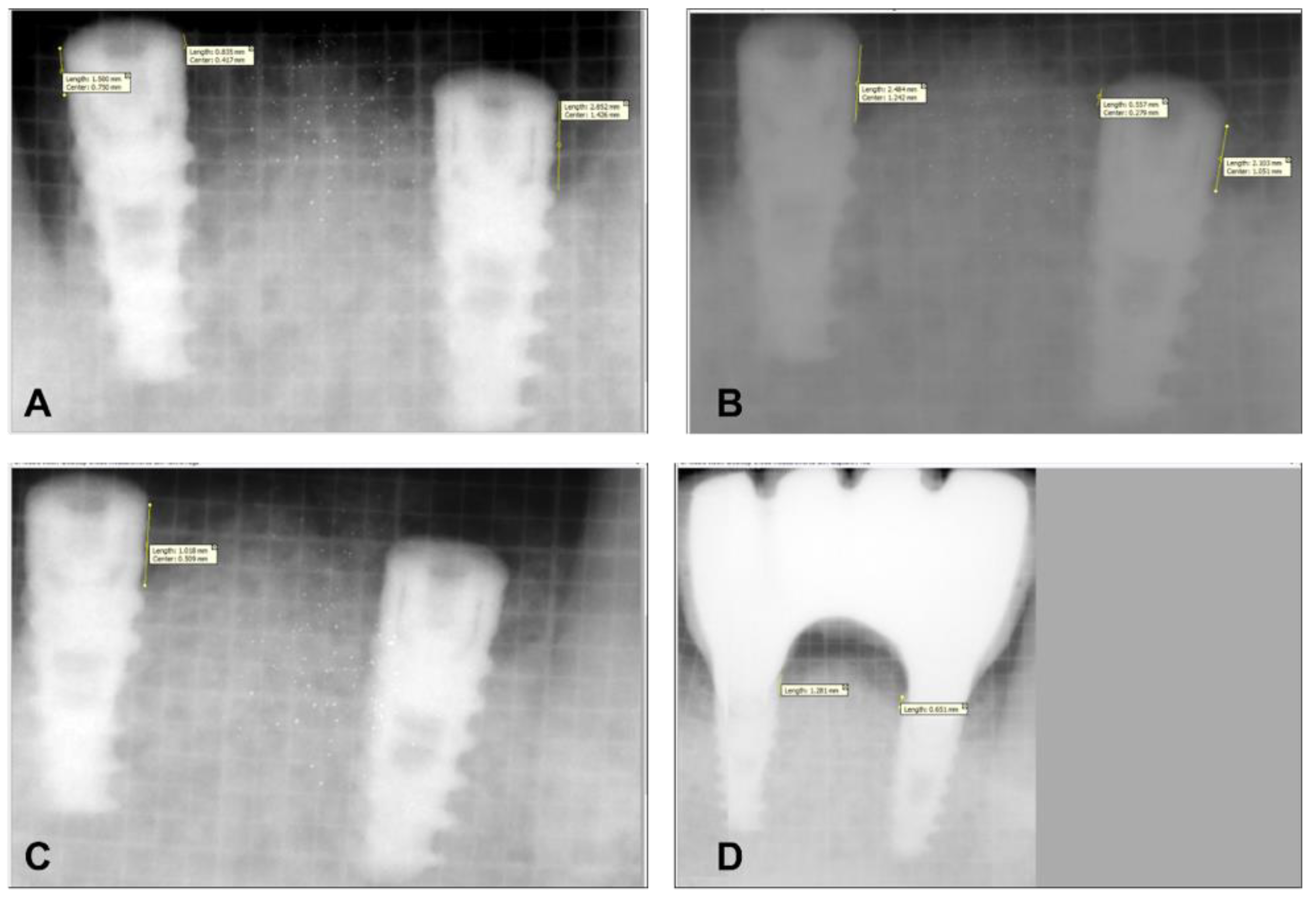

| Baseline | 3 Month | 6 Months | 9 Months | ANOVA F Value | p Value (Significance) | |
|---|---|---|---|---|---|---|
| Mean (S.D) | Mean (S.D) | Mean (S.D) | Mean (S.D) | |||
| Plaque index score (PI) | 2.51 (0.28) | 1.43 (0.27) | 1.53 (0.34) | 1.70 (0.25) | <0.001 ** | p < 0.001 |
| Gingival index score (GI) | 2.45 (0.18) | 1.34 (0.18) | 1.43 (0.34) | 1.6 (0.36) | <0.001 ** | p < 0.001 |
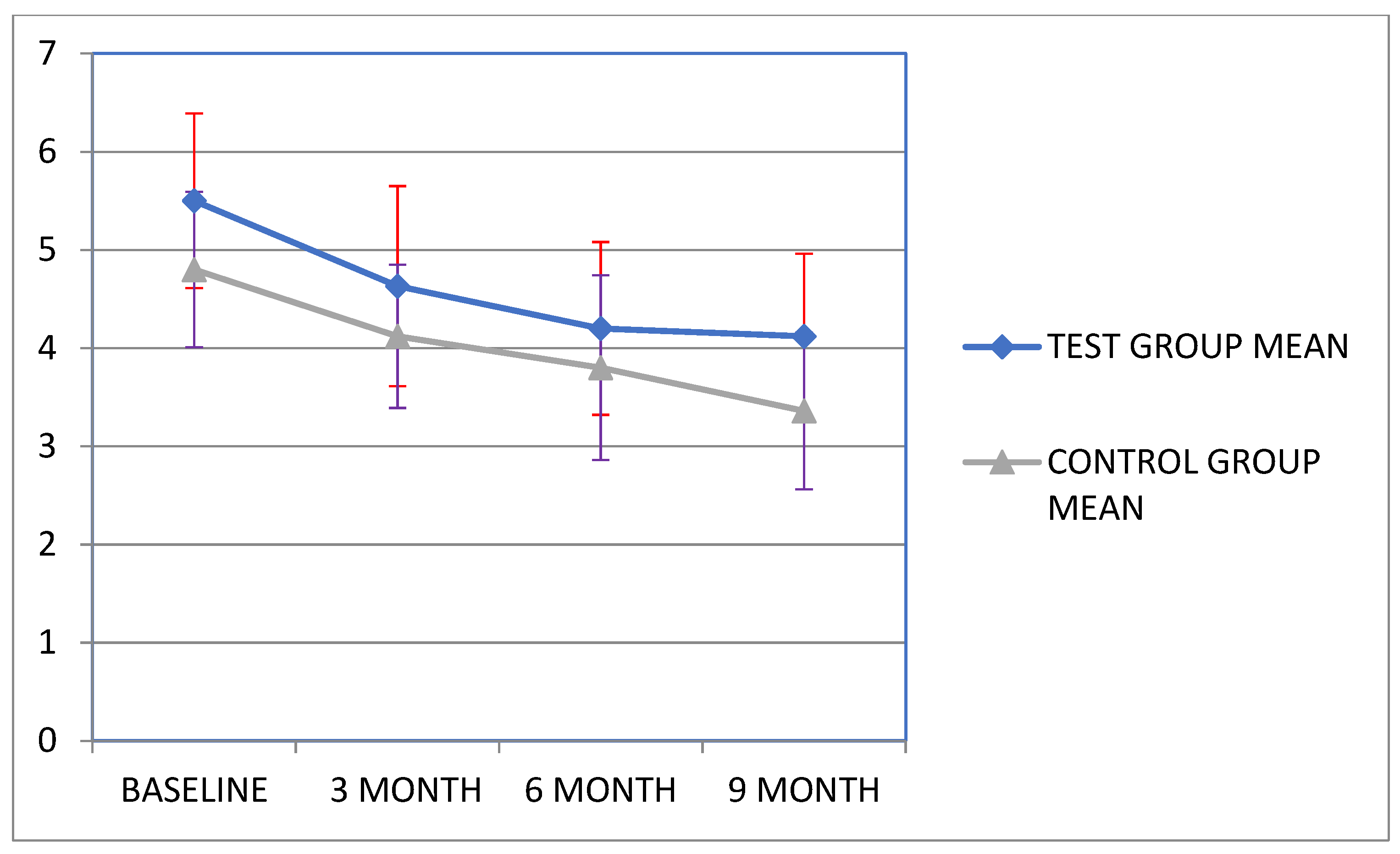
| Pocket depth (PD) | 5.55 (0.89) | 4.6 (1.02) | 4.2 (0.88) | 4.12 (0.84) | <0.001 ** | p = 0.002 |
| Clinical attachment loss (CAL) | 8.82 (0.99) | 7.88 (0.85) | 7.19 (1.07) | 6.9 (1.21) | <0.001 ** | p = 0.001 |
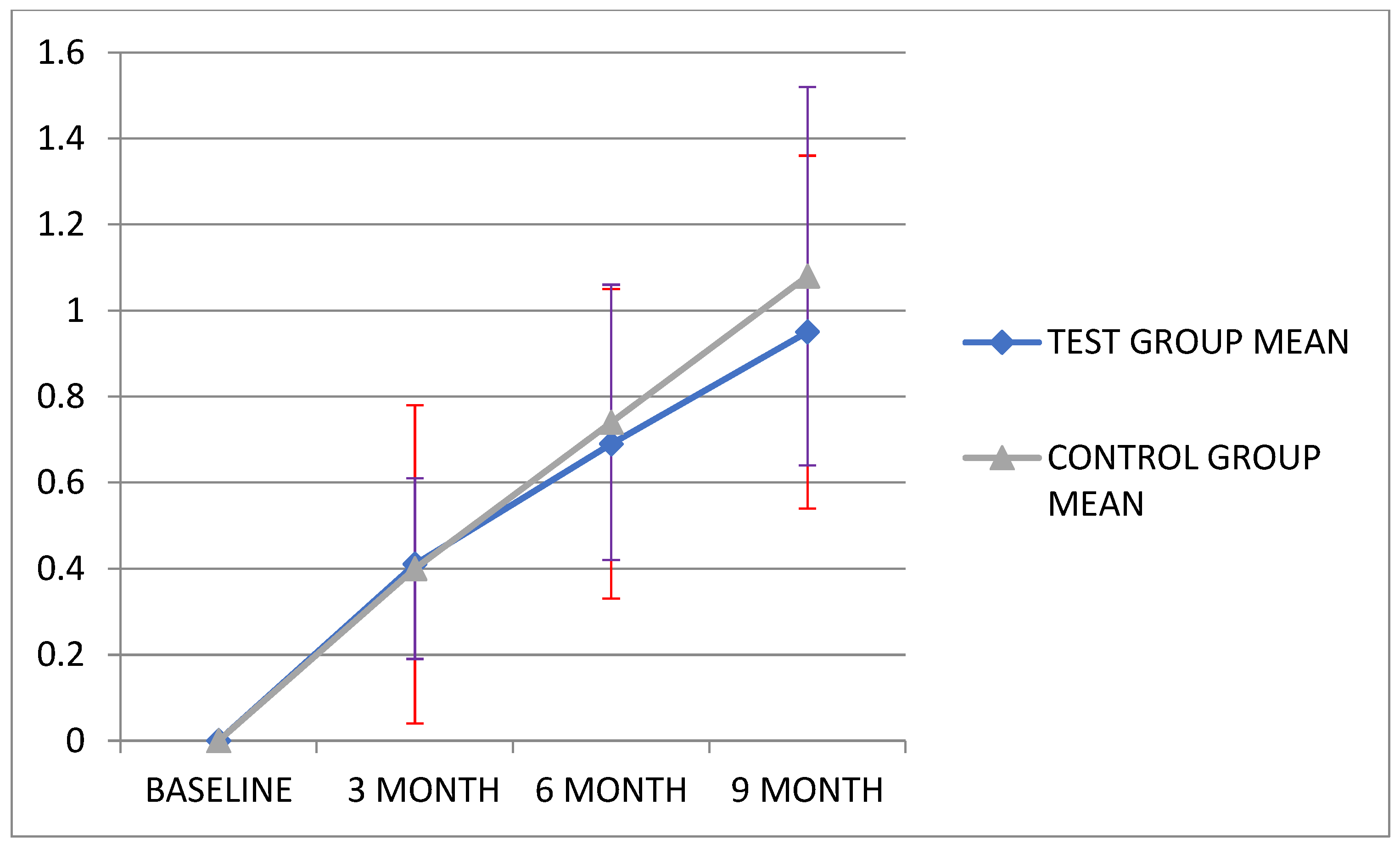
| MBL | 0.0 (0.0) | 0.41 (0.37) | 0.69 (0.36) | 0.95 (0.41) | <0.001 ** | p < 0.001 |
| Baseline vs. 3 Months | Baseline vs. 6 Months | Baseline vs. 9 Months | 3 Months vs. 6 Months | 3 Months vs. 9 Months | 6 Months vs. 9 Months | |
|---|---|---|---|---|---|---|
| Plaque index | ||||||
| Mean Difference | 1.08 | 0.98 | 0.80 | 0.1 | 0.27 | 0.17 |
| p value | p < 0.001 | p < 0.001 | p < 0.001 | 0.854 | 0.146 | 0.518 |
| GI score | ||||||
| Mean Difference | 1.109 | 1.018 | 0.854 | 0.09 | 0.25 | 0.16 |
| p value | p < 0.001 | p < 0.001 | p < 0.001 | 0.871 | 0.161 | 0.525 |
| PD score | ||||||
| Mean Difference | 0.91 | 1.35 | 1.42 | 0.43 | 0.50 | 0.07 |
| p value | p = 0.103 | p = 0.007 | p = 0.004 | 0.680 | 0.565 | 0.998 |
| CAL score | ||||||
| Mean Difference | 0.94 | 1.63 | 1.9 | 0.69 | 0.95 | 0.26 |
| p value | p = 0.163 | p = 0.004 | p = 0.001 | 0.416 | 0.156 | 0.934 |
| MBL score | ||||||
| Mean Difference | 0.41 | 0.69 | 0.95 | 0.27 | 0.53 | 0.26 |
| p value | p = 0.028 | p < 0.001 | p < 0.001 | 0.220 | 0.03 | 0.274 |
| Baseline | 3 Months | 6 Months | 9 Months | ANOVA F Value | p Value (Significance) | |
|---|---|---|---|---|---|---|
| Mean (S.D) | Mean (S.D) | Mean (S.D) | Mean (S.D) | |||
| Plaque index score (PI) | 2.68 (0.24) | 1.73 (0.34) | 1.87 (0.32) | 1.95 (0.26) | 24.644 | p < 0.001 |
| Gingival index score (GI) | 2.53 (0.28) | 1.36 (0.28) | 1.53 (0.32) | 1.79 (0.38) | 30.818 | p < 0.001 |
| Pocket depth (PD) | 4.82 (0.79) | 4.12 (0.73) | 3.8 (0.94) | 3.3 (0.80) | 6.697 | p = 0.001 |
| Clinical attachment loss (CAL) | 7.9 (0.81) | 7.21 (0.71) | 6.9 (0.88) | 6.3 (0.54) | 9.438 | p < 0.001 |
| MBL | 0.0 (0.0) | 0.40 (0.21) | 0.74 (0.32) | 1.08 (0.44) | 29.341 | p < 0.001 |
| Baseline vs. 3 Months | Baseline vs. 6 Months | Baseline vs. 9 Months | 3 Months vs. 6 Months | 3 Months vs. 9 Months | 6 Months vs. 9 Months | |
|---|---|---|---|---|---|---|
| Plaque index | ||||||
| Mean Difference | 0.95 | 0.80 | 0.72 | 0.14 | 0.22 | 0.083 |
| p value | p < 0.001 | p < 0.001 | p < 0.001 | 0.647 | 0.259 | 0.901 |
| GI score | ||||||
| Mean Difference | 1.16 | 1.0 | 0.741 | 0.16 | 0.425 | 0.25 |
| p value | p < 0.001 | p < 0.001 | p < 0.001 | 0.587 | 0.012 | 0.216 |
| PD score | ||||||
| Mean Difference | 0.70 | 1.01 | 1.45 | 0.31 | 0.75 | 0.07 |
| p value | p = 0.173 | p = 0.020 | p < 0.001 | 0.781 | 0.123 | 0.557 |
| CAL score | ||||||
| Mean Difference | 0.68 | 1.0 | 1.6 | 0.31 | 0.91 | 0.60 |
| p value | p = 0.132 | p = 0.011 | p < 0.001 | 0.732 | 0.023 | 0.220 |
| MBL score | ||||||
| Mean Difference | 0.40 | 0.74 | 1.08 | 0.335 | 0.684 | 0.348 |
| p value | p = 0.009 | p < 0.001 | p < 0.001 | 0.04 | 0.03 | 0.031 |
| Baseline | 3 Month | 6 Month | 9 Month | ||
|---|---|---|---|---|---|
| Plaque Index | |||||
| Test group (n = 11) | Mean (SD) | 2.51 (0.28) | 1.43 (0.27) | 1.53 (0.34) | 1.79 (0.25) |
| Control group (n = 12) | Mean (SD) | 2.68 (0.24) | 1.73 (0.34) | 1.87 (0.32) | 1.95 (0.26) |
| Independent t test | p value | 0.15 | 0.034 | 0.024 | 0.032 |
| Gingival Index | |||||
| Test group (n = 11) | Mean (SD) | 2.45 (0.18) | 1.34 (0.18) | 1.43 (0.34) | 1.6 (0.36) |
| Control group (n = 12) | Mean (SD) | 2.53 (0.28) | 1.36 (0.28) | 1.53 (0.32) | 1.79 (0.38) |
| Independent t test | p value | 0.437 | 0.835 | 0.493 | 0.234 |
| Pocket Depth | |||||
| Test group (n = 11) | Mean (SD) | 5.5 (0.89) | 4.63 (1.02) | 4.2 (0.88) | 4.12 (0.84) |
| Control group (n = 12) | Mean (SD) | 4.8 (0.79) | 4.12 (0.73) | 3.8 (0.94) | 3.36 (0.80) |
| Independent t test | p value | 0.051 | 0.181 | 0.317 | 0.038 |
| Clinical Attachment Level | |||||
| Test group (n = 11) | Mean (SD) | 8.8 (0.99) | 7.88 (0.85) | 7.19 (1.07) | 6.92 (1.21) |
| Control group (n = 12) | Mean (SD) | 7.9 (0.81) | 7.21 (0.71) | 6.9 (0.88) | 6.3 (0.54) |
| Independent t test | p value | 0.023 | 0.049 | 0.485 | 0.119 |
| Marginal Bone Loss | |||||
| Test group (n = 11) | Mean (SD) | 0.0 (0.0) | 0.41 (0.37) | 0.69 (0.36) | 0.95 (0.41) |
| Control group (n = 12) | Mean (SD) | 0.0 (0.0) | 0.40 (0.21) | 0.74 (0.32) | 1.08 (0.44) |
| Independent t test | p value | ------- | 0.930 | 0.750 | 0.462 |
| Groups | Baseline |
|---|---|
| Test Group (n = 11) Mean (SD) | 31.81 (7.83) |
| Control Group (n = 12) Mean (SD) | 37.08 (6.55) |
| Independent t test p value | 0.094 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardhan, P.K.; Paramashivaiah, R.; Prabhuji, M.L.V.; Bhavikatti, S.K.; Basha, S.; Arora, S.; Basheer, S.N.; Peeran, S.W.; Aldowah, O.; Heboyan, A. The Effect of Photodynamic Therapy on the Early Outcome of Implants Placed on Patients with Periodontitis. Photonics 2022, 9, 480. https://doi.org/10.3390/photonics9070480
Vardhan PK, Paramashivaiah R, Prabhuji MLV, Bhavikatti SK, Basha S, Arora S, Basheer SN, Peeran SW, Aldowah O, Heboyan A. The Effect of Photodynamic Therapy on the Early Outcome of Implants Placed on Patients with Periodontitis. Photonics. 2022; 9(7):480. https://doi.org/10.3390/photonics9070480
Chicago/Turabian StyleVardhan, Pabbati Krishna, Rashmi Paramashivaiah, Munivenkatappa Laxmaiah Venkatesh Prabhuji, Shaeesta Khaleelahmed Bhavikatti, Sakeenabi Basha, Suraj Arora, Syed Nahid Basheer, Syed Wali Peeran, Omir Aldowah, and Artak Heboyan. 2022. "The Effect of Photodynamic Therapy on the Early Outcome of Implants Placed on Patients with Periodontitis" Photonics 9, no. 7: 480. https://doi.org/10.3390/photonics9070480
APA StyleVardhan, P. K., Paramashivaiah, R., Prabhuji, M. L. V., Bhavikatti, S. K., Basha, S., Arora, S., Basheer, S. N., Peeran, S. W., Aldowah, O., & Heboyan, A. (2022). The Effect of Photodynamic Therapy on the Early Outcome of Implants Placed on Patients with Periodontitis. Photonics, 9(7), 480. https://doi.org/10.3390/photonics9070480






