Novel Elastography-Inspired Approach to Angiographic Visualization in Optical Coherence Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Method of Simulation of OCT-Scans with Arbitrary Motion of Scattering Particles
2.2. Optical Coherence Tomography Setup and Signal Acquisition
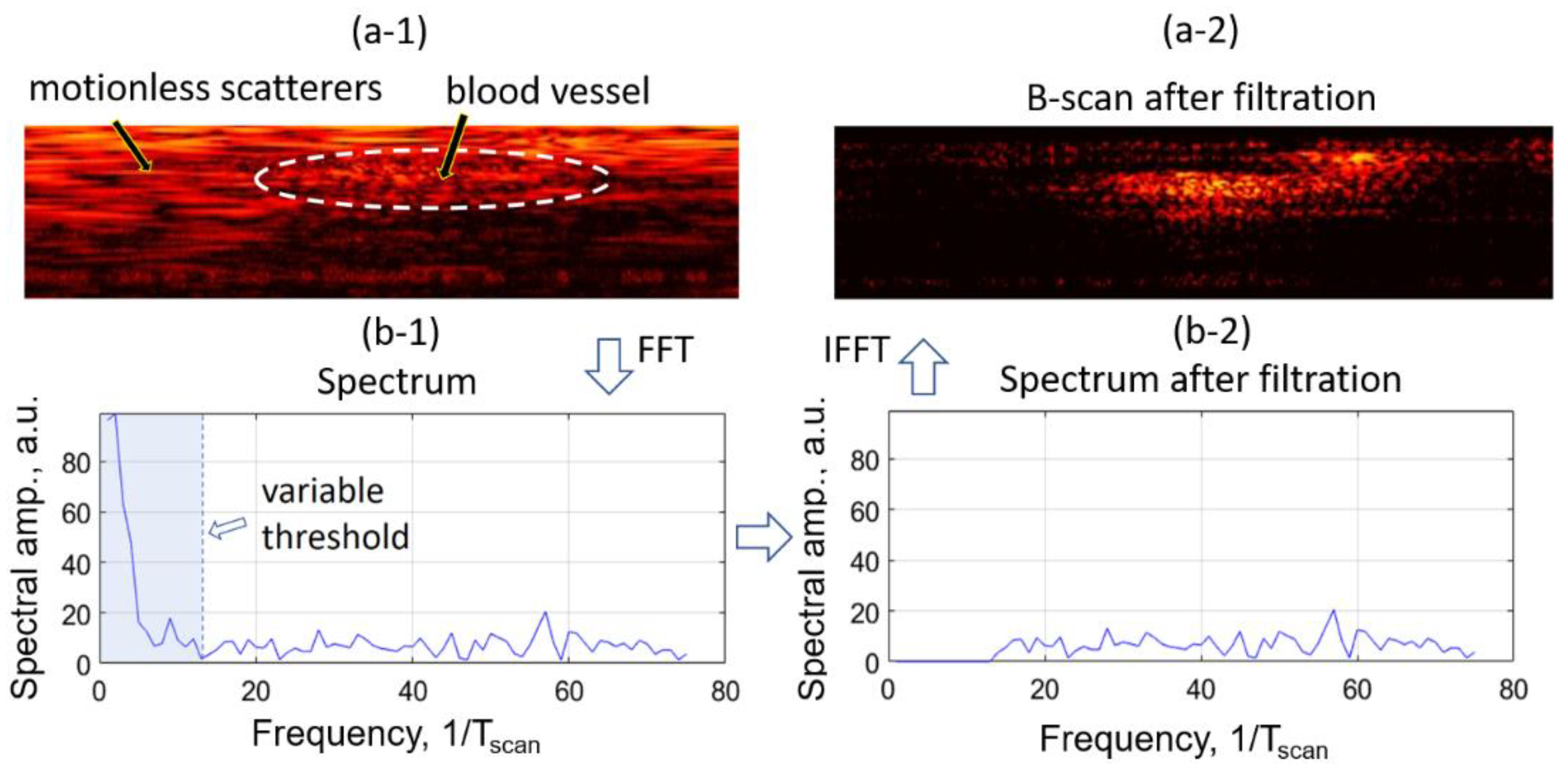
2.3. High Pass Filtering Angiography
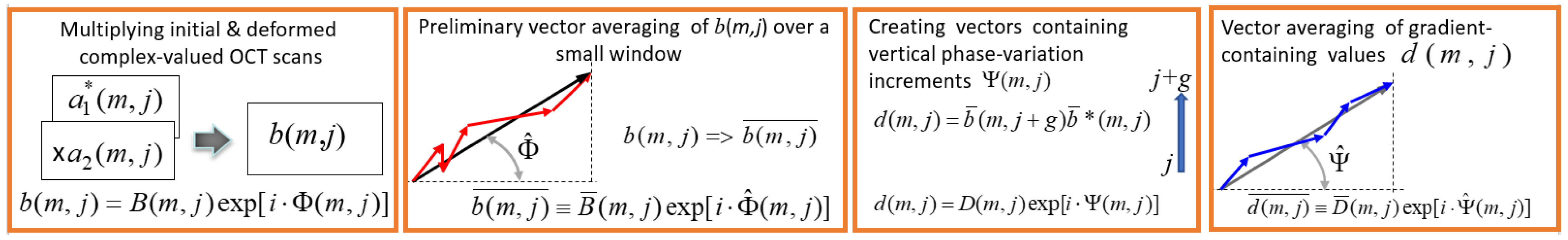
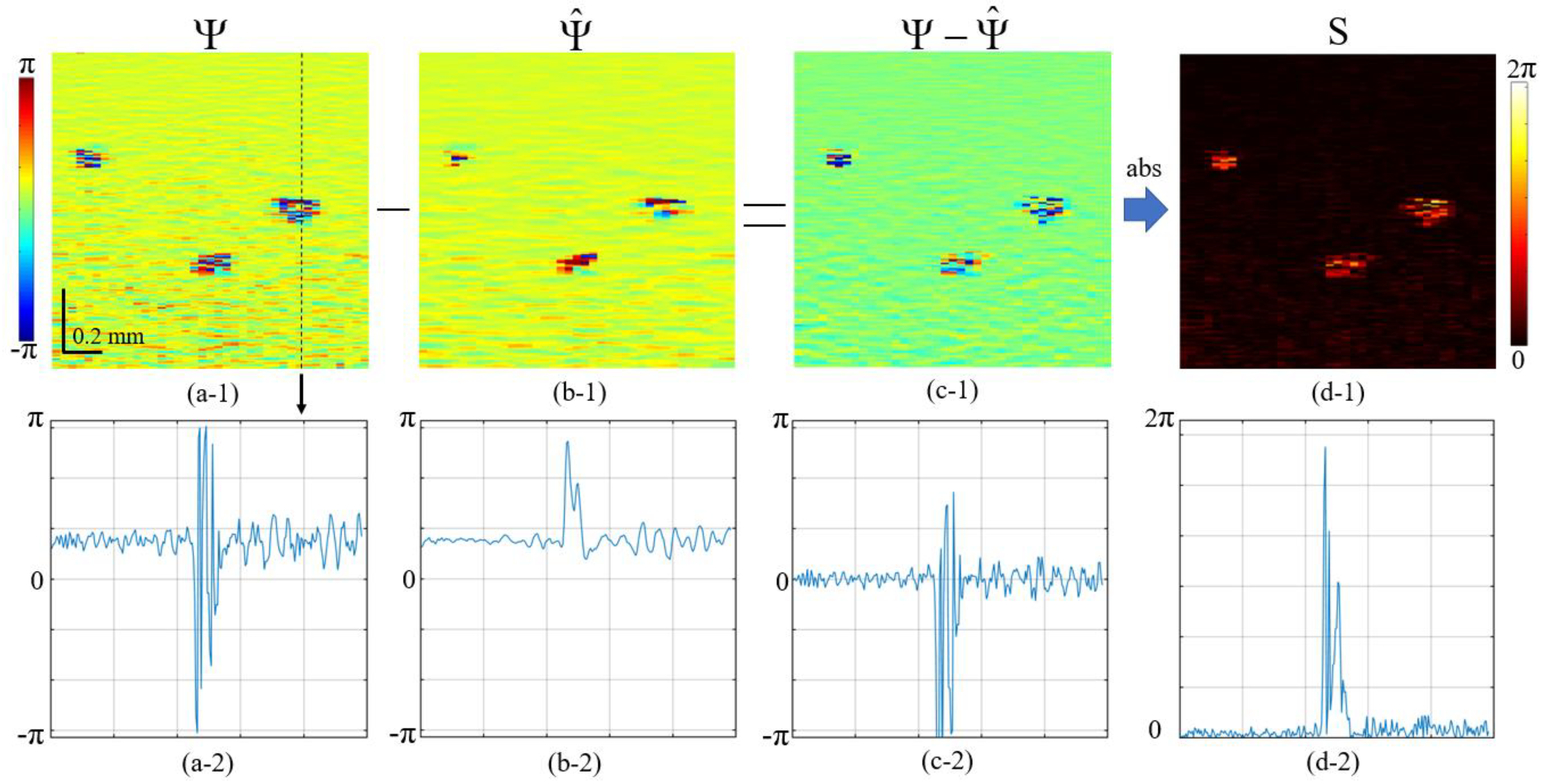
2.4. Local Strain Estimation Based Angiography (OCA-S)
- (a)
- Element-by-element multiplication of the deformed B-scan and initial complex-conjugated B-scan:where and . The asterisk means complex conjugation. The complex-valued matrix contains information about interframe phase difference in every pixel.
- (b)
- For improving the signal-to noise ratio without appreciable loss in resolution, the preliminary vector averaging of is made within a small sliding window in size (usually chosen around 2 × 2 pixels):where vector , found by summation of individual vectors , is characterized by the resultant amplitude and angle and shown in Figure 4 (second panel).The next step is finding the matrix containing axial phase-variation gradients:This quantity can be represented as . The argument of this complex-valued quantity is proportional to the axial phase-variation gradient and, correspondingly, to the local axial strain. In the simplest case, the vertical step for finding this gradient is g = 1, but if there is no phase wrapping on a scale of pixels, a larger chosen may significantly improve the quality of the gradient estimation. In the case of , the value should be used instead of for estimating the phase gradient.
- (c)
- Additional noise reduction may be obtained by vector averaging of quantity with a sliding window; the chosen size of this window usually may be larger than for the initial averaging in Equation (2), because the phase gradient often exhibits slower spatial variability than the initial phase variations .where, by analogy with quantity in Equation (2), quantity is the effective angle that is proportional to the sought averaged axial gradient of the interframe phase variation (see the last panel in Figure 4).
3. Results
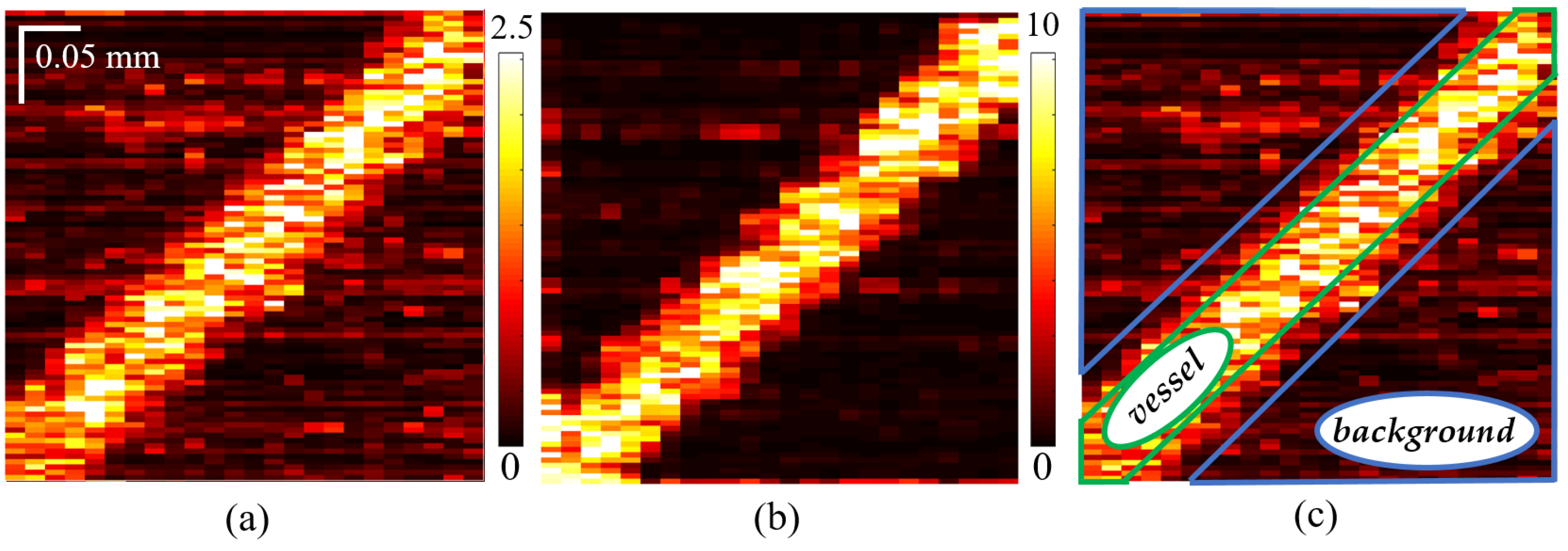
3.1. Elucidation of the Elastography-Inspired Angiography Using Simulated 2D OCA-S Images Containing Vessel Cross-Sections
3.2. Comparison of Simulated 3D OCA Images
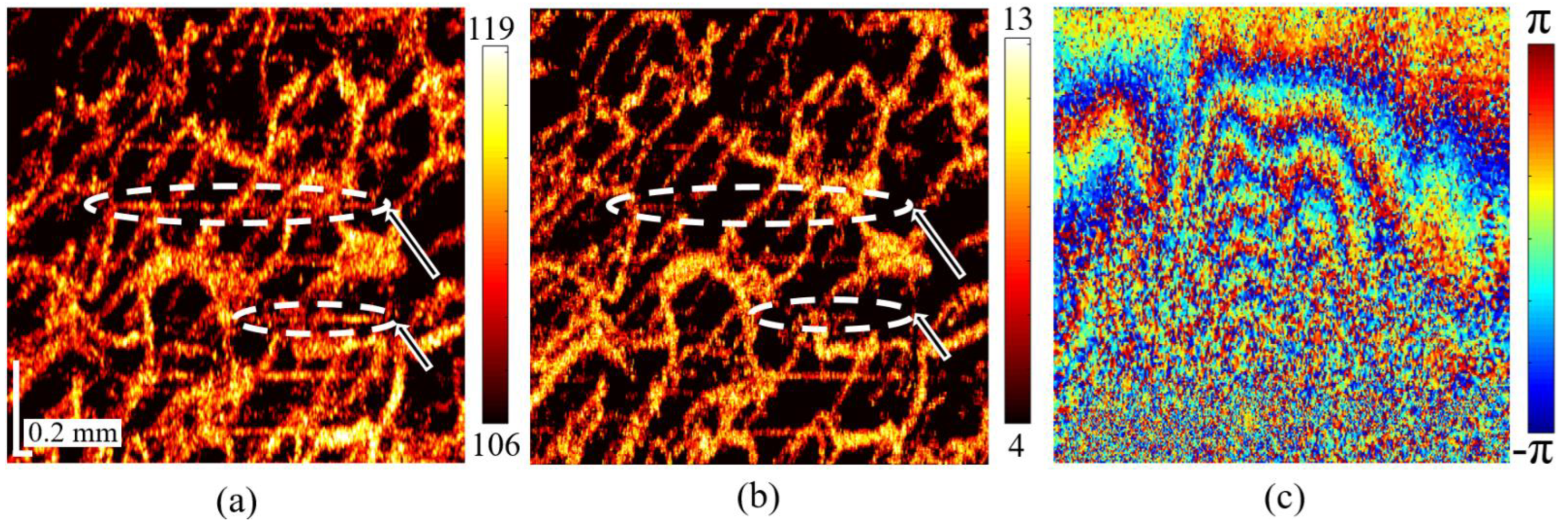
3.3. Comparison of Experimental 3D OCA Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leitgeb, R.; Placzek, F.; Rank, E.; Krainz, L.; Haindl, R.; Li, Q.; Liu, M.; Andreana, M.; Unterhuber, A.; Schmoll, T.; et al. Enhanced Medical Diagnosis for DOCTors: A Perspective of Optical Coherence Tomography. J. Biomed. Opt. 2021, 26, 100601. [Google Scholar] [CrossRef]
- Yang, V.X.D.; Gordon, M.L.; Qi, B.; Pekar, J.; Lo, S.; Seng-Yue, E.; Mok, A.; Wilson, B.C.; Vitkin, I.A. High Speed, Wide Velocity Dynamic Range Doppler Optical Coherence Tomography (Part I): System Design, Signal Processing, and Performance. Opt. Express 2003, 11, 794–809. [Google Scholar] [CrossRef] [Green Version]
- Leitgeb, R.A.; Schmetterer, L.; Drexler, W.; Fercher, A.F.; Zawadzki, R.J.; Bajraszewski, T. Real-Time Assessment of Retinal Blood Flow with Ultrafast Acquisition by Color Doppler Fourier Domain Optical Coherence Tomography. Opt. Express 2003, 11, 3116–3121. [Google Scholar] [CrossRef]
- Wang, R.K.; An, L. Doppler Optical Micro-Angiography for Volumetric Imaging of Vascular Perfusion in Vivo. Opt. Express 2009, 17, 8926–8940. [Google Scholar] [CrossRef] [Green Version]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical Coherence Tomography Angiography: A Comprehensive Review of Current Methods and Clinical Applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Mariampillai, A.; Standish, B.A.; Moriyama, E.H.; Khurana, M.; Munce, N.R.; Leung, M.K.K.; Jiang, J.; Cable, A.; Wilson, B.C.; Vitkin, I.A.; et al. Speckle Variance Detection of Microvasculature Using Swept-Source Optical Coherence Tomography. Opt. Lett. 2008, 33, 1530–1532. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, M.S.; Cadotte, D.W.; Vuong, B.; Sun, C.; Luk, T.W.H.; Mariampillai, A.; Yang, V.X.D. Review of Speckle and Phase Variance Optical Coherence Tomography to Visualize Microvascular Networks. J. Biomed. Opt. 2013, 18, 050901. [Google Scholar] [CrossRef] [Green Version]
- Enfield, J.; Jonathan, E.; Leahy, M. In Vivo Imaging of the Microcirculation of the Volar Forearm Using Correlation Mapping Optical Coherence Tomography (CmOCT). Biomed. Opt. Express 2011, 2, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.K.; Jacques, S.L.; Ma, Z.; Hurst, S.; Hanson, S.R.; Gruber, A. Three Dimensional Optical Angiography. Opt. Express 2007, 15, 4083–4097. [Google Scholar] [CrossRef]
- Makita, S.; Hong, Y.; Yamanari, M.; Yatagai, T.; Yasuno, Y. Optical Coherence Angiography. Opt. Express 2006, 14, 7821–7840. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-Spectrum Amplitude-Decorrelation Angiography with Optical Coherence Tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef] [Green Version]
- Postnov, D.D.; Tang, J.; Erdener, S.E.; Kılıç, K.; Boas, D.A. Dynamic Light Scattering Imaging. Sci. Adv. 2020, 6, eabc4628. [Google Scholar] [CrossRef]
- Tang, J.; Erdener, S.E.; Sunil, S.; Boas, D.A. Normalized Field Autocorrelation Function-Based Optical Coherence Tomography Three-Dimensional Angiography. J. Biomed. Opt. 2019, 24, 036005. [Google Scholar] [CrossRef] [Green Version]
- Matveev, L.A.; Zaitsev, V.Y.; Gelikonov, G.V.; Matveyev, A.L.; Moiseev, A.A.; Ksenofontov, S.Y.; Gelikonov, V.M.; Sirotkina, M.A.; Gladkova, N.D.; Demidov, V.; et al. Hybrid M-Mode-like OCT Imaging of Three-Dimensional Microvasculature in Vivo Using Reference-Free Processing of Complex Valued B-Scans. Opt. Lett. 2015, 40, 1472–1475. [Google Scholar] [CrossRef]
- Moiseev, A.; Ksenofontov, S.; Sirotkina, M.; Kiseleva, E.; Gorozhantseva, M.; Shakhova, N.; Matveev, L.; Zaitsev, V.; Matveyev, A.; Zagaynova, E.; et al. Optical Coherence Tomography-Based Angiography Device with Real-Time Angiography B-Scans Visualization and Hand-Held Probe for Everyday Clinical Use. J. Biophotonics 2018, 11, e201700292. [Google Scholar] [CrossRef]
- Demidov, V.; Matveev, L.A.; Demidova, O.; Matveyev, A.L.; Zaitsev, V.Y.; Flueraru, C.; Vitkin, I.A. Analysis of Low-Scattering Regions in Optical Coherence Tomography: Applications to Neurography and Lymphangiography. Biomed. Opt. Express 2019, 10, 4207–4219. [Google Scholar] [CrossRef]
- Sirotkina, M.A.; Moiseev, A.A.; Matveev, L.A.; Zaitsev, V.Y.; Elagin, V.V.; Kuznetsov, S.S.; Gelikonov, G.V.; Ksenofontov, S.Y.; Zagaynova, E.V.; Feldchtein, F.I.; et al. Accurate Early Prediction of Tumour Response to PDT Using Optical Coherence Angiography. Sci. Rep. 2019, 9, 6492. [Google Scholar] [CrossRef]
- Matveev, L.; Kiseleva, E.; Baleev, M.; Moiseev, A.; Ryabkov, M.; Potapov, A.; Bederina, E.; Sirotkina, M.; Shalin, V.; Smirnov, I.; et al. Optical Coherence Tomography Angiography and Attenuation Imaging for Label-Free Observation of Functional Changes in the Intestine after Sympathectomy: A Pilot Study. Photonics 2022, 9, 304. [Google Scholar] [CrossRef]
- Maslennikova, A.V.; Sirotkina, M.A.; Moiseev, A.A.; Finagina, E.S.; Ksenofontov, S.Y.; Gelikonov, G.V.; Matveev, L.A.; Kiseleva, E.B.; Zaitsev, V.Y.; Zagaynova, E.V.; et al. In-Vivo Longitudinal Imaging of Microvascular Changes in Irradiated Oral Mucosa of Radiotherapy Cancer Patients Using Optical Coherence Tomography. Sci. Rep. 2017, 7, 16505. [Google Scholar] [CrossRef]
- Gubarkova, E.V.; Feldchtein, F.I.; Zagaynova, E.V.; Gamayunov, S.V.; Sirotkina, M.A.; Sedova, E.S.; Kuznetsov, S.S.; Moiseev, A.A.; Matveev, L.A.; Zaitsev, V.Y.; et al. Optical Coherence Angiography for Pre-Treatment Assessment and Treatment Monitoring Following Photodynamic Therapy: A Basal Cell Carcinoma Patient Study. Sci. Rep. 2019, 9, 18670. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Hepburn, M.S.; Mowla, A.; Kennedy, B.F. Strain and Elasticity Imaging in Compression Optical Coherence Elastography: The Two-decade Perspective and Recent Advances. J. Biophotonics 2021, 14, e202000257. [Google Scholar] [CrossRef]
- Yao, G.; Wang, L.V. Monte Carlo Simulation of an Optical Coherence Tomography Signal in Homogeneous Turbid Media. Phys. Med. Biol. 1999, 44, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Kirillin, M.; Meglinski, I.; Kuzmin, V.; Sergeeva, E.; Myllylä, R. Simulation of Optical Coherence Tomography Images by Monte Carlo Modeling Based on Polarization Vector Approach. Opt. Express 2010, 18, 21714–21724. [Google Scholar] [CrossRef]
- Doronin, A.; Meglinski, I. Online Object Oriented Monte Carlo Computational Tool for the Needs of Biomedical Optics. Biomed. Opt. Express 2011, 2, 2461–2469. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, V.Y.; Matveev, L.A.; Matveyev, A.L.; Gelikonov, G.V.; Gelikonov, V.M. A Model for Simulating Speckle-Pattern Evolution Based on Close to Reality Procedures Used in Spectral-Domain OCT. Laser Phys. Lett. 2014, 11, 105601. [Google Scholar] [CrossRef] [Green Version]
- Zykov, A.; Matveyev, A.; Matveev, L.; Sovetsky, A.; Zaitsev, V. Flexible Computationally Efficient Platform for Simulating Scan Formation in Optical Coherence Tomography with Accounting for Arbitrary Motions of Scatterers. J. Biomed. Photon. Eng. 2021, 7, 010304. [Google Scholar] [CrossRef]
- Almasian, M.; van Leeuwen, T.G.; Faber, D.J. OCT Amplitude and Speckle Statistics of Discrete Random Media. Sci. Rep. 2017, 7, 14873. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Gelikonov, G.V.; Sovetsky, A.A.; Vitkin, A. Optimized Phase Gradient Measurements and Phase-Amplitude Interplay in Optical Coherence Elastography. J. Biomed. Opt 2016, 21, 116005. [Google Scholar] [CrossRef]
- Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Gelikonov, G.V.; Moiseev, A.A.; Zaitsev, V.Y. Vector Method for Strain Estimation in Phase-Sensitive Optical Coherence Elastography. Laser Phys. Lett. 2018, 15, 065603. [Google Scholar] [CrossRef]
- Li, J.; Pijewska, E.; Fang, Q.; Szkulmowski, M.; Kennedy, B.F. Analysis of Strain Estimation Methods in Phase-Sensitive Compression Optical Coherence Elastography. Biomed. Opt. Express 2022, 13, 2224. [Google Scholar] [CrossRef]
- Kling, S.; Khodadadi, H.; Goksel, O. Optical Coherence Elastography-Based Corneal Strain Imaging during Low-Amplitude Intraocular Pressure Modulation. Front. Bioeng. Biotechnol. 2020, 7, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kling, S. Optical Coherence Elastography by Ambient Pressure Modulation for High-Resolution Strain Mapping Applied to Patterned Cross-Linking. J. R. Soc. Interface 2020, 17, 20190786. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Ksenofontov, S.Y.; Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Zykov, A.A.; Gelikonov, G.V. Real-Time Strain and Elasticity Imaging in Phase-Sensitive Optical Coherence Elastography Using a Computationally Efficient Realization of the Vector Method. Photonics 2021, 8, 527. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zykov, A.A.; Matveyev, A.L.; Matveev, L.A.; Shabanov, D.V.; Zaitsev, V.Y. Novel Elastography-Inspired Approach to Angiographic Visualization in Optical Coherence Tomography. Photonics 2022, 9, 401. https://doi.org/10.3390/photonics9060401
Zykov AA, Matveyev AL, Matveev LA, Shabanov DV, Zaitsev VY. Novel Elastography-Inspired Approach to Angiographic Visualization in Optical Coherence Tomography. Photonics. 2022; 9(6):401. https://doi.org/10.3390/photonics9060401
Chicago/Turabian StyleZykov, Alexey A., Alexander L. Matveyev, Lev A. Matveev, Dmitry V. Shabanov, and Vladimir Y. Zaitsev. 2022. "Novel Elastography-Inspired Approach to Angiographic Visualization in Optical Coherence Tomography" Photonics 9, no. 6: 401. https://doi.org/10.3390/photonics9060401
APA StyleZykov, A. A., Matveyev, A. L., Matveev, L. A., Shabanov, D. V., & Zaitsev, V. Y. (2022). Novel Elastography-Inspired Approach to Angiographic Visualization in Optical Coherence Tomography. Photonics, 9(6), 401. https://doi.org/10.3390/photonics9060401





