Randomized and Controlled Clinical Studies on Antibacterial Photodynamic Therapy: An Overview
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- O’neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations: Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2018.
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 2017, 31, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy-what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef] [PubMed]
- Chandna, S.; Thakur, N.S.; Kaur, R.; Bhaumik, J. Lignin–Bimetallic Nanoconjugate Doped pH-Responsive Hydrogels for Laser-Assisted Antimicrobial Photodynamic Therapy. Biomacromolecules 2020, 21, 3216–3230. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2017; pp. 343–394. [Google Scholar]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef]
- Bacellar, I.O.L.; Oliveira, M.C.; Dantas, L.S.; Costa, E.B.; Junqueira, H.C.; Martins, W.K.; Durantini, A.M.; Cosa, G.; Di Mascio, P.; Wainwright, M.; et al. Photosensitized Membrane Permeabilization Requires Contact-Dependent Reactions between Photosensitizer and Lipids. J. Am. Chem. Soc. 2018, 140, 9606–9615. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.M.; Basset-Séguin, N.; Calzavara-Pinton, P.G.; Gilaberte, Y.; Hædersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 2: Emerging indications–field cancerization, photorejuvenation and inflammatory/infective dermatoses. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 17–29. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.M.; Basset-Seguin, N.; Calzavara-Pinton, P.; Gilaberte, Y.; Hædersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: Treatment delivery and established indications–actinic keratoses, Bowen’s disease and basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2225–2238. [Google Scholar] [CrossRef]
- Braathen, L.R.; Szeimies, R.-M.; Basset-Seguin, N.; Bissonnette, R.; Foley, P.; Pariser, D.; Roelandts, R.; Wennberg, A.-M.; Morton, C.A. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J. Am. Acad. Dermatol. 2007, 56, 125–143. [Google Scholar] [CrossRef]
- Mezzacappo, N.F.; Souza, L.M.; Inada, N.M.; Dias, L.D.; Garbuio, M.; Venturini, F.P.; Corrêa, T.Q.; Moura, L.; Blanco, K.C.; de Oliveira, K.T.; et al. Curcumin/d-mannitol as photolarvicide: Induced delay in larval development time, changes in sex ratio and reduced longevity of Aedes aegypti. Pest Manag. Sci. 2021, 77, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.M.; Inada, N.M.; Venturini, F.P.; Carmona-Vargas, C.C.; Pratavieira, S.; de Oliveira, K.T.; Kurachi, C.; Bagnato, V.S. Photolarvicidal effect of curcuminoids from Curcuma longa Linn. against Aedes aegypti larvae. J. Asia. Pac. Entomol. 2019, 22, 151–158. [Google Scholar] [CrossRef]
- Corrêa, T.Q.; Blanco, K.C.; Soares, J.M.; Inada, N.M.; Kurachi, C.; Golim, M.D.A.; Deffune, E.; Bagnato, V.S. Photodynamic inactivation for in vitro decontamination of Staphylococcus aureus in whole blood. Photodiagn. Photodyn. Ther. 2019, 28, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, C.; Wang, D. A novel ultraviolet illumination used in riboflavin photochemical method to inactivate drug-resistant bacteria in blood components. J. Photochem. Photobiol. B Biol. 2020, 204, 111782. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.Q.; Blanco, K.C.; Garcia, E.B.; Perez, S.M.L.; Chianfrone, D.J.; Morais, V.S.; Bagnato, V.S. Effects of ultraviolet light and curcumin-mediated photodynamic inactivation on microbiological food safety: A study in meat and fruit. Photodiagn. Photodyn. Ther. 2020, 30, 101678. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Magaraggia, M.; Fabris, C.; Soncin, M.; Camerin, M.; Tallandini, L.; Coppellotti, O.; Guidolin, L. Photodynamic Inactivation of Microbial Pathogens: Disinfection of Water and Prevention of Water-Borne Diseases. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Panhóca, V.H.; Luis Esteban Florez, F.; Quatrini Corrêa, T.; Paolillo, F.R.; Oliveira de Souza, C.W.; Bagnato, V.S. Oral decontamination of orthodontic patients using photodynamic therapy mediated by blue-light irradiation and curcumin associated with sodium dodecyl sulfate. Photomed. Laser Surg. 2016, 34, 411–417. [Google Scholar] [CrossRef]
- Al-Shammery, D.; Michelogiannakis, D.; Ahmed, Z.U.; Ahmed, H.B.; Rossouw, P.E.; Romanos, G.E.; Javed, F. Scope of antimicrobial photodynamic therapy in Orthodontics and related research: A review. Photodiagn. Photodyn. Ther. 2019, 25, 456–459. [Google Scholar] [CrossRef]
- Panhóca, V.H.; Carreira Geralde, M.; Corrêa, T.Q.; Carvalho, M.T.; Wesley, C.; Souza, O.; Bagnato, V.S. Enhancement of the Photodynamic Therapy Effect on Streptococcus Mutans Biofilm. J. Phys. Sci. Appl. 2014, 4, 107–114. [Google Scholar]
- Nie, M.; Deng, D.M.; Wu, Y.; de Oliveira, K.T.; Bagnato, V.S.; Crielaard, W.; de Souza Rastelli, A.N. Photodynamic inactivation mediated by methylene blue or chlorin e6 against Streptococcus mutans biofilm. Photodiagn. Photodyn. Ther. 2020, 31, 101817. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzzá, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-Pot Microwave-Assisted Synthesis of Carbon Dots and in vivo and in vitro Antimicrobial Photodynamic Applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef] [PubMed]
- Blanco, K.C.; Inada, N.M.; Carbinatto, F.M.; Giusti, A.L.; Bagnato, V.S. Treatment of recurrent pharyngotonsillitis by photodynamic therapy. Photodiagn. Photodyn. Ther. 2017, 18, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Inada, N.M.; Bagnato, V.S.; Blanco, K.C. Evolution of surviving Streptoccocus pyogenes from pharyngotonsillitis patients submit to multiple cycles of antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 210, 111985. [Google Scholar] [CrossRef]
- Geralde, M.C.; Leite, I.S.; Inada, N.M.; Salina, A.C.G.; Medeiros, A.I.; Kuebler, W.M.; Kurachi, C.; Bagnato, V.S. Pneumonia treatment by photodynamic therapy with extracorporeal illumination—An experimental model. Physiol. Rep. 2017, 5, e13190. [Google Scholar] [CrossRef] [PubMed]
- Leite, I.S.; Geralde, M.C.; Salina, A.C.G.; Medeiros, A.I.; Dovigo, L.N.; Bagnato, V.S.; Inada, N.M. Near–infrared photodynamic inactivation of S. pneumoniae and its interaction with RAW 264.7 macrophages. J. Biophotonics 2018, 11, e201600283. [Google Scholar] [CrossRef]
- Kassab, G.; Geralde, M.C.; Inada, N.M.; Achiles, A.E.; Guerra, V.G.; Bagnato, V.S. Nebulization as a tool for photosensitizer delivery to the respiratory tract. J. Biophotonics 2019, 12, e201800189. [Google Scholar] [CrossRef]
- Sousa, V.; Gomes, A.T.; Freitas, A.; Faustino, M.A.; Neves, M.G.; Almeida, A. Photodynamic inactivation of candida albicans in blood plasma and whole blood. Antibiotics 2019, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Spesia, M.B.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Escherichia coli and Streptococcus mitis by cationic zinc (II) phthalocyanines in media with blood derivatives. Eur. J. Med. Chem. 2010, 45, 2198–2205. [Google Scholar] [CrossRef]
- Dias, L.D.; Correa, T.Q.; Bagnato, V.S. Cooperative and competitive antimicrobial photodynamic effects induced by a combination of methylene blue and curcumin. Laser Phys. Lett. 2021, 18, 075601. [Google Scholar] [CrossRef]
- Pratavieira, S.; Uliana, M.P.; dos Santos Lopes, N.S.; Donatoni, M.C.; Linares, D.R.; de Freitas Anibal, F.; de Oliveira, K.T.; Kurachi, C.; de Souza, C.W.O. Photodynamic therapy with a new bacteriochlorin derivative: Characterization and in vitro studies. Photodiagn. Photodyn. Ther. 2021, 34, 102251. [Google Scholar] [CrossRef]
- Tonon, C.C.; Ashraf, S.; Alburquerque, J.Q.; Souza Rastelli, A.N.; Hasan, T.; Lyons, A.M.; Greer, A. Antimicrobial Photodynamic Inactivation Using Topical and Superhydrophobic Sensitizer Techniques: A Perspective from Diffusion in Biofilms. Photochem. Photobiol. 2021, 97, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Ghosh, G.; Xu, Q.; Liu, Y.; Ghogare, A.A.; Atem, C.; Greer, A.; Saxena, D.; Lyons, A.M. Superhydrophobic Photosensitizers: Airborne 1 O 2 Killing of an in Vitro Oral Biofilm at the Plastron Interface. ACS Appl. Mater. Interfaces 2018, 10, 25819–25829. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding randomised controlled trials. Arch. Dis. Child. 2005, 90, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christodoulides, N.; Nikolidakis, D.; Chondros, P.; Becker, J.; Schwarz, F.; Rössler, R.; Sculean, A. Photodynamic Therapy as an Adjunct to Nonsurgical Periodontal Treatment: A Randomized, Controlled Clinical Trial. J. Periodontol. 2008, 79, 1638–1644. [Google Scholar] [CrossRef]
- Chondros, P.; Nikolidakis, D.; Christodoulides, N.; Rössler, R.; Gutknecht, N.; Sculean, A. Photodynamic therapy as adjunct to nonsurgical periodontal treatment in patients on periodontal maintenance: A randomized controlled clinical trial. Lasers Med. Sci. 2009, 24, 681–688. [Google Scholar] [CrossRef]
- Lulic, M.; Leiggener Görög, I.; Salvi, G.E.; Ramseier, C.A.; Mattheos, N.; Lang, N.P. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: A proof-of-principle randomized-controlled clinical trial. J. Clin. Periodontol. 2009, 36, 661–666. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections-State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [Green Version]
- Rühling, A.; Fanghänel, J.; Houshmand, M.; Kuhr, A.; Meisel, P.; Schwahn, C.; Kocher, T. Photodynamic therapy of persistent pockets in maintenance patients—A clinical study. Clin. Oral Investig. 2010, 14, 637–644. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Silva, S.P.; Pires, J.R.; Soares, G.H.G.; Pontes, A.E.F.; Zuza, E.P.; Spolidório, D.M.P.; de Toledo, B.E.C.; Garcia, V.G. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med. Sci. 2012, 27, 687–693. [Google Scholar] [CrossRef]
- Balata, M.L.; Andrade, L.P.D.; Santos, D.B.N.; Cavalcanti, A.N.; Tunes, U.D.R.; Ribeiro, E.D.P.; Bittencourt, S. Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: A randomized-controlled clinical trial. J. Appl. Oral Sci. 2013, 21, 208–214. [Google Scholar] [CrossRef]
- Mongardini, C.; Di Tanna, G.L.; Pilloni, A. Light-activated disinfection using a light-emitting diode lamp in the red spectrum: Clinical and microbiological short-term findings on periodontitis patients in maintenance. A randomized controlled split-mouth clinical trial. Lasers Med. Sci. 2014, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Macedo, G.D.O.; Novaes, A.B.; Souza, S.L.S.; Taba, M.; Palioto, D.B.; Grisi, M.F.M. Additional effects of aPDT on nonsurgical periodontal treatment with doxycycline in type II diabetes: A randomized, controlled clinical trial. Lasers Med. Sci. 2014, 29, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.U.; Kim, J.W.; Kim, S.J.; Pang, E.K. Effects of adjunctive daily phototherapy on chronic periodontitis: A randomized single-blind controlled trial. J. Periodontal Implant Sci. 2014, 44, 280–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betsy, J.; Prasanth, C.S.; Baiju, K.V.; Prasanthila, J.; Subhash, N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.F.; Andrade, P.V.C.; Rodrigues, M.F.; Hirata, M.H.; Hirata, R.D.C.; Pannuti, C.M.; De Micheli, G.; Conde, M.C. Antimicrobial photodynamic effect to treat residual pockets in periodontal patients: A randomized controlled clinical trial. J. Clin. Periodontol. 2015, 42, 440–447. [Google Scholar] [CrossRef]
- Moreira, A.L.; Novaes, A.B.; Grisi, M.F.; Taba, M.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial Photodynamic Therapy as an Adjunct to Nonsurgical Treatment of Aggressive Periodontitis: A Split-Mouth Randomized Controlled Trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef]
- Birang, R.; Shahaboui, M.; Kiani, S.; Shadmehr, E.; Naghsh, N. Effect of nonsurgical periodontal treatment combined with diode laser or photodynamic therapy on chronic periodontitis: A randomized controlled split-mouth clinical trial. J. Lasers Med. Sci. 2015, 6, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, K.; Chandra, R.V.; Reddy, A.A.; Reddy, B.H.; Reddy, C.; Naveen, A. Effect of a single session of antimicrobial photodynamic therapy using indocyanine green in the treatment of chronic periodontitis: A randomized controlled pilot trial. Quintessence Int. 2015, 46, 391–400. [Google Scholar] [CrossRef]
- Giannelli, M.; Formigli, L.; Lorenzini, L.; Bani, D. Efficacy of Combined Photoablative-Photodynamic Diode Laser Therapy Adjunctive to Scaling and Root Planing in Periodontitis: Randomized Split-Mouth Trial with 4-Year Follow-Up. Photomed. Laser Surg. 2015, 33, 473–480. [Google Scholar] [CrossRef]
- Pulikkotil, S.J.; Toh, C.G.; Mohandas, K.; Leong, K.V.G. Effect of photodynamic therapy adjunct to scaling and root planing in periodontitis patients: A randomized clinical trial. Aust. Dent. J. 2016, 61, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Castro dos Santos, N.C.; Andere, N.M.R.B.; Araujo, C.F.; de Marco, A.C.; dos Santos, L.M.; Jardini, M.A.N.; Santamaria, M.P. Local adjunct effect of antimicrobial photodynamic therapy for the treatment of chronic periodontitis in type 2 diabetics: Split-mouth double-blind randomized controlled clinical trial. Lasers Med. Sci. 2016, 31, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Taliee, R.; Mojahedi, M.; Meymandi, M.; Torshabi, M. Microbiological efficacy of photodynamic therapy as an adjunct to nonsurgical periodontal treatment: A clinical trial. J. Lasers Med. Sci. 2016, 7, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, F.; Simões, A.; Oliveira, M.; Luiz, A.C.; Gallottini, M.; Pannuti, C. Efficacy of antimicrobial photodynamic therapy as an adjuvant in periodontal treatment in Down syndrome patients. Lasers Med. Sci. 2016, 31, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Ramaglia, L.; Cicciu, M.; Cordasco, G.; Isola, G. The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: A 1-Year Randomized Controlled Clinical Trial. Photomed. Laser Surg. 2017, 35, 702–709. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Williams, R.C.; Siciliano, V.I.; Alibrandi, A.; Cordasco, G.; Ramaglia, L. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef]
- Cadore, U.B.; Reis, M.B.L.; Martins, S.H.L.; Invernici, M.D.M.; Novaes, A.B., Jr.; Taba, M., Jr.; Palioto, D.B.; Messora, M.R.; Souza, S.L.S. Multiple sessions of antimicrobial photodynamic therapy associated with surgical periodontal treatment in patients with chronic periodontitis. J. Periodontol. 2019, 90, 339–349. [Google Scholar] [CrossRef]
- Borekci, T.; Meseli, S.E.; Noyan, U.; Kuru, B.E.; Kuru, L. Efficacy of adjunctive photodynamic therapy in the treatment of generalized aggressive periodontitis: A randomized controlled clinical trial. Lasers Surg. Med. 2019, 51, 167–175. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Gaspirc, B.; Sculean, A. Clinical and microbiological effects of multiple applications of antibacterial photodynamic therapy in periodontal maintenance patients. A randomized controlled clinical study. Photodiagn. Photodyn. Ther. 2019, 27, 44–50. [Google Scholar] [CrossRef]
- Niazi, F.H.; Koppolu, P.; Tanvir, S.B.; Samran, A.; Alqerban, A. Clinical efficacy of photodynamic therapy in the treatment of necrotizing ulcerative periodontitis among HIV seropositive patients: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2020, 29, 101608. [Google Scholar] [CrossRef]
- Rosa, E.P.; Murakami-Malaquias-Silva, F.; Schalch, T.O.; Teixeira, D.B.; Horliana, R.F.; Tortamano, A.; Tortamano, I.P.; Buscariolo, I.A.; Longo, P.L.; Negreiros, R.M.; et al. Efficacy of photodynamic therapy and periodontal treatment in patients with gingivitis and fixed orthodontic appliances: Protocol of randomized, controlled, double-blind study. Medicine 2020, 99, e19429. [Google Scholar] [CrossRef]
- Al Nazeh, A.; Alshahrani, A.; Almoammar, S.; Kamran, M.A.; Togoo, R.A.; Alshahrani, I. Application of photodynamic therapy against periodontal bacteria in established gingivitis lesions in adolescent patients undergoing fixed orthodontic treatment. Photodiagn. Photodyn. Ther. 2020, 31, 101904. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Prakash, S.; Jagadeson, M.; Namachivayam, A.; Das, D.; Sarkar, S. Clinico-microbiological efficacy of indocyanine green as a novel photosensitizer for photodynamic therapy among patients with chronic periodontitis: A split-mouth randomized controlled clinical trial. J. Pharm. Bioallied Sci. 2021, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Patyna, M.; Ehlers, V.; Bahlmann, B.; Kasaj, A. Effects of adjunctive light-activated disinfection and probiotics on clinical and microbiological parameters in periodontal treatment: A randomized, controlled, clinical pilot study. Clin. Oral Investig. 2021, 25, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Llanos do Vale, K.; Ratto Tempestini Horliana, A.C.; Romero dos Santos, S.; Oppido Schalch, T.; Melo de Ana, A.; Agnelli Mesquita Ferrari, R.; Kalil Bussadori, S.; Porta Santos Fernandes, K. Treatment of halitosis with photodynamic therapy in older adults with complete dentures: A randomized, controlled, clinical trial. Photodiagn. Photodyn. Ther. 2021, 33, 102128. [Google Scholar] [CrossRef]
- Al-Momani, M.M. Indocyanine-mediated antimicrobial photodynamic therapy promotes superior clinical effects in stage III and grade C chronic periodontitis among controlled and uncontrolled diabetes mellitus: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2021, 35, 102379. [Google Scholar] [CrossRef]
- Lopes, R.G.; de Godoy, C.H.L.; Deana, A.M.; de Santi, M.E.S.O.; Prates, R.A.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Bussadori, S.K. Photodynamic therapy as a novel treatment for halitosis in adolescents: Study protocol for a randomized controlled trial. Trials 2014, 15, 443. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.G.; da Mota, A.C.C.; Soares, C.; Tarzia, O.; Deana, A.M.; Prates, R.A.; França, C.M.; Fernandes, K.P.S.; Ferrari, R.A.M.; Bussadori, S.K. Immediate results of photodynamic therapy for the treatment of halitosis in adolescents: A randomized, controlled, clinical trial. Lasers Med. Sci. 2016, 31, 41–47. [Google Scholar] [CrossRef]
- Da Mota Ciarcia, A.C.C.; Gonçalves, M.L.L.; Horliana, A.C.R.T.; Suguimoto, E.S.A.; Araujo, L.; Laselva, A.; Mayer, M.P.A.; Motta, L.J.; Deana, A.M.; Mesquita-Ferrari, R.A.; et al. Action of antimicrobial photodynamic therapy with red leds in microorganisms related to halitose: Controlled and randomized clinical trial. Medicine 2019, 98, e13939. [Google Scholar] [CrossRef]
- Romero, S.D.S.; Schalch, T.O.; Do Vale, K.L.; Ando, E.S.; Mayer, M.P.A.; Feniar, J.P.G.; Fernandes, K.P.S.; Bussadori, S.K.; Motta, L.J.; Negreiros, R.M.; et al. Evaluation of halitosis in adult patients after treatment with photodynamic therapy associated with periodontal treatment: Protocol for a randomized, controlled, single-blinded trial with 3-month follow up. Medicine 2019, 98, e16976. [Google Scholar] [CrossRef]
- Gonçalves, M.L.L.; da Mota, A.C.C.; Deana, A.M.; de Souza Cavalcante, L.A.; Horliana, A.C.R.T.; Pavani, C.; Motta, L.J.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; da Silva, D.F.T.; et al. Antimicrobial photodynamic therapy with Bixa orellana extract and blue LED in the reduction of halitosis—A randomized, controlled clinical trial. Photodiagn. Photodyn. Ther. 2020, 30, 101751. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alhaizaey, A.; Kamran, M.A.; Alshahrani, I. Efficacy of antimicrobial photodynamic therapy against halitosis in adolescent patients undergoing orthodontic treatment. Photodiagn. Photodyn. Ther. 2020, 32, 102019. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.S.; do Vale, K.L.; Remolina, V.G.; Silva, T.G.; Schalch, T.O.; Ramalho, K.M.; Negreiros, R.M.; Ando, E.S.; Mayer, M.P.A.; Mesquita Ferrari, R.A.; et al. Oral hygiene associated with antimicrobial photodynamic therapy or lingual scraper in the reduction of halitosis after 90 days follow up: A randomized, controlled, single-blinded trial. Photodiagn. Photodyn. Ther. 2021, 33, 102057. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Schär, D.; Wicki, B.; Eick, S.; Ramseier, C.A.; Arweiler, N.B.; Sculean, A.; Salvi, G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Rakašević, D.; Lazic, Z.; Rakonjac, B.; Soldatovic, I.; Jankovic, S.; Magic, M.; Aleksic, Z. Efficiency of photodynamic therapy in the treatment of peri-implantitis: A three-month randomized controlled clinical trial. Srp. Arh. Celok. Lek. 2016, 144, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.R.; Hasani, A.; Khosroshahian, S. Efficacy of Antimicrobial Photodynamic Therapy as an Adjunctive to Mechanical Debridement in the Treatment of Peri-implant Diseases: A Randomized Controlled Clinical Trial. J. Lasers Med. Sci. 2016, 7, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Ichinose-Tsuno, A.; Aoki, A.; Takeuchi, Y.; Kirikae, T.; Shimbo, T.; Lee, M.-C.; Yoshino, F.; Maruoka, Y.; Itoh, T.; Ishikawa, I.; et al. Antimicrobial photodynamic therapy suppresses dental plaque formation in healthy adults: A randomized controlled clinical trial. BMC Oral Health 2014, 14, 152. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Rolim, J.P.M.L.; Passos, V.F.; Lima, R.A.; Zanin, I.C.J.; Codes, B.M.; Rocha, S.S.; Rodrigues, L.K.A. Photodynamic antimicrobial chemotherapy and ultraconservative caries removal linked for management of deep caries lesions. Photodiagn. Photodyn. Ther. 2015, 12, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Asnaashari, M.; Ashraf, H.; Rahmati, A.; Amini, N. A comparison between effect of photodynamic therapy by LED and calcium hydroxide therapy for root canal disinfection against Enterococcus faecalis: A randomized controlled trial. Photodiagn. Photodyn. Ther. 2017, 17, 226–232. [Google Scholar] [CrossRef]
- Costa-Santos, L.; Silva-Júnior, Z.S.; Sfalcin, R.A.; da Mota, A.C.C.; Horliana, A.C.R.T.; Motta, L.J.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Prates, R.A.; Silva, D.F.T.; et al. The effect of antimicrobial photodynamic therapy on infected dentin in primary teeth: A randomized controlled clinical trial protocol. Medicine 2019, 98, e15110. [Google Scholar] [CrossRef]
- Okamoto, C.B.; Bussadori, S.K.; Prates, R.A.; da Mota, A.C.C.; Tempestini Horliana, A.C.R.; Fernandes, K.P.S.; Motta, L.J. Photodynamic therapy for endodontic treatment of primary teeth: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2020, 30, 101732. [Google Scholar] [CrossRef]
- Leite, D.P.V.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of Photodynamic Therapy with Blue Light and Curcumin as Mouth Rinse for Oral Disinfection: A Randomized Controlled Trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, S.; Griffiths, J.; Philips, G.; Moseley, H.; O’Grady, C.; Mellish, K.; Lankester, C.L.; Faris, B.; Young, R.J.; Brown, S.B.; et al. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: A new approach to antimicrobial therapy. Br. J. Dermatol. 2013, 168, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C. Periodontal Disease. N. Engl. J. Med. 1990, 322, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Cortelli, J.R.; Barbosa, M.D.S.; Westphal, M.A. Halitosis: A review of associated factors and therapeutic approach. Braz. Oral Res. 2008, 22, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef] [Green Version]
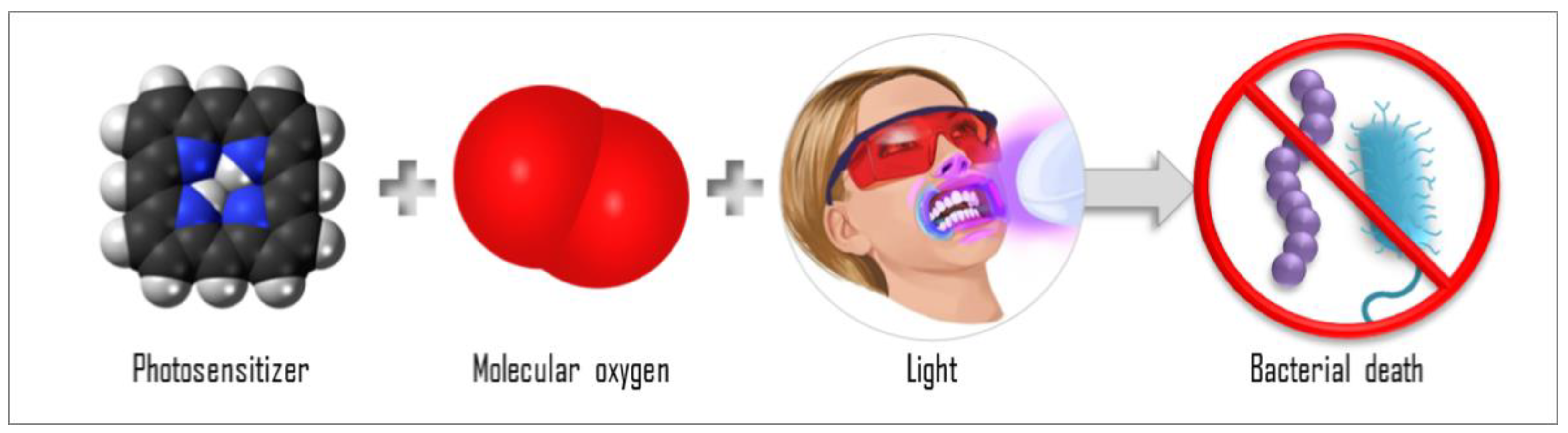
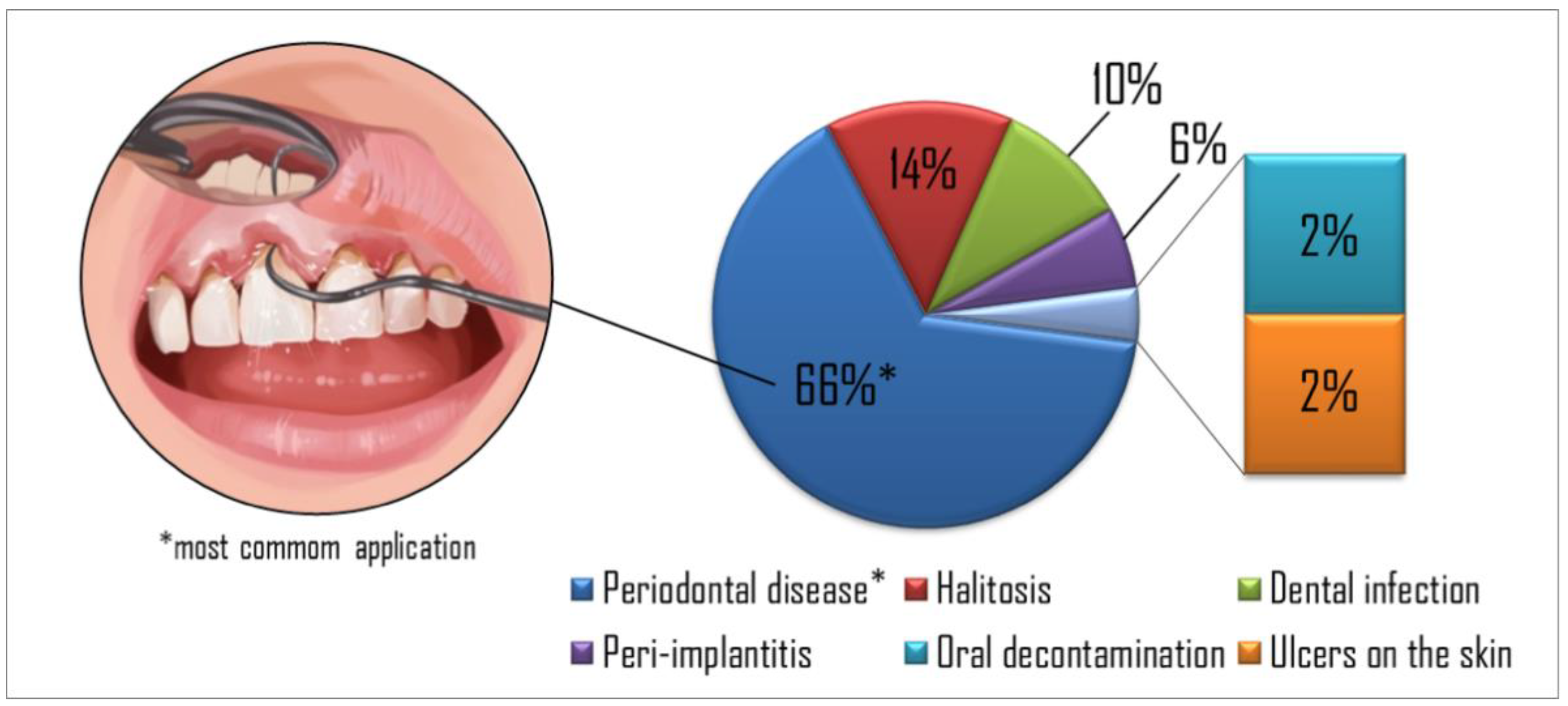
| Ref. | Year of Publication | Country | Number of Patients | Treatment Site | Goal | Clinical Condition | Protocols | Light Parameters | PS, Concentration, and DLI | Follow-Up | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [37] | 2008 | Netherlands | 24 | Periodontal pocket and gingiva | Evaluate the clinical and microbiological adjunctive uses of aPDT for nonsurgical periodontal treatment in chronic periodontitis | Chronic periodontitis | Control Group (n = 12): SRP using hand instruments and sonic instrumentation; aPDT Group (n = 12) | 670 nm, 75 mW, for 1 min | HELBO Blue Photosensitizer, (HELBO Photodynamic Systems, Austria) for 3 min | 3 and 6 months after treatment | A single aPDT session added to SRP failed to improve probing depth reduction and clinical attachment level gain, but it resulted in a greater reduction in bleeding scores compared with SRP alone |
| [38] | 2009 | Germany | 24 | Periodontal pocket | Evaluate the clinical and microbiological effects of the adjunctive use of aPDT in nonsurgical periodontal treatment | Patients receiving supportive periodontal therapy | Randomly treated with either subgingival SRP followed by a single episode of aPDT (test) or subgingival SRP alone (control) | 670 nm, power density of 75 mW/cm2 | Phenothiazine chloride (HELBO Blue Photosensitizer®, HELBO Photodynamic Systems) | 6 months | The additional application of a single episode of PDT to SRP failed to result in an additional improvement in terms of PPD reduction and CAL gain, but it resulted in a significantly higher reduction in bleeding scores than following SRP alone |
| [39] | 2009 | China | 10 | Periodontal pocket | Possible added benefits of repeated adjunctive aPDT to conventional treatment of residual pockets in patients enrolled in periodontal maintenance | Patients with residual pockets | Treatment randomly assigned 5 times in 2 weeks (Days 0, 1, 2, 7, 14) with aPDT (test) or nonactivated laser (control) following debridement | 670 nm, irradiance of 75 mW/cm2 | Phenothiazine chloride (HELBO Blue Photosensitizer, HELBO® Photodynamic Systems GmbH) for 3 min | 12 months | Repeated (5 times) aPDT adjunctive to debridement yielded improved clinical outcomes in residual pockets in maintenance patients |
| [40] | 2009 | Austria | 58 | Periodontal sites | To evaluate aPDT for its bactericidal potential and clinical effect in the treatment of periodontitis | Periodontitis | Group 1 (control group) was managed with an EMS Piezon Master 600 ultrasonic system (EMS Electro Medical Systems, Nyon, Switzerland); Group 2 (laser group) was managed by aPDT in addition to ultrasonic treatment | 680 nm, 75 mW | Methylene blue at 0.005% for 3 min | 3 months after treatment | The application of a single cycle of aPDT was not effective as an adjunct to ultrasonic periodontal treatment |
| [41] | 2010 | Germany | 60 | Periodontal pocket | Evaluate whether aPDT can reduce probing depth in persistent periodontal pockets, change the microbial composition, and decrease the total load of subgingival bacteria more than conventional mechanical debridement | Chronic periodontitis | Control Group (n = 29) treated with conventional ultrasonic debridement; aPDT Group (n = 25) treated with PS and light | 635 nm, 100 mW, for 1 min | Tolonium chloride (Asclepion-Meditec, UK) at 5% concentration for 30 s | 3 months after treatment | Both therapies resulted in the same clinical effect; however, aPDT is less harmful to the teeth and microbial counts were reduced by about 30%–40%, but returned to baseline values after 3 months, irrespective of treatment |
| [42] | 2012 | Brazil | 33 | Periodontal sites | Evaluate the long-term clinical and microbiological effects of aPDT associated with nonsurgical periodontal treatment | Chronic periodontitis | (1) SRP group; (2) SRP and irrigation with toluidine blue O (TBO group); and (3) SRP, irrigation with TBO, and low level laser irradiation (aPDT group) | 660 nm, power 30 mW, spot size 0.07 cm2, energy 4.5 J | Toluidine blue O phenothiazine dye (100 μg/mL; Sigma Chemical Co., St Louis, MO), for 1 min | 180 days | aPDT as an adjunct to periodontal treatment produced statistically significant reductions in some of the key periodontal pathogens but produced no statistically significant benefit in terms of clinical outcome |
| [43] | 2013 | Brazil | 22 | Periodontal sites | To evaluate an aPDT protocol as an adjunct to ultrasonic debridement in patients with severe chronic periodontitis | Chronic periodontitis | (1) Patients were submitted to full-mouth ultrasonic debridement with an ultrasonic scaler; (2) aPDT protocol was carried out (one side of the mouth) with 0.005% methylene blue; (3) after 2 min, the light (660 nm) was applied | 660 nm, 100 mW, light dose = 320 J/cm2 | 0.005% methylene blue | 3 months | aPDT and ultrasonic debridement showed good clinical improvements; however, aPDT did not provide any additional benefit when used with ultrasonic debridement |
| [44] | 2014 | Italy | 30 | Periodontal sites | To study the potential adjunctive effect of microbiological/clinical photodynamic protocol using an LED lamp (red spectrum) and to compare it to SRP | Chronic periodontitis | (1) Patients were treated with SRP; (2) treatment sites were allocated by a toss; (3) toluidine blue was applied and the location was illuminated by using an LED lamp (red spectrum—628 nm) | 628 nm, 2000 mW/cm2, light dose = 20 J/cm2 | Toluidine blue-Fotosan Agent®-(0.1 mg/mL) in 1% xanthan gel | - | A single protocol using an LED light system (red spectrum) and toluidine blue enhance short-term clinical and microbiological outcomes of the mechanical procedure |
| [45] | 2014 | Brazil | 30 | Periodontal sites | To evaluate the aPDT combined with nonsurgical periodontal and doxycycline on clinical and metabolic effects in patients that show type 2 diabetes mellitus | Chronic periodontitis | All subjects of both groups (SRP and SRP + aPDT) were treated with SRP in combination with doxycycline (100 mg/day, for 2 weeks); (1) in the SRP + aPDT group, a diode laser (660 nm) for 1 min and phenothiazine chloride as PS were applied | 660 nm, 28 mW/cm2, light dose = 16.72 J/cm2 | Phenothiazine chloride (10 mg/mL) | 3 months | aPDT (single use) did not show any clinical improvement as an adjunct to SRP but significantly reduced the glycated hemoglobin levels (HbA1c) |
| [46] | 2014 | Korea | 41 | Periodontal sites | To elucidate clinical and antimicrobial effects of daily phototherapy (PT) as an adjunct to SRP in patients with chronic periodontitis | Chronic periodontitis | All participants underwent full-mouth SRP with periodontal curettes and an ultrasonic device: (1) SRP + PT group assigned electric toothbrushes with embedded LEDs (single frequency, 635 nm wavelength, 13 mW/cm2); (2) SRP group assigned electric toothbrushes without LEDs; irradiation time was 3 min per session | Single frequency, 635 nm, 13 mW/cm2, 3 min | - | 4 weeks | The clinical parameters were improved in both groups; probing pocket depth (PPD) was significantly decreased in the SRP + PT group at the follow-up; furthermore, PPD and clinical attachment levels showed greater changes in the SRP + PT group than in the SRP group; no significant antimicrobial intergroup differences were noted |
| [47] | 2014 | India | 88 | Periodontal pocket and gingiva | Evaluate whether adjunctive use of aPDT to SRP has any short-term effectiveness in chronic periodontitis | Chronic periodontitis | Group 1(n = 44) assigned SRP by hand scalers, universal curettes, and ultrasonic scaler; Group 2 (n = 44) assigned SRP according to Group 1 + aPDT | 655 nm, 60 mW/cm2, for 60 s | Methylene blue (Sigma-Aldrich, St. Louis, MO, USA) at 10 mg/mL concentration for 3 min | 1, 3, and 6 months after treatment | A single application of this aPDT protocol was found to be effective in reducing gingival inflammation and probing pocket depth, evaluated over 6 months |
| [48] | 2015 | Brazil | 34 | Periodontal pocket | Evaluate the clinical and microbiological effects of aPDT in the treatment of residual pockets of patients with chronic periodontitis subjected to supportive therapy | Chronic periodontitis | Control group (n = 16) assigned saline solution; aPDT group (n = 18) assigned PS + light | 660 nm, 90 J/cm2, 40 mW, for 90 s | Methylene blue (Chimiolux®, Hypofarma, Brazil) at 0.01% concentration for 5 min | 7 days, 3, 6, and 12 months after treatment | aPDT protocol used failed to demonstrate additional clinical and bacteriological benefits in residual pockets treatment |
| [49] | 2015 | Brazil | 20 | Periodontal sites | To study the efficiency of multiple sessions of aPDT in combination with SRP versus SRP in patients that show AgP | Aggressive periodontitis | (1) Patients received full-mouth supragingival scaling; (2) SRP was carried out; (3) phenothiazine chloride was added; (4) a diode soft-laser light (670 nm) was applied subgingivally | 670 nm, 0.25 W/cm2, light dose = 2.49 J/cm2 | Phenothiazine chloride (10 mg/mL) | - | The application of aPDT (4 sessions) as an adjunctive protocol to SRP, promotes additional microbiologic, clinical, and immunologic benefits |
| [50] | 2015 | Iran | 20 | Periodontal sites | To evaluate the impact of adjunctive laser therapy (LT) and aPDT on patients with chronic periodontitis. | Chronic periodontitis | All patients received SRP; (1) only SRP; (2) SRP with laser therapy—810 nm; (3) SRP + aPDT mediated by Emundo® mixture | Step 1—transgingival irradiation by bleaching handpiece (0.5 W, 10 s); Step 2—irradiation by a 300 µm bare fiber in a circular pattern (0.5 W, 15 s); (c) Step 3—Granulation tissue removal using a 300 µm bare fiber (0.5 W, 25 s) | Emundo® mixture | 3 months | All groups showed improvements in terms of clinical attachment level (CAL) gain, periodontal pocket depth (PPD) reduction, papilla bleeding index, and microbial count compared with baseline; the results showed more significant improvement in the 6-week evaluation in terms of CAL in groups 2 and 3 than in group 1; group 2 also revealed a greater reduction in PPD than the other treatment modalities |
| [51] | 2015 | India | 60 | Periodontal sites | To evaluate the effects of indocyanine green as an adjunct to nonsurgical periodontal therapy in terms of reduction in the percentage of viable bacteria and host tissue injury | Periodontitis | SRP group (only SRP); laser group—SRP and application of diode laser at 810 nm for 5 s;test group—indocyanine green (SRP) and application of diode laser beam at 810 nm in a continuous wave mode with 0.7 W output for 5 s along with 0.5 mL of 5 mg/mL ICG solution | 810 nm with 0.7 W output for 5 s | 0.5 mL indocyanine green, 5 mg/mL injected through a blunt end cannula till the pocket was overfilled | 6-month period after treatment | Laser-activated ICG dye may enhance the potential benefits of SRP and can be used as an adjunct to nonsurgical periodontal therapy |
| [52] | 2015 | Italy | 26 | Periodontal pocket | Report the 4-year follow-up results of multiple aPDT cycles (PAPD) associated with SRP compared to sham treatment associated with SRP alone | Chronic periodontitis | Control group (n = 138 teeth)—sham + SRP; aPDT group (n = 138 teeth)—PAPD + SRP | aPDT: 635 nm, 100 mW Noncontact—gingival pocket external 11.6 W/cm2, 3.8 J/cm2 each passage Photoablative 810 nm, 1 W Contact—gingival pocket internal + external: 353.4 W/cm2, 66.7 J/cm2 Contact—gingival pocket internal: 35.3 W/cm2, 6.7 J/cm2 each passage | Methylene blue at 0.3% concentration for 5 min | 4 years (every 3 months during the 1st year and then every 6 months until the end) | PAPD + SRP provided a significant and durable improvement compared with sham + SRP alone |
| [53] | 2016 | Malaysia | 20 | Periodontal sites | To evaluate the efficacy of aPDT in reducing Aggregatibacter actinomycetemcomitans (Aa) in periodontitis patients | Periodontitis | Conventional nonsurgical periodontal therapy (NSPT) was performed; in addition, the test side received adjunct aPDT | A red LED lamp with a frequency of 628 Hz; gingiva and pocket were irradiated for 10 s | Methylene blue, 1 min of DLI | 7 days, 1 and 3 months | There was a clinical improvement in 1 and 3 months compared with baseline, while the bleeding on probing was reduced only in the aPDT group in month 3; however, no difference in the quantification of Aa was detected between the groups |
| [54] | 2016 | Brazil | 20 | Periodontal pockets | Investigate the local effect of adjunct aPDT to ultrasonic periodontal debridement (UPD) and compare it to UD only | Moderate to severe generalized chronic periodontitis in type 2 diabetic patients | Control group (n = 20)—UPDT; test group (n = 20)—UPD + aPDT | 660 nm, 60 mW with irradiance of 2.15 W/cm2, total energy of 3.6 J and fluency of 129 J/cm2 | 0.005% methylene blue—Chimiolux DMC for 60 s | 180 days | After 180 days, there were statistically in the UPD group and the UPD + aPDT group; however, the intergroup analysis did not reveal statistically significant differences in any of the evaluated clinical parameters |
| [55] | 2016 | Iran | 18 | Periodontal pocket | Compare the microbiologic effectiveness of the aPDT as an adjunctive treatment modality for nonsurgical treatment in chronic periodontitis | Moderate–severe chronic periodontitis, presence of at least 2 teeth with a pocket depth of 4–10 mm in each quadrant, gingival bleeding, and presence of at least 5 natural teeth in each quadrant | Four quadrants were randomly treated by SRP, diode laser (810 nm wavelength, 1.5 W, and 320 μm fiber, contact, and sweeping technique), SRP + aPDT (with diode laser 808 nm, 0.5 W), and laser + SRP (with diode laser 808 nm, 1 W) in each patient | 808 nm, 0.2 W power | - | 3 months | aPDT was more effective as an adjunctive treatment to SRP than SRP alone; however, no distinct differences were found between both treatment modalities regarding the reduction in certain pathogen bacteria |
| [56] | 2016 | Brazil | 13 | Periodontal pocket | Evaluate the efficacy of aPDT as an adjuvant to conventional periodontal treatment in down syndrome patients | Down syndrome patients who presented at least one tooth in each quadrant of the mouth with probing pocket depth equal to or greater than 5 mm were included | Conventional treatment with SRP + a sham procedure and the experimental treatment SRP + aPDT | 660 nm, 120 J/cm2 divided into 4 points of 30 J/cm2 per tooth (1.2 J per point); the application time was 30 s/point with a spot size of 0.04 cm2 | Methylene blue 0.01% (Chimiolux®, DMC, São Carlos, SP, Brazil), for 4 min | 1 month | Both types of periodontal treatment, with and without aPDT, were similarly effective and were associated with good clinical response |
| [57] | 2017 | Italy | 31 | Periodontal sites | To further evaluate the effects of SRP + diode laser for the treatment of generalized aggressive periodontitis. | Generalized aggressive periodontitis | SRP + diode laser or SRP alone | 810 nm laser, set at 1 W in pulsating mode at 50 Hz, toff = 100 msec, ton = 100 msec, and an energy density of 24.84 J/cm2, with a 300 µm fiber optic delivery system | - | 1 year | Both treatments demonstrated an improvement in periodontal parameters at 1 year; however, SRP + diode laser produced a significant improvement in probing depth and in clinical attachment level; however, microbial and inflammatory mediator changes were not significantly reduced compared to SRP alone |
| [58] | 2018 | Italy | 36 | Periodontal sites | To investigate and compare a desiccant agent as an adjunct to SRP versus SRP alone for the treatment of chronic periodontitis | Chronic periodontitis | - | - | - | - | No aPDT or light therapy was performed |
| [59] | 2019 | Brazil | 16 | Periodontal sites | To evaluate the clinical effects and the subgingival microbiota after multiple sessions of aPDT associated with surgical treatment of severe chronic periodontitis (SCP) | Chronic periodontitis | All participants underwent 4 sessions of full-mouth SRP: test group (TG)—multiple sessions of aPDT and surgical periodontal treatment (ST); control group (CG)—ST only, in a split-mouth design | Laser diode, 660 nm, 60 mW/cm2, 0.6 J/cm2, 60 s per site | 10 mg/mL of phenothiazine chloride for 5 min | Baseline (preintervention), 60 days (30 days after the end of nonsurgical therapy), and at 150 days (90 days after surgery) | A reduction in probing depth was observed at 150 days for the TG, when compared with the CG; clinical attachment level gain was higher in the TG at 60 and 150 days; changes in the subgingival microbiota were similar between the groups, but the TG revealed a larger number of bacteria associated with periodontal disease at the end of the experiment |
| [60] | 2019 | Turkey | 24 | Periodontal sites | To evaluate the microbiological and clinical effects of aPDT as an adjunctive tool to the nonsurgical periodontal protocol in patients that show aggressive periodontitis (AgP) | Generalized aggressive periodontitis | (1) SRP was applied to 12 subjects with ultrasonic; (2) toluidine blue was applied at the bottom of the periodontal pocket; (3) an LED source (625–635 nm) was inserted parallel to the root surface and the illumination was performed | 625–635 nm, 2000 mW/cm2, light dose = 20 J/cm2 | Toluidine blue O (0.1 mg/mL) | - | The use of the aPDT (two sessions) as an adjunct to SRP did not show superior to SRP regarding microbiological and clinical results |
| [61] | 2019 | Finland | 20 | Periodontal sites | To evaluate clinically and microbiologically the outcomes following one single session of subgingival mechanical debridement | Periodontitis | 40 patients were randomly assigned 2 treatments: 1. SRP using ultrasonic and hand instruments followed by one single session of SRP followed by 1 x immediate application of aPDT and 2 x subsequent applications of aPDT without SRP (test); 2. SRP alone (control) | 635 nm, 117.64 J/mm | Toluidine blue 0.1% for 1 min | 6 months after treatment | Enhanced the clinical and microbiological outcomes compared with SRP alone |
| [62] | 2020 | Finland | 30 | Periodontal sites | To evaluate clinical periodontal and microbiological parameters after the treatment with adjunctive antimicrobial aPDT among HIV-seropositive and -seronegative patients with necrotizing ulcerative periodontitis | Necrotizing ulcerative periodontitis | Group I—provision of treatment through aPDT on the dorsum of tongue; group II—provision of treatment with the help of tongue scrappers (TS); group III—provision of treatment with the help of TS and adjunctive aPDT | 670 nm, 22 J/cm2 | Methylene blue (Helbo Blue photosensitizer) with 0.005%. | 3 and 6 months after treatment | aPDT was effective in improving clinical periodontal parameters and bacterial levels |
| [63] | 2020 | Brazil | - | Periodontal sites | To evaluate the impact of photodynamic therapy (aPDT) as an adjuvant treatment in patients with gingivitis and fixed orthodontic appliances | Gingivitis and fixed orthodontic appliances | - | - | - | 21 days | Not performed |
| [64] | 2020 | Saudi Arabia | 22 | Periodontal sites | To evaluate the effectiveness of aPDT as an adjunct to ultrasonic scaling (in the reduction in gingival inflammatory parameters and periodontal pathogens | Gingivitis lesions | US group—patients receiving ultrasonic scaling (US) with usual oral hygiene in- instructions; aPDT group—in which patients received adjunctive aPDT with US | 670 nm, 22 J/cm2 | Methylene blue (0.0005%) photo- sensitizer (HELBO Blue) for 3 min | 6 months or 12 months | aPDT was effective in significantly reducing periodontal pathogens in established gingivitis lesions |
| [65] | 2021 | India | 20 | Periodontal sites | To determine the clinical and microbiological efficacy of aPDT using Indocyanine green (ICG) as a novel PS for the treatment of chronic periodontitis | Chronic periodontitis | All patients received full-mouth supragingival scaling; (1) SRP + aPDT mediated by ICG; (2) only SRP | Soft-tissue diode laser unit (300 mW, 810 nm); each site was irradiated for 30 s | ICG tablet was suspended in distilled water at a concentration of 1 mg/mL, with a DLI of 2 min | 3 months | Sites additionally treated with ICG-mediated aPDT presented a statistically significant reduction in PD and CAL when compared with sites treated with only SRP after 3 months of treatment; adjunctive aPDT can be advocated as a treatment option for chronic periodontitis |
| [66] | 2021 | Germany | 48 | Periodontal sites | To evaluate the microbiological and clinical effects of aPDT procedure alone or in combination with probiotics as an adjunct to nonsurgical periodontal treatment | Chronic periodontitis | (1) Subgingival mechanical debridement was carried out; (2) toluidine blue was applied in the periodontal pockets; (3) an LED device (628 nm) (2000–4000 mW/cm2) was applied subgingivally for 10 s at each side of the tooth | 628 nm, 2000–4000 mW/cm2, time of application = 10 s | Toluidine blue O, Fotosan Agent®, (0.1 mg/mL) | 3–6 months | The combined use of subgingival mechanical debridement, aPDT, and probiotics did not lead to significant improvements in the treatment of chronic periodontitis when compared to subgingival mechanical debridement plus aPDT and subgingival mechanical debridement alone |
| [67] | 2021 | Brazil | 62 | Periodontal sites and gingiva | To compare the effect of aPDT and tongue scraping (standard treatment) in older people with complete dentures diagnosed with halitosis | Periodontitis | Group I—provision of treatment through aPDT on the dorsum of tongue; group II—provision of treatment with the help of tongue scrappers (TS); group III—provision of treatment with the help of TS and adjunctive aPDT | 660 nm, 3183 J/cm2 | Methylene blue 0.005%, Brazil) for 5 min | 3 and 6 months after treatment | The oral hygiene behavior associated with aPDT or tongue scraper was not able to reduce halitosis after a 90-day follow-up |
| [68] | 2021 | Saudi Arabia | 51 | Periodontal pocket and papilla | Evaluate the efficacy of ICG/aPDT in the treatment of chronic periodontitis in terms of clinical, microbiological, and immune-inflammatory parameters in patients with well-controlled and poorly controlled forms of type-2 diabetes mellitus (T2DM) | Chronic periodontitis | Split-mouth design—one site for control and the other for treatment (n = 17); control group—only root surface debridement (RSD); treatment group—ICG/aPDT + RSD
| 810 nm, 200 mW, 4 J | Indocyanine green (Sigma Aldrich, SA, St. Louis, MO, USA) at 0.5 mg/mL concentration for 30 s in the papilla and 10 s inside the periodontal pocket depth | 3 and 6 months after treatment | ICG/aPDT improved clinical and antimicrobial parameters in well-controlled and poorly controlled T2DM; glycemic status did not interfere with the reduction in periodontal parameters in either type of T2DM |
| Ref. | Year of Publication | Country | Number of Patients | Treatment Site | Goal | Clinical Condition | Protocols | Light Parameters | PS, Concentration, and DLI | Follow-Up | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [69] | 2014 | Brazil | - | Tongue | To evaluate the antimicrobial effect of aPDT on halitosis in adolescents | Halitosis | - | - | - | - | Not performed |
| [70] | 2016 | Brazil | 45 | Tongue surface | Evaluate the aPDT effect for halitosis in adolescents through the analysis of volatile sulfur compounds | Halitosis stemming from lingual bacteria | Group 1 (n = 16)—aPDT; group 2 (n = 15)—tongue scraper; group 3 (n = 14)—tongue scraper and aPDT | 660.52 nm, 3537 mW/cm2 for 90 s/region; 6 regions | Methylene blue at 0.005% (165 µM) for 5 min | Immediately after treatment | A novel option (Group 2) for the treatment of halitosis with an immediate effect without involving mechanical aggression of the lingual papillae |
| [71] | 2019 | Brazil | 39 | Tongue | To evaluate the effectiveness of the application of aPDT in the tongue coating as a new way to control halitosis | Halitosis | (1) aPDT in 4 points of the tongue, E = 36 J, T = 90 s/point; (2) tongue scraper—10 scrapes in the tongue dorsum (Halitus); (3) tongue scraper—10 scrapes in the tongue dorsum and aPDT in 4 points, E = 36 J, T = 90 s/point | Red LED (660 nm) and tip of 2.84 cm2 in diameter; power of 400 mW, E = 36 J, T = 90 s/point | Methylene blue 0.005% (165 µM), 2 min of DLI | 7, 14, and 30 days | Not performed |
| [72] | 2019 | Brazil | 40 | Tongue | To treat oral halitosis in healthy adults with aPDT, associated with periodontal treatment | Halitosis | The participants (n = 40) with halitosis will be randomized into 2 groups: G1—treatment with aPDT (n = 20); G2—cleaning of the tongue with a tongue scraper (n = 20) | 660 nm, 318 J/cm2 | Methylene blue, 0.005% for 3 min | 3 months after treatment | This protocol determined the effectiveness of aPDT in the reduction in halitosis in adults |
| [73] | 2020 | Brazil | 44 | Six points on the back of the tongue | Evaluate the reduction in halitosis using aPDT with Bixa orellana extract and blue LED, compare it to the tongue scraping, and verify the association of both treatments | Diagnosis of sulfide (H2S) ≥ 112 ppb in gas chromatography | Group 1 (n = 15)—aPDT with annatto and LED; group 2 (n = 14)—tongue scraping; group 3 (n = 15)—tongue scraping and aPDT | 395−480 nm for 20 s, 9.6 J per point | Bixa orellana extract in spray at a concentration of 20% w/v for 2 min | 7 days | There was an immediate reduction in halitosis, but the reduction was not maintained after 7 days |
| [74] | 2020 | Saudi Arabia | 45 | Tongue | To evaluate the efficacy of aPDT on halitosis in adolescent patients undergoing fixed orthodontic treatment | Halitosis | Group I—provision of treatment through aPDT on the dorsum of tongue; group II—provision of treatment with the help of tongue scrappers (TS); group III—provision of treatment with the help of TS and adjunctive aPDT | 660 nm, 317.43 J/cm2 was kept 0.028 cm | Methylene blue at 0.005% for 5 min | 2 weeks after treatment | aPDT along with tongue scraping showed effective immediate reduction in H2S concentration and reduction in oral pathogens |
| [75] | 2021 | Brazil | 40 | Tongue | Verify whether modification of oral hygiene behavior associated with aPDT or lingual scraper can reduce halitosis after a 90-day follow-up | Halitosis | Split-mouth design—one site for control and the other for treatment (n = 17); control group—only root surface debridement (RSD); treatment group—ICG/aPDT + RSD; controlled T2DM; uncontrolled T2DM; nondiabetic | 660 nm, 318 J/cm2 | Methylene blue, 0.005% | 90 days after treatment. | aPDT improved clinical and antimicrobial parameters in well-controlled and poorly controlled T2DM |
| Ref. | Year of Publication | Country | Number of Patients | Treatment Site | Goal | Clinical Condition | Protocols | Light Parameters | PS, Concentration, and DLI | Follow-Up | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [76] | 2014 | Switzerland | 40 | Dental implants | To compare the clinical, microbiological, and host-derived effects in the nonsurgical treatment of initial peri-implantitis with either adjunctive local drug delivery or adjunctive aPDT after 12 months | Initial peri-implantitis | (1) aPDT group—the dye phenothiazine chloride was used as PS (3 min), then the pockets were irrigated with 3% hydrogen peroxide and exposed to the laser light for 10 s and aPDT was repeated 1 week later; (2) one unit dosage of minocycline hydrochloride microspheres (1 mg) | Hand-held diode laser, 660 nm, power density of 100 mW, for 10 s | Phenothiazine chloride (3 min) | 3, 6, 9, and 12 months from baseline | Nonsurgical mechanical debridement with adjunctive aPDT was equally as effective in the reduction in mucosal inflammation as with adjunctive delivery of minocycline microspheres up to 12 months |
| [77] | 2016 | Serbia | 52 | Peri-implantitis sites | Evaluate early clinical and microbiological outcomes of peri-implantitis after surgical therapy with adjuvant aPDT | Decontamination of the implant surface | Control group used chlorhexidine gel (CHX) followed by saline irrigation; study group used aPDT for decontamination of the implant surface | 660 nm, 100 mW for 30 s/spot | Phenothiazine chloride (HELBOR Blue Photosensitizer, bredent medical GmbH&Co. KG) was applied onto implant surface, bone, and peri-implant soft tissue, for 3 min | 3 months | aPDT resulted in a significant decrease in bleeding on probing in comparison with CHX (p < 0.001) and showed significant decontamination of implant surfaces with complete elimination of anaerobic bacteria immediately after surgical procedure and three months later |
| [78] | 2016 | Iran | 10 | 30 dental implants | Assess the clinical effects of aPDT after closed surface scaling in the treatment of peri-implant diseases | Peri-implant diseases | Control group (n = 15)—only closed-surface scaling; aPDT group (n = 15)—aPDT after closed-surface scaling | 630 nm, 2000 mW/cm2 for 120 s | Fotösan (CMS Dental, Denmark) for 3 min | 1.5 and 3 months after treatment | Improvement of clinical parameters, in the treatment of peri-implant diseases |
| Ref. | Year of Publication | Country | Number of Patients | Treatment Site | Goal | Clinical Condition | Protocols | Light Parameters | PS, Concentration, and DLI | Follow-Up | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [79] | 2014 | Japan | 11 | Premolar surfaces | Investigate the inhibitory effects of aPDT in the oral cavity of healthy volunteers | Dental plaque deposition | Control group (n = 11)—no treatment; aPDT group (n = 11)—PS + light | 660 nm, 1100 mW/cm2 for 20 s/surface of each tooth | Toluidine blue ortho (Sigma-Aldrich, USA) at 1 mg/mL concentration for 10 s | Every day until 4 days after treatment | The plaque formation on the aPDT group was inhibited after day 4 and the percentages of plaque deposition areas to total buccal and lingual tooth surfaces were significantly reduced compared with the control group |
| [80] | 2015 | Brazil | 45 | 90 deep carious lesions | Test whether photochemistry-based treatment (PACT) reduces bacterial viability in remaining dentin | Deep carious lesions | Control group (n = 45)—0.89% NaCl solution + light; experimental group PACT (n = 45)—PS + light | 630 nm, 150 mW, 94 J/cm2 | Toluidine blue ortho (Sigma, St. Louis, MO, USA) at 100 g/mL concentration for 5 min | Immediately after treatment | PACT led to statistically significant reductions in mutans streptococci, Lactobacillus spp. and total viable bacteria compared with the control |
| [81] | 2017 | Iran | 20 | Molars | Investigate the role of aPDT as a bactericidal agent in infected canals compared with calcium hydroxide therapy | Molars requiring endodontic retreatment | Group 1 (n = 10)—aPDT; group 2 (n = 10)—calcium hydroxide therapy | 635 nm, 200 mW/cm2 for 60 s | Toluidine blue (MDD, CMS Dental Denmark, Korea) at 0.1 mg/mL concentration for 5 min | Immediately after treatment | aPDT presented better disinfectant performance than calcium hydroxide therapy |
| [82] | 2019 | Brazil | 32 | Teeth | To evaluate the clinical effect of aPDT on infected dentin in dental caries lesions in primary teeth | Infection | 32 primary molars with deep occlusal dental caries will be selected and divided into 2 groups: G1—caries removal with a low-speed drill; G2—application of aPDT with PapacarieMBlue | 660 nm, 6 J, 60 s | Methylene blue for 5 min | 12 months after treatment | Adding methylene blue dye to the formula of PapacarieMBlue might potentiate the antimicrobial action of aPDT and work more effectively on the infected dentin combined with a conservative, minimally invasive treatment |
| [83] | 2020 | Brazil | 30 | Teeth | To evaluate the reduction in bacterial load following conventional endodontic treatment with and without antimicrobial aPDT in primary teeth | Endodontic treatment of primary teeth | Group I—patients undergoing conventional root canal therapy (n = 15); group II—patients undergoing conventional root canal therapy combined with antimicrobial aPDT (n = 15) | 660 nm, J/cm2 | Methylene blue, 0.005% for 3 min | 3 months after treatment | This study proved effective (aPDT) but presented the equal efficacious capability to conventional endodontic treatment alone |
| Ref. | Year of Publication | Country | Number of Patients | Treatment Site | Goal | Clinical Condition | Protocols | Light Parameters | PS, Concentration, and DLI | Follow-Up | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [84] | 2014 | Brazil | 27 | Oral cavity | Evaluate the effects of the aPDT with blue light and curcumin on oral disinfection for 2 h after treatment | Oral cavity decontamination | Light group (n = 9)—only light; Curcumin group (n = 9); aPDT group (n = 9) | 455 nm, 300 mW/cm2, 5 min, 200 J/cm2 | Curcumin (aPDT Pharma, Brazil) at 30 mg/L concentration for 5 min | Immediately, 1 h, and 2 h after treatment | Curcumin has the potential to disaggregate oral plaque; aPDT protocol may be used for the reduction in salivary microorganisms to overall mouth disinfection before intraoral surgical procedures |
| Ref. | Year of Publication | Country | Number of Patients | Treatment Site | Goal | Clinical Condition | Protocols | Light Parameters | PS, Concentration, and DLI | Follow-Up | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [85] | 2013 | Scotland | 32 | Legs and foot | To determine whether aPDT in bacterially colonized chronic leg ulcers and chronic diabetic foot ulcers can reduce bacterial load, and potentially lead to accelerated wound healing | Chronic leg and diabetic foot ulcers | All patients (cationic photosensitizer-PPA); G1—placebo; G2—patients with leg ulcer PPA904; G3—patients with diabetic foot ulcer PPA904 | 570–670 nm at a total dose of 50 J/cm2. | [3,7-bis (N,N-dibutylamino) pheno- thiazin-5-ium bromide] for 15 min; 500 μmol/L | 3 months after treatment | This first controlled study of aPDT in chronic wounds demonstrated a significant reduction in bacterial load |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, F.; Stringasci, M.D.; Requena, M.B.; Blanco, K.C.; Dias, L.D.; Corrêa, T.Q.; Bagnato, V.S. Randomized and Controlled Clinical Studies on Antibacterial Photodynamic Therapy: An Overview. Photonics 2022, 9, 340. https://doi.org/10.3390/photonics9050340
Alves F, Stringasci MD, Requena MB, Blanco KC, Dias LD, Corrêa TQ, Bagnato VS. Randomized and Controlled Clinical Studies on Antibacterial Photodynamic Therapy: An Overview. Photonics. 2022; 9(5):340. https://doi.org/10.3390/photonics9050340
Chicago/Turabian StyleAlves, Fernanda, Mirian D. Stringasci, Michelle B. Requena, Kate C. Blanco, Lucas D. Dias, Thaila Q. Corrêa, and Vanderlei S. Bagnato. 2022. "Randomized and Controlled Clinical Studies on Antibacterial Photodynamic Therapy: An Overview" Photonics 9, no. 5: 340. https://doi.org/10.3390/photonics9050340
APA StyleAlves, F., Stringasci, M. D., Requena, M. B., Blanco, K. C., Dias, L. D., Corrêa, T. Q., & Bagnato, V. S. (2022). Randomized and Controlled Clinical Studies on Antibacterial Photodynamic Therapy: An Overview. Photonics, 9(5), 340. https://doi.org/10.3390/photonics9050340





