Quantitative Analysis of Industrial Solid Waste Based on Terahertz Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Instruments and Method
3. Results and Discussions
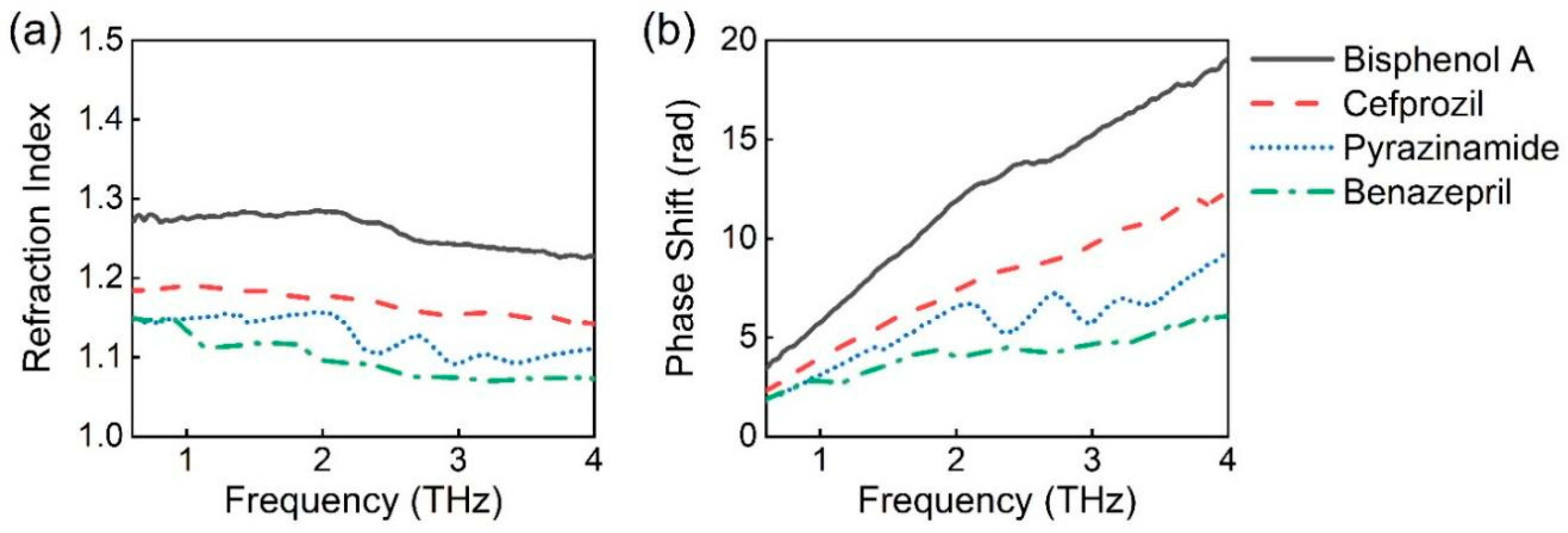
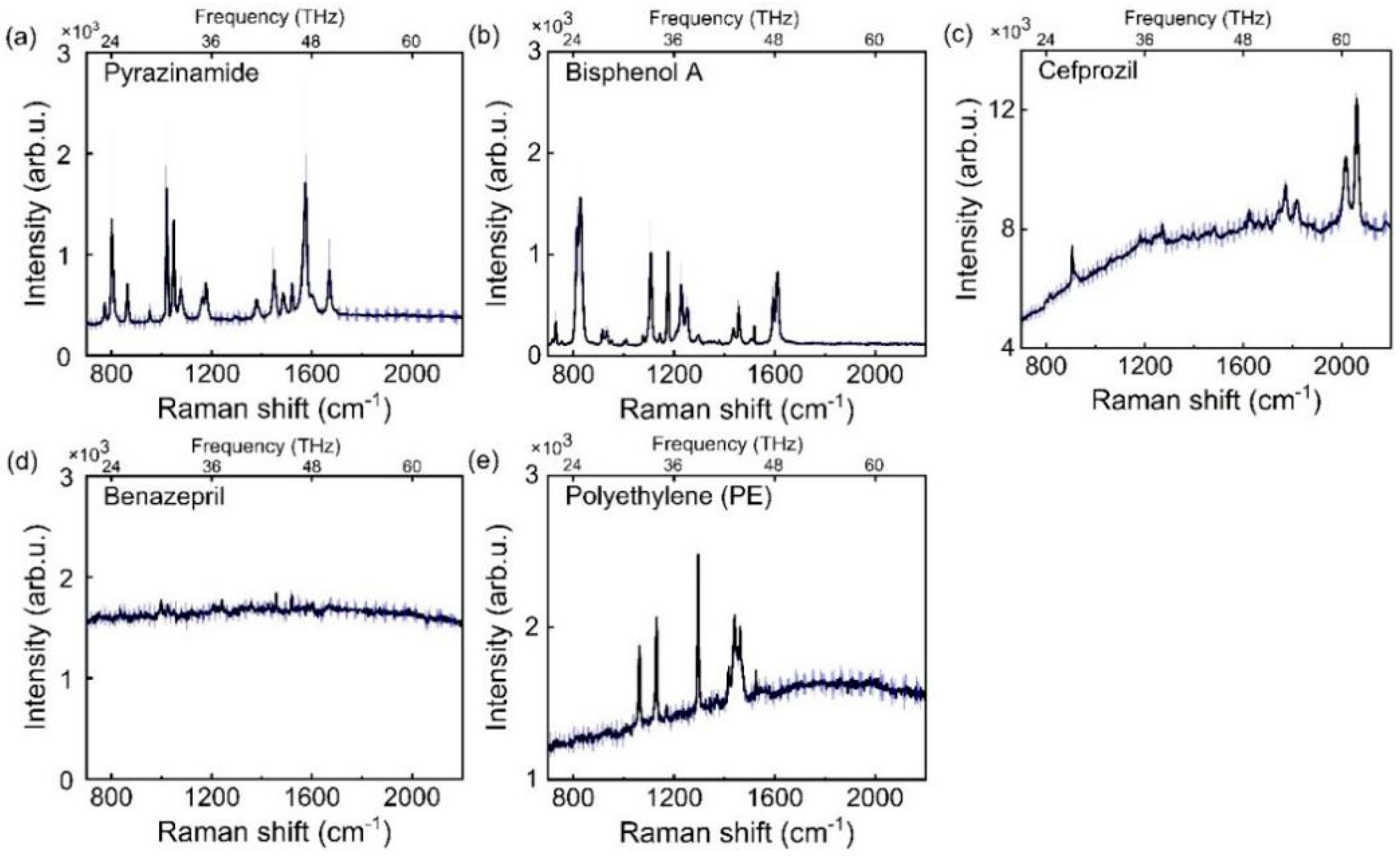
3.1. THz Test Results
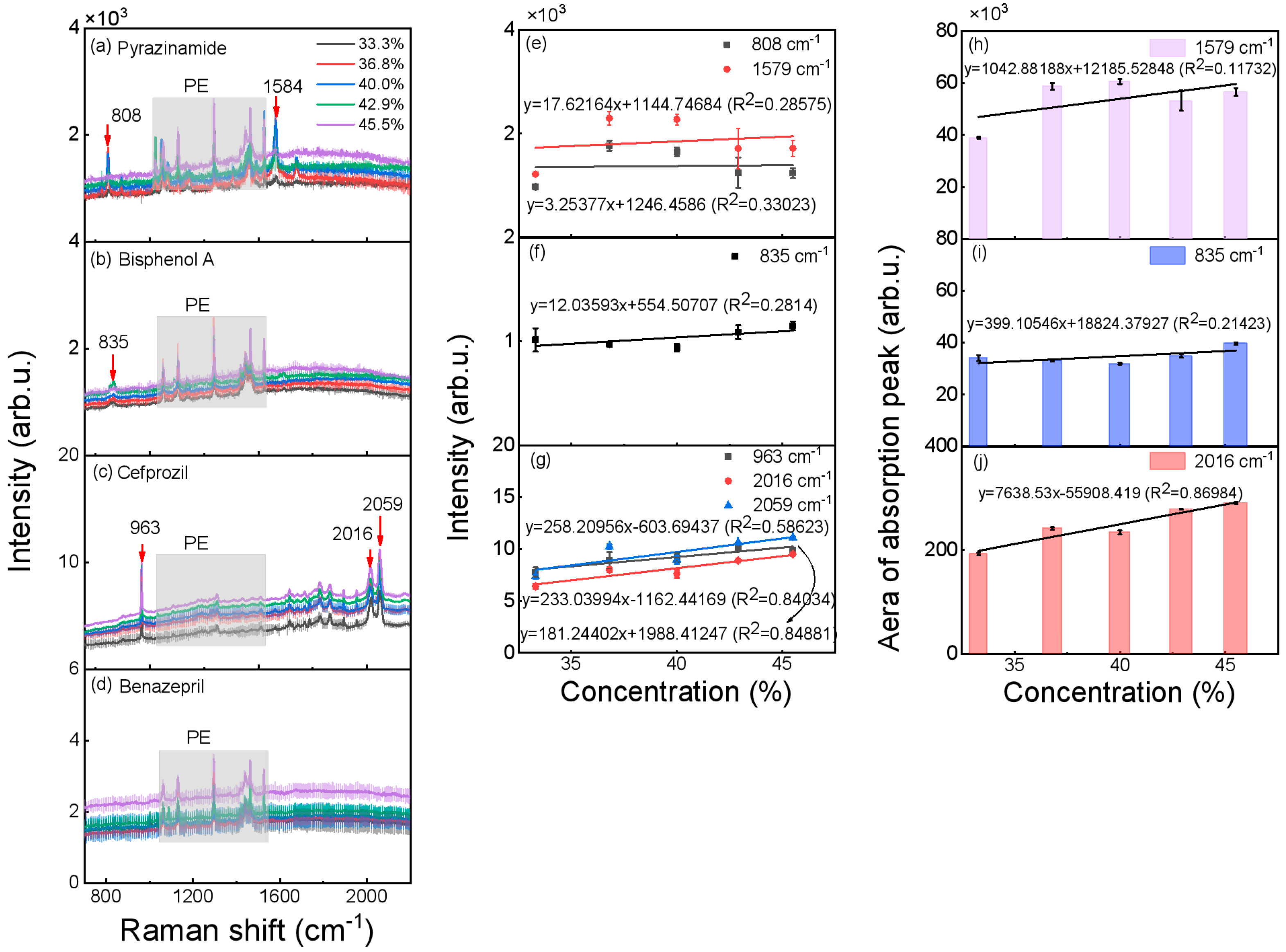
3.2. Concentration Analysis
3.3. Quantitative Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngoc, U.N.; Schnitzer, H. Sustainable solutions for solid waste management in Southeast Asian countries. Waste Manag. 2009, 29, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Tostmann, A.; Aarnoutse, R.E.; Peters, W.H.M.; Richard, P.N.R.; Boeree, M.J. Xanthine oxidase inhibition by allopurinol increasesin vitropyrazinamide-induced hepatotoxicity in HepG2 cells. Drug. Chem. Toxicol. 2010, 33, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Li, L.-M.; Cao, W.-H.; Zhan, S.-Y.; Hu, Y.-H. Postmarketing surveillance of benazepril-related cough and related risk factors analysis on hypertensives. Chin. J. Epidemiol. 2003, 24, 401–405. [Google Scholar]
- Bakris, G.L.; Sarafidis, P.A.; Weir, M.R.; Dahlöf, B.; Pitt, B.; Jamerson, K.; Velazquez, E.J.; Staikos-Byrne, L.; Kelly, R.Y.; Shi, V.; et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): A prespecified secondary analysis of a randomised controlled trial. Lancet 2010, 375, 1173–1181. [Google Scholar] [CrossRef]
- Wilber, R.B.; Doyle, C.A.; Durham, S.J.; Conetta, B.J.; DeGraw, S.S.; Leigh, A. Safety Profile of Cefprozil. Clin. Infect. Dis. 1992, 14, S264–S271. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, R.; Marco-Ferreira, A.; Antunes, L.C.; Komatsu, A.K. Socio-productive inclusion of scavengers in municipal solid waste management in Brazil: Practices, paradigms and future prospects. Resour. Conserv. Recycl. 2019, 154, 104594. [Google Scholar] [CrossRef]
- Lin, A.; Yu, T.-H.; Lin, C.-F. Pharmaceutical contamination in residential, industrial, and agricultural waste streams: Risk to aqueous environments in Taiwan. Chemosphere 2008, 74, 131–141. [Google Scholar] [CrossRef]
- Intrakamhaeng, V.; Clavier, K.A.; Townsend, T.G. Hazardous waste characterization implications of updating the toxicity characteristic list. J. Hazard. Mater. 2020, 383, 121171. [Google Scholar] [CrossRef]
- Ebdon, L.; Hill, S.J.; Rivas, C. Organotin compounds in solid waste: A review of their properties and determination using high-performance liquid chromatography. TrAC Trends Anal. Chem. 1998, 17, 277–288. [Google Scholar] [CrossRef]
- Mendes, L.D.; Frena, M.; Madureira, L.A.; Richter, P.; Rosero-Moreano, M.; Carasek, E. Development of an eco-friendly method for the determination of total polychlorinated biphenyls in solid waste by gas chromatography-mass spectrometry. J. Environ. Chem. Eng. 2018, 6, 5042–5048. [Google Scholar] [CrossRef]
- Rogalski, A.; Sizov, F. Terahertz detectors and focal plane arrays. Opto-Electron. Rev. 2011, 19, 346–404. [Google Scholar] [CrossRef]
- Fan, S.; He, Y.; Ung, B.; Pickwell-MacPherson, E. The growth of biomedical terahertz research. J. Phys. D Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Li, T.; Ma, H.; Peng, Y.; Chen, X.; Zhu, Z.; Wu, X.; Kou, T.; Song, B.; Guo, S.; Liu, L.; et al. Gaussian numerical analysis and terahertz spectroscopic measurement of homocysteine. Biomed. Opt. Express 2018, 9, 5467–5476. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Shi, C.; Zhu, Y.; Gu, M.; Zhuang, S. Terahertz spectroscopy in biomedical field: A review on signal-to-noise ratio improvement. PhotoniX 2020, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhou, H.; Liu, M.; Li, X.; Hu, Q. Study of the skin depth and defect detection in carbon fiber composites with Terahertz waves. Optik 2018, 178, 1035–1044. [Google Scholar] [CrossRef]
- Li, B.; Bai, J.; Zhang, S. Low concentration noroxin detection using terahertz spectroscopy combined with metamaterial. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Tang, P.; Yu, L.; Jiang, T.; Tian, L. Channel measurements and models for 6G: Current status and future outlook. Front. Inf. Technol. Electron. Eng. 2020, 21, 39–61. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Huang, P.; Ge, W.; Hou, D.; Zhang, G. Inspecting human colon adenocarcinoma cell lines by using terahertz time-domain reflection spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 211, 356–362. [Google Scholar] [CrossRef]
- Chen, W.Q.; Peng, Y.; Jiang, X.K.; Zhao, J.Y.; Zhao, H.W.; Zhu, Y.M. Isomers Identification of 2-hydroxyglutarate acid disodium salt (2HG) by Terahertz Time-domain Spectroscopy. Sci. Rep. 2017, 7, 12166. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Shi, C.; Xu, M.; Kou, T.; Wu, X.; Song, B.; Ma, H.; Guo, S.; Liu, L.; Zhu, Y. Qualitative and Quantitative Identification of Components in Mixture by Terahertz Spectroscopy. IEEE Trans. Terahertz Sci. Technol. 2018, 8, 696–701. [Google Scholar] [CrossRef]
- Duvillaret, L.; Garet, F.; Coutaz, J.-L. A reliable method for extraction of material parameters in terahertz time-domain spectroscopy. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Dorney, T.D.; Baraniuk, R.G.; Mittleman, D.M. Material parameter estimation with terahertz time-domain spectroscopy. J. Opt. Soc. Am. A 2001, 18, 1562–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitley, V.H.; Hooks, D.E.; Ramos, K.J.; O’Hara, J.F.; Azad, A.K.; Taylor, A.J.; Barber, J.; Averitt, R.D. Polarization orientation dependence of the far infrared spectra of oriented single crystals of 1,3,5-trinitro-S-triazine (RDX) using terahertz time–domain spectroscopy. Anal. Bioanal. Chem. 2009, 395, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Yuan, X.; Zou, X.; Chen, W.; Huang, H.; Zhao, H.; Song, B.; Chen, L.; Zhu, Y. Terahertz identification and quantification of neurotransmitter and neurotrophy mixture. Biomed. Opt. Express 2016, 7, 4472–4479. [Google Scholar] [CrossRef] [PubMed] [Green Version]


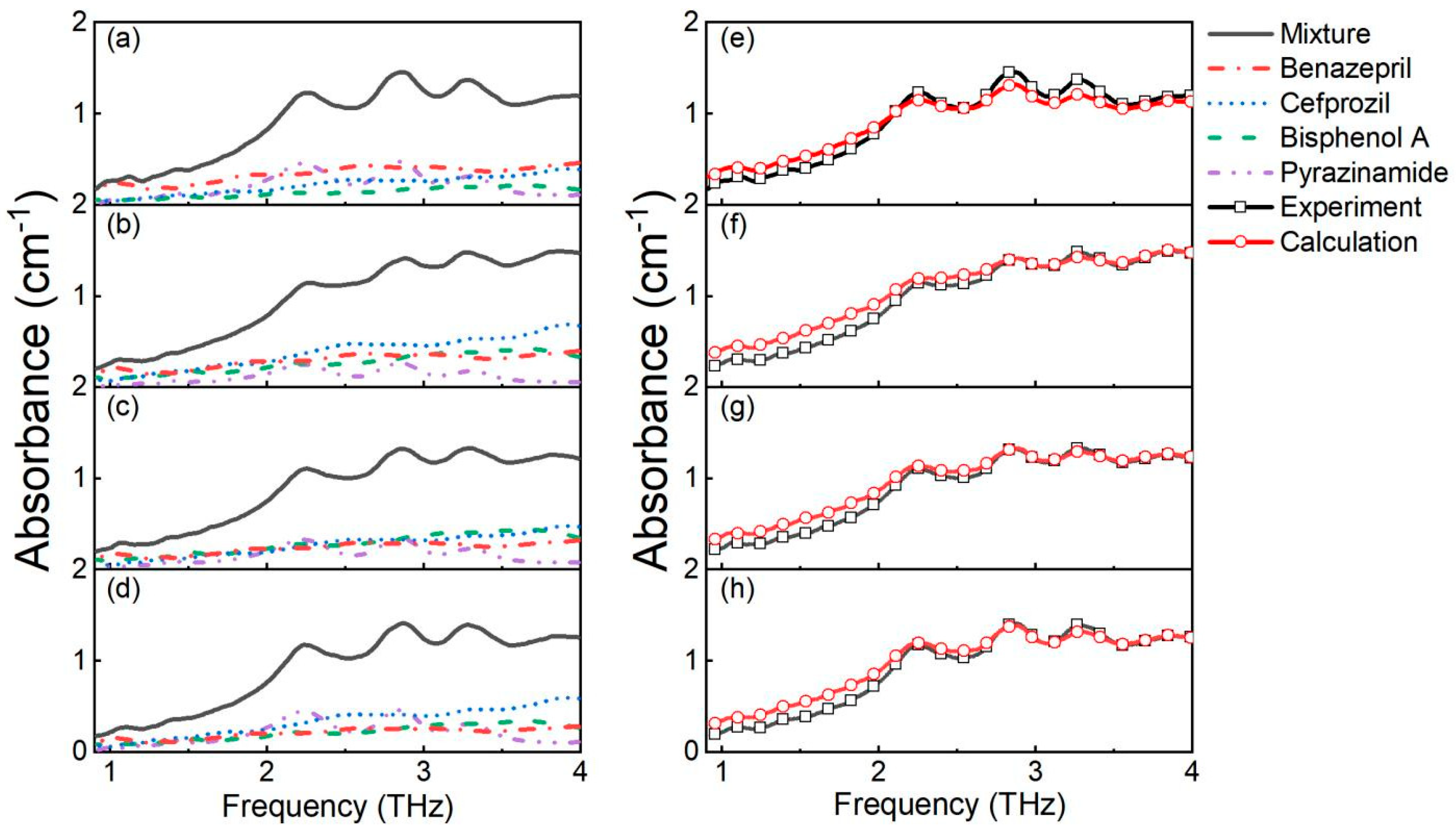
| Groups | Substance | Pyrazinamide | Bisphenol A | Cefprozil | Benazepril | |
|---|---|---|---|---|---|---|
| Ratio | ||||||
| (a) | Ideal | 6.0 | 7.0 | 5.0 | 7.0 | |
| Actual | 5.6 | 6.5 | 4.6 | 6.5 | ||
| Presumptive | 5.4 | 6.1 | 4.3 | 6.5 | ||
| Relative error | 3.6% | 6.2% | 8.5% | 0% | ||
| Average error | 4.575% | |||||
| (b) | Ideal | 3 | 11 | 8 | 5 | |
| Actual | 2.9 | 10.8 | 7.8 | 4.9 | ||
| Presumptive | 2.9 | 11.1 | 7.3 | 5.1 | ||
| Relative error | 0% | 2.8% | 6.4% | 4.1% | ||
| Average error | 3.325% | |||||
| (c) | Ideal | 4 | 12 | 5 | 5 | |
| Actual | 3.8 | 11.4 | 4.8 | 4.8 | ||
| Presumptive | 3.6 | 11.6 | 4.6 | 4.6 | ||
| Relative error | 5.3% | 1.8% | 4.2% | 4.2% | ||
| Average error | 3.875% | |||||
| (d) | Ideal | 5 | 9 | 7 | 4 | |
| Actual | 4.8 | 8.6 | 6.7 | 3.8 | ||
| Presumptive | 4.8 | 9.0 | 6.2 | 3.9 | ||
| Relative error | 0% | 4.7% | 7.5% | 2.6% | ||
| Average error | 3.7% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, Q.; Yang, Z.; Wu, X.; Peng, Y. Quantitative Analysis of Industrial Solid Waste Based on Terahertz Spectroscopy. Photonics 2022, 9, 184. https://doi.org/10.3390/photonics9030184
Wang Q, Wang Q, Yang Z, Wu X, Peng Y. Quantitative Analysis of Industrial Solid Waste Based on Terahertz Spectroscopy. Photonics. 2022; 9(3):184. https://doi.org/10.3390/photonics9030184
Chicago/Turabian StyleWang, Qingfang, Qichao Wang, Zhangfan Yang, Xu Wu, and Yan Peng. 2022. "Quantitative Analysis of Industrial Solid Waste Based on Terahertz Spectroscopy" Photonics 9, no. 3: 184. https://doi.org/10.3390/photonics9030184
APA StyleWang, Q., Wang, Q., Yang, Z., Wu, X., & Peng, Y. (2022). Quantitative Analysis of Industrial Solid Waste Based on Terahertz Spectroscopy. Photonics, 9(3), 184. https://doi.org/10.3390/photonics9030184






