Detection of Changes in Macrophage Polarization as a Result of 5-Aminolevulinic Acid Photodynamic Therapy Using Fluorescence-Lifetime Imaging Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Polarized Macrophages from Human Monocyte Culture THP-1
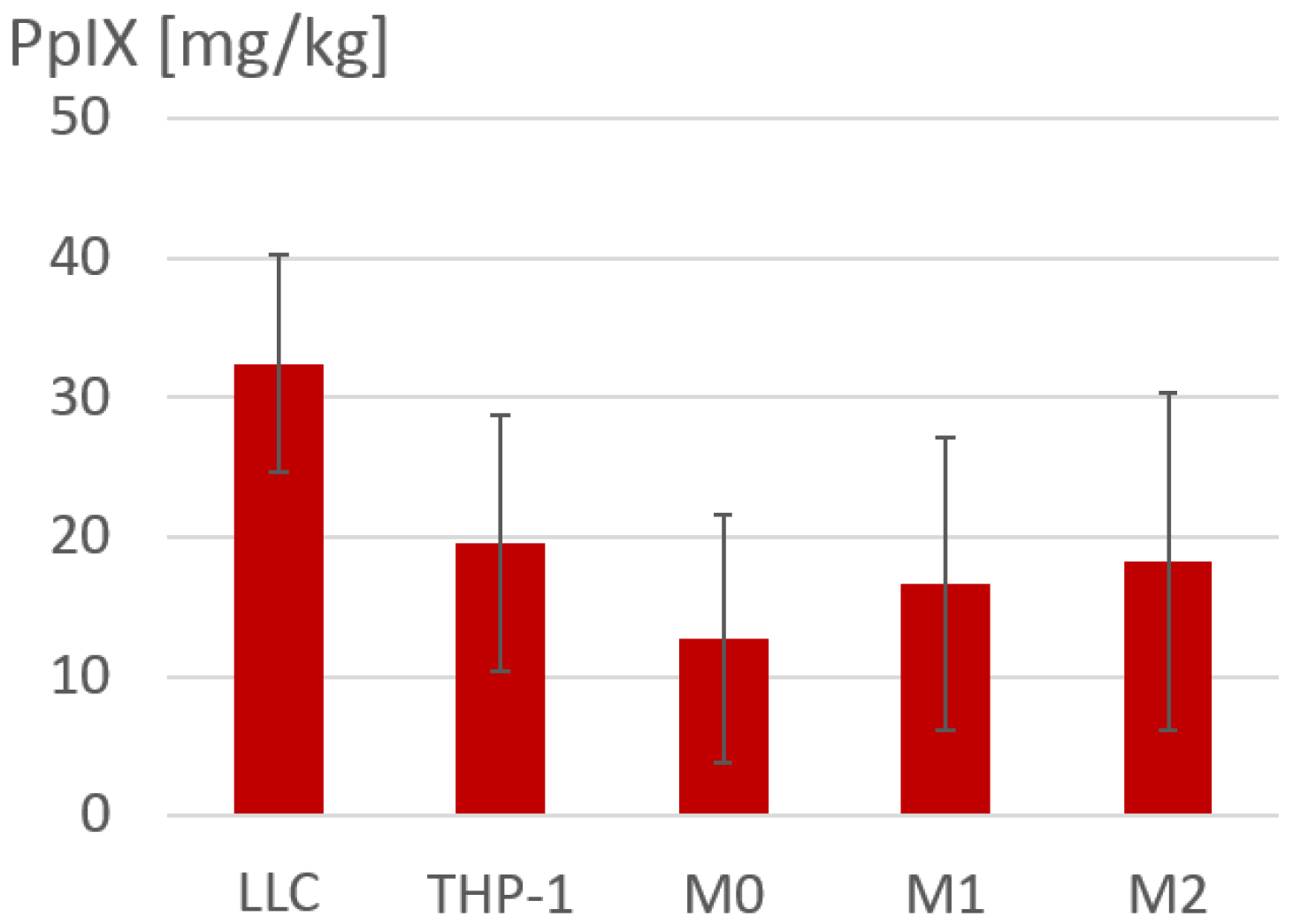
2.2. Determination of 5-ALA-Induced PpIX Accumulation in Macrophage Cultures
2.3. ALA PDT Procedure on Cells In Vitro
2.4. ALA–PDT Procedure on Tumor Model In Vivo
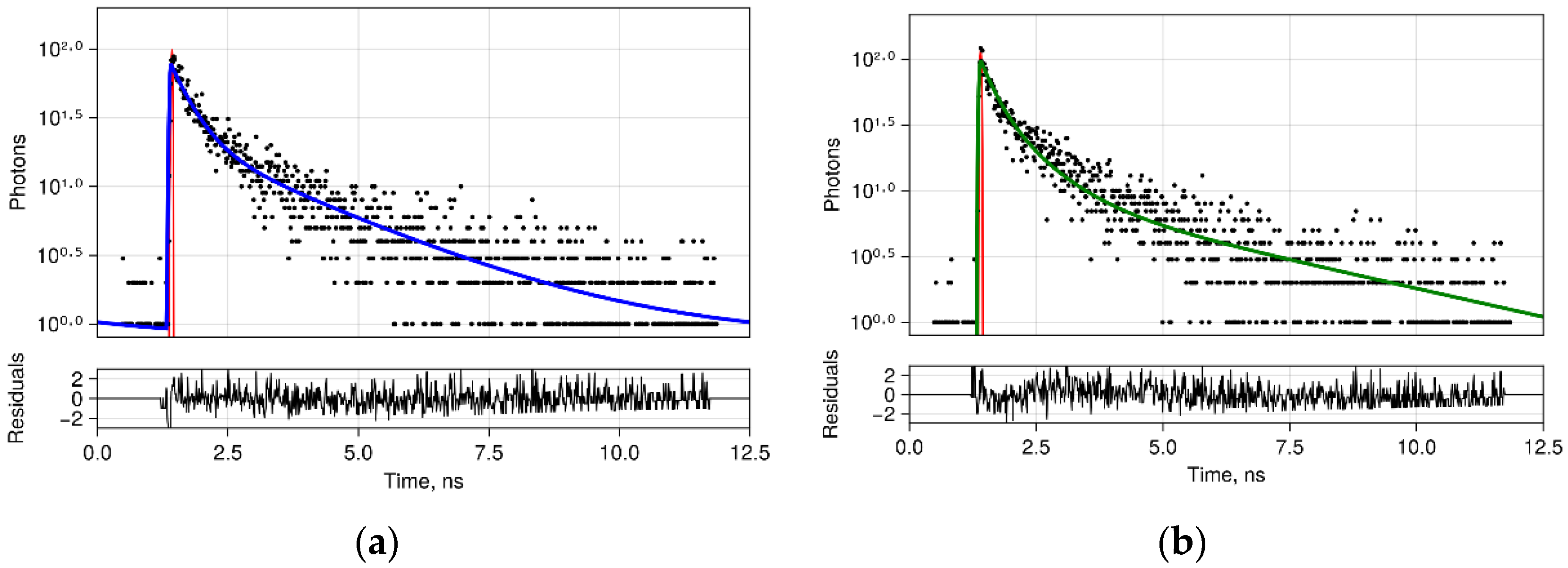
2.5. FLIM Procedure
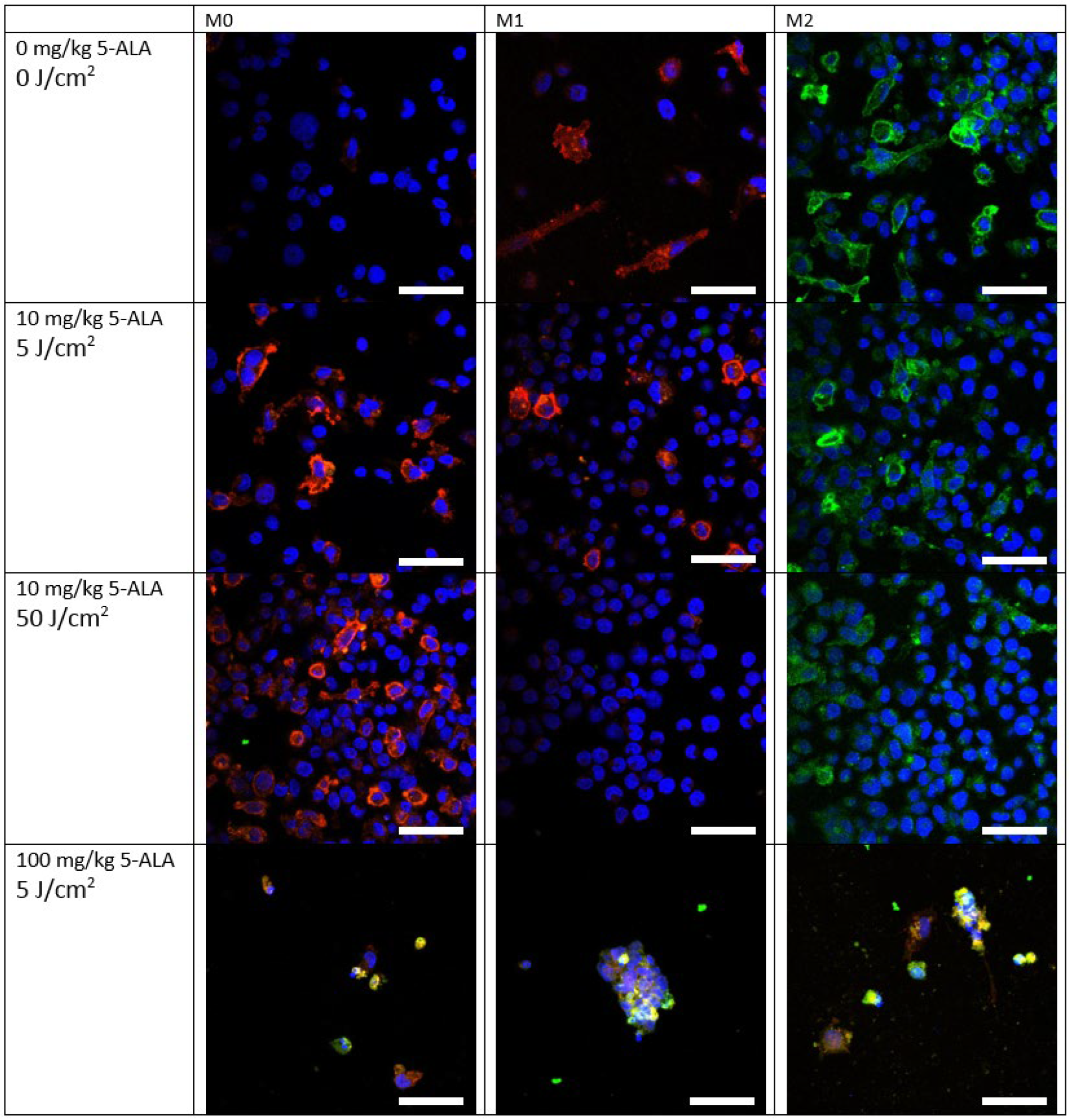
2.6. Immunofluorescent Staining
3. Results
3.1. Accumulation of 5-ALA-Induced PpIX in Polarized Macrophages
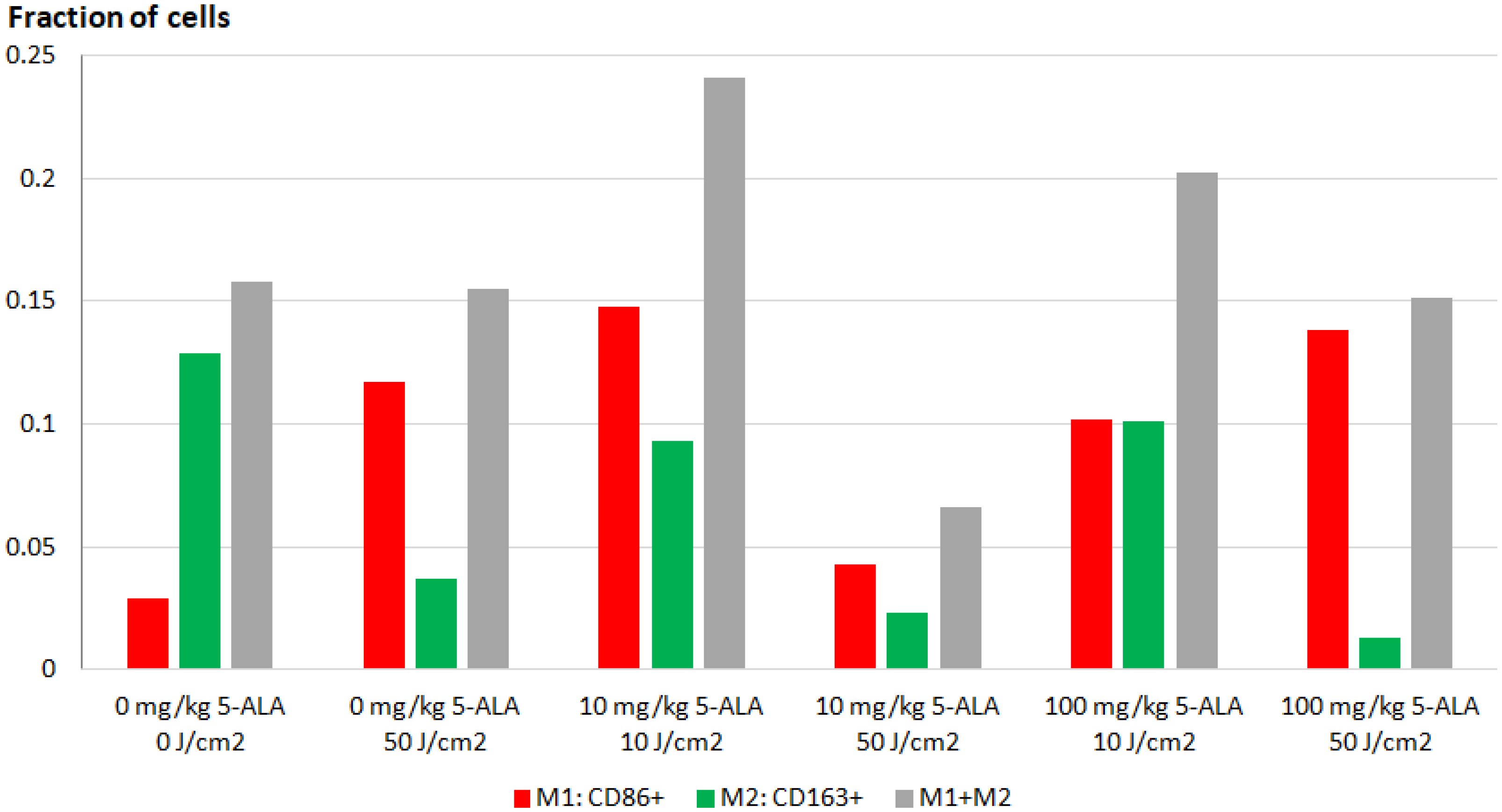
3.2. Immunofluorescence Staining of Macrophage Cultures after 5-ALA–PDT
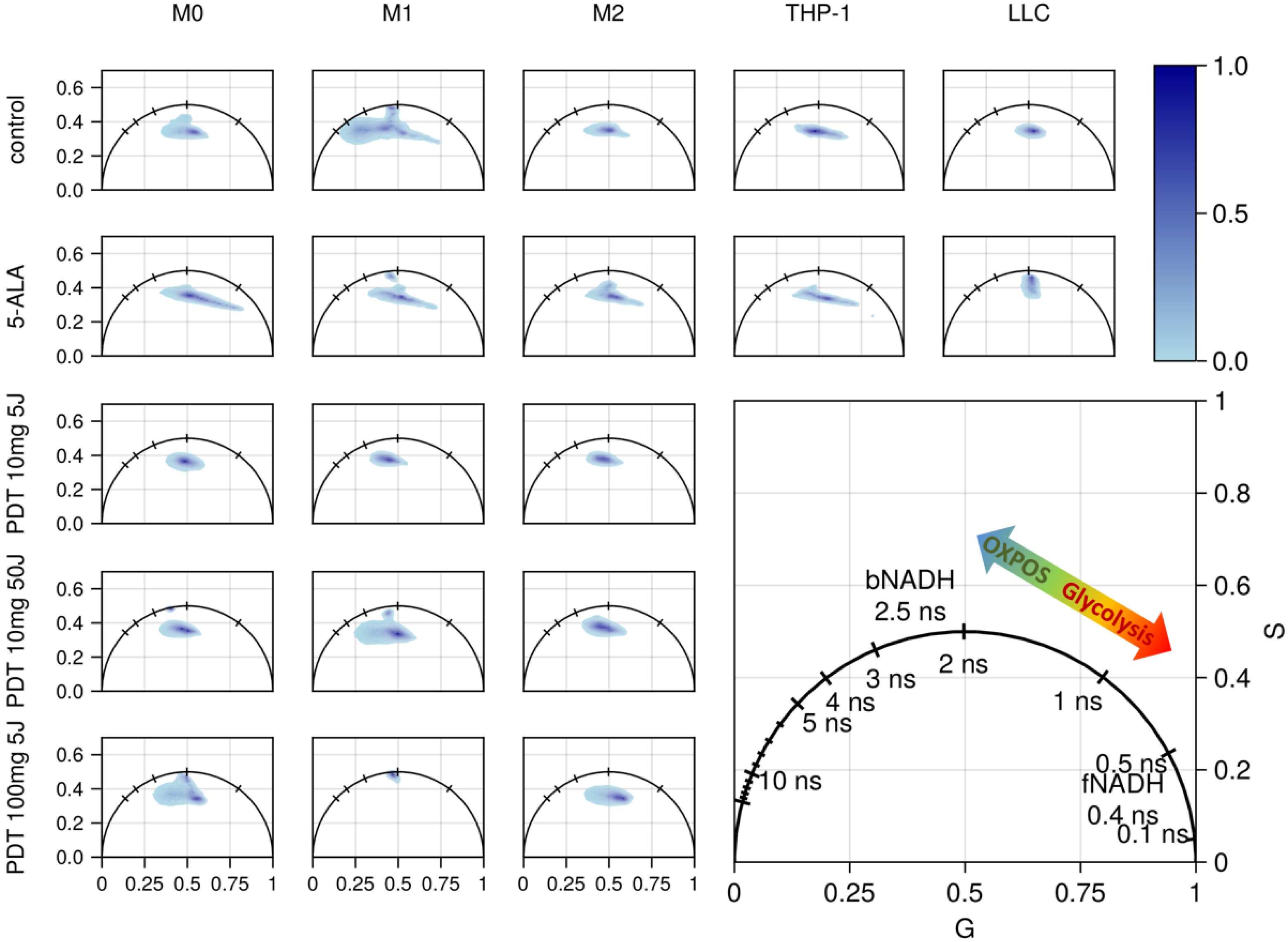
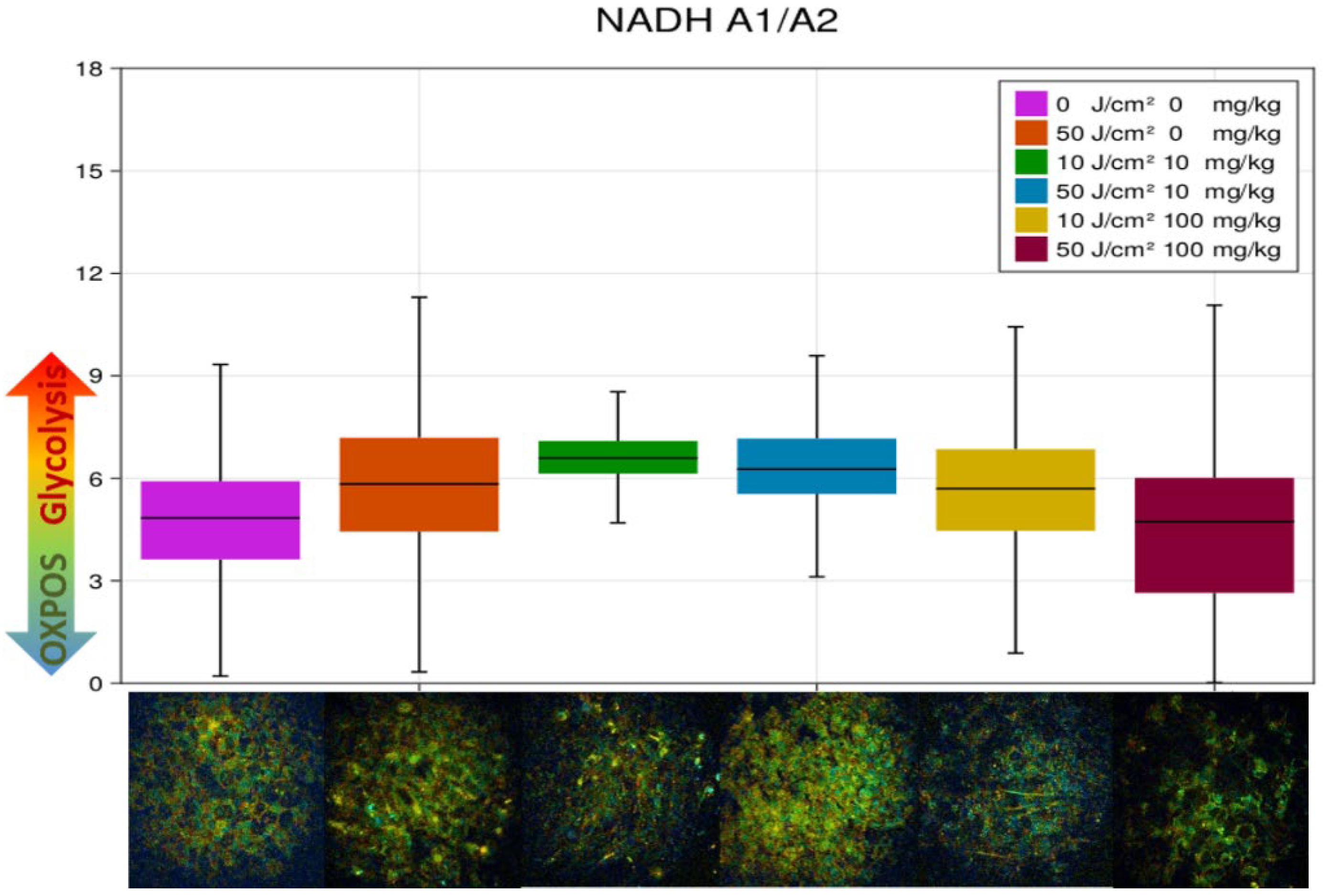
3.3. FLIM of Polarized Macrophages In Vitro: Effect of ALA–PDT
3.4. FLIM of Tumor Model In Vivo: Effect of ALA–PDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The Role of Myeloid Cells in Cancer Therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Haas, L.; Obenauf, A.C. Allies or Enemies—The Multifaceted Role of Myeloid Cells in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2746. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, J.L. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol. Med. 2018, 24, 472–489. [Google Scholar] [CrossRef] [PubMed]
- House, I.G.; Savas, P.; Lai, J.; Chen, A.X.Y.; Oliver, A.J.; Teo, Z.L.; Todd, K.L.; Henderson, M.A.; Giuffrida, L.; Petley, E.V.; et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin. Cancer Res. 2020, 26, 487–504. [Google Scholar] [CrossRef]
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018, 8, 49. [Google Scholar] [CrossRef]
- Netea-Maier, R.T.; Smit, J.W.A.; Netea, M.G. Metabolic Changes in Tumor Cells and Tumor-Associated Macrophages: A Mutual Relationship. Cancer Lett. 2018, 413, 102–109. [Google Scholar] [CrossRef]
- Rabold, K.; Netea, M.G.; Adema, G.J.; Netea-Maier, R.T. Cellular Metabolism of Tumor-Associated Macrophages—Functional Impact and Consequences. FEBS Lett. 2017, 591, 3022–3041. [Google Scholar] [CrossRef]
- Leone, R.D.; Powell, J.D. Metabolism of Immune Cells in Cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Meleppat, R.K.; Ronning, K.E.; Karlen, S.J.; Kothandath, K.K.; Burns, M.E.; Pugh, E.N., Jr.; Zawadzki, R.J. In Situ Morphologic and Spectral Characterization of Retinal Pigment Epithelium Organelles in Mice Using Multicolor Confocal Fluorescence Imaging. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Meleppat, R.K.; Ronning, K.E.; Karlen, S.J.; Burns, M.E.; Pugh, E.N.; Zawadzki, R.J. In Vivo Multimodal Retinal Imaging of Disease-Related Pigmentary Changes in Retinal Pigment Epithelium. Sci. Rep. 2021, 11, 16252. [Google Scholar] [CrossRef]
- Suhling, K.; Hirvonen, L.M.; Levitt, J.A.; Chung, P.-H.; Tregido, C.; le Marois, A.; Rusakov, D.A.; Zheng, K.; Ameer-Beg, S.; Poland, S.; et al. Fluorescence Lifetime Imaging (FLIM): Basic Concepts and Recent Applications. In Advanced Time-Correlated Single Photon Counting Applications; Becker, W., Ed.; Springer Series in Chemical Physics; Springer International Publishing: Cham, Switzerland, 2015; pp. 119–188. ISBN 978-3-319-14929-5. [Google Scholar]
- Weijer, R.; Clavier, S.; Zaal, E.A.; Pijls, M.M.E.; van Kooten, R.T.; Vermaas, K.; Leen, R.; Jongejan, A.; Moerland, P.D.; van Kampen, A.H.C.; et al. Multi-OMIC Profiling of Survival and Metabolic Signaling Networks in Cells Subjected to Photodynamic Therapy. Cell Mol. Life Sci. 2017, 74, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pouli, D.; Alonzo, C.A.; Varone, A.; Karaliota, S.; Quinn, K.P.; Münger, K.; Karalis, K.P.; Georgakoudi, I. Mapping Metabolic Changes by Noninvasive, Multiparametric, High-Resolution Imaging Using Endogenous Contrast. Sci. Adv. 2018, 4, eaap9302. [Google Scholar] [CrossRef] [PubMed]
- Sharick, J.T.; Favreau, P.F.; Gillette, A.A.; Sdao, S.M.; Merrins, M.J.; Skala, M.C. Protein-Bound NAD(P)H Lifetime Is Sensitive to Multiple Fates of Glucose Carbon. Sci. Rep. 2018, 8, 5456. [Google Scholar] [CrossRef]
- Pastore, M.N.; Studier, H.; Bonder, C.S.; Roberts, M.S. Non-Invasive Metabolic Imaging of Melanoma Progression. Exp. Dermatol. 2017, 26, 607–614. [Google Scholar] [CrossRef]
- Heaster, T.M.; Heaton, A.R.; Sondel, P.M.; Skala, M.C. Intravital Metabolic Autofluorescence Imaging Captures Macrophage Heterogeneity Across Normal and Cancerous Tissue. Front. Bioeng. Biotechnol. 2021, 9, 644648. [Google Scholar] [CrossRef]
- Miskolci, V.; Tweed, K.E.; Lasarev, M.R.; Britt, E.C.; Walsh, A.J.; Zimmerman, L.J.; McDougal, C.E.; Cronan, M.R.; Fan, J.; Sauer, J.-D.; et al. In Vivo Fluorescence Lifetime Imaging of Macrophage Intracellular Metabolism during Wound Responses in Zebrafish. eLife 2022, 11, e66080. [Google Scholar] [CrossRef]
- DeCamp, S.J.; Tsuda, V.M.K.; Ferruzzi, J.; Koehler, S.A.; Giblin, J.T.; Roblyer, D.; Zaman, M.H.; Weiss, S.T.; Kılıç, A.; De Marzio, M.; et al. Epithelial Layer Unjamming Shifts Energy Metabolism toward Glycolysis. Sci. Rep. 2020, 10, 18302. [Google Scholar] [CrossRef]
- Walsh, A.J.; Cook, R.S.; Sanders, M.E.; Aurisicchio, L.; Ciliberto, G.; Arteaga, C.L.; Skala, M.C. Quantitative Optical Imaging of Primary Tumor Organoid Metabolism Predicts Drug Response in Breast Cancer. Cancer Res. 2014, 74, 5184–5194. [Google Scholar] [CrossRef]
- Alhallak, K.; Jenkins, S.V.; Lee, D.E.; Greene, N.P.; Quinn, K.P.; Griffin, R.J.; Dings, R.P.M.; Rajaram, N. Optical Imaging of Radiation-Induced Metabolic Changes in Radiation-Sensitive and Resistant Cancer Cells. J. Biomed. Opt. 2017, 22, 60502. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One—Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which Cell Death Modality Wins the Contest for Photodynamic Therapy of Cancer? Cell Death Dis. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an INOS+/M1 Phenotype That Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef]
- Korbelik, M.; Hamblin, M.R. The Impact of Macrophage-Cancer Cell Interaction on the Efficacy of Photodynamic Therapy. Photochem. Photobiol. Sci. 2015, 14, 1403–1409. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Kiening, M.; Lange, N. A Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7974. [Google Scholar] [CrossRef]
- Hebeda, K.M.; Saarnak, A.E.; Olivo, M.; Sterenborg, H.J.; Wolbers, J.G. 5-Aminolevulinic Acid Induced Endogenous Porphyrin Fluorescence in 9L and C6 Brain Tumours and in the Normal Rat Brain. Acta Neurochir. 1998, 140, 503–512, discussion 512–513. [Google Scholar] [CrossRef]
- Kelty, C.J.; Brown, N.J.; Reed, M.W.R.; Ackroyd, R. The Use of 5-Aminolaevulinic Acid as a Photosensitiser in Photodynamic Therapy and Photodiagnosis. Photochem. Photobiol. Sci. 2002, 1, 158–168. [Google Scholar] [CrossRef]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef]
- Schneckenburger, H.; König, K.; Kunzi-Rapp, K.; Westphal-Frösch, C.; Rück, A. Time-Resolved in-Vivo Fluorescence of Photosensitizing Porphyrins. J. Photochem. Photobiol. B 1993, 21, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the Selectivity of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Formation. Curr. Med. Chem. Anticancer. Agents 2004, 4, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, J.; Zhang, H.; Fan, Z.; Zhang, L.; Shi, L.; Zhou, F.; Chen, W.R.; Wang, H.; Wang, X. Stimulation of Dendritic Cells by DAMPs in ALA-PDT Treated SCC Tumor Cells. Oncotarget 2015, 6, 44688–44702. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and Characterization of a Human Acute Monocytic Leukemia Cell Line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 Macrophages Derived from THP-1 Cells Differentially Modulate the Response of Cancer Cells to Etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive Machine Learning for (Bio)Image Analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Ranjit, S.; Malacrida, L.; Jameson, D.M.; Gratton, E. Fit-Free Analysis of Fluorescence Lifetime Imaging Data Using the Phasor Approach. Nat. Protoc. 2018, 13, 1979–2004. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, S.; Freymueller, C.; Naskar, N.; von Einem, B.; Reess, K.; Sroka, R.; Rueck, A. Bioenergetic Alterations of Metabolic Redox Coenzymes as NADH, FAD and FMN by Means of Fluorescence Lifetime Imaging Techniques. Int. J. Mol. Sci. 2021, 22, 5952. [Google Scholar] [CrossRef]
- Muldoon, J.J.; Chuang, Y.; Bagheri, N.; Leonard, J.N. Macrophages Employ Quorum Licensing to Regulate Collective Activation. Nat. Commun. 2020, 11, 878. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Brantsch, M.; Niu, C.; Moses, Z.B.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. δ-Aminolevulinic Acid-Induced Protoporphyrin IX Concentration Correlates with Histopathologic Markers of Malignancy in Human Gliomas: The Need for Quantitative Fluorescence-Guided Resection to Identify Regions of Increasing Malignancy. Neuro. Oncol. 2011, 13, 846–856. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.A.; Veltrini, V.C. Photodynamic Therapy in Oral Potentially Malignant Disorders—Critical Literature Review of Existing Protocols. Photodiagnosis Photodyn. Ther. 2017, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Scalfi-Happ, C.; Ryabova, A.; Gräfe, S.; Wiehe, A.; Peter, R.-U.; Loschenov, V.; Steiner, R.; Wittig, R. Photodynamic Activity of Temoporfin Nanoparticles Induces a Shift to the M1-like Phenotype in M2-Polarized Macrophages. J. Photochem. Photobiol. B Biol. 2018, 185, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, P.M.; Kalinina, S.; Rueck, A.; von Arnim, C.A.F.; von Einem, B. NADH Autofluorescence-A Marker on Its Way to Boost Bioenergetic Research: NADH Autofluorescence. Cytometry 2019, 95, 34–46. [Google Scholar] [CrossRef]
- Blacker, T.S.; Duchen, M.R. Investigating Mitochondrial Redox State Using NADH and NADPH Autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef]
- Blinova, K.; Carroll, S.; Bose, S.; Smirnov, A.V.; Harvey, J.J.; Knutson, J.R.; Balaban, R.S. Distribution of Mitochondrial NADH Fluorescence Lifetimes: Steady-State Kinetics of Matrix NADH Interactions. Biochemistry 2005, 44, 2585–2594. [Google Scholar] [CrossRef]
- Ranjit, S.; Malacrida, L.; Stakic, M.; Gratton, E. Determination of the Metabolic Index Using the Fluorescence Lifetime of Free and Bound NADH in the Phasor Approach. J. Biophotonics 2019, 12, e201900156. [Google Scholar] [CrossRef]
- Ciccarese, F.; Ciminale, V. Escaping Death: Mitochondrial Redox Homeostasis in Cancer Cells. Front. Oncol. 2017, 7, 117. [Google Scholar] [CrossRef]
- Stringari, C.; Edwards, R.A.; Pate, K.T.; Waterman, M.L.; Donovan, P.J.; Gratton, E. Metabolic Trajectory of Cellular Differentiation in Small Intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci. Rep. 2012, 2, 568. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef]
- Rück, A.; Dolp, F.; Hülshoff, C.; Hauser, C.; Scalfi-Happ, C. Fluorescence Lifetime Imaging in PDT. An Overview. Med. Laser Appl. 2005, 20, 125–129. [Google Scholar] [CrossRef]
- Schneckenburger, H.; Gschwend, M.H.; Sailer, R.; Rück, A.; Strauβ, W.S.L. Time-Resolved PH-Dependent Fluorescence of Hydrophilic Porphyrins in Solution and in Cultivated Cells. J. Photochem. Photobiol. B Biol. 1995, 27, 251–255. [Google Scholar] [CrossRef]
- Brancaleon, L.; Magennis, S.W.; Samuel, I.D.W.; Namdas, E.; Lesar, A.; Moseley, H. Characterization of the Photoproducts of Protoporphyrin IX Bound to Human Serum Albumin and Immunoglobulin G. Biophys. Chem. 2004, 109, 351–360. [Google Scholar] [CrossRef]
- Stringari, C.; Nourse, J.L.; Flanagan, L.A.; Gratton, E. Phasor Fluorescence Lifetime Microscopy of Free and Protein-Bound NADH Reveals Neural Stem Cell Differentiation Potential. PLoS ONE 2012, 7, e48014. [Google Scholar] [CrossRef]
- Ranjit, S.; Datta, R.; Dvornikov, A.; Gratton, E. Multicomponent Analysis of Phasor Plot in a Single Pixel to Calculate Changes of Metabolic Trajectory in Biological Systems. J. Phys. Chem. A 2019, 123, 9865–9873. [Google Scholar] [CrossRef]
- Grigalavicius, M.; Ezzatpanah, S.; Papakyriakou, A.; Raabe, T.T.H.; Yannakopoulou, K.; Theodossiou, T.A. 5-ALA Is a Potent Lactate Dehydrogenase Inhibitor but Not a Substrate: Implications for Cell Glycolysis and New Avenues in 5-ALA-Mediated Anticancer Action. Cancers 2022, 14, 4003. [Google Scholar] [CrossRef]
- Shinoda, Y.; Kato, D.; Ando, R.; Endo, H.; Takahashi, T.; Tsuneoka, Y.; Fujiwara, Y. Systematic Review and Meta-Analysis of In Vitro Anti-Human Cancer Experiments Investigating the Use of 5-Aminolevulinic Acid (5-ALA) for Photodynamic Therapy. Pharmaceuticals 2021, 14, 229. [Google Scholar] [CrossRef]
- Mathews, M.S.; Angell-Petersen, E.; Sanchez, R.; Sun, C.-H.; Vo, V.; Hirschberg, H.; Madsen, S.J. The Effects of Ultra Low Fluence Rate Single and Repetitive Photodynamic Therapy on Glioma Spheroids. Lasers Surg. Med. 2009, 41, 578–584. [Google Scholar] [CrossRef]
- Beltrán Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef]
- Ji, J.; Wang, P.; Zhou, Q.; Zhu, L.; Zhang, H.; Zhang, Y.; Zheng, Z.; Bhatta, A.K.; Zhang, G.; Wang, X. CCL8 Enhances Sensitivity of Cutaneous Squamous Cell Carcinoma to Photodynamic Therapy by Recruiting M1 Macrophages. Photodiagnosis Photodyn. Ther. 2019, 26, 235–243. [Google Scholar] [CrossRef]
- Henderson, B.W.; Busch, T.M.; Vaughan, L.A.; Frawley, N.P.; Babich, D.; Sosa, T.A.; Zollo, J.D.; Dee, A.S.; Cooper, M.T.; Bellnier, D.A.; et al. Photofrin Photodynamic Therapy Can Significantly Deplete or Preserve Oxygenation in Human Basal Cell Carcinomas during Treatment, Depending on Fluence Rate. Cancer Res. 2000, 60, 525–529. [Google Scholar]
- Kabingu, E.; Oseroff, A.R.; Wilding, G.E.; Gollnick, S.O. Enhanced Systemic Immune Reactivity to a Basal Cell Carcinoma Associated Antigen Following Photodynamic Therapy. Clin. Cancer Res. 2009, 15, 4460–4466. [Google Scholar] [CrossRef]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of Oxygen-Conserving Treatment Regimen Determines the Inflammatory Response and Outcome of Photodynamic Therapy of Tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef]
- de Vijlder, H.C.; Sterenborg, H.J.C.M.; Neumann, H.A.M.; Robinson, D.J.; de Haas, E.R.M. Light Fractionation Significantly Improves the Response of Superficial Basal Cell Carcinoma to Aminolaevulinic Acid Photodynamic Therapy: Five-Year Follow-up of a Randomized, Prospective Trial. Acta Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef]
- Shams, M.; Owczarczak, B.; Manderscheid-Kern, P.; Bellnier, D.A.; Gollnick, S.O. Development of Photodynamic Therapy Regimens That Control Primary Tumor Growth and Inhibit Secondary Disease. Cancer Immunol. Immunother. 2015, 64, 287–297. [Google Scholar] [CrossRef]
- Walsh, A.J.; Shah, A.T.; Sharick, J.T.; Skala, M.C. Fluorescence Lifetime Measurements of NAD(P)H in Live Cells and Tissue. In Advanced Time-Correlated Single Photon Counting Applications; Becker, W., Ed.; Springer Series in Chemical Physics; Springer International Publishing: Cham, Switzerland, 2015; pp. 435–456. ISBN 978-3-319-14929-5. [Google Scholar]
- Wang, H.-W.; Gukassyan, V.; Chen, C.-T.; Wei, Y.-H.; Guo, H.-W.; Yu, J.-S.; Kao, F.-J. Differentiation of Apoptosis from Necrosis by Dynamic Changes of Reduced Nicotinamide Adenine Dinucleotide Fluorescence Lifetime in Live Cells. J. Biomed. Opt. 2008, 13, 054011. [Google Scholar] [CrossRef]
- Becker, W.; Shcheslavskiy, V.; Studier, H. TCSPC FLIM with Different Optical Scanning Techniques. In Advanced Time-Correlated Single Photon Counting Applications; Becker, W., Ed.; Springer Series in Chemical Physics; Springer International Publishing: Cham, Switzerland, 2015; pp. 65–117. ISBN 978-3-319-14929-5. [Google Scholar]
- Sitiwin, E.; Madigan, M.C.; Gratton, E.; Cherepanoff, S.; Conway, R.M.; Whan, R.; Macmillan, A. Shedding Light on Melanins within in Situ Human Eye Melanocytes Using 2-Photon Microscopy Profiling Techniques. Sci. Rep. 2019, 9, 18585. [Google Scholar] [CrossRef]




| Spectral Range | Cellular Components | Decay Time [ns] | References |
|---|---|---|---|
| Blue 410–490 nm | NADH | free NADH—τ1 = 0.4 bound NADH—τ2 = 1.0–4.0 | [39] [14,45] |
| NAD(P)H | bound NAD(P)H—τ2 = 1.9–5.7 | [46,47,48,49,50] | |
| Green–orange 510–590 nm | FAD | bound FAD—τ1 = 0.25 free FAD—τ2 = 1.4 | [39] |
| FMN | 5.0 | [39] | |
| Red 610–670 nm | PpIX | monomer, τ1 = 12.8–17.8 aggregates, τ2 = 3.5–3.9 | [51] |
| Uro-porphyrin I | 1.7 | [52] | |
| Uroporphyrin III | τ1 = 8.4 τ2 = 16.5 | [53] | |
| PpIX’ photoproducts | 1.5–6, 2.6 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryabova, A.; Romanishkin, I.; Skobeltsin, A.; Markova, I.; Pominova, D.; Linkov, K.; Loschenov, V. Detection of Changes in Macrophage Polarization as a Result of 5-Aminolevulinic Acid Photodynamic Therapy Using Fluorescence-Lifetime Imaging Microscopy. Photonics 2022, 9, 961. https://doi.org/10.3390/photonics9120961
Ryabova A, Romanishkin I, Skobeltsin A, Markova I, Pominova D, Linkov K, Loschenov V. Detection of Changes in Macrophage Polarization as a Result of 5-Aminolevulinic Acid Photodynamic Therapy Using Fluorescence-Lifetime Imaging Microscopy. Photonics. 2022; 9(12):961. https://doi.org/10.3390/photonics9120961
Chicago/Turabian StyleRyabova, Anastasia, Igor Romanishkin, Alexey Skobeltsin, Inessa Markova, Daria Pominova, Kirill Linkov, and Victor Loschenov. 2022. "Detection of Changes in Macrophage Polarization as a Result of 5-Aminolevulinic Acid Photodynamic Therapy Using Fluorescence-Lifetime Imaging Microscopy" Photonics 9, no. 12: 961. https://doi.org/10.3390/photonics9120961
APA StyleRyabova, A., Romanishkin, I., Skobeltsin, A., Markova, I., Pominova, D., Linkov, K., & Loschenov, V. (2022). Detection of Changes in Macrophage Polarization as a Result of 5-Aminolevulinic Acid Photodynamic Therapy Using Fluorescence-Lifetime Imaging Microscopy. Photonics, 9(12), 961. https://doi.org/10.3390/photonics9120961







