Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation
Abstract
1. Introduction
2. Experiments and Methodology
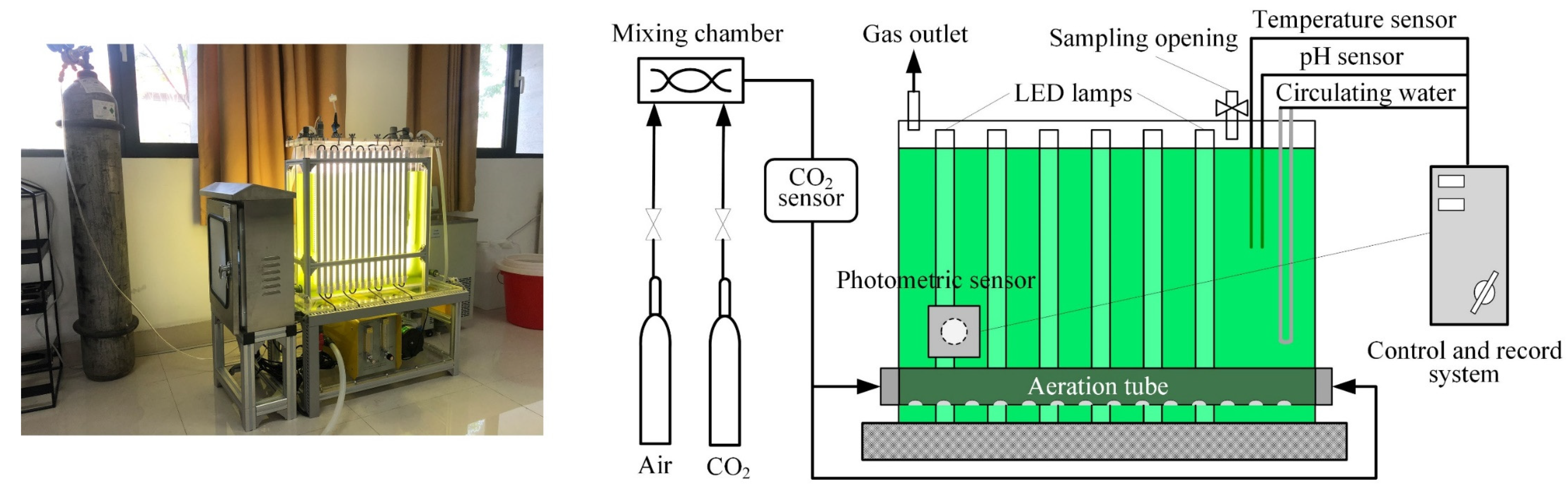
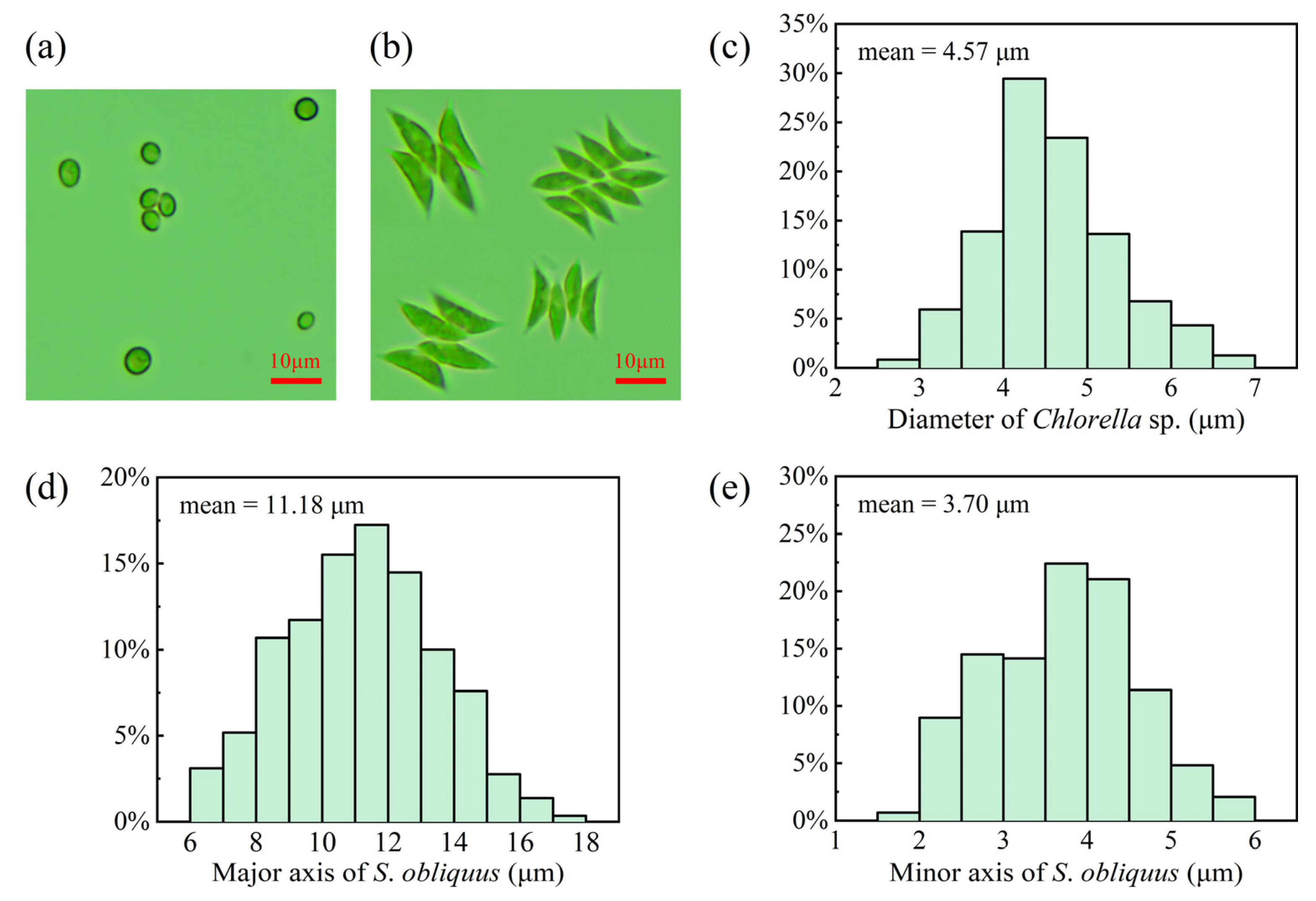
2.1. Cultivation and Sample Preparation
2.2. Radiation Characteristics Measurements
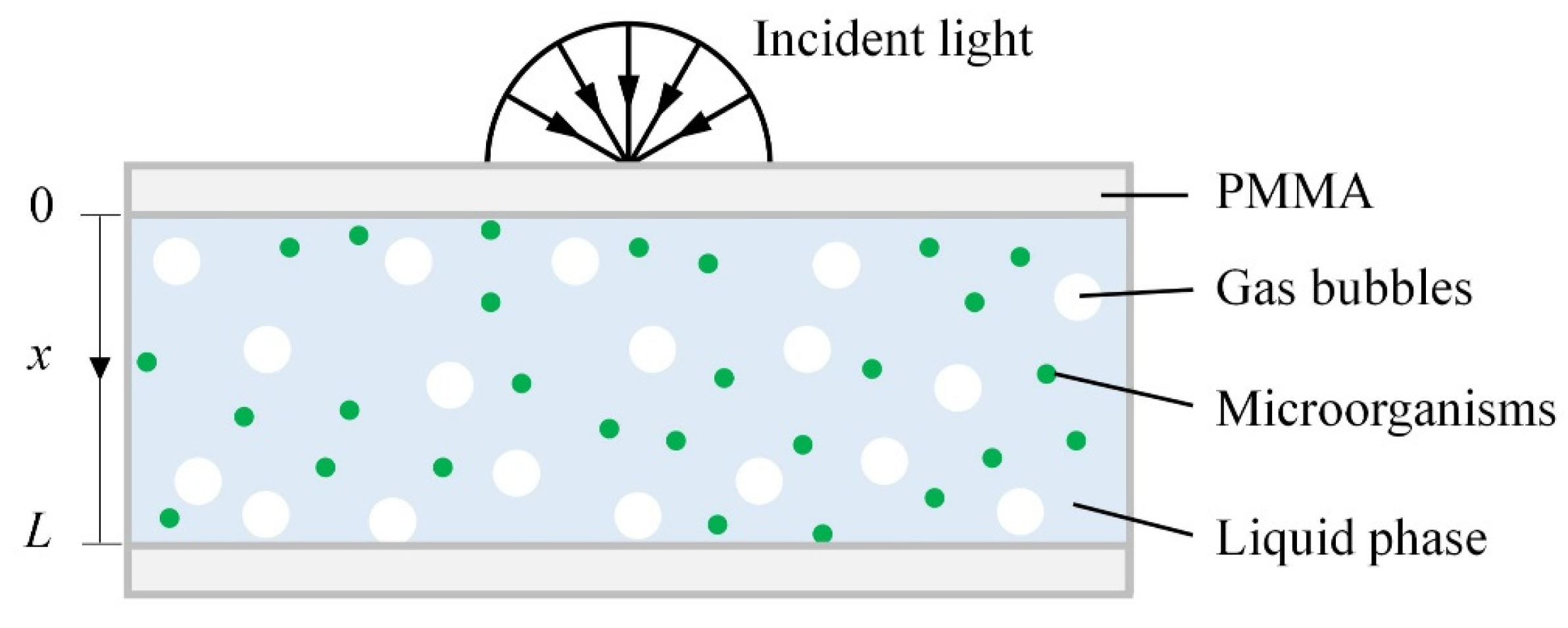
2.3. Radiation Transfer Modeling of the PBR
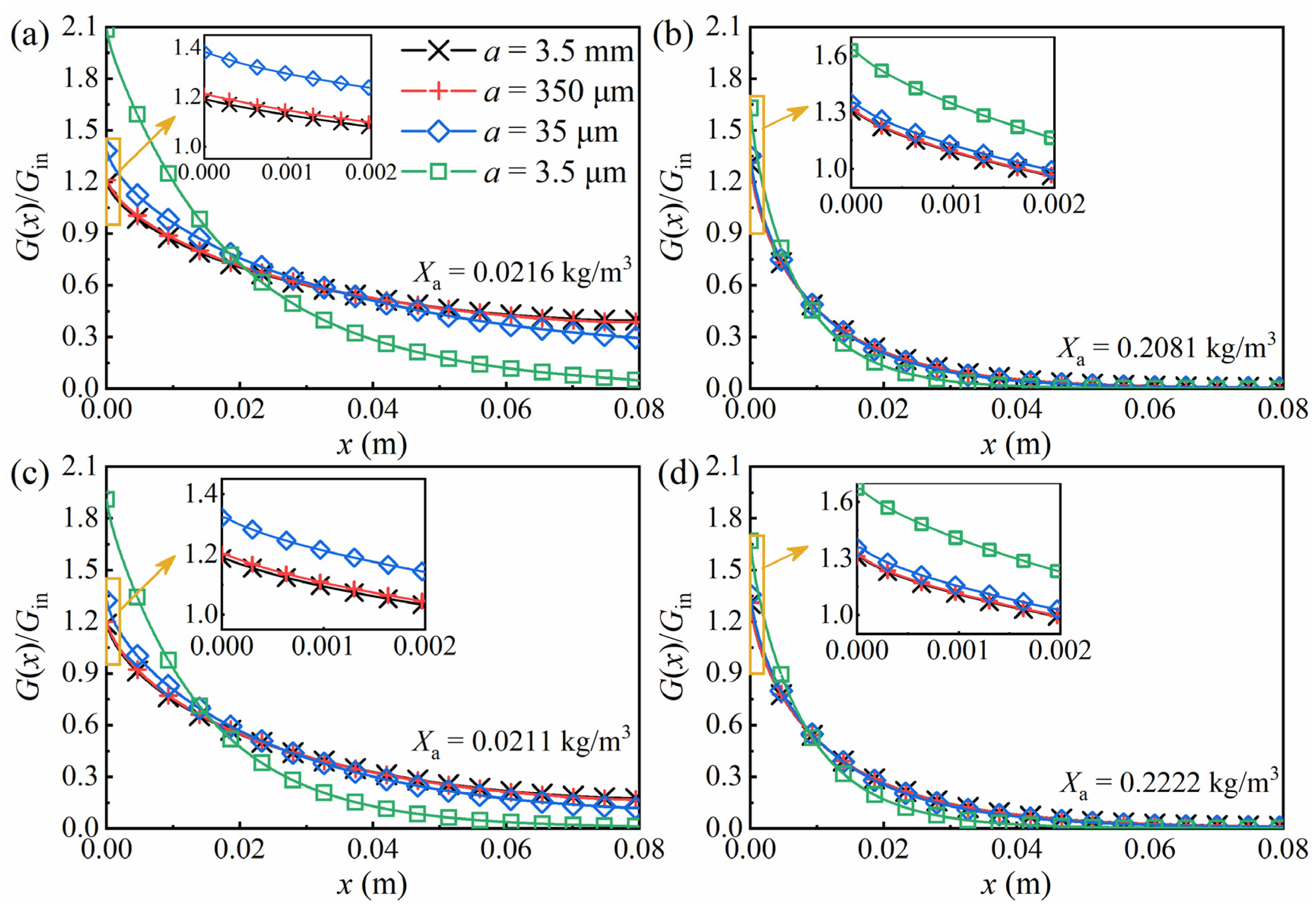
3. Results and Discussion
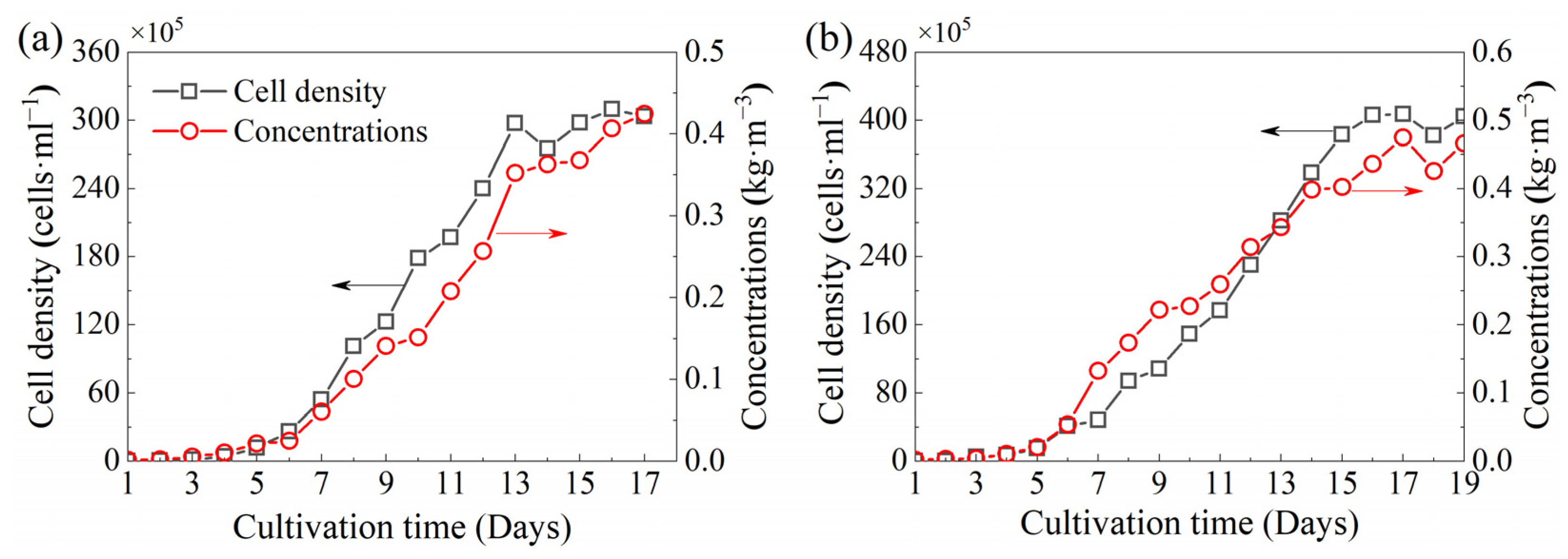
3.1. Cell Growth
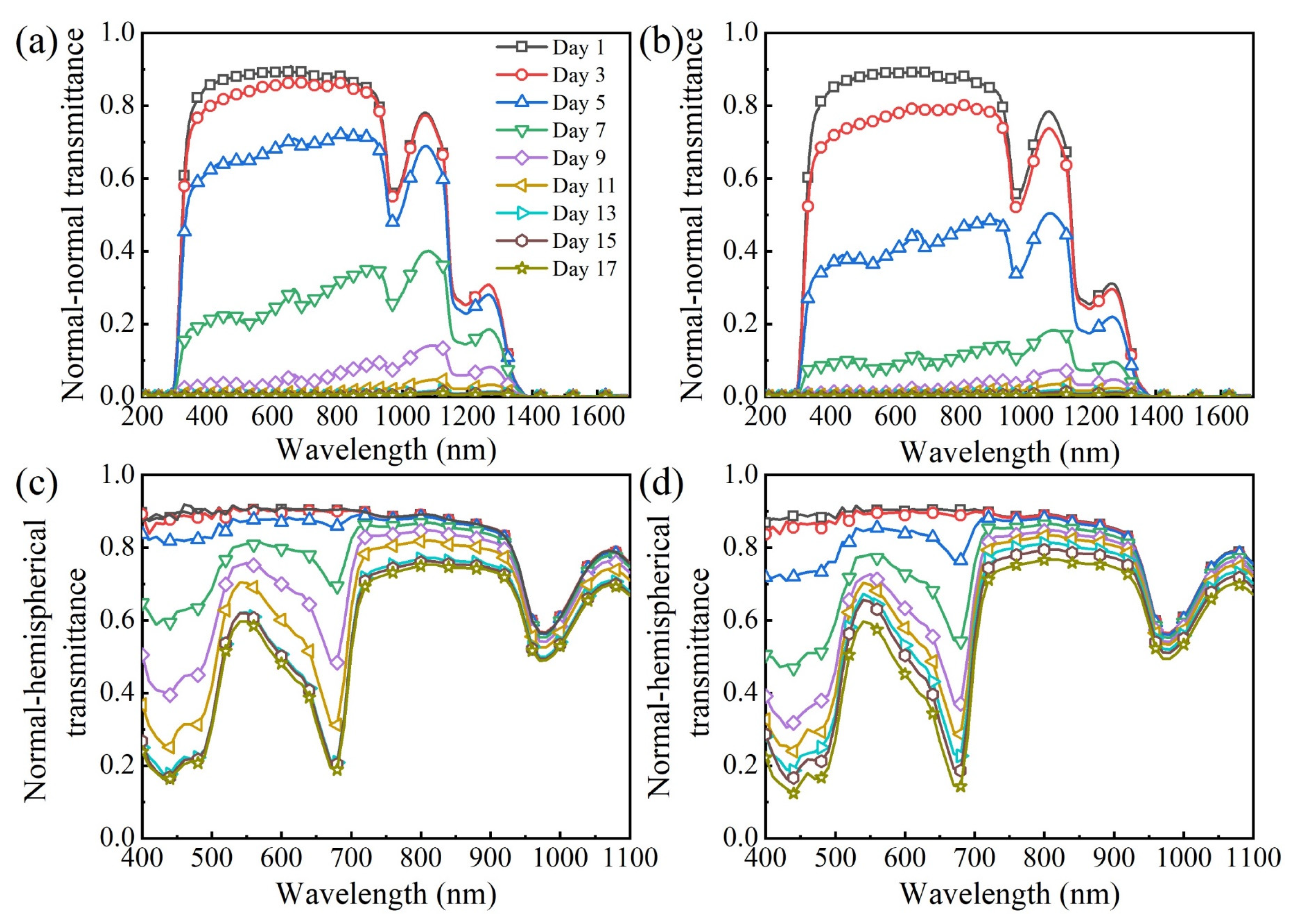
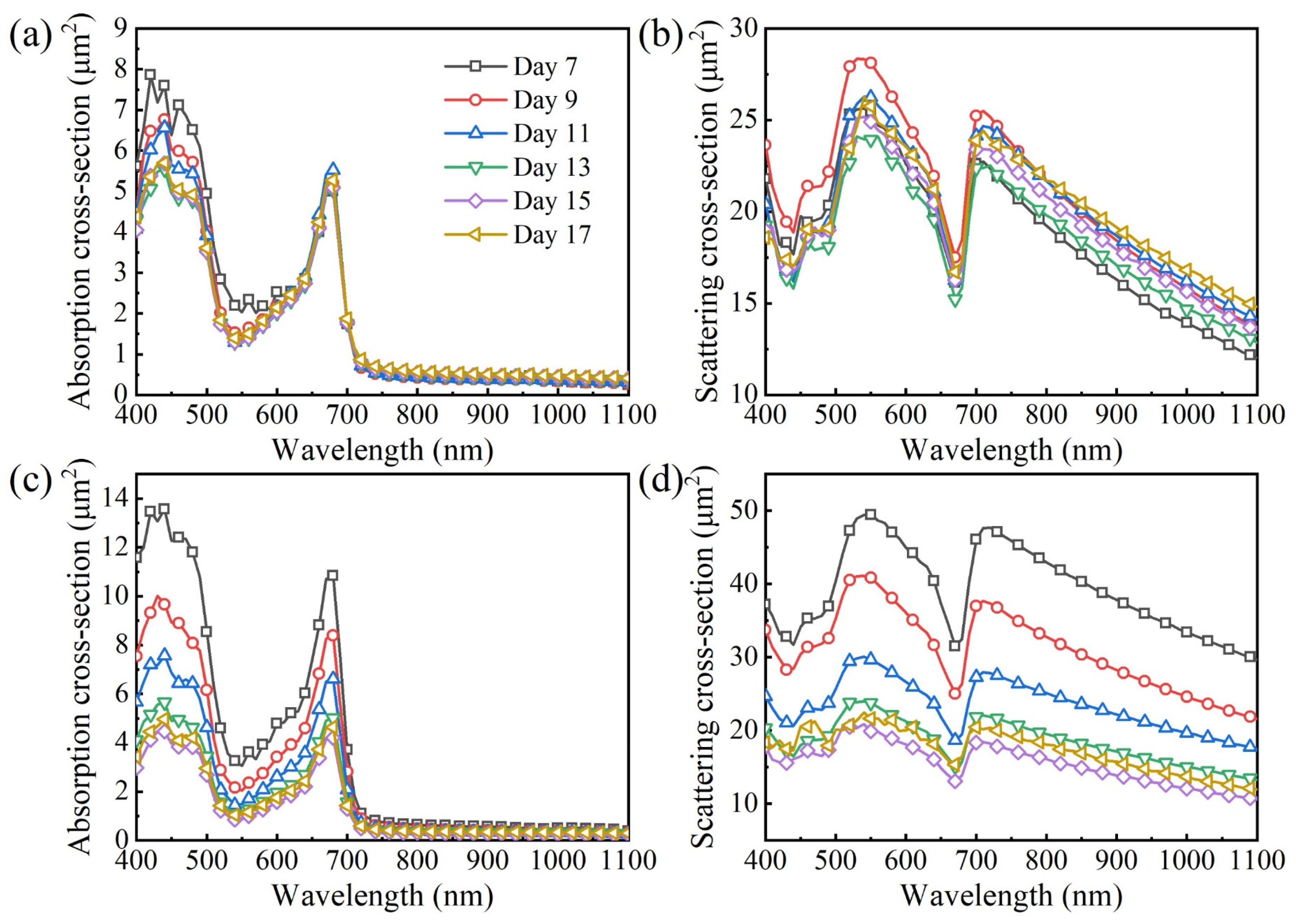
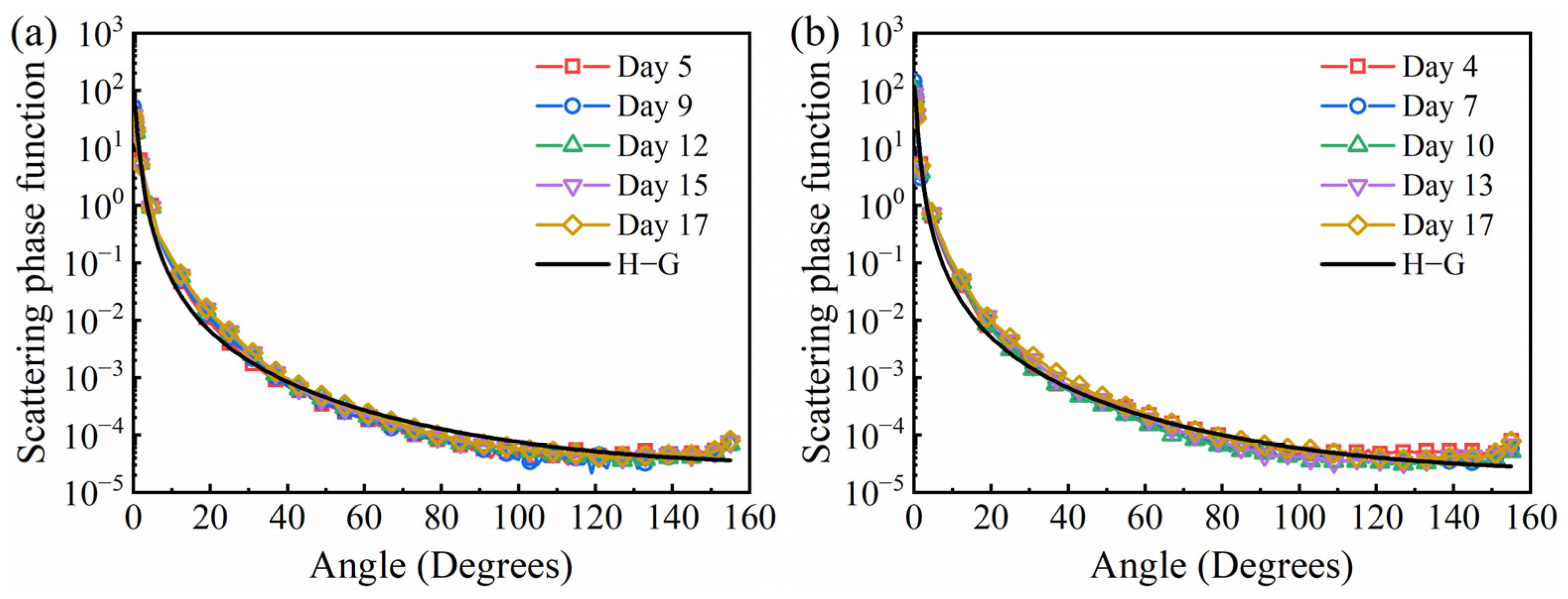
3.2. Time-Dependent Radiation Characteristics of Microalgae
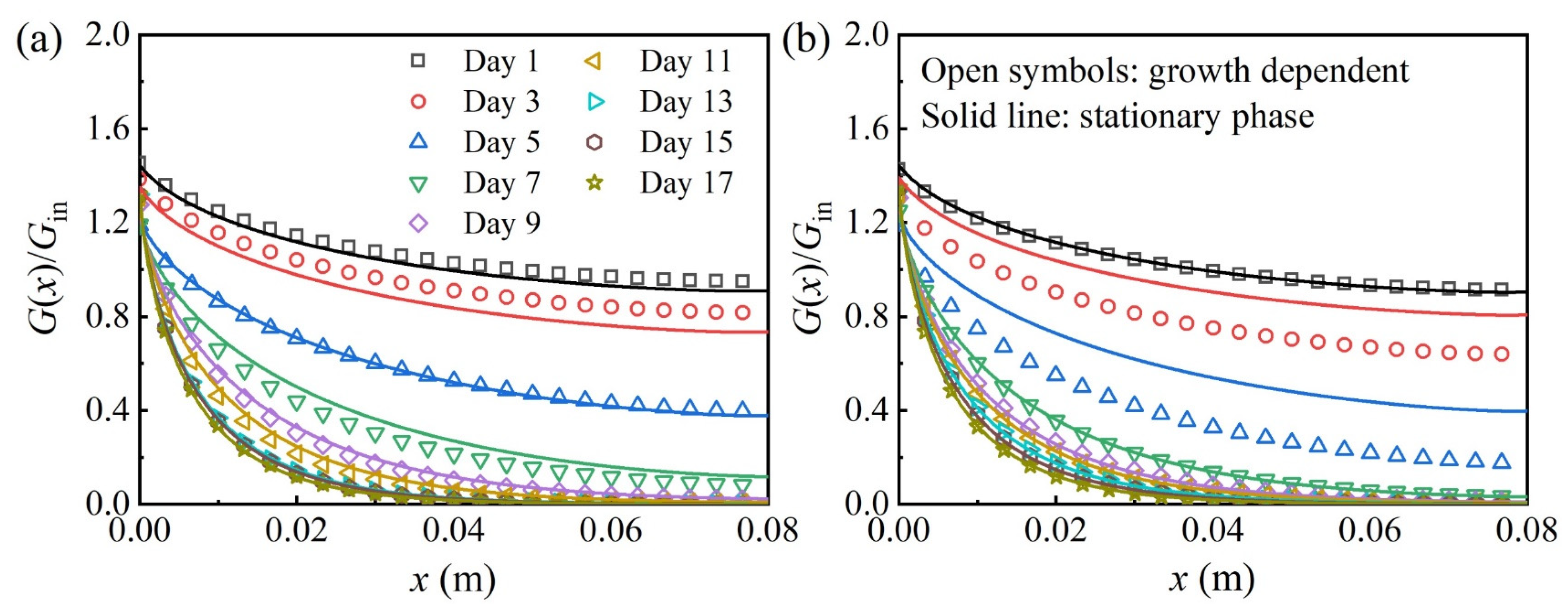
3.3. Time-Dependent Radiation Transfer in the PBR
3.4. Bubble Volume Fraction and Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreira, D.; Pires, J.C.M. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef]
- Brilman, W.; Alba, L.G.; Veneman, R. Capturing atmospheric CO2 using supported amine sorbents for microalgae cultivation. Biomass Bioenergy 2013, 53, 39–47. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Du, Z.; Hu, B.; Ma, X.; Cheng, Y.; Liu, Y.; Lin, X.; Wan, Y.; Lei, H.; Chen, P.; Ruan, R. Catalytic pyrolysis of microalgae and their three major components: Carbohydrates, proteins, and lipids. Bioresour. Technol. 2013, 130, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Tourlouki, K.; Tsavatopoulou, V.; Alexandropoulos, D.; Manariotis, I.D.; Mazzucato, S. A novel microalgae harvesting method using laser micromachined glass fiber reinforced polymers. Photonics 2020, 7, 42. [Google Scholar] [CrossRef]
- Legrand, J.; Artu, A.; Pruvost, J. A review on photobioreactor design and modelling for microalgae production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Powtongsook, S.; Pavasant, P. Enhanced productivity of Chaetoceros calcitrans in airlift photobioreactors. Bioresour. Technol. 2007, 98, 2123–2130. [Google Scholar] [CrossRef]
- Benemann, J.R. Hydrogen production by microalgae. J. Appl. Phycol. 2000, 12, 291–300. [Google Scholar] [CrossRef]
- Cheng, J.; Song, Y.; Guo, W.; Miao, Y.; Chen, S.; Zhou, J. Developing microporous fibrous-diaphragm aerator to decrease bubble generation diameter for improving microalgal growth with CO2 fixation in a raceway pond. Bioresour. Technol. 2019, 276, 28–34. [Google Scholar] [CrossRef]
- Le Gouic, B.; Marec, H.; Pruvost, J.; Cornet, J.F. Investigation of growth limitation by CO2 mass transfer and inorganic carbon source for the microalga Chlorella vulgaris in a dedicated photobioreactor. Chem. Eng. Sci. 2021, 233, 116388. [Google Scholar] [CrossRef]
- Guo, W.B.; Cheng, J.; Liu, S.Z.; Feng, L.C.; Su, Y.N.; Li, Y.G. A novel porous nickel-foam filled CO2 absorptive photobioreactor system to promote CO2 conversion by microalgal biomass. Sci. Total Environ. 2020, 713, 136593. [Google Scholar] [CrossRef] [PubMed]
- Pruvost, J.; Legrand, J.; Legentilhomme, P.; Muller-Feuga, A. Simulation of microalgae growth in limiting light conditions: Flow effect. AICHE J. 2002, 48, 1109–1120. [Google Scholar] [CrossRef]
- Fernandez, F.G.A.; Camacho, F.G.; Perez, J.A.S.; Sevilla, J.M.F.; Grima, E.M. A model for light distribution and average solar irradiance inside outdoor tubular photobioreactors for the microalgal mass culture. Biotechnol. Bioeng. 1997, 55, 701–714. [Google Scholar] [CrossRef]
- Cornet, J.F.; Dussap, C.G.; Dubertret, G. A structured model for simulation of cultures of the cyanobacterium Spirulina platensis in photobioreactors: I. Coupling between light transfer and growth kinetics. Biotechnol. Bioeng. 1992, 40, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Cornet, J.F.; Dussap, C.G.; Gros, J.B.; Binois, C.; Lasseur, C. A simplified monodimensional approach for modeling coupling between radiant light transfer and growth kinetics in photobioreactors. Chem. Eng. Sci. 1995, 50, 1489–1500. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.F.; Goetz, V.; Legrand, J. Modeling dynamic functioning of rectangular photobioreactors in solar conditions. AICHE J. 2011, 57, 1947–1960. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, T.; Yang, J.; Yao, L.; Gao, L. Evaluation of radiative transfer using the finite volume method in cylindrical photoreactors. Chem. Eng. Sci. 2011, 66, 3930–3940. [Google Scholar] [CrossRef]
- Lee, E.; Pruvost, J.; He, X.; Munipalli, R.; Pilon, L. Design tool and guidelines for outdoor photobioreactors. Chem. Eng. Sci. 2014, 106, 18–29. [Google Scholar] [CrossRef]
- Kandilian, R.; Pruvost, J.; Artu, A.; Lemasson, C.; Legrand, J.; Pilon, L. Comparison of experimentally and theoretically determined radiation characteristics of photosynthetic microorganisms. J. Quant. Spectros. Radiat. Transfer 2016, 175, 30–45. [Google Scholar] [CrossRef]
- Ohi, N.; Ishiwata, Y.; Taguchi, S. Diel patterns in light absorption and absorption efficiency factors of Isochrysis galbana (Prymnesiophyceae). J. Phycol. 2002, 38, 730–737. [Google Scholar] [CrossRef]
- DuRand, M.D.; Green, R.E.; Sosik, H.M.; Olson, R.J. Diel variations in optical properties of Micromonas pusilla (Prasinophyceae). J. Phycol. 2002, 38, 1132–1142. [Google Scholar] [CrossRef]
- Heng, R.-L.; Pilon, L. Time-dependent radiation characteristics of Nannochloropsis oculata during batch culture. J. Quant. Spectros. Radiat. Transfer 2014, 144, 154–163. [Google Scholar] [CrossRef]
- Heng, R.-L.; Lee, E.; Pilon, L. Radiation characteristics and optical properties of filamentous cyanobacterium Anabaena cylindrica. J. Opt. Soc. Am. A 2014, 31, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.M.; Ma, C.Y.; Liu, L.H. Temporal scaling of the growth dependent optical properties of microalgae. J. Quant. Spectros. Radiat. Transfer. 2018, 214, 61–70. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhao, J.M.; Liu, L.H.; Zhang, L. Growth-dependent radiative properties of Chlorella vulgaris and its influence on prediction of light fluence rate in photobioreactor. J. Appl. Phycol. 2019, 31, 235–247. [Google Scholar] [CrossRef]
- Araujo, S.D.; Garcia, V.M.T. Growth and biochemical composition of the diatom Chaetoceros cf. wighamii brightwell under different temperature, salinity and carbon dioxide levels. I. Protein, carbohydrates and lipids. Aquaculture 2005, 246, 405–412. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, S.; Guo, W.; Song, Y.; Kumar, S.; Kubar, A.A.; Su, Y.; Li, Y. Developing staggered woven mesh aerator with three variable-micropore layers in recycling water pipeline to enhance CO2 conversion for improving Arthrospira growth. Sci. Total Environ. 2021, 760, 143941. [Google Scholar] [CrossRef]
- Cheng, J.; Miao, Y.; Guo, W.; Song, Y.; Tian, J.; Zhou, J. Reduced generation time and size of carbon dioxide bubbles in a volute aerator for improving Spirulina sp. growth. Bioresour. Technol. 2018, 270, 352–358. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, S.; Ding, Y.-d.; Liao, Q.; Huang, Y.; Zhu, X. Optimizing the gas distributor based on CO2 bubble dynamic behaviors to improve microalgal biomass production in an air-lift photo-bioreactor. Bioresour. Technol. 2017, 233, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Berberoglu, H.; Yin, J.; Pilon, L. Light transfer in bubble sparged photobioreactors for H2 production and CO2 mitigation. Int. J. Hydrog. Energy 2007, 32, 2273–2285. [Google Scholar] [CrossRef]
- Berberoglu, H.; Pilon, L.; Melis, A. Radiation characteristics of Chlamydomonas reinhardtii CC125 and its truncated chlorophyll antenna transformants tla1, tlaX and tla1-CW+. Int. J. Hydrog. Energy 2008, 33, 6467–6483. [Google Scholar] [CrossRef]
- Berberoglu, H.; Gomez, P.S.; Pilon, L. Radiation characteristics of Botryococcus braunii, Chlorococcum littorale, and Chlorella sp. used for CO2 fixation and biofuel production. J. Quant. Spectros. Radiat. Transfer 2009, 110, 1879–1893. [Google Scholar] [CrossRef]
- Wheaton, Z.C.; Krishnamoorthy, G. Modeling radiative transfer in photobioreactors for algal growth. Comput. Electron. Agric. 2012, 87, 64–73. [Google Scholar] [CrossRef]
- McHardy, C.; Luzi, G.; Lindenberger, C.; Agudo, J.R.; Delgado, A.; Rauh, C. Numerical analysis of the effects of air on light distribution in a bubble column photobioreactor. Algal Res. 2018, 31, 311–325. [Google Scholar] [CrossRef]
- Luzi, G.; McHardy, C.; Lindenberger, C.; Rauh, C.; Delgado, A. Comparison between different strategies for the realization of flashing-light effects—Pneumatic mixing and flashing illumination. Algal Res. 2019, 38, 101404. [Google Scholar] [CrossRef]
- Watanabe, K.; Imase, M.; Sasaki, K.; Ohmura, N.; Saiki, H.; Tanaka, H. Composition of the sheath produced by the green alga Chlorella sorokiniana. Lett. Appl. Microbiol. 2006, 42, 538–543. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.Y.; Lee, J.Y.; Ahn, C.Y.; Oh, H.M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef]
- Ansari, F.A.; Shriwastav, A.; Gupta, S.K.; Rawat, I.; Bux, F. Exploration of Microalgae Biorefinery by Optimizing Sequential Extraction of Major Metabolites from Scenedesmus obliquus. Ind. Eng. Chem. Res. 2017, 56, 3407–3412. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Li, X.C.; Zhao, J.M.; Wang, C.C.; Liu, L.H. Improved transmission method for measuring the optical extinction coefficient of micro/nano particle suspensions. Appl. Opt. 2016, 55, 8171–8179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Zhao, J.; Liu, L. A new method for determining the optical constants of highly transparent solids. Appl. Spectrosc. 2017, 71, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Zhao, J.; Tan, J. Optical properties of sodium chloride solution within the spectral range from 300 to 2500 nm at room temperature. Appl. Spectrosc. 2015, 69, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Palik, E.D. Handbook of Optical Constants of Solids; Academic Press: San Diego, CA, USA, 1985. [Google Scholar]
- Modest, M.F. Radiative Heat Transfer, 3rd ed.; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Raithby, G.D.; Chui, E.H. A finite-volume method for predicting a radiant-heat transfer in enclosures with participating media. J. Heat Transf. -Trans. Asme 1990, 112, 415–423. [Google Scholar] [CrossRef]
- Chai, J.C. One-dimensional transient radiation heat transfer modeling using a finite-volume method. Numer. HeatTransf. Part B Fundam. 2003, 44, 187–208. [Google Scholar] [CrossRef]
- Henyey, L.G.; Greenstein, J.L. Diffuse radiation in the galaxy. Astrophys. J. 1941, 93, 70–83. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A.V. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Chen, H.; Gao, C. Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Pilon, L.; Berberoglu, H.; Kandilian, R. Radiation transfer in photobiological carbon dioxide fixation and fuel production by microalgae. J. Quant. Spectros. Radiat. Transfer 2011, 112, 2639–2660. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhao, J.M.; Liu, L.H.; Zhang, L. The growth dependent radiative properties of microalgae and light field distribution within photobioreactors. In Proceedings of the Progress in Electromagnetics Research Symposium-Fall (PIERS-FALL), Singapore, 19–22 November 2017. [Google Scholar]
- Pilon, L.; Kandilian, R. Interaction between light and photosynthetic microorganisms. In Advances in Chemical Engineering; Legrand, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 107–149. [Google Scholar]
- Kandilian, R.; Lee, E.; Pilon, L. Radiation and optical properties of Nannochloropsis oculata grown under different irradiances and spectra. Bioresour. Technol. 2013, 137, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Pico-Marco, E.; Navarro, J.L.; Bruno-Barcena, J.M. A closed loop exponential feeding law: Invariance and global stability analysis. J. Process Control 2006, 16, 395–402. [Google Scholar] [CrossRef]
- Pandey, S.S.; Kumar, D.; Tiwari, B.S. Chloroplast metabolic engineering for sustainable agriculture. In Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production; Dubey, S.K., Pandey, A., Sangwan, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 149–162. [Google Scholar]
- Stenzel, O. The Physics of Thin Film Optical Spectra; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhang, Z.M. Nano/Microscale Heat Transfer; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Gershun, A. Fresnel reflection of diffusely incident light. J. Opt. Soc. Am. 1945, 35, 162–163. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, J.; Li, X.; Zhao, J.; Liu, L. Complex refractive indices measurements of polymers in visible and near-infrared bands. Appl. Opt. 2020, 59, 2337–2344. [Google Scholar] [CrossRef]
- Sorokin, C.; Krauss, R.W. The effects of light intensity on the growth rates of green algae. Plant Physiol. 1958, 33, 109–113. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1998. [Google Scholar]



| ωeff | [G(0) − Gin]/Gin, % | |||||||
|---|---|---|---|---|---|---|---|---|
| fb = 0 | fb = 0.003 | fb = 0.03 | fb = 0.3 | fb = 0 | fb = 0.003 | fb = 0.03 | fb = 0.3 | |
| Chlorella sp. (Xa = 0.0216 kg/m3) | 0.745 | 0.751 | 0.793 | 0.930 | 18.6 | 18.9 | 21.2 | 42.2 |
| Chlorella sp. (Xa = 0.2081 kg/m3) | 0.879 | 0.879 | 0.881 | 0.896 | 30.5 | 30.6 | 31.3 | 38.7 |
| S. obliquus (Xa = 0.0211 kg/m3) | 0.835 | 0.836 | 0.848 | 0.917 | 18.4 | 18.6 | 20.2 | 36.5 |
| S. obliquus (Xa = 0.2222 kg/m3) | 0.879 | 0.879 | 0.881 | 0.899 | 30.5 | 30.6 | 31.4 | 40.2 |
| ωeff | [G(0) − Gin]/Gin, % | |||||||
|---|---|---|---|---|---|---|---|---|
| a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | |
| Chlorella sp. (Xa = 0.0216 kg/m3) | 0.751 | 0.790 | 0.919 | 0.989 | 18.9 | 21.1 | 38.0 | 108.5 |
| Chlorella sp. (Xa = 0.2081 kg/m3) | 0.879 | 0.880 | 0.894 | 0.950 | 30.6 | 31.0 | 35.0 | 62.9 |
| S. obliquus (Xa = 0.0211 kg/m3) | 0.836 | 0.847 | 0.909 | 0.982 | 18.6 | 20.0 | 32.2 | 90.9 |
| S. obliquus (Xa = 0.2222 kg/m3) | 0.879 | 0.881 | 0.896 | 0.955 | 30.6 | 31.1 | 35.8 | 66.9 |
| fb = 0.003 | fb = 0.03 | fb = 0.3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | a = 3.5 mm | a = 350 μm | a = 35 μm | a = 3.5 μm | |
| Chlorella sp. (Xa = 0.0216 kg/m3) | 1.6492 | 1.6468 | 1.6107 | 1.2296 | 1.6507 | 1.6139 | 1.2284 | 0.5322 | 1.6614 | 1.2714 | 0.5480 | 0.2325 |
| Chlorella sp. (Xa = 0.2081 kg/m3) | 0.6412 | 0.6386 | 0.6144 | 0.4729 | 0.6457 | 0.6208 | 0.4760 | 0.2307 | 0.7010 | 0.5302 | 0.2522 | 0.1207 |
| S. obliquus (Xa = 0.0211 kg/m3) | 0.5978 | 0.5978 | 0.5872 | 0.4483 | 0.6013 | 0.5905 | 0.4490 | 0.2056 | 0.6349 | 0.4789 | 0.2163 | 0.0905 |
| S. obliquus (Xa = 0.2222 kg/m3) | 0.1841 | 0.1837 | 0.1793 | 0.1504 | 0.1858 | 0.1813 | 0.1516 | 0.0850 | 0.2057 | 0.1696 | 0.0929 | 0.0441 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, T.; Lin, L.; Li, X.; Yang, J.-Y.; Zhao, J.; Liu, L. Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation. Photonics 2022, 9, 864. https://doi.org/10.3390/photonics9110864
Fei T, Lin L, Li X, Yang J-Y, Zhao J, Liu L. Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation. Photonics. 2022; 9(11):864. https://doi.org/10.3390/photonics9110864
Chicago/Turabian StyleFei, Tianhao, Li Lin, Xingcan Li, Jia-Yue Yang, Junming Zhao, and Linhua Liu. 2022. "Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation" Photonics 9, no. 11: 864. https://doi.org/10.3390/photonics9110864
APA StyleFei, T., Lin, L., Li, X., Yang, J.-Y., Zhao, J., & Liu, L. (2022). Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation. Photonics, 9(11), 864. https://doi.org/10.3390/photonics9110864





