UV-Induced Gold Nanoparticle Growth in Polystyrene Matrix with Soluble Precursor
Abstract
1. Introduction
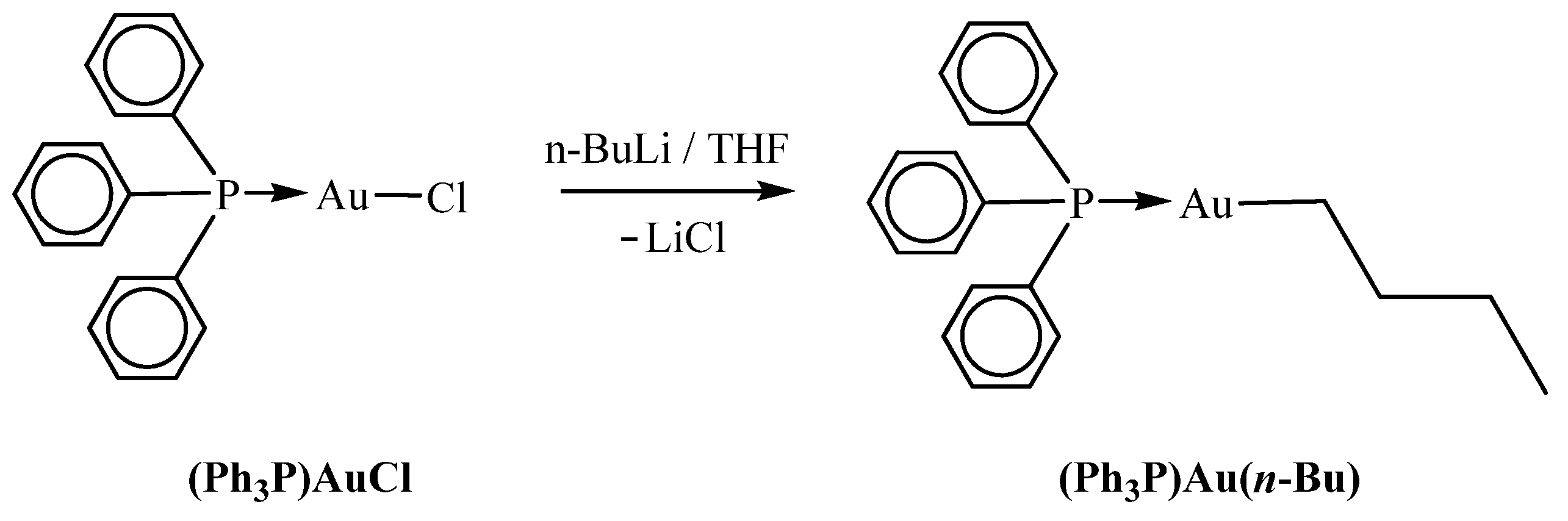
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- We demonstrate good-quality polystyrene films with up to 5 wt% of (Ph3P)Au(n-Bu) obtained by casting technique from the solution in toluene. The films are transparent in the optical region and show no signs of scattering.
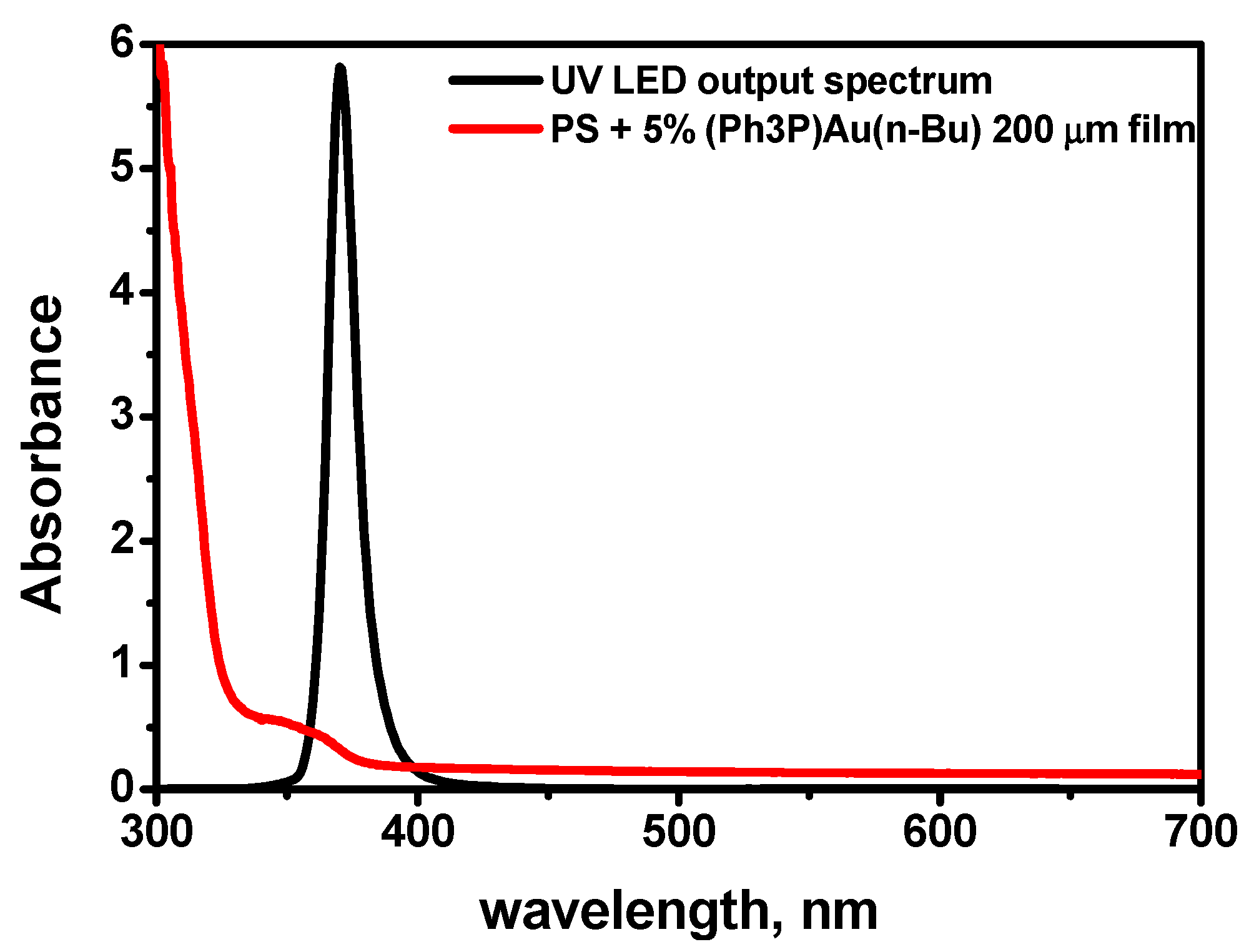
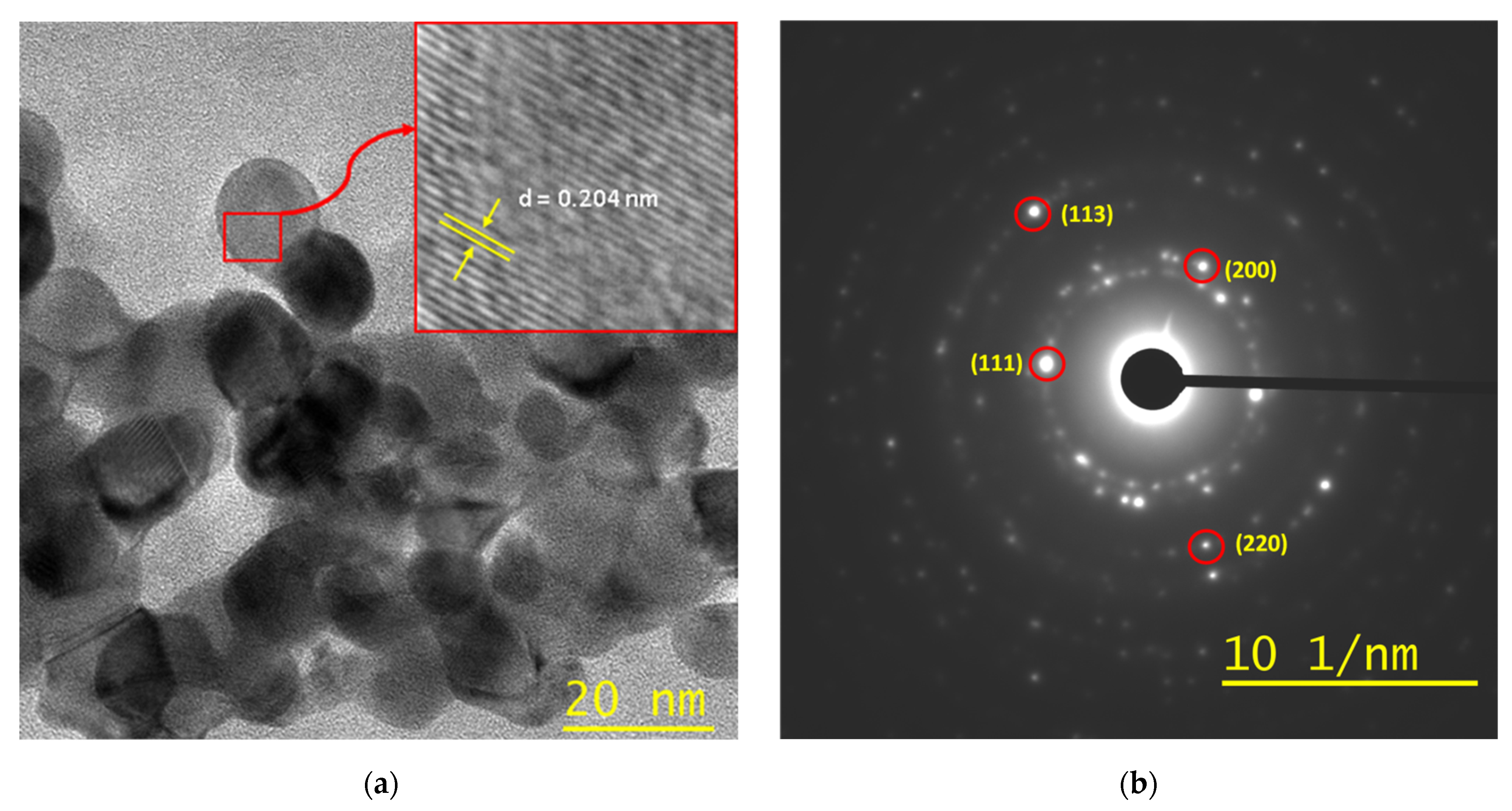
- The UV irradiation of the films at room temperature provides the formation of an extinction band in the optical region. Subsequent heating of the UV-irradiated films results in an absorption band with a maximum near 540 nm, which is characteristic of the plasmon resonance band of gold nanoparticles in polystyrene. TEM microscopy confirms the existence of gold nanoparticles within the irradiated and heated region.
- Without UV irradiation, the heating does not lead to any transformation of the film’s optical properties during the same period of treatment. This makes it possible to obtain patterns by means of UV irradiation of the samples through a mask (see Figure 5) employing the exposure–development process when the development is the heating of the exposed film at temperatures of approximately 110 °C. These structures can be used in photonics devices because plasmon nanoparticles strongly change the optical properties of the original material, which remains intact in unexposed parts.
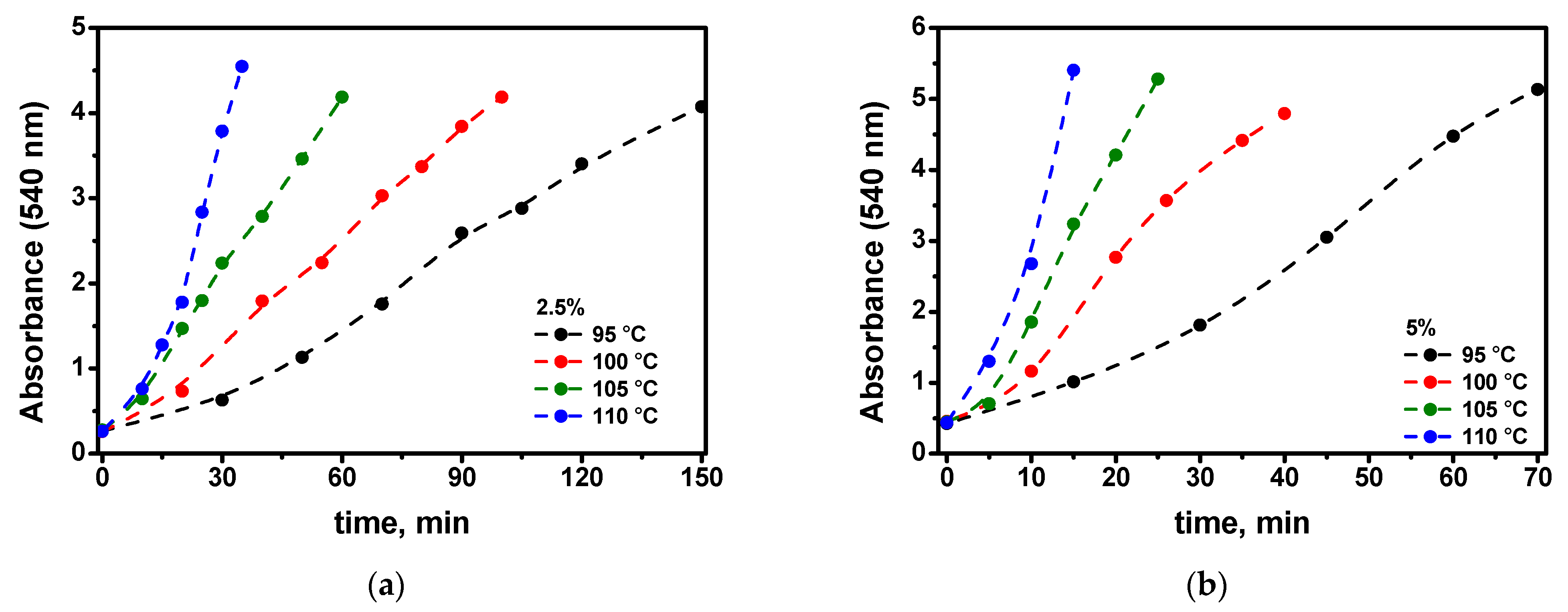
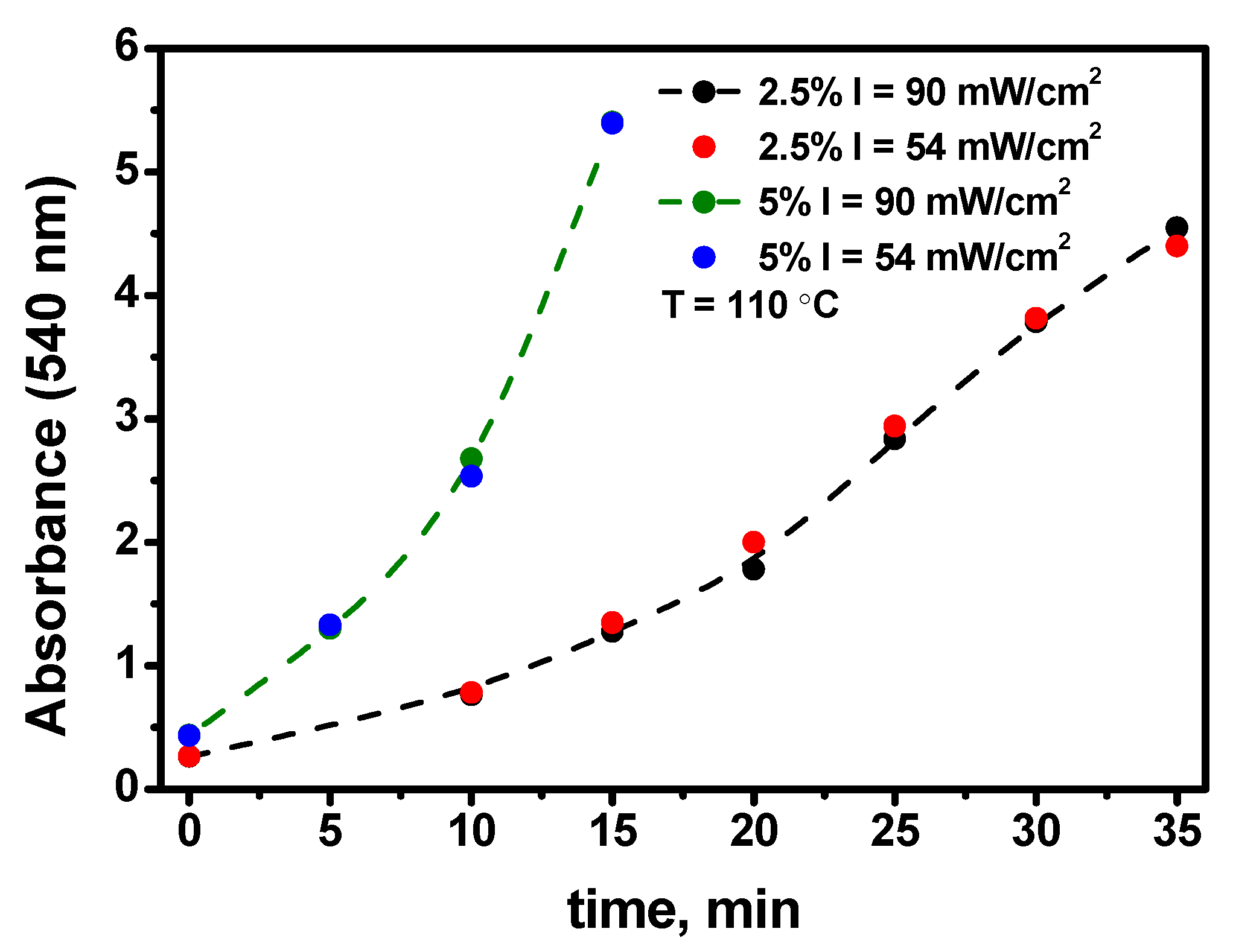
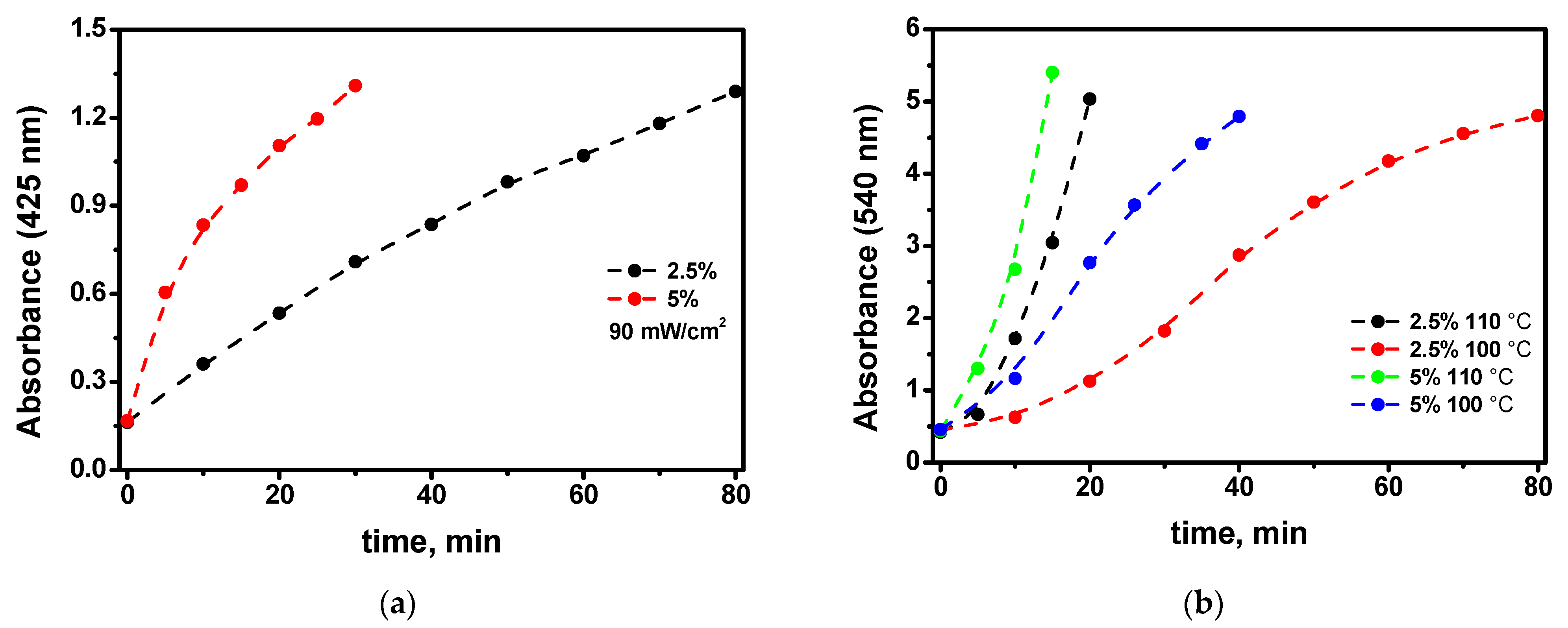
- The elucidation of the kinetics of the nanoparticle growth at different precursor percentages in the films allows us to conclude that the process of the self-catalytic deposition of the gold atoms to the gold nanoparticle directly from the precursor molecule is significant (see Figure 9). This challenges existing ideas about the mechanism of the growth of gold nanoparticles in polymer films.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Liu, D.; Khan, K.; Shao, J.; Liu, X.; Cao, R.; Ma, C.; Chong, F.; Tareen, A.K.; Hu, F.; et al. Two-dimensional metal organic frameworks for photonic applications. Opt. Mater. Express 2022, 12, 1102–1121. [Google Scholar] [CrossRef]
- Ma, N.; Horike, S. Metal−Organic Network-Forming Glasses. Chem. Rev. 2022, 122, 4163–4203. [Google Scholar]
- Castel, N.; Coudert, F.-X. Atomistic Models of Amorphous Metal−Organic Frameworks. J. Phys. Chem. C 2022, 126, 6905–6914. [Google Scholar] [CrossRef]
- Loste, J.; Lopez-Cuesta, J.-M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Prog. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Bityurin, N.; Alexandrov, A.; Afanasiev, A.; Agareva, N.; Pikulin, A.; Sapogova, N.; Soustov, L.; Salomatina, E.; Gorshkova, E.; Tsverova, N.; et al. Photoinduced nanocomposites—creation, modification, linear and nonlinear optical properties. Appl. Phys. A 2013, 112, 135–138. [Google Scholar] [CrossRef]
- Antolini, F.; Orazi, L. Quantum dots synthesis through direct laser patterning: A review. Front. Chem. 2019, 7, 252. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Elagin, V.; Afanasiev, A.; Pikulin, A.; Bityurin, N. Luminescent patterns recorded by laser irradiation of a PMMA matrix with a soluble CdS precursor. Opt. Mater. Express 2020, 10, 2114–2125. [Google Scholar] [CrossRef]
- Bityurin, N.; Ermolaev, N.; Smirnov, A.A.; Afanasiev, A.; Agareva, N.; Koryukina, T.; Bredikhin, V.; Kamenskiy, V.; Pikulin, A.; Sapogova, N. Plasmonic, excitonic and exciton-plasmonic photoinduced nanocomposites. Appl. Phys. A 2016, 122, 193. [Google Scholar] [CrossRef]
- Lippman, D.H.; Kochan, N.S.; Yang, T.; Schmidt, G.R.; Bentley, J.L.; Moore, D.T. Freeform gradient-index media: A new frontier in freeform optics. Opt. Express 2021, 29, 36997–37012. [Google Scholar] [CrossRef]
- Bityurin, N.; Kudryashov, A. Diffusion-assisted ultrashort laser pulse induced photothermal growth of core-shell nanoparticles in polymer matrix. Opt. Express 2021, 29, 37376–37398. [Google Scholar] [CrossRef]
- Antolini, F.; Pentimalli, M.; Di Luccio, T.; Terzi, R.; Schioppa, M.; Re, M.; Mirenghi, L.; Tapfer, L. Structural characterization of CdS nanoparticles grown in polystyrene matrix by thermolytic synthesis. Mater. Lett. 2005, 59, 3181–3187. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Afanasiev, A.; Ermolaev, N.; Bityurin, N. LED induced green luminescence in visually transparent PMMA films with CdS precursor. Opt. Mater. Express 2016, 6, 290–295. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Afanasiev, A.; Gusev, S.; Tatarskiy, D.; Ermolaev, N.; Bityurin, N. Exposure dependence of the UV initiated optical absorption increase in polymer films with a soluble CdS precursor and its relation to the photoinduced nanoparticle growth. Opt. Mater. Express 2018, 8, 1603–1612. [Google Scholar] [CrossRef]
- Huang, R.; Fu, Y.; Zeng, W.; Zhang, L.; Wang, D. The facile approach to fabricate gold nanoparticles and their application on the hydration and dehydrogenation reactions. J. Organomet. Chem. 2017, 851, 46–51. [Google Scholar] [CrossRef]
- Peña-López, M.; Ayán-Varela, M.; Sarandeses, L.A.; Pérez Sestelo, J. Palladium-catalyzed cross-coupling reactions of organogold(I) phosphanes with allylic electrophiles. Org. Biomol. Chem. 2012, 10, 1686–1694. [Google Scholar] [CrossRef]
- Alexandrov, A.; Smirnova, L.; Yakimovich, N.; Sapogova, N.; Soustov, L.; Kirsanov, A.; Bityurin, N. UV initiated growth of gold nanoparticles in PMMA matrix. Appl. Surf. Sci. 2005, 248, 181–184. [Google Scholar] [CrossRef]
- Carotenuto, G.; Martorana, B.; Perlo, P.B.; Nicolais, L. A universal method for the synthesis of metal and metal sulfide clusters embedded in polymer matrices. J. Mater. Chem. 2003, 13, 2927–2930. [Google Scholar] [CrossRef]
- Carotenuto, G.; Nicolais, L.; Perlo, P. Synthesis of polymer-embedded noble metal clusters by thermolysis of mercaptides dissolved in polymers. Polym. Eng. Sci. 2006, 46, 1016–1021. [Google Scholar] [CrossRef]
- Carotenuto, G.; Longo, A.; Repetto, P.; Perlo, P.; Ambrosio, L. New polymer additives for photoelectric sensing. Sens. Actuators B Chem. 2007, 125, 202–206. [Google Scholar] [CrossRef]
- Longo, A.; Pepe, G.P.; Carotenuto, G.; Ruotolo, A.; De Nicola, S.; Belotelov, V.I.; Zvezdin, A.K. Optical emission studies in Au/Ag nanoparticles. Nanotechnology 2007, 18, 365701. [Google Scholar] [CrossRef]
- Susha, A.S.; Ringler, M.; Ohlinger, A.; Paderi, M.; Li Pira, N.; Carotenuto, G.; Rogach, A.L.; Feldmann, J. Strongly Luminescent Films Fabricated by Thermolysis of Gold-Thiolate Complexes in a Polymer Matrix. Chem. Mater. 2008, 20, 6169–6175. [Google Scholar] [CrossRef]
- Carotenuto, G.; Longo, A.; Hison, C.L. Tuned linear optical properties of gold-polymer nanocomposites. J. Mater. Chem. 2009, 19, 5744–5750. [Google Scholar] [CrossRef]
- Pramanik, S.; Das, P. Metal-Based Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites: Raw Materials to Applications; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 91–121. [Google Scholar]
- Bityurin, N.; Smirnov, A.A. Model for UV induced growth of semiconductor nanoparticles in polymer films. Appl. Surf. Sci. 2019, 487, 678–691. [Google Scholar] [CrossRef]
- Pikulin, A.; Bityurin, N. Homogeneous Model for the Nanoparticle Growth in Polymer Matrices. J. Phys. Chem. C 2020, 124, 16136–16142. [Google Scholar] [CrossRef]
- Streszewski, B.; Jaworski, W.; Pacławski, K.; Csapó, E.; Dékány, I.; Fitzner, K. Gold nanoparticles formation in the aqueous system of gold(III) chloride complex ions and hydrazine sulfate—Kinetic studies. Colloids Surf. A Physicochem. Eng. Asp. 2012, 397, 63–72. [Google Scholar] [CrossRef]
- Harada, M.; Kizaki, S. Formation Mechanism of Gold Nanoparticles Synthesized by Photoreduction in Aqueous Ethanol Solutions of Polymers Using In Situ Quick Scanning X-ray Absorption Fine Structure and Small-Angle X-ray Scattering. Cryst. Growth Des. 2016, 16, 1200–1212. [Google Scholar] [CrossRef]
- Luty-Błocho, M.; Wojnicki, M.; Fitzner, K. Gold Nanoparticles Formation via Au(III) Complex Ions Reduction with L-Ascorbic Acid. Int. J. Chem. Kin. 2017, 49, 789–797. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryashov, A.; Baryshnikova, S.; Gusev, S.; Tatarskiy, D.; Lukichev, I.; Agareva, N.; Poddel’sky, A.; Bityurin, N. UV-Induced Gold Nanoparticle Growth in Polystyrene Matrix with Soluble Precursor. Photonics 2022, 9, 776. https://doi.org/10.3390/photonics9100776
Kudryashov A, Baryshnikova S, Gusev S, Tatarskiy D, Lukichev I, Agareva N, Poddel’sky A, Bityurin N. UV-Induced Gold Nanoparticle Growth in Polystyrene Matrix with Soluble Precursor. Photonics. 2022; 9(10):776. https://doi.org/10.3390/photonics9100776
Chicago/Turabian StyleKudryashov, Andrey, Svetlana Baryshnikova, Sergey Gusev, Dmitry Tatarskiy, Ivan Lukichev, Nadezhda Agareva, Andrey Poddel’sky, and Nikita Bityurin. 2022. "UV-Induced Gold Nanoparticle Growth in Polystyrene Matrix with Soluble Precursor" Photonics 9, no. 10: 776. https://doi.org/10.3390/photonics9100776
APA StyleKudryashov, A., Baryshnikova, S., Gusev, S., Tatarskiy, D., Lukichev, I., Agareva, N., Poddel’sky, A., & Bityurin, N. (2022). UV-Induced Gold Nanoparticle Growth in Polystyrene Matrix with Soluble Precursor. Photonics, 9(10), 776. https://doi.org/10.3390/photonics9100776






