Computational Design of Highly-Sensitive Graphene-Based Multilayer SPR Biosensor
Abstract
:1. Introduction
2. Theoretical Background
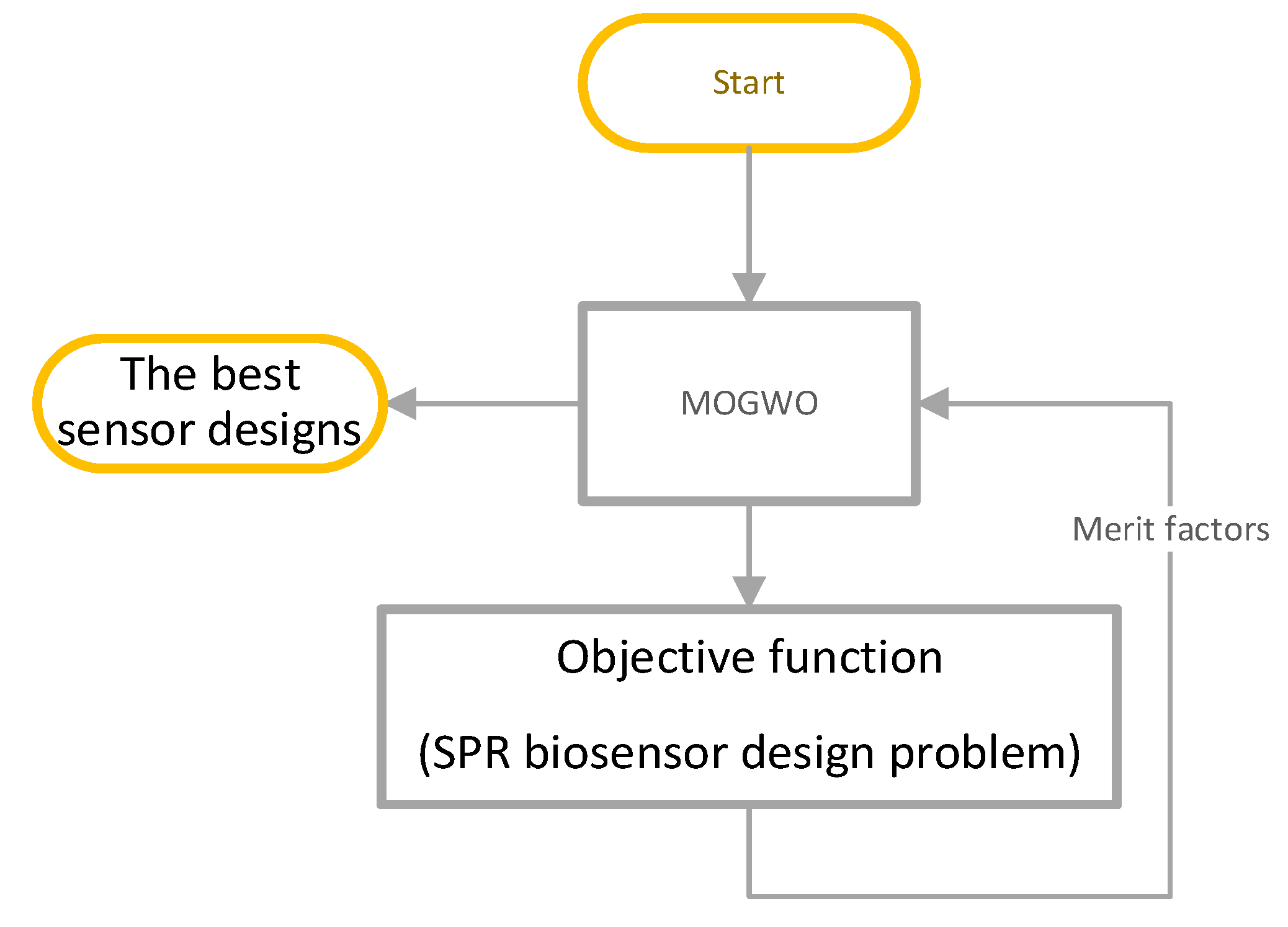
3. Multi-Objective Optimization Framework
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Jia, Y.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity improved SPR biosensor based on the MoS2/graphene–aluminum hybrid structure. J. Lightwave Technol. 2017, 35, 82–87. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ho, H.P.; Wu, S.Y.; Kong, S.K.; Wong, W.W.; Shum, P. Phase sensitive SPR sensor for wide dynamic range detection. Opt. Lett. 2011, 36, 4092–4094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface Plasmon Resonance Biosensor Based on Smart Phone Platforms. Sci. Rep. 2015, 5, 12864. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hsieh, C.-Y.; Wu, L.; Ang, L. Two-dimensional transition metal dichalcogenides mediated long range surface plasmon resonance biosensors. J. Phys. D Appl. Phys. 2018, 52, 065101. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zeng, S.; Jiang, L.; Qu, J.; Dinh, X.Q.; Qian, J.; He, S.; Coquet, P.; Yong, K.T. Two-dimensional transition metal dichalcogenide enhanced phase-sensitive plasmonic biosensors: Theoretical insight. J. Phys. Chem. C 2017, 121, 6282–6289. [Google Scholar] [CrossRef]
- Johansen, K.; Arwin, H.; Lundström, I.; Liedberg, B. Imaging surface plasmon resonance sensor based on multiple wavelengths: Sensitivity considerations. Rev. Sci. Instrum. 2000, 71, 3530–3538. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, D.; Qi, W.; Elstner, M.; Fan, C.; Fang, H. Graphene on Au(111): A Highly Conductive Material with Excellent Adsorption Properties for High-Resolution Bio/Nanodetection and Identification. ChemPhysChem 2010, 11, 585–589. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, G.B.; Gagné, M.; Rappé, A.K. pi-Stacking interactions. Alive and well in proteins. J. Biol. Chem. 1998, 273, 15458–15463. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, H.; Cort, J.R.; Buchko, G.W.; Zhang, Y.; Shao, Y.; Aksay, I.A.; Liu, J.; Lin, Y. Constraint of DNA on functionalized graphene improves its biostability and specificity. Small 2010, 6, 1205–1209. [Google Scholar] [CrossRef]
- Faramarzi, V.; Ahmadi, V.; Hwang, M.T.; Snapp, P. Highly sensitive crumpled 2D material-based plasmonic biosensors. Biomed. Opt. Express 2021, 12, 4544–4559. [Google Scholar] [CrossRef]
- Faramarzi, V.; Ahmadi, V.; Golmohamadi, F.G.; Fotouhi, B. A biosensor based on plasmonic wave excitation with diffractive grating structure. Sci. Iran. 2017, 24, 3441–3447. [Google Scholar] [CrossRef]
- Lei, Z.-L.; Guo, B. 2D material-based optical biosensor: Status and prospect. Adv. Sci. 2022, 9, 2102924. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, Y.L.; Byun, K.M. Graphene-on-silver substrates for sensitive surface plasmon resonance imaging biosensors. Opt. Express 2011, 19, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Gupta, B.D.; Jha, R. Sensitivity enhancement of a surface plasmon resonance based biomolecules sensor using graphene and silicon layers. Sens. Actuators B Chem. 2011, 160, 623–631. [Google Scholar] [CrossRef]
- Zeng, S.; Hu, S.; Xia, J.; Anderson, T.; Dinh, X.Q.; Meng, X.M.; Coquet, P.; Yong, K.T. Graphene-MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A.; Kaźmierczak, A.; Piramidowicz, R. A numerical investigation of a plasmonic sensor based on a metal-insulator-metal waveguide for simultaneous detection of biological analytes and ambient temperature. Nanomaterials 2021, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Thomas, P.A.; Kravets, V.G.; Arola, H.O.; Soikkeli, M.; Iljin, K.; Kim, G.; Kim, M.; Shin, H.S.; Andreeva, D.V.; et al. Layered material platform for surface plasmon resonance biosensing. Sci. Rep. 2019, 9, 20286. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.G.; Wu, F.; Yu, T.; Grigorenko, A.N. Metal-dielectric-graphene hybrid heterostructures with enhanced surface plasmon resonance sensitivity based on amplitude and phase measurements. Plasmonics 2022, 17, 973–987. [Google Scholar] [CrossRef]
- Bruna, M.; Borini, S. Optical constants of graphene layers in the visible range. Appl. Phys. Lett. 2009, 94, 031901. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Rashidi, K.; Mirjalili, S.M.; Taleb, H.; Fathi, D. Optimal Design of Large Mode Area Photonic Crystal Fibers Using a Multiobjective Gray Wolf Optimization Technique. J. Lightwave Technol. 2018, 36, 5626–5632. [Google Scholar] [CrossRef]
- Mirjalili, S.; Mirjalili, S.M.; Lewis, A. Grey wolf optimizer. Adv. Eng. Softw. 2014, 69, 46–61. [Google Scholar] [CrossRef]
- Maharana, P.K.; Jha, R. Chalcogenide prism and graphene multilayer based surface plasmon resonance affinity biosensor for high performance. Sens. Actuators B Chem. 2012, 169, 161–166. [Google Scholar] [CrossRef]
- Safdari, M.; Mirjalili, S.; Bianucci, P.; Zhang, X. Multi-objective optimization framework for designing photonic crystal sensors. Appl. Opt. 2018, 57, 1950. [Google Scholar] [CrossRef] [PubMed]
- Siefke, T.; Kroker, S.; Pfeiffer, K.; Puffky, O.; Dietrich, K.; Franta, D.; Ohlídal, I.; Szeghalmi, A.; Kley, E.-B.; Tünnermann, A. Materials Pushing the Application Limits of Wire Grid Polarizers further into the Deep Ultraviolet Spectral Range. Adv. Opt. Mater. 2016, 4, 1780–1786. [Google Scholar] [CrossRef]
- Marcos, L.V.R.; Larruquert, J.I.; Méndez, J.A.; Aznárez, J.A. Self-consistent optical constants of SiO2 and Ta2O5 films. Opt. Mater. Express 2016, 6, 3622–3637. [Google Scholar] [CrossRef]
- Mirjalili, S.; Saremi, S.; Mirjalili, S.M.; Coelho, L.d.S. Multi-objective grey wolf optimizer: A novel algorithm for multi-criterion optimization. Expert Syst. Appl. 2016, 47, 106–119. [Google Scholar] [CrossRef]
- Saremi, S.; Mirjalili, S.Z.; Mirjalili, S.M. Evolutionary population dynamics and grey wolf optimizer. Neural Comput. Appl. 2015, 26, 1257–1263. [Google Scholar] [CrossRef]
- Faris, H.; Aljarah, I.; Al-Betar, M.A.; Mirjalili, S. Grey wolf optimizer: A review of recent variants and applications. Neural Comput. Appl. 2018, 30, 413–435. [Google Scholar] [CrossRef]
- Sun, P.; Wang, M.; Liu, L.; Jiao, L.; Du, W.; Xia, F.; Liu, M.; Kong, W.; Dong, L.; Yun, M. Sensitivity enhancement of surface plasmon resonance biosensor based on graphene and barium titanate layers. Appl. Surf. Sci. 2019, 475, 342–347. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, S.K. Gas sensing in Kretschmann configuration utilizing bi-metallic layer of Rhodium-Silver in visible region. Sens. Actuators B Chem. 2016, 237, 969–973. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zeng, S.; Jiang, L.; Hong, L.; Xu, G.; Dinh, X.-Q.; Qian, J.; He, S.; Qu, J.; Coquet, P.; et al. Sensitivity enhancement of transition metal dichalcogenides/silicon nanostructure-based surface plasmon resonance biosensor. Sci. Rep. 2016, 6, 28190. [Google Scholar] [CrossRef]
- Gupta, G.; Kondoh, J. Tuning and sensitivity enhancement of surface plasmon resonance sensor. Sens. Actuators B Chem. 2007, 122, 381–388. [Google Scholar] [CrossRef]

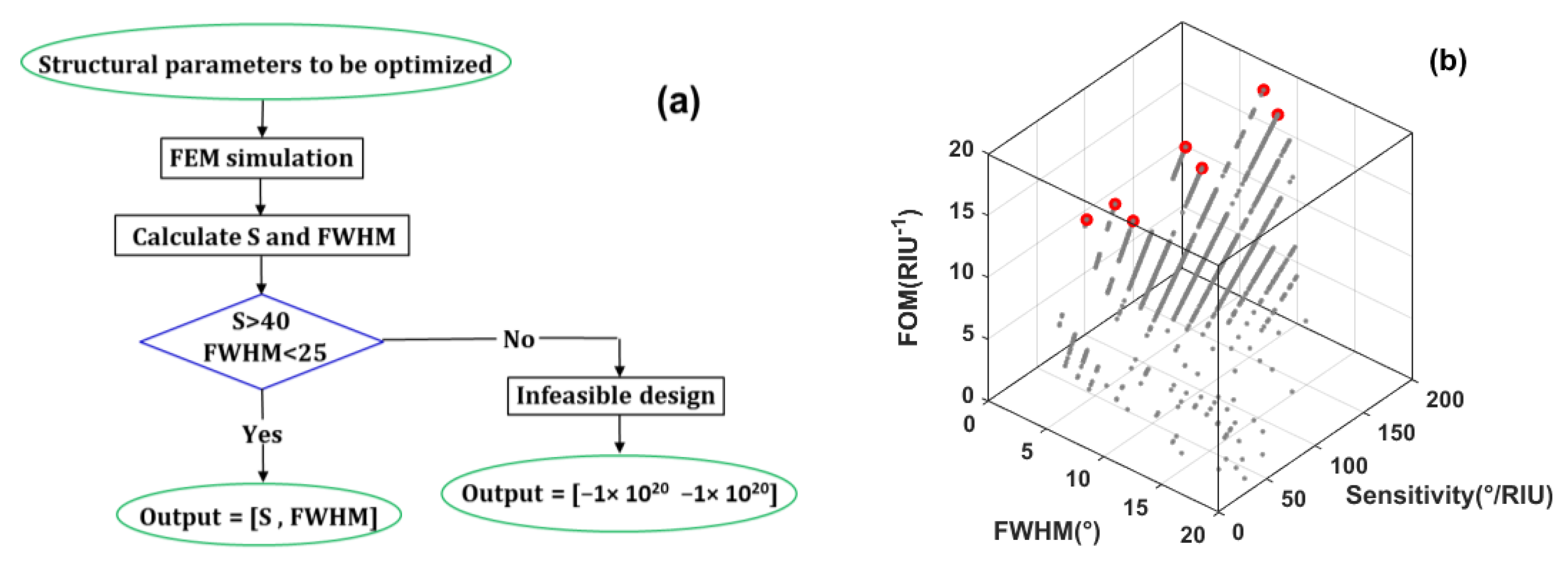
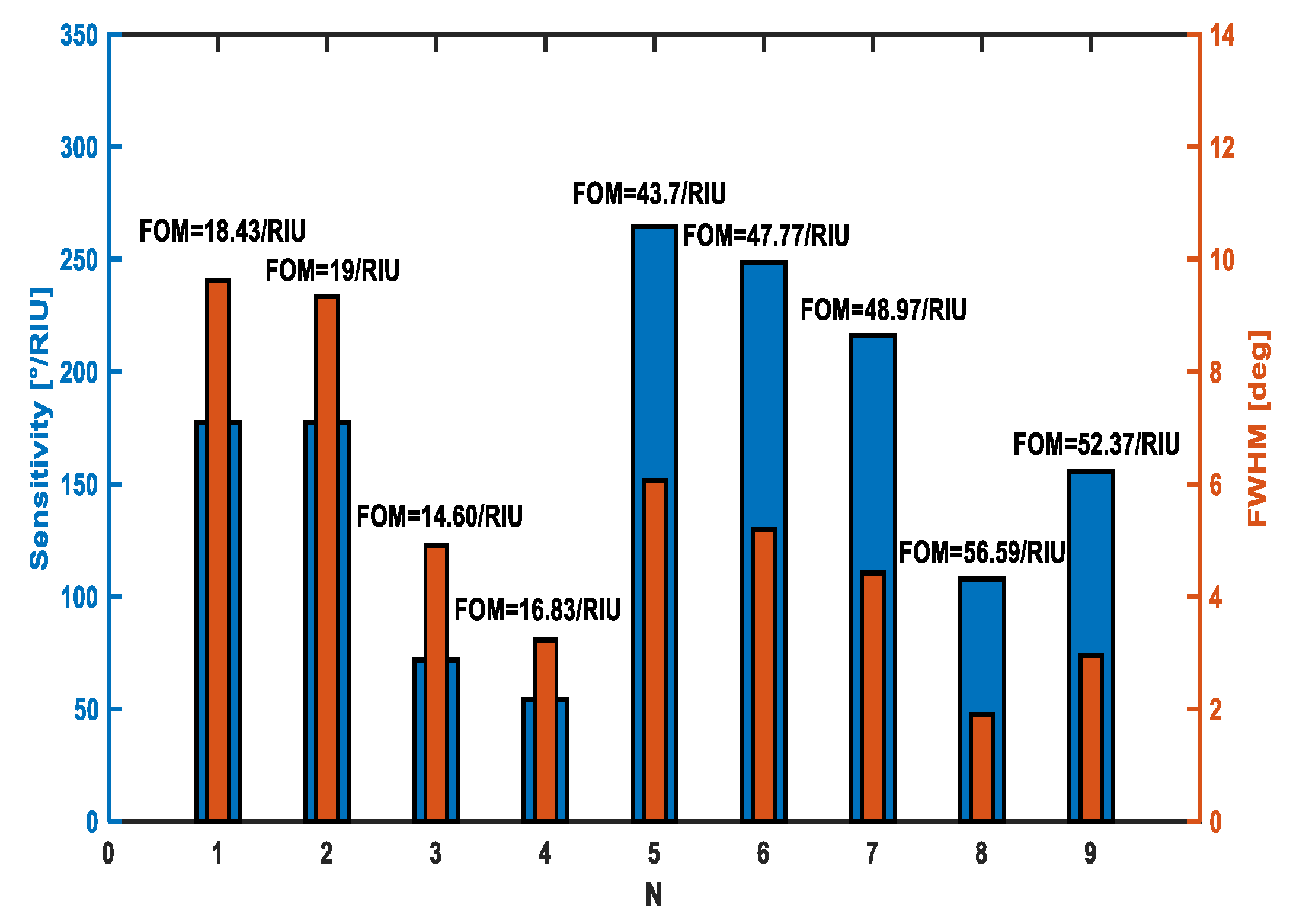
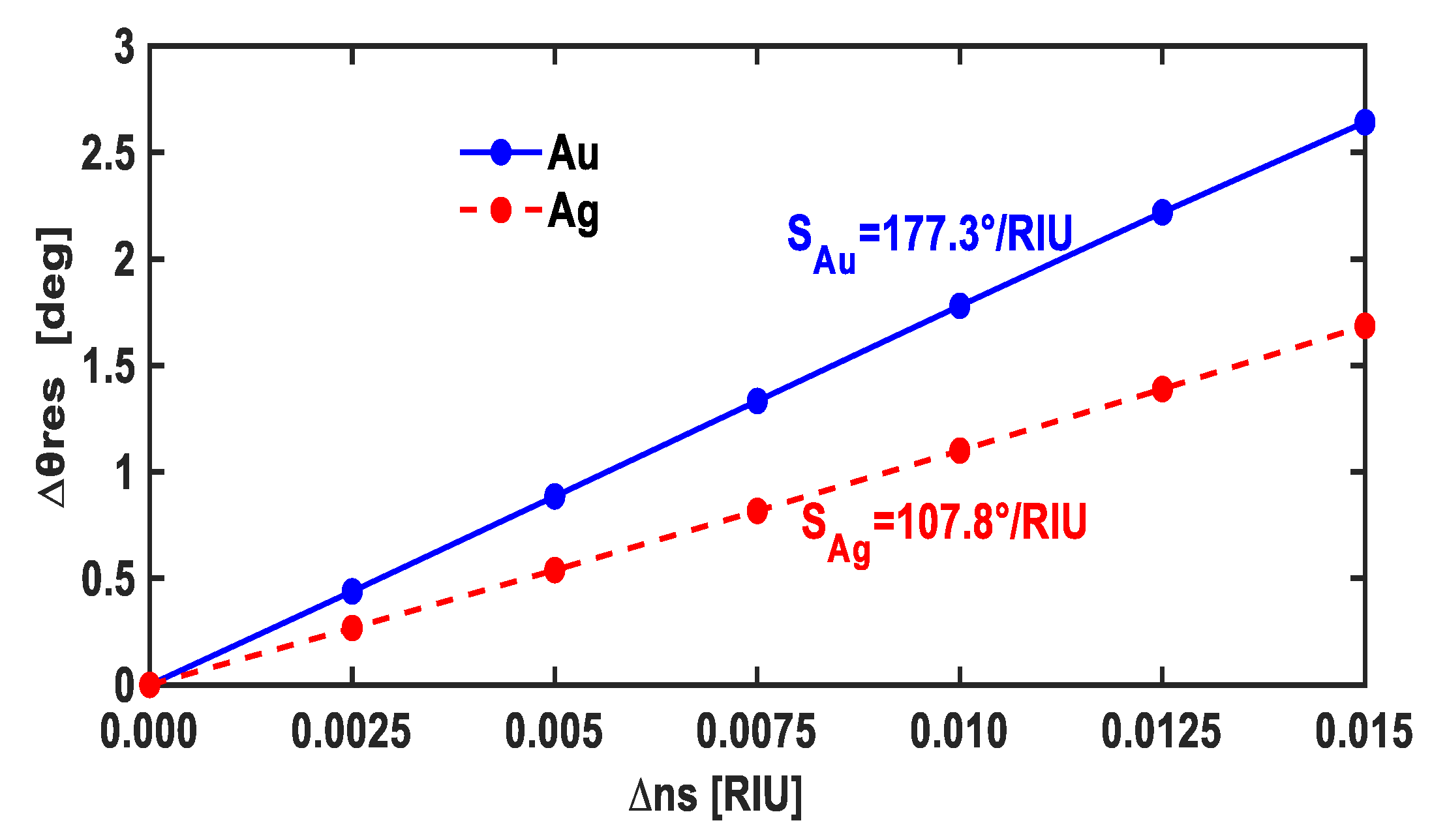
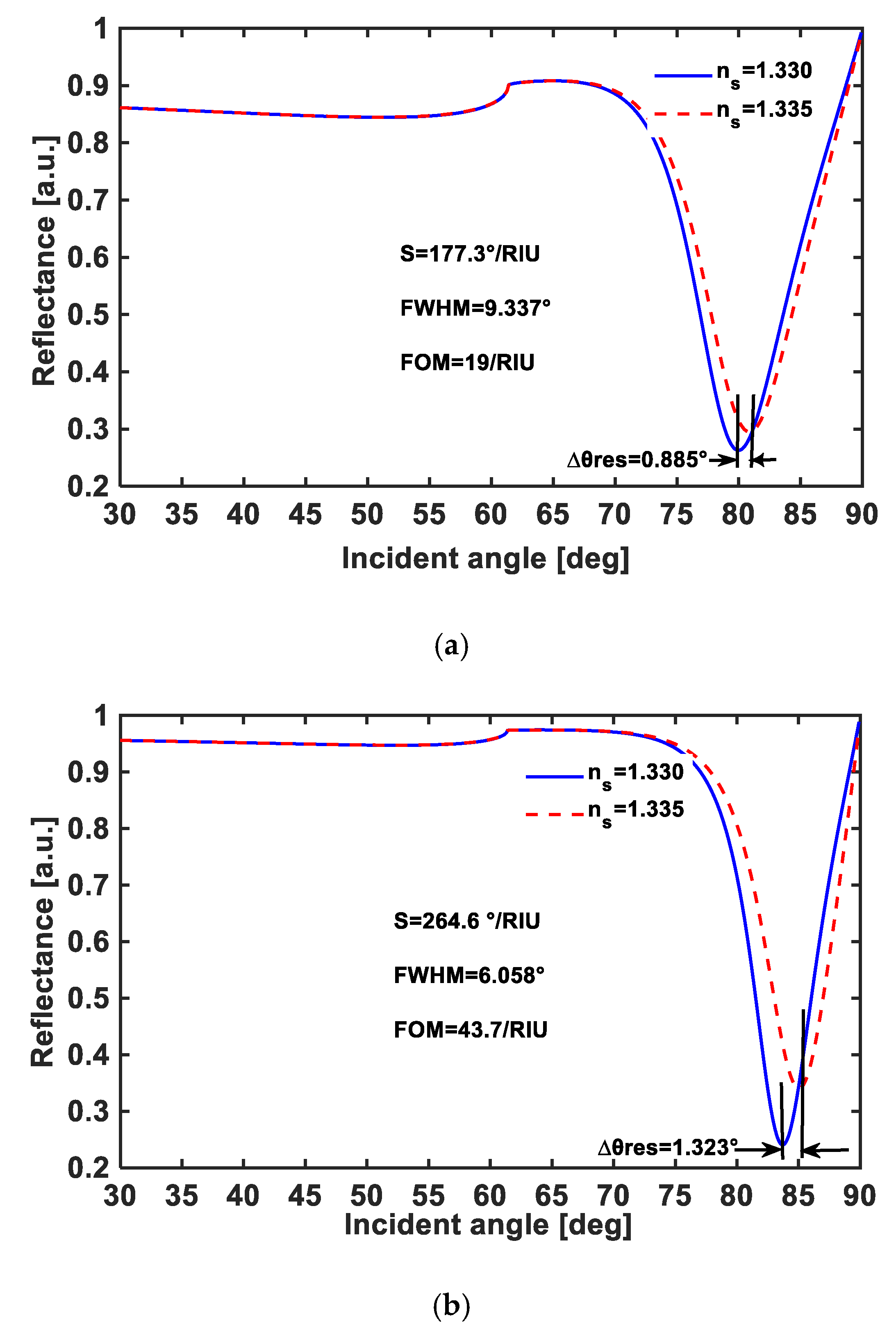
| # | Tmetal (nm) | IL | tIL (nm) | Prism | S (°/RIU) | FWHM (°) | FOM |
|---|---|---|---|---|---|---|---|
| 1 | Au = 53 | TiO2 | 5 | BK7 | 177.3 | 9.622 | 18.43 |
| 2 | Au = 56 | TiO2 | 5 | BK7 | 177.3 | 9.337 | 19 |
| 3 | Au = 63 | TiO2 | 5 | SF10 | 71.69 | 4.909 | 14.60 |
| 4 | Au = 62 | SiO2 | 6 | SF10 | 54.27 | 3.225 | 16.83 |
| 5 | Ag = 52 | Ta2O5 | 16 | BK7 | 264.6 | 6.058 | 43.7 |
| 6 | Ag = 55 | Ta2O5 | 16 | BK7 | 248.4 | 5.2 | 47.77 |
| 7 | Ag = 56 | Ta2O5 | 15 | BK7 | 216.2 | 4.415 | 48.97 |
| 8 | Ag = 58 | Ta2O5 | 8 | BK7 | 107.8 | 1.905 | 56.6 |
| 9 | Ag = 59 | Ta2O5 | 13 | BK7 | 155.8 | 2.95 | 52.37 |
| Structure | Wavelength (nm) | S (°/RIU) | FWHM (°) | FOM (1/RIU) | REF |
|---|---|---|---|---|---|
| Ag (45 nm)-BaTiO3(10 nm)-graphene (1 L) | 633 | 257 | 5.705 | 45.05 | [31] |
| RH (10 nm)- Ag (45 nm)- Si (14 nm)-graphene (1 L) | 632 | 220 | 10.204 | 21.56 | [32] |
| Au (35 nm)-Si (7 nm)-WS2 (1 L) | 600 | 155.68 | 17.464 | 8.914 | [33] |
| Au (40 nm)-Si (5 nm)-WS2 (13 L) | 785 | 127.90 | 15.523 | 8.239 | [33] |
| Au (40 nm)-Si (7 nm)-graphene (2 L) | 633 | 134.60 | 17.975 | 7.488 | [15] |
| Air (35 nm)-MoS2(6 L)-AL (35 nm)- Graphene (1 L) | 633 | 190.83 | 19 | 10.05 | [1] |
| This work | 633 | 107.8 | 1.905 | 56.59 | |
| This work | 633 | 216.2 | 4.415 | 48.97 | |
| This work | 633 | 264.6 | 6.058 | 43.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi-Kiasari, S.M.G.; Rashidi, K.; Fathi, D.; Taleb, H.; Mirjalili, S.M.; Faramarzi, V. Computational Design of Highly-Sensitive Graphene-Based Multilayer SPR Biosensor. Photonics 2022, 9, 688. https://doi.org/10.3390/photonics9100688
Mousavi-Kiasari SMG, Rashidi K, Fathi D, Taleb H, Mirjalili SM, Faramarzi V. Computational Design of Highly-Sensitive Graphene-Based Multilayer SPR Biosensor. Photonics. 2022; 9(10):688. https://doi.org/10.3390/photonics9100688
Chicago/Turabian StyleMousavi-Kiasari, Seyyed Mohammad Ghasem, Kamyar Rashidi, Davood Fathi, Hussein Taleb, Seyed Mohammad Mirjalili, and Vahid Faramarzi. 2022. "Computational Design of Highly-Sensitive Graphene-Based Multilayer SPR Biosensor" Photonics 9, no. 10: 688. https://doi.org/10.3390/photonics9100688
APA StyleMousavi-Kiasari, S. M. G., Rashidi, K., Fathi, D., Taleb, H., Mirjalili, S. M., & Faramarzi, V. (2022). Computational Design of Highly-Sensitive Graphene-Based Multilayer SPR Biosensor. Photonics, 9(10), 688. https://doi.org/10.3390/photonics9100688





