Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. What Is Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy?
3. Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy
3.1. SHINERS Application to the Reactions with CO Molecules
3.2. SHINERS Application to Other Surface Catalytic Reactions
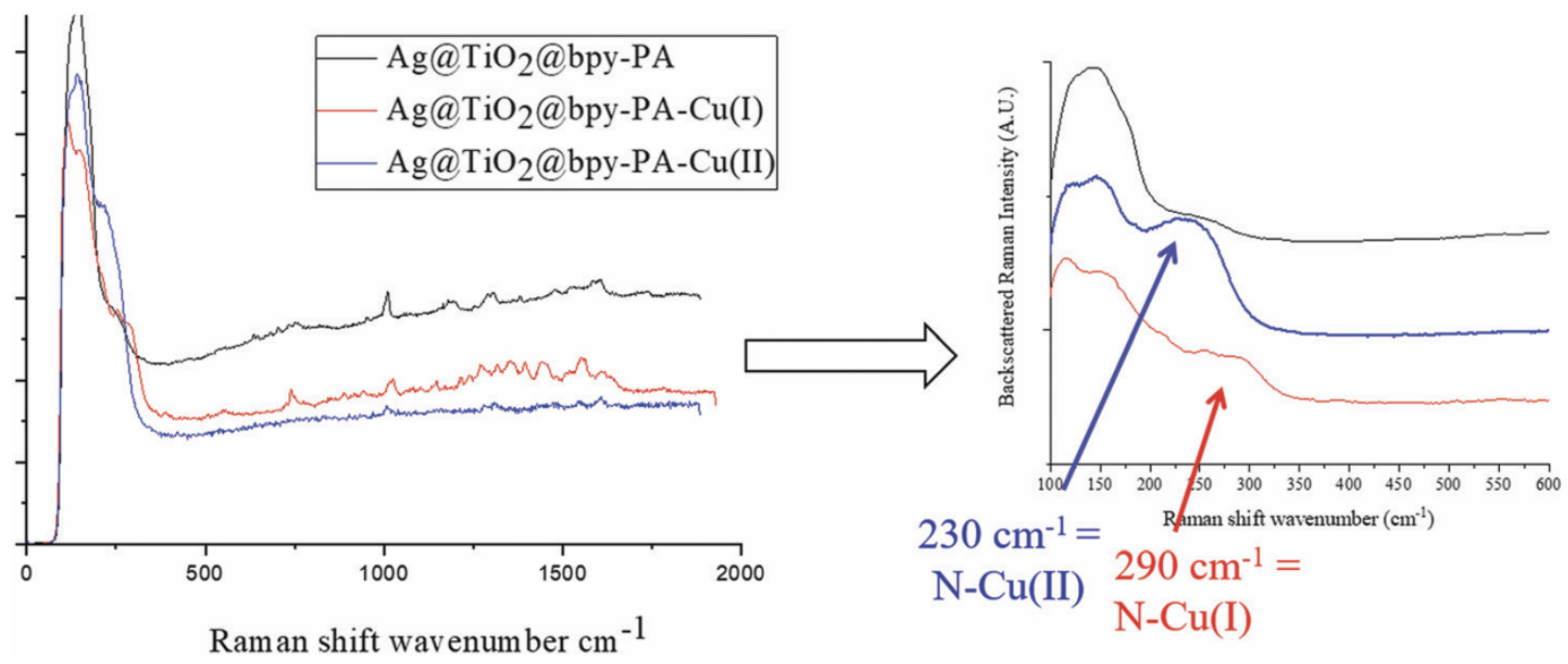
3.3. SHINERS Application for the Detection of Molecules and Ions
4. Discussions and Future Directions
5. Conclusions
Funding
Conflicts of Interest
References
- Castro-Grijalba, A.; Montes-Garcia, V.; Cordero-Ferradas, M.J.; Coronado, E.; Perez-Juste, J.; Pastoriza-Santos, I. SERS-Based Molecularly Imprinted Plasmonic Sensor for Highly Sensitive PAH Detection. ACS Sens. 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Blanco-Formoso, M.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Fabrication and SERS properties of complex and organized nanoparticle plasmonic clusters stable in solution. Nanoscale 2020, 12, 14948–14956. [Google Scholar] [CrossRef]
- Lan, L.; Fan, X.; Gao, Y.; Li, G.; Hao, Q.; Qiu, T. Plasmonic metal carbide SERS chips. J. Mater. Chem. C 2020, 8, 14523–14530. [Google Scholar] [CrossRef]
- Huang, J.A.; Mousavi, M.Z.; Zhao, Y.Q.; Hubarevich, A.; Omeis, F.; Giovannini, G.; Schutte, M.; Garoli, D.; De Angelis, F. SERS discrimination of single DNA bases in single oligonucleotides by electro-plasmonic trapping. Nat. Commun. 2019, 10, 5321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.T.; Lee, J.H.; Rathnam, C.; Hou, Y.N.; Choi, J.W.; Lee, K.B. Dual-Enhanced Raman Scattering-Based Characterization of Stem Cell Differentiation Using Graphene-Plasmonic Hybrid Nanoarray. Nano Lett. 2019, 19, 8138–8148. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Vogelsang, J.; Yi, J.M.; Wang, D.; Wittenbecher, L.; Mikaelsson, S.; Korte, A.; Chimeh, A.; Arnold, C.L.; Schaaf, P.; et al. Nonlinear plasmon-exciton coupling enhances sum-frequency generation from a hybrid metal/semiconductor nanostructure. Nat. Commun. 2020, 11, 1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; He, Y.H.; Chen, Y.; Shih, T.M.; Yang, W.M.; Chen, H.Y.; Yang, Z.L.; Wang, Z.H. Enhanced sum frequency generation for ultrasensitive characterization of plasmonic modes. Nanophotonics 2020, 9, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Dalstein, L.; Humbert, C.; Ben Haddada, M.; Boujday, S.; Barbillon, G.; Busson, B. The Prevailing Role of Hotspots in Plasmon-Enhanced Sum-Frequency Generation Spectroscopy. J. Phys. Chem. Lett. 2019, 10, 7706–7711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbillon, G.; Noblet, T.; Busson, B.; Tadjeddine, A.; Humbert, C. Localised detection of thiophenol with gold nanotriangles highly structured as honeycombs by nonlinear sum frequency generation spectroscopy. J. Mater. Sci. 2018, 53, 4554–4562. [Google Scholar] [CrossRef]
- Dalstein, L.; Ben Haddada, M.; Barbillon, G.; Humbert, C.; Tadjeddine, A.; Boujday, S.; Busson, B. Revealing the Interplay between Adsorbed Molecular Layers and Gold Nanoparticles by Linear and Nonlinear Optical Properties. J. Phys. Chem. C 2015, 115, 17146–17155. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Sun, M.; Xu, L.; Wang, L.; Kuang, H.; Xu, C. A SERS active gold nanostar dimer for mercury ion detection. Chem. Commun. 2013, 49, 4989–4991. [Google Scholar] [CrossRef] [PubMed]
- Kessentini, S.; Barchiesi, D.; D’Andrea, C.; Toma, A.; Guillot, N.; Di Fabrizio, E.; Fazio, B.; Marago, O.M.; Gucciardi, P.G.; Lamy de la Chapelle, M. Gold Dimer Nanoantenna with Slanted Gap for Tunable LSPR and Improved SERS. J. Phys. Chem. C 2014, 118, 3209–3219. [Google Scholar] [CrossRef]
- Hakonen, A.; Svedendahl, M.; Ogier, R.; Yang, Z.-Y.; Lodewijks, K.; Verre, R.; Shegai, T.; Andersson, P.O.; Käll, M. Dimer-on-mirror SERS substrates with attogram sensitivity fabricated by colloidal lithography. Nanoscale 2015, 7, 9405–9410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz, J.; Heck, C.; Ellerik, L.; Merk, V.; Bald, I. DNA origami based Au–Ag-core–shell nanoparticle dimers with single-molecule SERS sensitivity. Nanoscale 2016, 8, 5612–5620. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Paria, D.; Balasubramanian, K.; Ghosh, A.; Narayanan, R.; Raghavan, S. Directed Microwave-Assisted Self-Assembly of Au–Graphene–Au Plasmonic Dimers for SERS Applications. Adv. Mater. Interfaces 2019, 6, 1900629. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Ren, C.; Ju, P.; Huang, Y.; Zhang, F.; Zhao, F.; Zhang, Z.; Zhang, D. Detection of corrosion inhibitor adsorption via a surface-enhanced Raman spectroscopy (SERS) silver nanorods tape sensor. Sens. Actuators B 2020, 321, 128617. [Google Scholar] [CrossRef]
- Kuttner, C.; Höller, R.P.M.; Quintanilla, M.; Schnepf, M.J.; Dulle, M.; Fery, A.; Liz-Marzan, L.M. SERS and plasmonic heating efficiency from anisotropic core/satellite superstructures. Nanoscale 2019, 11, 19561–19570. [Google Scholar] [CrossRef] [Green Version]
- Reguera, J.; Langer, J.; Jimenez de Aberasturi, D.; Liz-Marzan, L.M. Anistropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef]
- Tang, L.; Li, S.; Han, F.; Liu, L.; Xu, L.; Ma, W.; Kuang, H.; Li, A.; Wang, L.; Xu, C. SERS-active Au@Ag nanorod dimers for ultrasensitive dopamine detection. Biosens. Bioelectron. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Alexander, K.D.; Skinner, K.; Zhang, S.; Wei, H.; Lopez, R. Tunable SERS in Gold Nanorod Dimers through Strain Control on an Elastomeric Substrate. Nano Lett. 2010, 10, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Höller, R.P.M.; Kuttner, C.; Mayer, M.; Wang, R.; Dulle, M.; Contreras-Caceres, R.; Fery, A.; Liz-Marzan, L.M. Colloidal Superstructures with Triangular Cores: Size Effects on SERS Efficiency. ACS Photonics 2020, 7, 1839–1848. [Google Scholar] [CrossRef]
- Koetz, J. The Effect of Surface Modification of Gold Nanotriangles for Surface-Enhanced Raman Scattering Performance. Nanomaterials 2020, 10, 2187. [Google Scholar] [CrossRef] [PubMed]
- Liebig, F.; Sarhan, R.M.; Bargheer, M.; Schmitt, C.N.Z.; Poghasyan, A.H.; Shahinsyan, A.A.; Koetz, J. Spiked gold nanotriangles: Formation, characterization and applications in surface-enhanced Raman spectroscopy and plasmon-enhanced catalysis. RSC Adv. 2020, 10, 8152–8160. [Google Scholar] [CrossRef]
- Kuttner, C.; Mayer, M.; Dulle, M.; Moscoso, A.; Lopez-Romero, J.M.; Forster, S.; Fery, A.; Perez-Juste, J.; Contreras-Caceres, R. Seeded Growth Synthesis of Gold Nanotriangles: Size Control, SAXS Analysis, and SERS Performance. ACS Appl. Mater. Interfaces 2018, 10, 11152–11163. [Google Scholar] [CrossRef] [PubMed]
- Bryche, J.-F.; Tsigara, A.; Bélier, B.; Lamy de la Chapelle, M.; Canva, M.; Bartenlian, B.; Barbillon, G. Surface enhanced Raman scattering improvement of gold triangular nanoprisms by a gold reflective underlayer for chemical sensing. Sens. Actuators B 2016, 228, 31–35. [Google Scholar] [CrossRef]
- Su, Q.; Ma, X.; Dong, J.; Jiang, C.; Qian, W. A Reproducible SERS Substrate Based on Electrostatically Assisted APTES-Functionalized Self-Assembly of Gold Nanostars. ACS Appl. Mater. Interfaces 2011, 3, 1873–1879. [Google Scholar] [CrossRef]
- Wang, Y.; Polavarapu, L.; Liz-Marzan, L.M. Reduced Graphene Oxide-Supported Gold Nanostars for Improved SERS Sensing and Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 21798–21805. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Meyers, S.; Shubeita, S.; Feldman, L.C.; Gustafsson, T.; Fabris, L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale 2014, 6, 8891–8899. [Google Scholar] [CrossRef]
- Jimenez de Aberasturi, D.; Serrano-Montes, A.B.; Langer, J.; Henriksen-Lacey, M.; Parak, W.J.; Liz-Marzan, L.M. Surface enhanced Raman scattering encoded gold nanostars for multiplexed cell discrimination. Chem. Mater. 2016, 28, 6779–6790. [Google Scholar] [CrossRef]
- Serrano-Montes, A.B.; Langer, J.; Henriksen-Lacey, M.; Jimenez de Aberasturi, D.; Solis, D.M.; Taboada, J.M.; Obelleiro, F.; Sentosun, K.; Bals, S.; Bekdemir, A.; et al. Gold nanostar-coated polystyrene beads as multifunctional nanoprobes for SERS bioimaging. J. Phys. Chem. C 2016, 120, 20860–20868. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lang, X.; Hirata, A.; Chen, M. Wrinkled Nanoporous Gold Films with Ultrahigh Surface-Enhanced Raman Scattering Enhancement. ACS Nano 2011, 5, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Liu, X.; Lu, W.; Dai, J.; Lei, D.; MacFarlane, D.R. Hierarchical Porous Plasmonic Metamaterials for Reproducible Ultrasensitive Surface-Enhanced Raman Spectroscopy. Adv. Mater. 2015, 27, 1090–1096. [Google Scholar] [CrossRef]
- Hubarevich, A.; Huang, J.-A.; Giovanni, G.; Schirato, A.; Zhao, Y.; Maccaferri, N.; De Angelis, F.; Alabastri, A.; Garoli, D. λ-DNA through Porous Materials–Surface-Enhanced Raman Scattering in a Single Plasmonic Nanopore. J. Phys. Chem. C 2020, 124, 22663–22670. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.-L.; Yang, J.-M.; Li, Z.-Q.; Yang, D.-R.; Ji, L.-N.; Wang, K.; Xia, X.-H. SERS Detection of Nucleobases in Single Silver Plasmonic Nanopores. ACS Sens. 2020, 5, 2198–2204. [Google Scholar] [CrossRef]
- Lum, W.; Bruzas, I.; Gorunmez, Z.; Unser, S.; Beck, T.; Sagle, L. Novel Liposome-Based Surface-Enhanced Raman Spectroscopy (SERS) Substrate. J. Phys. Chem. Lett. 2017, 8, 2639–2646. [Google Scholar] [CrossRef] [Green Version]
- Yue, W.; Wang, Z.; Whittaker, J.; Lopez-Royo, F.; Yang, Y.; Zayats, A.V. Amplification of surface-enhanced Raman scattering due to substrate-mediated localized surface plasmons in gold nanodimers. J. Mater. Chem. C 2017, 5, 4075–4084. [Google Scholar] [CrossRef] [Green Version]
- Benz, F.; Chikkaraddy, R.; Salmon, A.; Ohadi, H.; de Nijs, B.; Mertens, J.; Carnegie, C.; Bowman, R.W.; Baumberg, J.J. SERS of Individual Nanoparticles on a Mirror: Size Does Matter, but so Does Shape. J. Phys. Chem. Lett. 2016, 7, 2264–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ma, L.; Hou, M.; Li, J.; Xie, Z.; Zhang, Z. Hybridized plasmon modes and near-field enhancement of metallic nanoparticle-dimer on a mirror. Sci. Rep. 2016, 6, 30011. [Google Scholar] [CrossRef] [Green Version]
- Sobhani, A.; Manjavacas, A.; Cao, Y.; McClain, M.J.; Javier Garcia de Abajo, F.; Nordlander, P.; Halas, N.J. Pronounced Linewidth Narrowing of an Aluminum Nanoparticle Plasmon Resonance by Interaction with an Aluminum Metallic Film. Nano Lett. 2015, 15, 6946–6951. [Google Scholar] [CrossRef] [PubMed]
- Convertino, A.; Mussi, V.; Maiolo, L. Disordered array of Au covered silicon nanowires for SERS biosensing combined with electrochemical detection. Sci. Rep. 2016, 6, 25099. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jiang, X.; Lee, S.T.; He, Y. Silicon nanohybrid-based surface-enhanced Raman scattering sensors. Small 2014, 10, 4455–4468. [Google Scholar] [CrossRef]
- Akin, M.S.; Yilmaz, M.; Babur, E.; Ozdemur, B.; Erdogan, H.; Tamer, U.; Demirel, G. Large area uniform deposition of silver nanoparticles through bio-inspired polydopamine coating on silicon nanowire arrays for practical SERS applications. J. Mater. Chem. B 2014, 10, 4455–4468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryche, J.-F.; Bélier, B.; Bartenlian, B.; Barbillon, G. Low-cost SERS substrates composed of hybrid nanoskittles for a highly sensitive sensing of chemical molecules. Sens. Actuators B 2017, 239, 795–799. [Google Scholar] [CrossRef]
- Barbillon, G.; Ivanov, A.; Sarychev, A.K. Hybrid Au/Si Disk-Shaped Nanoresonators on Gold Film for Amplified SERS Chemical Sensing. Nanomaterials 2019, 9, 1588. [Google Scholar] [CrossRef] [Green Version]
- Graniel, O.; Iatsunskyi, I.; Coy, E.; Humbert, C.; Barbillon, G.; Michel, T.; Maurin, D.; Balme, S.; Miele, P.; Bechelany, M. Au-covered hollow urchin-like ZnO nanostructures for surface-enhanced Raman scattering sensing. J. Mater. Chem. C 2019, 7, 15066–15073. [Google Scholar] [CrossRef]
- Cui, S.; Dai, Z.; Tian, Q.; Liu, J.; Xiao, X.; Jiang, C.; Wu, W.; Roy, V.A.L. Wetting properties and SERS applications of ZnO/Ag nanowire arrays patterned by a screen printing method. J. Mater. Chem. C 2016, 4, 6371–6379. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Lee, T.K.; Park, J.; Ha, M.; Kwak, S.K.; Ko, H. Particle-on-film gap plasmons on antireflective ZnO nanocone arrays for molecular-level surface-enhanced Raman scattering sensors. ACS Appl. Mater. Interfaces 2015, 7, 26421–26429. [Google Scholar] [CrossRef]
- Song, W.; Ji, W.; Vantasin, S.; Tanabe, I.; Zhao, B.; Ozaki, Y. Fabrication of a highly sensitive surface-enhanced Raman scattering substrate for monitoring the catalytic degradation of organic pollutants. J. Mater. Chem. A 2015, 3, 13556–13562. [Google Scholar] [CrossRef]
- He, X.; Yue, C.; Zang, Y.; Yin, J.; Sun, S.; Li, J.; Kang, J. Multi-hotspot configuration on urchin-like Ag nanoparticle/ZnO hollow nanosphere arrays for highly sensitive SERS. J. Mater. Chem. A 2013, 1, 15010–15015. [Google Scholar] [CrossRef]
- Liu, X.W.; Wang, D.S.; Li, Y.D. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 2012, 7, 448–466. [Google Scholar] [CrossRef]
- Su, Y.; Xu, S.; Zhang, J.; Chen, X.; Jiang, L.-P.; Zheng, T.; Zhu, J.-J. Plasmon Near-Field Coupling of Bimetallic Nanostars and a Hierarchical Bimetallic SERS “Hot Field”: Toward Ultrasensitive Simultaneous Detection of Multiple Cardiorenal Syndrome Biomarkers. Anal. Chem. 2019, 91, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.; Kwak, C.H.; Huh, Y.S.; Han, Y.-K. Synthesis of AuAg@Ag core@shell hollow cubic nanostructures as SERS substrates for attomolar chemical sensing. Sens. Actuators B 2019, 281, 471–477. [Google Scholar] [CrossRef]
- Vu, T.D.; Duy, P.K.; Chung, H. Nickel foam-caged Ag-Au bimetallic nanostructure as a highly rugged and durable SERS substrate. Sens. Actuators B 2019, 282, 535–540. [Google Scholar] [CrossRef]
- Barbillon, G. Latest Novelties on Plasmonic and Non-Plasmonic Nanomaterials for SERS Sensing. Nanomaterials 2020, 10, 1200. [Google Scholar] [CrossRef]
- Hussain, A.; Sun, D.-W.; Pu, H. Bimetallic core shelled nanoparticles (Au@AgNPs) for rapid detection of thiram and dicyandiamide contaminants in liquid milk using SERS. Food Chem. 2020, 317, 126429. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef]
- Liu, W.; Bai, H.; Li, X.; Li, W.; Zhai, J.; Li, J.; Xi, G. Improved Surface-Enhanced Raman Spectroscopy Sensitivity on Metallic Tungsten Oxide by the Synergistic Effect of Surface Plasmon Resonance Coupling and Charge Transfer. J. Phys. Chem. Lett. 2018, 9, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Fan, X.; Wei, P.; Qiu, T. Planar transition metal oxides SERS chips: A general strategy. J. Mater. Chem. C 2019, 7, 11134–11141. [Google Scholar] [CrossRef]
- Wei, W.; Yao, Y.; Zhao, Q.; Xu, Z.; Wang, Q.; Zhang, Z.; Gao, Y. Oxygen defect-induced localized surface plasmon resonance at the WO3−x quantum dot/silver nanowire interface: SERS and photocatalysis. Nanoscale 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- He, R.; Lai, H.; Wang, S.; Chen, T.; Xie, F.; Chen, Q.; Liu, P.; Chen, J.; Xie, W. Few-layered vdW MoO3 for sensitive, uniform and stable SERS applications. Appl. Surf. Sci. 2020, 507, 145116. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Shen, Y.; Xie, A. An assembled ordered W18O49 nanowire film with high SERS sensitivity and stability for the detection of RB. Appl. Surf. Sci. 2020, 504, 144073. [Google Scholar] [CrossRef]
- Ben-Jaber, S.; Peveler, W.J.; Quesada-Cabrera, R.; Cortés, E.; Sotelo-Vazquez, C.; Abdul-Karim, N.; Maier, S.A.; Parkin, I.P. Photo-induced enhanced Raman spectroscopy for universal ultra-trace detection of explosives, pollutants and biomolecules. Nat. Commun. 2016, 7, 12189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, D.; Cortés, E.; Ben-Jaber, S.; Brick, T.; Peveler, W.J.; Blackman, C.S.; Howle, C.R.; Quesada-Cabrera, R.; Parkin, I.P.; Maier, S.A. Dynamics of Photo-Induced Surface Oxygen Vacancies in Metal-Oxide Semiconductors Studied Under Ambient Conditions. Adv. Sci. 2019, 6, 1901841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbillon, G.; Noblet, T.; Humbert, C. Highly crystalline ZnO film decorated with gold nanospheres for PIERS chemical sensing. Phys. Chem. Chem. Phys. 2020, 22, 21000–21004. [Google Scholar] [CrossRef]
- Sun, H.H.; Yao, M.G.; Song, Y.P.; Zhu, L.Y.; Dong, J.J.; Liu, R.; Li, P.; Zhao, B.; Liu, B.B. Pressure-induced SERS enhancement in a MoS2/Au/R6G system by a two-step charge transfer process. Nanoscale 2019, 11, 21493–21501. [Google Scholar] [CrossRef]
- Barbillon, G. Nanoplasmonics in High Pressure Environment. Photonics 2020, 7, 53. [Google Scholar] [CrossRef]
- Li, J.-F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 111, 5002–5069. [Google Scholar] [CrossRef]
- Li, C.-Y.; Meng, M.; Huang, S.-C.; Li, L.; Huang, S.-R.; Chen, S.; Meng, L.-Y.; Panneerselvam, R.; Zhang, S.-J.; Ren, B.; et al. “Smart” Ag Nanostructures for Plasmon-Enhanced Spectroscopies. J. Am. Chem. Soc. 2015, 137, 13784–13787. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.; Weckhuysen, B.M. Thermally Stable TiO2- and SiO2-Shell-Isolated Au Nanoparticles for In Situ Plasmon-Enhanced Raman Spectroscopy of Hydrogenation Catalysts. Chem. Eur. J. 2018, 24, 3733–3741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, T.; Wondergem, C.S.; Weckhuysen, B.M. Pratical Guidelines for Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Heterogeneous Catalysts. ChemPhysChem 2018, 19, 2461–2467. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.G.; Wei, J.; Wang, C.; Chen, S.; Sun, H.L.; Wang, Y.H.; Chen, B.H.; Yang, Z.L.; Wu, D.Y.; et al. Revealing the Role of Interfacial Properties on Catalytic Behaviors by In-Situ Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2017, 139, 10339–10346. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Sun, H.L.; Fu, G.; Chen, S.; Zhang, Y.J.; Chen, B.H.; Anema, J.R.; Yang, Z.L.; Li, J.F.; et al. In Situ Dynamic Tracking of Heterogeneous Nanocatalytic Processes by Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nat. Commun. 2017, 8, 15447. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.-D.; Li, J.-F.; Huang, Y.-F.; Tian, X.-D.; Uzayisenga, V.; Li, S.-B.; Ren, B.; Tian, Z.-Q. Shell-isolated nanoparticle-enhanced Raman spectroscopy: Nanoparticle synthesis, characterization and applications in electrochemistry. J. Electroanal. Chem. 2013, 688, 5–11. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Zhang, J. Few-Layer Graphene-Encapsulated Metal Nanoparticles for Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2014, 118, 8993–8998. [Google Scholar] [CrossRef]
- Chen, S.N.; Li, X.; Zhao, Y.Y.; Chang, L.M.; Qi, J.Y. Graphene oxide shell-isolated Ag nanoparticles for surface-enhanced Raman scattering. Carbon 2015, 81, 767–772. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, C.; Huo, Y.Y.; Jiang, S.Z.; Qiu, H.W.; Xu, Y.Y.; Li, X.H.; Man, B.Y. Shell-isolated graphene@Cu nanoparticles on graphene@Cu substrates for the application in SERS. Carbon 2016, 98, 526–533. [Google Scholar] [CrossRef]
- Lin, X.-D.; Uzayisenga, V.; Li, J.-F.; Fang, P.-P.; Wu, D.-Y.; Ren, B.; Tian, Z.-Q. Synthesis of ultrathin and compact Au@MnO2 nanoparticles for shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 40–45. [Google Scholar] [CrossRef]
- Honesty, N.R.; Gewirth, A.A. Shell-isolated nanoparticle enhanced Raman spectroscopy (SHINERS) investigation of benzotriazole film formation on Cu(100), Cu(111), and Cu(poly). J. Raman Spectrosc. 2012, 43, 46–50. [Google Scholar] [CrossRef]
- Li, S.-B.; Li, L.-M.; Anema, J.R.; Li, J.-F.; Yang, Z.-L.; Ren, B.; Sun, J.-J.; Tian, Z.-Q. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy (SHINERS) Based on Gold-Core Silica-Shell Nanorods. Z. Phys. Chem. 2011, 225, 775–783. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Wei, J.; Radjenovic, P.; Tian, Z.-Q.; Li, J.-F. In Situ Analysis of Surface Catalytic Reactions Using Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Anal. Chem. 2019, 91, 1675–1685. [Google Scholar] [CrossRef]
- Su, M.; Dong, J.-C.; Le, J.-B.; Zhao, Y.; Yang, W.-M.; Yang, Z.-L.; Attard, G.; Liu, G.-K.; Cheng, J.; Wei, Y.-M.; et al. In Situ Raman Study of CO Electrooxidation Pt(Hkl) Single-Cryst. Surfaces Acidic Solution. Angew. Chem. Int. Ed. 2020, 59, 1–6. [Google Scholar] [CrossRef]
- Wondergem, C.S.; Hartman, T.; Weckhuysen, B.M. In Situ Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy to Unravel Sequential Hydrogenation of Phenylacetylene over Platinum Nanoparticles. ACS Catal. 2019, 9, 10794–10802. [Google Scholar] [CrossRef]
- Wondergem, C.S.; Kromwijk, J.J.G.; Slagter, M.; Vrijburg, W.L.; Hensen, E.J.M.; Monai, M.; Vogt, C.; Weckhuysen, B.M. In Situ Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Nickel-Catalyzed Hydrogenation Reactions. ChemPhysChem 2020, 21, 625–632. [Google Scholar] [CrossRef]
- Hartman, T.; Geitenbeek, R.G.; Whiting, G.T.; Weckhuysen, B.M. Operando monitoring of temperature and active species at the single catalyst particle level. Nat. Catal. 2019, 2, 986–996. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Chen, Q.-Q.; Chen, X.; Wang, A.; Tian, Z.-Q.; Li, J.-F. Graphene-coated Au nanoparticle-enhanced Raman spectroscopy. J. Raman Spectrosc. 2020, 1–7. [Google Scholar] [CrossRef]
- Kuruvinashetti, K.; Zhang, Y.; Li, J.; Kornienko, N. Shell isolated nanoparticle enhanced Raman spectroscopy for renewable energy electorcatalysis. New J. Chem. 2020, 44, 19953–19960. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, X.; Malkani, A.S.; Yang, X.; Thompson, L.; Jiao, F.; Xu, B. Speciation of Cu Surfaces During the Electrochemical CO Reduction Reaction. J. Am. Chem. Soc. 2020, 142, 9735–9743. [Google Scholar] [CrossRef]
- Guan, S.; Attard, G.A.; Wain, A.J. Observation of Substituent Effects in the Electrochemical Adsorption and Hydrogenation of Alkynes on Pt{Hkl} Using SHINERS. ACS Catal. 2020, 10, 10999–11010. [Google Scholar] [CrossRef]
- Saeed, K.H.; Forster, M.; Li, J.-F.; Hardwick, L.J.; Cowan, A.J. Water oxidation intermediates on iridium oxide electrodes probed by in situ electrochemical SHINERS. Chem. Commun. 2020, 56, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Le, J.-B.; Wang, Y.-H.; Chen, S.; Yang, Z.-L.; Li, J.-F.; Cheng, J.; Tian, Z.-Q. In situ probing electrified interfacial water structures at atomically flat surfaces. Nat. Mater. 2019, 18, 697–701. [Google Scholar] [CrossRef]
- Guo, B.; Lin, X.; Burgess, I.J.; Yu, C. Electrochemical SHINERS investigation of the adsorption of butyl xanthate and 2-mercaptobenzothiazole on pyrite. Appl. Surf. Sci. 2020, 529, 147118. [Google Scholar] [CrossRef]
- Bodappa, N.; Su, M.; Zhao, Y.; Le, J.-B.; Yang, W.-M.; Radjenovic, P.; Dong, J.-C.; Cheng, J.; Tian, Z.-Q.; Li, J.-F. Early Stages of Electrochemical Oxydation of Cu(111) and Polycrystalline Cu Surfaces Revealed by in Situ Raman Spectroscopy. J. Am. Chem. Soc. 2019, 141, 12192–12196. [Google Scholar] [CrossRef]
- Galloway, T.A.; Dong, J.-C.; Li, J.-F.; Attard, G.; Hardwick, L.J. Oxygen reactions on Pthkl in a non-aqueous Na+ electrolyte: Site selective stabilisation of a sodium peroxy species. Chem. Sci. 2019, 10, 2956–2964. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.-C.; Zhang, X.-G.; Briega-Martos, V.; Jin, X.; Yang, J.; Chen, S.; Yang, Z.-L.; Wu, D.-Y.; Feliu, J.M.; Williams, C.T.; et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermadiates at platinum single-crystal surfaces. Nat. Energy 2019, 4, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.-C.; Su, M.; Briega-Martos, V.; Li, L.; Le, J.-B.; Radjenovic, P.; Zhou, X.-S.; Feliu, J.M.; Tian, Z.-Q.; Li, J.-F. Direct in Situ Raman Spectroscopic Evidence of Oxygen Reduction Reaction Intermadiates at High-Index Pt(hkl) Surfaces. J. Am. Chem. Soc. 2020, 142, 715–719. [Google Scholar] [CrossRef]
- Barlow, B.C.; Guo, B.; Situm, A.; Grosvenor, A.P.; Burgess, I.J. Shell isolated nanoparticle enhanced Raman spectroscopy (SHINERS) studies of steel surface corrosion. J. Electroanal. Chem. 2019, 853, 113559. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Chen, T.-M.; Wei, J.; Qin, S.-N.; Zheng, J.-F.; Zhang, H.; Tian, Z.-Q.; Li, J.-F. In-situ SHINERS Study of the Size and Composition Effect of Pt-Based Nanocatalysts in Catalytic Hydrogenation. ChemCatChem 2020, 12, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Wang, M.; Zhang, L.; Cao, M.; Yang, Z.; Dou, J.; Ji, S.; Ji, Y.; Kou, S.; Bhatti, A.S. Insights into the role of graphene in hybrid photocatalytic system by in-situ shell-isolated nanoparticle-enhanced Raman Spectroscopy. Carbon 2019, 152, 305–315. [Google Scholar] [CrossRef]
- Wondergem, C.S.; van Swieten, T.P.; Geitenbeek, R.G.; Erné, B.H.; Weckhuysen, B.M. Extented Surface-Enhanced Raman Spectroscopy to Liquids Using Shell-Isolated Plasmonic Superstructures. Chem. Eur. J. 2019, 25, 15772–15778. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-P.; Lin, J.-S.; Lin, R.-K.; Radjenovic, P.M.; Yang, W.-M.; Xu, J.; Dong, J.-C.; Yang, Z.-L.; Hang, W.; Tian, Z.-Q.; et al. In situ Raman study of the photoinduced behavior of dye molecules on TiO2(hkl) single crystal surfaces. Chem. Sci. 2020, 11, 6431–6435. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Li, B.; Liu, X.; Chen, J.; Mu, T.; Zhu, L.; Guo, J.; Ma, X. Performance enhancement of paper-based SERS chips by shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Mater. Sci. Technol. 2019, 35, 2207–2212. [Google Scholar] [CrossRef]
- Forato, F.; Talebzadeh, S.; Rousseau, N.; Mevellec, J.-Y.; Bujoli, B.; Knight, D.A.; Queffélec, C.; Humbert, B. Functionalized core–shell Ag@TiO2 nanoparticles for enhanced Raman spectroscopy: A sensitive detection method for Cu(II) ions. Phys. Chem. Chem. Phys. 2019, 21, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Queffélec, C.; Forato, F.; Bujoli, B.; Knight, D.A.; Fonda, E.; Humbert, B. Investigation of copper oxidation states in plasmonic nanomaterials by XAS and Raman spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 2193–2199. [Google Scholar] [CrossRef]
- Krajczewski, J.; Kedziora, M.; Kołataj, K.; Kudelski, A. Improved synthesis of concave cubic gold nanoparticles and their applications for Raman analysis of surfaces. RSC Adv. 2019, 9, 18609–18618. [Google Scholar] [CrossRef] [Green Version]
- Nicinski, K.; Krajczewski, J.; Kudelski, A.; Witkowska, E.; Trzcinska-Danielewicz, J.; Girstun, A.; Kaminska, A. Detection of circulating tumor cells in blood by shell-isolated nanoparticle—Enhanced Raman spectroscopy (SHINERS) in microfluidic device. Sci. Rep. 2019, 9, 9267. [Google Scholar] [CrossRef] [Green Version]
- El-Said, W.A.; Alshitari, W.; Choi, J.-W. Controlled fabrication of gold nanobipyramids/polypyrrole for shell-isolated nanoparticle-enhanced Raman spectroscopy to detect γ-aminobutyric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117890. [Google Scholar] [CrossRef] [PubMed]
- Zdaniauskienė, A.; Charkova, T.; Ignatjev, I.; Melvydas, V.; Garjonytė, R.; Matulaitienė, I.; Talaikis, M.; Niaura, G. Shell-isolated nanoparticle-enhanced Raman spectroscopy for characterization of living yeast cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118560. [Google Scholar] [CrossRef]
- Zheng, C.; Jia, H.Y.; Liu, L.Y.; Wang, Q.; Jiang, H.C.; Teng, L.S.; Geng, C.Z.; Jin, F.; Tang, L.L.; Zhang, J.G.; et al. Molecular fingerprint of precancerous lesions in breast atypical hyperplasia. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Hartman, T.; Geitenbeek, R.G.; Wondergem, C.S.; van der Stam, W.; Weckhuysen, B.M. Operando Nanoscale Sensors in Catalysis: All Eyes on Catalyst Particles. ACS Nano 2020, 14, 3725–3735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Duan, S.; Radjenovic, P.M.; Tian, Z.-Q.; Li, J.-F. Core–Shell Nanostructure-Enhanced Raman Spectroscopy for Surface Catalysis. Acc. Chem. Res. 2020, 53, 729–739. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ren, H.; You, E.-M.; Radjenovic, P.M.; Sun, S.-G.; Tian, Z.-Q.; Li, J.-F.; Wang, Z. Polarization- and Wavelength-Dependent Shell-Isolated-Nanoparticle-Enhanced Sum-Frequency Generation with High Sensitivity. Phys. Rev. Lett. 2020, 125, 047401. [Google Scholar] [CrossRef] [PubMed]
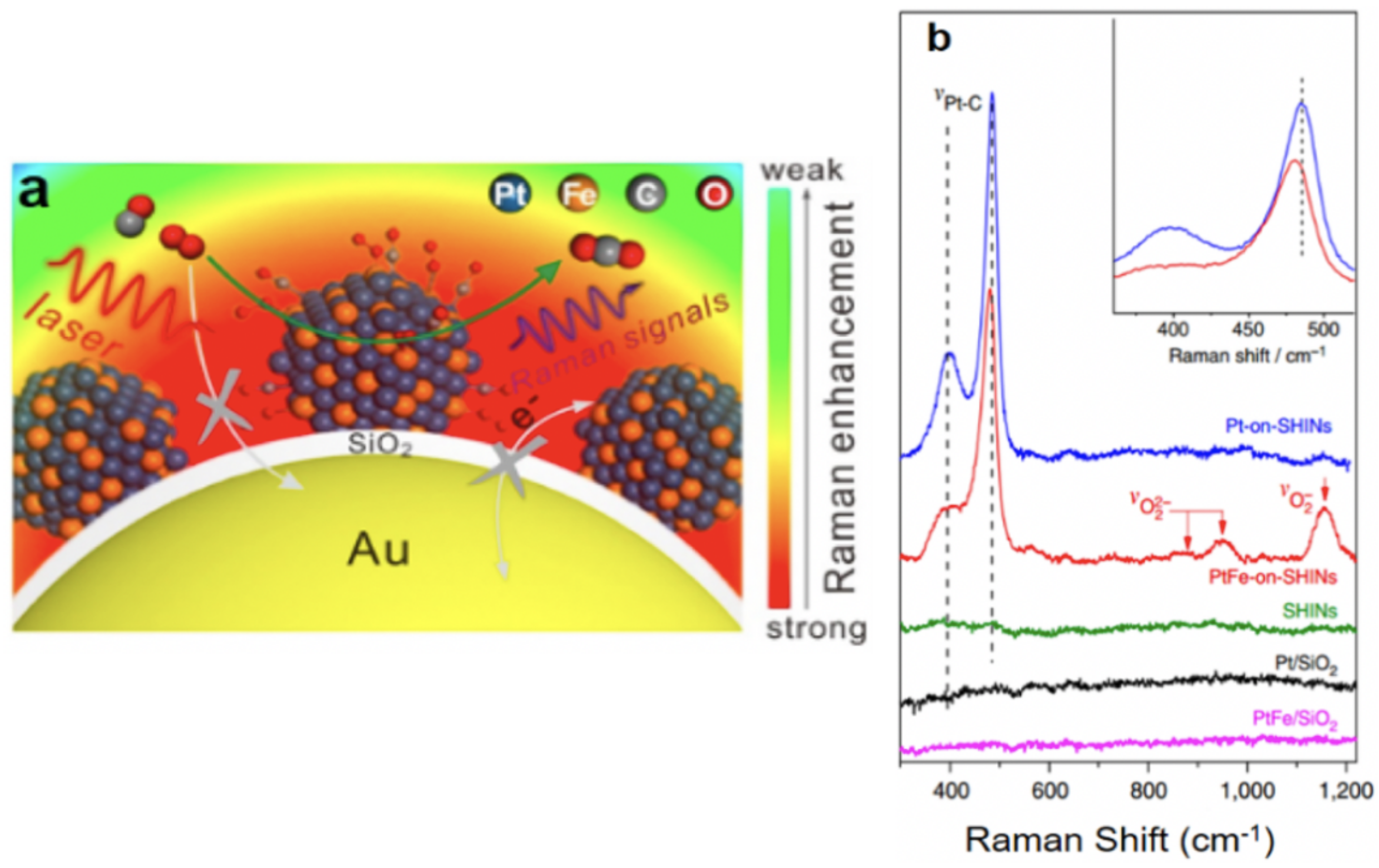
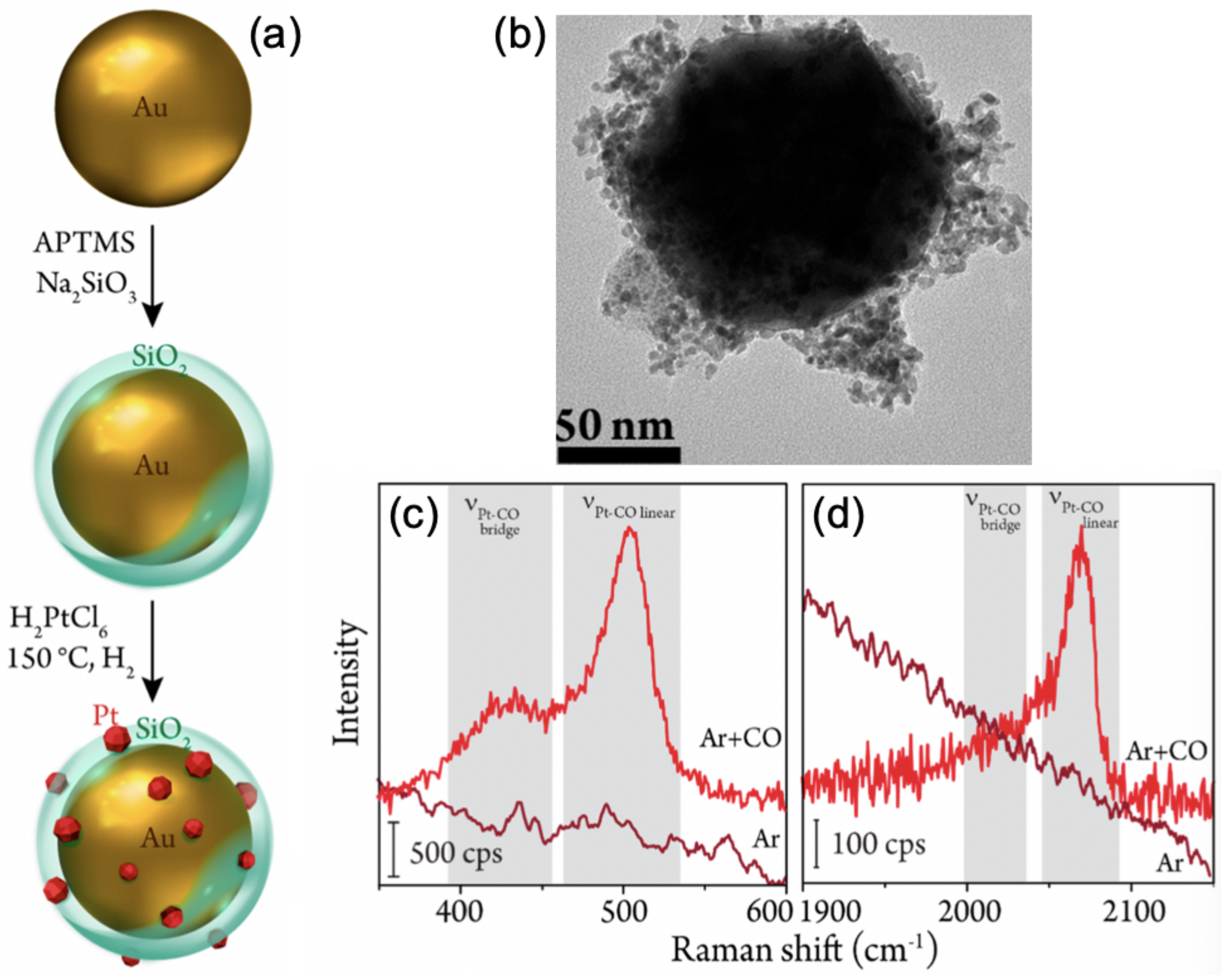



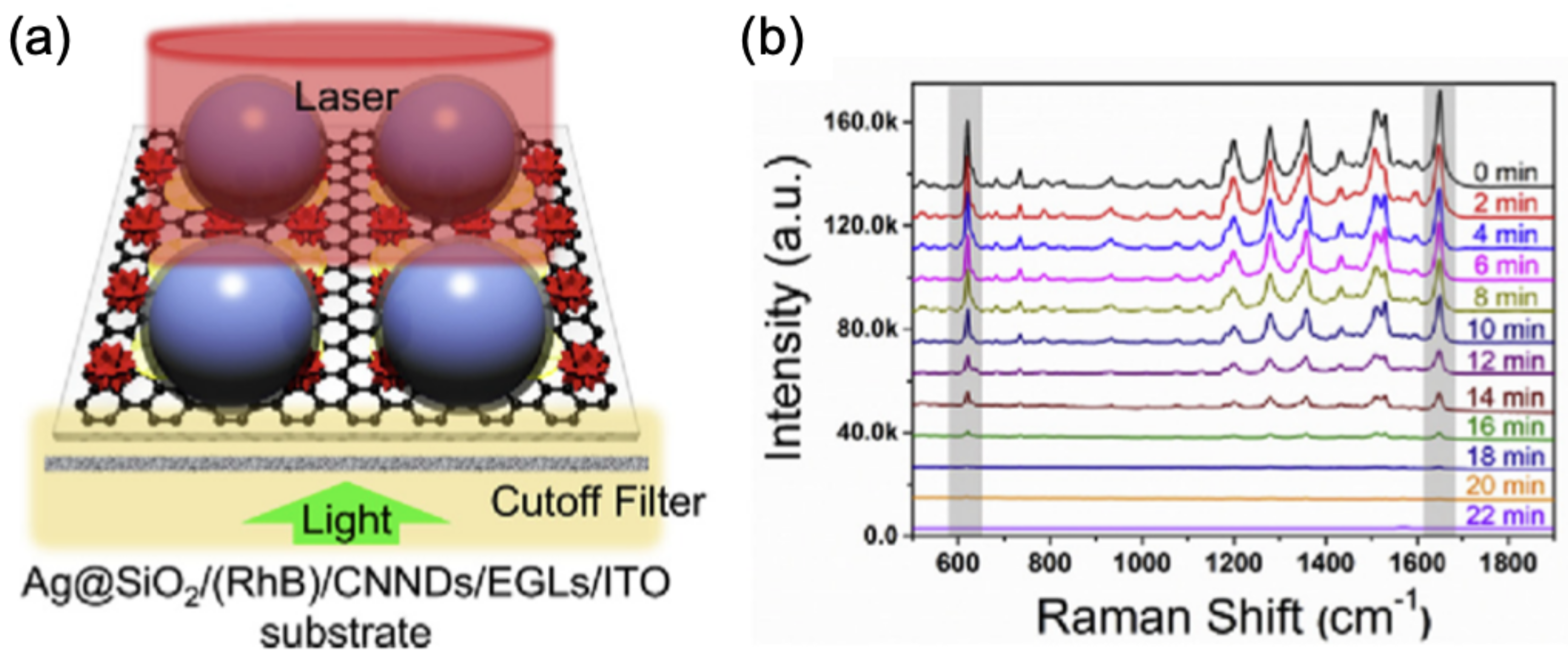
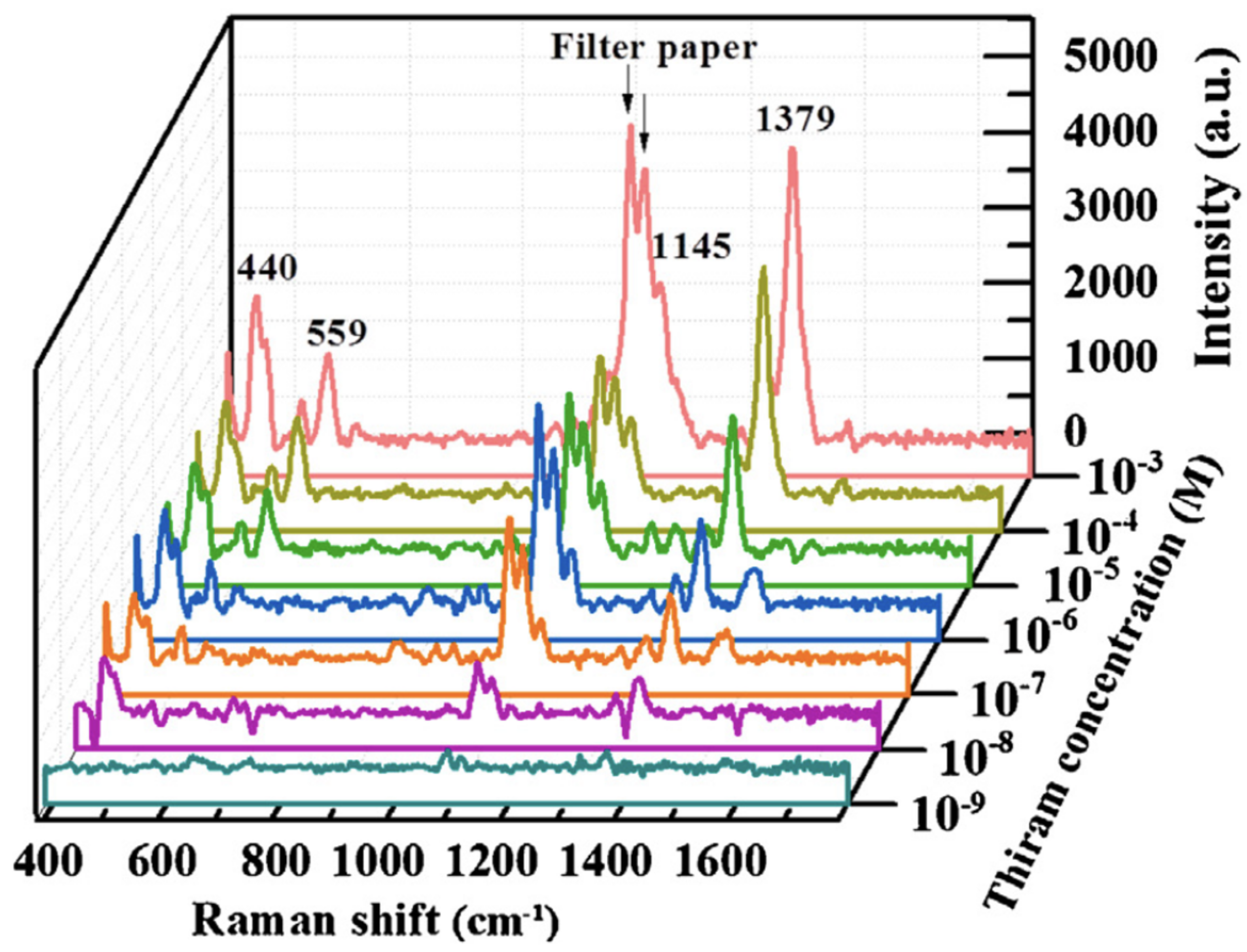
| Samples | Core/Shell Size (nm) | Reaction | Refs |
|---|---|---|---|
| PtFe/Au@SiO NPs | 120/2 | CO oxidation | [82] |
| Pt/Au@SiO NPs | 55/2 | CO electrooxidation | [83] |
| Pt/Au@SiO NPs | 101/2 | CO adsorption | [84] |
| Ni/Au@SiO NPs | 90/2 | CO adsorption | [85] |
| Au@SiO NPs, Au@TiO NPs | 90/3–105/2 | Hydrogenation/dissociation of CO | [86] |
| Pt/Au@G NPs | 500/5 layers of G | CO adsorption | [87] |
| Au@SiO NPs on Cu foil | 50/2 | CO adsorption | [88,89] |
| Samples | Core/Shell Size (nm) | Reaction | Refs |
|---|---|---|---|
| Au@SiO NPs/Pt | 90/2 | Adsorption of Propargyl Alcohol | [90] |
| Au@SiO NPs/IrO surface | 55/2 | Water oxidation | [91] |
| Au@SiO NPs/Au(111) | 55/2 | Configuration of interfacial water | [92] |
| Au@SiO NPs/pyrite | 55/2 | Adsorption of MBT | [93] |
| Au@SiO NPs/Cu surface | 55/2 | Oxidation of Cu surfaces | [94] |
| Au@SiO NPs/Pt() | 55/2–3 | Oxygen reduction reaction | [95] |
| Au@SiO NPs/Pt() | 55/2 | Oxygen reduction reaction | [96] |
| Au@SiO NPs/Pt() | 55/2 | Oxygen reduction reaction | [97] |
| Au@SnO NPs/steel surface | 35/4 | Steel surface corrosion | [98] |
| Pt/Au@SiO NPs | 120/2 | Hydrogenation of pNTP | [99] |
| Ag@SiO/RhB/CNND/EGL/ITO | 75/3 | Photodegradation of RhB | [100] |
| Samples | Core/Shell Size (nm) | Detection | Refs |
|---|---|---|---|
| (Au/SiO)@SiO NPs on Si | (61/900)/1–2 | Rhodamine 6G | [101] |
| Au@SiO NPs on TiO(hkl) | 55/2 | Photoinduced behavior of dyes | [102] |
| Ag@SiO NPs on filter paper | 45/3 | Thiram | [103] |
| Ag@TiO NPs | 10-30/2–10 | Copper ions | [104] |
| Ag@TiO NPs | 20/2–10 | Copper oxidation states | [105] |
| cc-Au@Ag@SiO NPs | 50/4/2–5 | 4-mercaptobenzoic acid | [106] |
| Au@PPy bipyramids | 100 (length)/1 | -aminobutyric acid | [108] |
| Au@SiO NPs | 40/5 | Metschnikowia pulcherrima yeast cells | [109] |
| Au@SiO NPs | 55/2 | Atypical hyperplasia | [110] |
| Ag@SiO NPs | 53/2–5 | Tumor cells in blood | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbillon, G. Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Photonics 2021, 8, 46. https://doi.org/10.3390/photonics8020046
Barbillon G. Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Photonics. 2021; 8(2):46. https://doi.org/10.3390/photonics8020046
Chicago/Turabian StyleBarbillon, Grégory. 2021. "Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy" Photonics 8, no. 2: 46. https://doi.org/10.3390/photonics8020046
APA StyleBarbillon, G. (2021). Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Photonics, 8(2), 46. https://doi.org/10.3390/photonics8020046




