Optogenetics in Brain Research: From a Strategy to Investigate Physiological Function to a Therapeutic Tool
Abstract
1. Introduction
1.1. Opsins: An Optical Tool to Manipulate In-Vivo Neural Networks
1.2. Light Sources and Light Delivery Systems
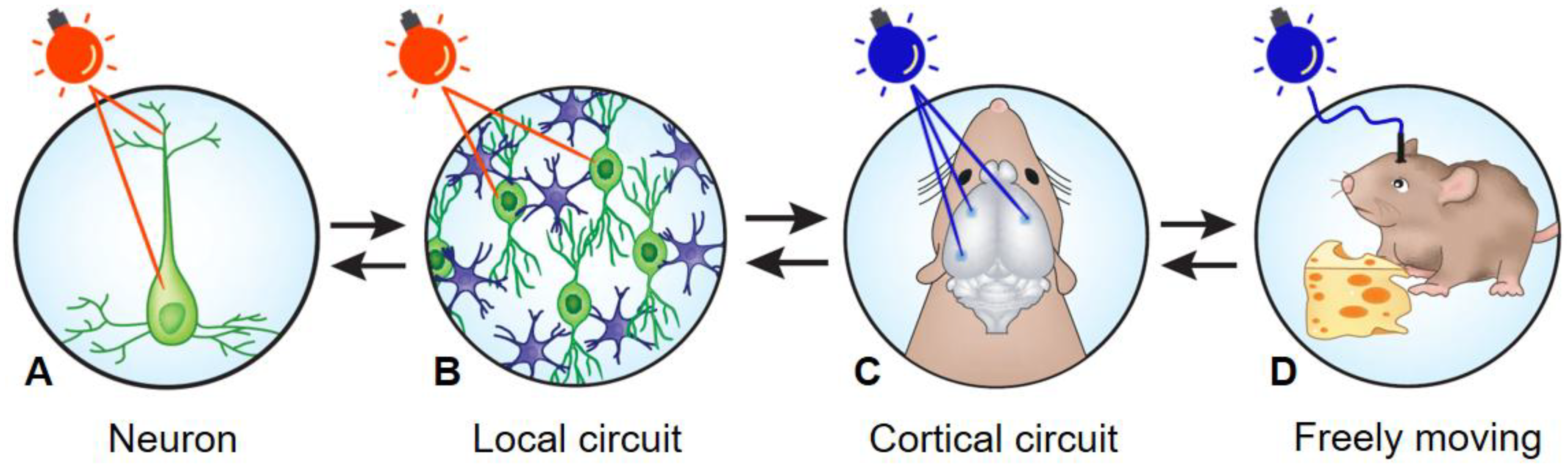
2. Towards a Deeper Understanding of Brain Circuits Functions, Using Optogenetics
3. Optogenetics to Dissect Disease Neural Circuitry
4. Light as Rehabilitative Strategy in Pre-Clinical Models
4.1. Parkinson’s Disease
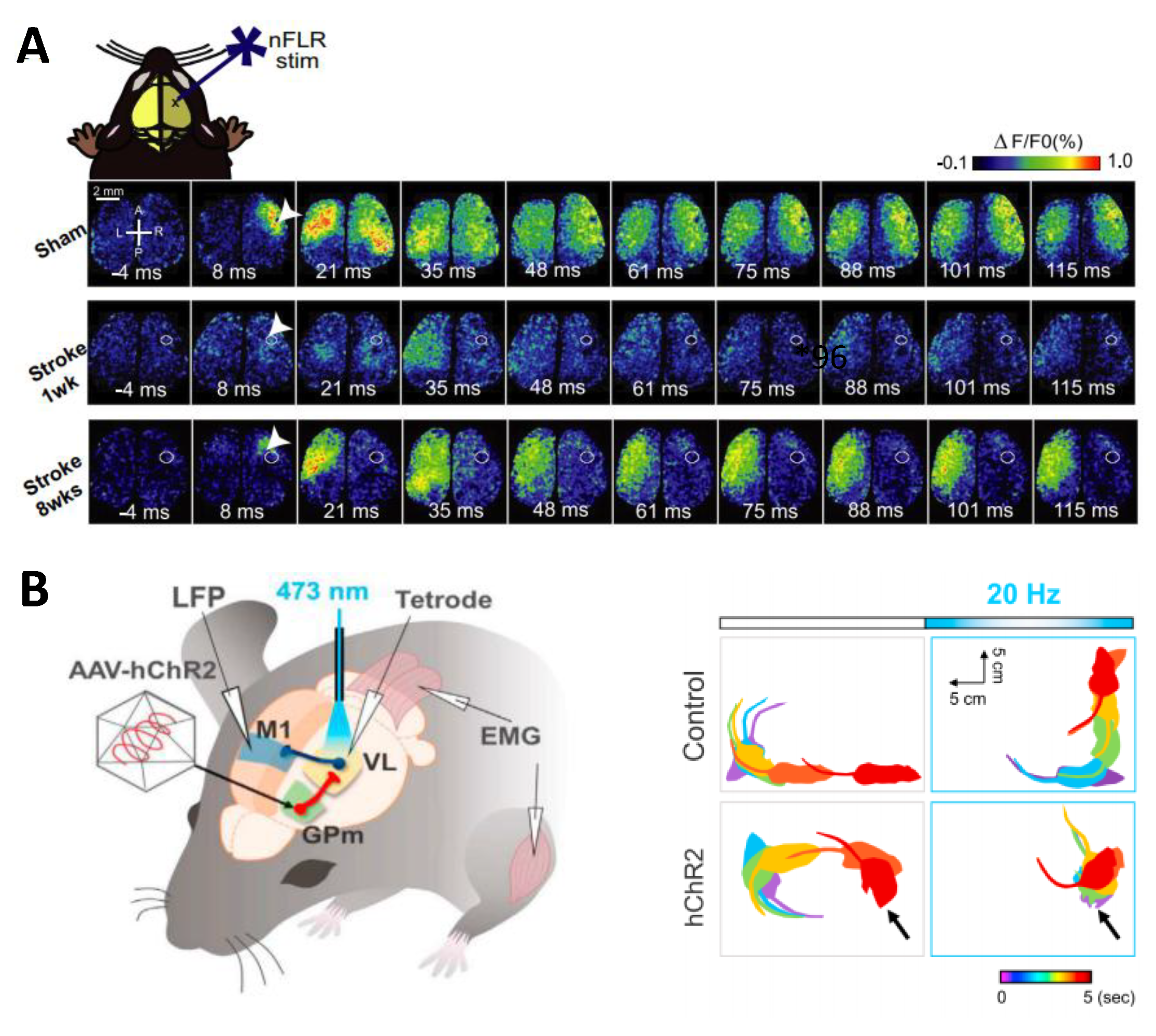
4.2. Stroke
4.3. Other Therapeutic Applications
5. The Future of Optogenetics
Author Contributions
Funding
Conflicts of Interest
References
- Kravitz, A.V.; Kreitzer, A.C. Optogenetic manipulation of neural circuitry in vivo. Curr. Opin. Neurobiol. 2011, 21, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.E.; de Lecea, L. Optogenetic investigation of neural circuits in vivo. Trends Mol. Med. 2011, 17, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Deisseroth, K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 2012, 13, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Tang, B.; Jiang, H. Optogenetic investigation of neuropsychiatric diseases. Int. J. Neurosci. 2013, 123, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, P.; Sigrist, S. Optogenetics; De gruyter: Vienna, Austria, 2013. [Google Scholar]
- Eickelbeck, D.; Karapinar, R.; Herlitze, S.; Spoida, K. Optogenetic Approaches for Controlling Neuronal Activity and Plasticity. In Handbook of In Vivo Neural Plasticity Techniques; Elsevier: Amsterdam, The Netherlands, 2018; pp. 285–310. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.P.; Brauner, M.; Liewald, J.F.; Kay, K.; Watzke, N.; Wood, P.G.; Bamberg, E.; Nagel, G.; Gottschalk, A.; et al. Multimodal fast optical interrogation of neural circuitry. Nature 2007, 446, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y. A user’s guide to channelrhodopsin variants: Features, limitations and future developments. Exp. Physiol. 2011, 96, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y. Optogenetic excitation of neurons with channelrhodopsins: Light instrumentation, expression systems, and channelrhodopsin variants. Prog. Brain Res. 2012, 196, 29–47. [Google Scholar] [CrossRef]
- Raimondo, J.V.; Kay, L.; Ellender, T.J.; Akerman, C.J. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat. Neurosci. 2012, 15, 1102–1104. [Google Scholar] [CrossRef]
- Karra, D.; Dahm, R. Transfection techniques for neuronal cells. J. Neurosci. 2010, 30, 6171–6177. [Google Scholar] [CrossRef]
- Murlidharan, G.; Samulski, R.J.; Asokan, A. Biology of adeno-associated viral vectors in the central nervous system. Front. Mol. Neurosci. 2014, 7, 76. [Google Scholar] [CrossRef]
- Zingg, B.; Chou, X.L.; Zhang, Z.G.; Mesik, L.; Liang, F.; Tao, H.W.; Zhang, L.I. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 2017, 93, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.H.; Jaeger, D. Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol. Dis. 2016, 95, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Madisen, L. Mouse transgenic approaches in optogenetics. Prog. Brain Res. 2012, 196, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.C.; Ayling, O.G.; Murphy, T.H. Distinct cortical circuit mechanisms for complex forelimb movement and motor map topography. Neuron 2012, 74, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Hausser, M. Optogenetics: The age of light. Nat. Methods 2014, 11, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, N.A.; Buetfering, C.; Lecoq, J.; Lee, C.R.; Peters, A.J.; Jacobs, E.A.K.; Coen, P.; Ollerenshaw, D.R.; Valley, M.T.; de Vries, S.E.J.; et al. Aberrant Cortical Activity in Multiple GCaMP6-Expressing Transgenic Mouse Lines. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Shao, Y.R.; Chung, J.; Pourzia, O.; Feldman, D.E. Long-term channelrhodopsin-2 (ChR2) expression can induce abnormal axonal morphology and targeting in cerebral cortex. Front. Neural Circuits 2013, 7, 8. [Google Scholar] [CrossRef]
- Wang, X. Cre transgenic mouse lines. Methods Mol. Biol. 2009, 561, 265–273. [Google Scholar] [CrossRef]
- Ferenczi, E.A.; Vierock, J.; Atsuta-Tsunoda, K.; Tsunoda, S.P.; Ramakrishnan, C.; Gorini, C.; Thompson, K.; Lee, S.Y.; Berndt, A.; Perry, C.; et al. Optogenetic approaches addressing extracellular modulation of neural excitability. Sci. Rep. 2016, 6, 23947. [Google Scholar] [CrossRef]
- Forli, A.; Vecchia, D.; Binini, N.; Succol, F.; Bovetti, S.; Moretti, C.; Nespoli, F.; Mahn, M.; Baker, C.A.; Bolton, M.M.; et al. Two-Photon Bidirectional Control and Imaging of Neuronal Excitability with High Spatial Resolution In Vivo. Cell Rep. 2018, 22, 3087–3098. [Google Scholar] [CrossRef]
- Simonova, N.A.; Bal, N.V.; Balaban, P.M.; Volgushev, M.A.; Malyshev, A.Y. An Optogenetic Approach to Studies of the Mechanisms of Heterosynaptic Plasticity in Neocortical Neurons. Neurosci. Behav. Physiol. 2019, 49, 208–215. [Google Scholar] [CrossRef]
- Hira, R.; Terada, S.; Kondo, M.; Matsuzaki, M. Distinct Functional Modules for Discrete and Rhythmic Forelimb Movements in the Mouse Motor Cortex. J. Neurosci. 2015, 35, 13311–13322. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.T. The ripple effect of a single neuron. Nature 2019, 567, 320–321. [Google Scholar] [CrossRef]
- Papagiakoumou, E. Optical developments for optogenetics. Biol. Cell 2013, 105, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Oron, D.; Papagiakoumou, E.; Anselmi, F.; Emiliani, V. Two-photon optogenetics. Prog. Brain Res. 2012, 196, 119–143. [Google Scholar] [CrossRef]
- Packer, A.M.; Roska, B.; Hausser, M. Targeting neurons and photons for optogenetics. Nat. Neurosci. 2013, 16, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, S.B.; Ribeiro, J.F.; Silva, A.F.; Costa, R.M.; Correia, J.H. Design and manufacturing challenges of optogenetic neural interfaces: A review. J. Neural Eng. 2017, 14, 041001. [Google Scholar] [CrossRef]
- Grossman, N.; Poher, V.; Grubb, M.S.; Kennedy, G.T.; Nikolic, K.; McGovern, B.; Berlinguer Palmini, R.; Gong, Z.; Drakakis, E.M.; Neil, M.A.; et al. Multi-site optical excitation using ChR2 and micro-LED array. J. Neural Eng. 2010, 7, 16004. [Google Scholar] [CrossRef] [PubMed]
- Scharf, R.; Tsunematsu, T.; McAlinden, N.; Dawson, M.D.; Sakata, S.; Mathieson, K. Depth-specific optogenetic control in vivo with a scalable, high-density muLED neural probe. Sci. Rep. 2016, 6, 28381. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, N.; Gu, E.; Dawson, M.D.; Sakata, S.; Mathieson, K. Optogenetic activation of neocortical neurons in vivo with a sapphire-based micro-scale LED probe. Front. Neural Circuits 2015, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Stark, E.; Ku, P.C.; Wise, K.D.; Buzsaki, G.; Yoon, E. Monolithically Integrated muLEDs on Silicon Neural Probes for High-Resolution Optogenetic Studies in Behaving Animals. Neuron 2015, 88, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Silasi, G.; Boyd, J.D.; Ledue, J.; Murphy, T.H. Improved methods for chronic light-based motor mapping in mice: Automated movement tracking with accelerometers, and chronic EEG recording in a bilateral thin-skull preparation. Front. Neural Circuits 2013, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Vanni, M.P.; Chan, A.W.; Balbi, M.; Silasi, G.; Murphy, T.H. Mesoscale Mapping of Mouse Cortex Reveals Frequency-Dependent Cycling between Distinct Macroscale Functional Modules. J. Neurosci. 2017, 37, 7513–7533. [Google Scholar] [CrossRef] [PubMed]
- Papagiakoumou, E.; Bègue, A.; Leshem, B.; Schwartz, O.; Stell, B.M.; Bradley, J.; Oron, D.; Emiliani, V. Functional patterned multiphoton excitation deep inside scattering tissue. Nat. Photonics 2013, 7, 274–278. [Google Scholar] [CrossRef]
- Portugues, R.; Severi, K.E.; Wyart, C.; Ahrens, M.B. Optogenetics in a transparent animal: Circuit function in the larval zebrafish. Curr. Opin. Neurobiol. 2013, 23, 119–126. [Google Scholar] [CrossRef]
- Jacques, S.L. Corrigendum: Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 5007–5008. [Google Scholar] [CrossRef]
- Schultz, S.R.; Copeland, C.S.; Foust, A.J.; Quicke, P.; Schuck, R. Advances in two photon scanning and scanless microscopy technologies for functional neural circuit imaging. Proc. IEEE Inst. Electr. Electron. Eng. 2017, 105, 139–157. [Google Scholar] [CrossRef]
- Saggau, P. New methods and uses for fast optical scanning. Curr. Opin. Neurobiol. 2006, 16, 543–550. [Google Scholar] [CrossRef]
- Packer, A.M.; Peterka, D.S.; Hirtz, J.; Prakash, R.; Deisseroth, K.; Yuste, R. Two-photon optogenetics of dendritic spines and neural circuits in 3D. Nat. Methods 2012, 9, 1202–1205. [Google Scholar] [CrossRef]
- Papagiakoumou, E.; Anselmi, F.; Begue, A.; de Sars, V.; Gluckstad, J.; Isacoff, E.Y.; Emiliani, V. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 2010, 7, 848–854. [Google Scholar] [CrossRef]
- Rickgauer, J.P.; Tank, D.W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 2009, 106, 15025–15030. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, O.A.; Tanese, D.; Zampini, V.; Linghu, C.; Piatkevich, K.; Ronzitti, E.; Papagiakoumou, E.; Boyden, E.S.; Emiliani, V. Temporally precise single-cell-resolution optogenetics. Nat. Neurosci. 2017, 20, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Ronzitti, E.; Conti, R.; Zampini, V.; Tanese, D.; Foust, A.J.; Klapoetke, N.; Boyden, E.S.; Papagiakoumou, E.; Emiliani, V. Submillisecond Optogenetic Control of Neuronal Firing with Two-Photon Holographic Photoactivation of Chronos. J. Neurosci. 2017, 37, 10679–10689. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; LeDue, J.M.; Mohajerani, M.H.; Murphy, T.H. Optogenetic mapping after stroke reveals network-wide scaling of functional connections and heterogeneous recovery of the peri-infarct. J. Neurosci. 2014, 34, 16455–16466. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Allegra Mascaro, A.; Pavone, F. Large Scale Double-Path Illumination System with Split Field of View for the All-Optical Study of Inter-and Intra-Hemispheric Functional Connectivity on Mice. Methods Protoc. 2019, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.V.; Hart, A.C.; Ramanathan, S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat. Methods 2009, 6, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Ledue, J.; Mohajerani, M.H.; Vanni, M.P.; Murphy, T.H. Optogenetic approaches for functional mouse brain mapping. Front. Neurosci. 2013, 7, 54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prakash, R.; Yizhar, O.; Grewe, B.; Ramakrishnan, C.; Wang, N.; Goshen, I.; Packer, A.M.; Peterka, D.S.; Yuste, R.; Schnitzer, M.J.; et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods 2012, 9, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Carmi, I.; De Battista, M.; Maddalena, L.; Carroll, E.C.; Kienzler, M.A.; Berlin, S. Holographic two-photon activation for synthetic optogenetics. Nat. Protoc. 2019, 14, 864–900. [Google Scholar] [CrossRef] [PubMed]
- Nikolenko, V.; Watson, B.O.; Araya, R.; Woodruff, A.; Peterka, D.S.; Yuste, R. SLM Microscopy: Scanless Two-Photon Imaging and Photostimulation with Spatial Light Modulators. Front. Neural Circuits 2008, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Huang, R.-P.; Tsai, P.-S.; Lee, C.-H. Wide-field super-resolution optical sectioning microscopy using a single spatial light modulator. J. Opt. A Pure Appl. Opt. 2009, 11. [Google Scholar] [CrossRef]
- Yang, W.; Carrillo-Reid, L.; Bando, Y.; Peterka, D.S.; Yuste, R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. eLife 2018, 7, e32671. [Google Scholar] [CrossRef] [PubMed]
- Dal Maschio, M.; Donovan, J.C.; Helmbrecht, T.O.; Baier, H. Linking Neurons to Network Function and Behavior by Two-Photon Holographic Optogenetics and Volumetric Imaging. Neuron 2017, 94, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Rickgauer, J.P.; Deisseroth, K.; Tank, D.W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 2014, 17, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Pegard, N.C.; Mardinly, A.R.; Oldenburg, I.A.; Sridharan, S.; Waller, L.; Adesnik, H. Three-dimensional scanless holographic optogenetics with temporal focusing (3D-SHOT). Nat. Commun. 2017, 8, 1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.W.; Ronzitti, E.; Lee, B.R.; Daigle, T.L.; Dalkara, D.; Zeng, H.; Emiliani, V.; Papagiakoumou, E. In vivo sub-millisecond two-photon optogenetics with temporally focused patterned light. J. Neurosci. 2019. [Google Scholar] [CrossRef]
- Sileo, L.; Pisanello, M.; della Patria, A.; Emhara, M.S.; Pisanello, F.; De Vittorio, M. Optical Fiber Technologies for in-vivo Light Delivery and Optogenetics. In Proceedings of the 2015 17th International Conference on Transparent Optical Networks (ICTON), Budapest, Hungary, 5–9 July 2015. [Google Scholar] [CrossRef]
- Nazempour, R.; Zhang, Q.; Fu, R.; Sheng, X. Biocompatible and Implantable Optical Fibers and Waveguides for Biomedicine. Materials 2018, 11, 1283. [Google Scholar] [CrossRef]
- Yoon, H.H.; Park, J.H.; Kim, Y.H.; Min, J.; Hwang, E.; Lee, C.J.; Suh, J.K.; Hwang, O.; Jeon, S.R. Optogenetic inactivation of the subthalamic nucleus improves forelimb akinesia in a rat model of Parkinson disease. Neurosurgery 2014, 74, 533–540. [Google Scholar] [CrossRef]
- Pisanello, M.; Pisano, F.; Sileo, L.; Maglie, E.; Bellistri, E.; Spagnolo, B.; Mandelbaum, G.; Sabatini, B.L.; De Vittorio, M.; Pisanello, F. Tailoring light delivery for optogenetics by modal demultiplexing in tapered optical fibers. Sci. Rep. 2018, 8, 4467. [Google Scholar] [CrossRef]
- Pisanello, F.; Mandelbaum, G.; Pisanello, M.; Oldenburg, I.A.; Sileo, L.; Markowitz, J.E.; Peterson, R.E.; Della Patria, A.; Haynes, T.M.; Emara, M.S.; et al. Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nat. Neurosci. 2017, 20, 1180–1188. [Google Scholar] [CrossRef]
- Arlow, R.L.; Foutz, T.J.; McIntyre, C.C. Theoretical principles underlying optical stimulation of myelinated axons expressing channelrhodopsin-2. Neuroscience 2013, 248, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, K.L.; Yeh, A.J.; Ho, J.S.; Tsao, V.; Mohan Iyer, S.; Grosenick, L.; Ferenczi, E.A.; Tanabe, Y.; Deisseroth, K.; Delp, S.L.; et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 2015, 12, 969–974. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.G.; Kim, T.I.; Shin, G.; Huang, X.; Jung, Y.H.; Al-Hasani, R.; Omenetto, F.G.; Bruchas, M.R.; Rogers, J.A. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat. Protoc. 2013, 8, 2413–2428. [Google Scholar] [CrossRef] [PubMed]
- Kale, R.P.; Kouzani, A.Z.; Berk, M.; Walder, K.; Berk, J.; Tye, S.J. Wireless Optogenetics: An Exploration of Portable Microdevices for Small Animal Photostimulation. Procedia Technol. 2015, 20, 225–230. [Google Scholar] [CrossRef][Green Version]
- Kwon, K.Y.; Lee, H.M.; Ghovanloo, M.; Weber, A.; Li, W. Design, fabrication, and packaging of an integrated, wirelessly-powered optrode array for optogenetics application. Front. Syst. Neurosci. 2015, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, C.; Liu, Z.; Luo, W.; Li, L.; Cai, X.; Liang, D.; Su, Y.; Ding, H.; Wang, Q.; et al. Wirelessly Operated, Implantable Optoelectronic Probes for Optogenetics in Freely Moving Animals. IEEE Trans. Electron Devices 2019, 66, 785–792. [Google Scholar] [CrossRef]
- Emara, M.S.; Pisanello, M.; Sileo, L.; De Vittorio, M.; Pisanello, F. A Wireless Head-mountable Device with Tapered Optical Fiber-coupled Laser Diode for Light Delivery in Deep Brain Regions. IEEE Trans. Biomed. Eng. 2018, 66, 1996–2009. [Google Scholar] [CrossRef]
- Owen, S.F.; Liu, M.H.; Kreitzer, A.C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 2019, 22, 1061–1065. [Google Scholar] [CrossRef]
- Gysbrechts, B.; Wang, L.; Trong, N.N.; Cabral, H.; Navratilova, Z.; Battaglia, F.; Saeys, W.; Bartic, C. Light distribution and thermal effects in the rat brain under optogenetic stimulation. J. Biophotonics 2016, 9, 576–585. [Google Scholar] [CrossRef]
- Ait Ouares, K.; Beurrier, C.; Canepari, M.; Laverne, G.; Kuczewski, N. Opto nongenetics inhibition of neuronal firing. Eur. J. Neurosci. 2019, 49, 6–26. [Google Scholar] [CrossRef]
- Jazayeri, M.; Lindbloom-Brown, Z.; Horwitz, G.D. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat. Neurosci. 2012, 15, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Soma, S.; Yoshida, J.; Kato, S.; Takahashi, Y.; Nonomura, S.; Sugimura, Y.K.; Rios, A.; Kawabata, M.; Kobayashi, K.; Kato, F.; et al. Ipsilateral-Dominant Control of Limb Movements in Rodent Posterior Parietal Cortex. J. Neurosci. 2019, 39, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Ren, C.; Liu, H.; Kim, A.N.; Kondapaneni, N.; Liu, X.; Kuzum, D.; Komiyama, T. Transformation of Cortex-wide Emergent Properties during Motor Learning. Neuron 2017, 94, 880–890.e888. [Google Scholar] [CrossRef] [PubMed]
- Montagni, E.; Resta, F.; Conti, E.; Scaglione, A.; Pasquini, M.; Micera, S.; Mascaro, A.L.A.; Pavone, F.S. Wide-field imaging of cortical neuronal activity with red-shifted functional indicators during motor task execution. J. Phys. D Appl. Phys. 2019, 52. [Google Scholar] [CrossRef]
- Choi, J.H.; Koch, K.P.; Poppendieck, W.; Lee, M.; Shin, H.S. High resolution electroencephalography in freely moving mice. J. Neurophysiol. 2010, 104, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Ferezou, I.; Haiss, F.; Gentet, L.J.; Aronoff, R.; Weber, B.; Petersen, C.C. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 2007, 56, 907–923. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef]
- Akerboom, J.; Carreras Calderon, N.; Tian, L.; Wabnig, S.; Prigge, M.; Tolo, J.; Gordus, A.; Orger, M.B.; Severi, K.E.; Macklin, J.J.; et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013, 6, 2. [Google Scholar] [CrossRef]
- Miesenböck, G. The Optogenetic Catechism. Science 2009, 326, 395–399. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef]
- Britt, J.P.; McDevitt, R.A.; Bonci, A. Use of channelrhodopsin for activation of CNS neurons. Curr. Protoc. Neurosci. 2012. [Google Scholar] [CrossRef]
- Freeze, B.S.; Kravitz, A.V.; Hammack, N.; Berke, J.D.; Kreitzer, A.C. Control of basal ganglia output by direct and indirect pathway projection neurons. J. Neurosci. 2013, 33, 18531–18539. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.W.; Dombeck, D.A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 2016, 535, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Warden, M.R.; Selimbeyoglu, A.; Mirzabekov, J.J.; Lo, M.; Thompson, K.R.; Kim, S.Y.; Adhikari, A.; Tye, K.M.; Frank, L.M.; Deisseroth, K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 2012, 492, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.E.; Li, H.; Jin, X. Optogenetic Editing Reveals the Hierarchical Organization of Learned Action Sequences. Cell 2018, 174, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiao, Y.; Dai, Z.; Sui, N.; Shen, F.; Zhang, J.; Liang, J. Medium spiny neurons of the anterior dorsomedial striatum mediate reversal learning in a cell-type-dependent manner. Brain Struct. Funct. 2018, 224, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Knopfel, T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 2012, 13, 687–700. [Google Scholar] [CrossRef]
- Emiliani, V.; Cohen, A.E.; Deisseroth, K.; Hausser, M. All-Optical Interrogation of Neural Circuits. J. Neurosci. 2015, 35, 13917–13926. [Google Scholar] [CrossRef]
- Bando, Y.; Sakamoto, M.; Kim, S.; Ayzenshtat, I.; Yuste, R. Comparative Evaluation of Genetically Encoded Voltage Indicators. Cell Rep. 2019, 26, 802–813. [Google Scholar] [CrossRef]
- Tian, L.; Akerboom, J.; Schreiter, E.R.; Looger, L.L. Neural activity imaging with genetically encoded calcium indicators. Prog. Brain Res. 2012, 196, 79–94. [Google Scholar] [CrossRef]
- Kim, C.K.; Adhikari, A.; Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 2017, 18, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Chemla, S.; Chavane, F. Voltage-sensitive dye imaging: Technique review and models. J. Physiol. Paris 2010, 104, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Labat-gest, V.; Tomasi, S. Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, S.; Anenberg, E.; Murphy, T.H. Resistance of optogenetically evoked motor function to global ischemia and reperfusion in mouse in vivo. J. Cereb. Blood Flow Metab. 2013, 33, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.; Nakajima, R.; Shin, A.; Jeong, M.; Park, A.H.; Jeong, Y.; Jo, S.; Yang, S.; Park, H.; et al. Inhibitory Basal Ganglia Inputs Induce Excitatory Motor Signals in the Thalamus. Neuron 2017, 95, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Burguiere, E.; Monteiro, P.; Feng, G.; Graybiel, A.M. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science 2013, 340, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Cela, E.; McFarlan, A.R.; Chung, A.J.; Wang, T.; Chierzi, S.; Murai, K.K.; Sjöström, P.J. An Optogenetic Kindling Model of Neocortical Epilepsy. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013, 493, 532–536. [Google Scholar] [CrossRef]
- Lomarev, M.P.; Kanchana, S.; Bara-Jimenez, W.; Iyer, M.; Wassermann, E.M.; Hallett, M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov. Disord. 2006, 21, 325–331. [Google Scholar] [CrossRef]
- Kubis, N. Non-Invasive Brain Stimulation to Enhance Post-Stroke Recovery. Front. Neural Circuits 2016, 10, 56. [Google Scholar] [CrossRef]
- Pallanti, S.; Bernardi, S. Neurobiology of repeated transcranial magnetic stimulation in the treatment of anxiety: A critical review. Int. Clin. Psychopharmacol. 2009, 24, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.J.; Taylor, J.P. Transcranial magnetic stimulation and transcranial direct current stimulation: Treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers Res. Ther. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Stilling, J.M.; Monchi, O.; Amoozegar, F.; Debert, C.T. Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review. Headache 2019, 59, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Guggisberg, A.G.; Koch, P.J.; Hummel, F.C.; Buetefisch, C. Brain networks and their relevance for stroke rehabilitation. Clin. Neurophysiol. 2019, 130, 1098–1124. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov. Disord. 2006, 21, 1693–1702. [Google Scholar] [CrossRef]
- Naro, A.; Milardi, D.; Russo, M.; Terranova, C.; Rizzo, V.; Cacciola, A.; Marino, S.; Calabro, R.S.; Quartarone, A. Non-invasive Brain Stimulation, a Tool to Revert Maladaptive Plasticity in Neuropathic Pain. Front. Hum. Neurosci. 2016, 10, 376. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Aswendt, M.; Steinberg, G.K. Optogenetic Approaches to Target Specific Neural Circuits in Post-stroke Recovery. Neurotherapeutics 2016, 13, 325–340. [Google Scholar] [CrossRef]
- Benabid, A.L.; Chabardes, S.; Mitrofanis, J.; Pollak, P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 2009, 8, 67–81. [Google Scholar] [CrossRef]
- Wichmann, T.; DeLong, M.R.; Guridi, J.; Obeso, J.A. Milestones in research on the pathophysiology of Parkinson’s disease. Mov. Disord. 2011, 26, 1032–1041. [Google Scholar] [CrossRef]
- Ztaou, S.; Amalric, M. Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochem. Int. 2019, 126, 1–10. [Google Scholar] [CrossRef]
- Seeger-Armbruster, S.; Bosch-Bouju, C.; Little, S.T.; Smither, R.A.; Hughes, S.M.; Hyland, B.I.; Parr-Brownlie, L.C. Patterned, but not tonic, optogenetic stimulation in motor thalamus improves reaching in acute drug-induced Parkinsonian rats. J. Neurosci. 2015, 35, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Viana Magno, L.A.; Tenza-Ferrer, H.; Collodetti, M.; Felipe Guimaraes Aguiar, M.; Paula Carneiro Rodrigues, A.; Souza da Silva, R.; do Prado Silva, J.; Ferreira Nicolau, N.; Valadao Freitas Rosa, D.; Birbrair, A.; et al. Optogenetic stimulation of the M2 cortex reverts motor dysfunction in a mouse model of Parkinson’s Disease. J. Neurosci. 2019. [Google Scholar] [CrossRef]
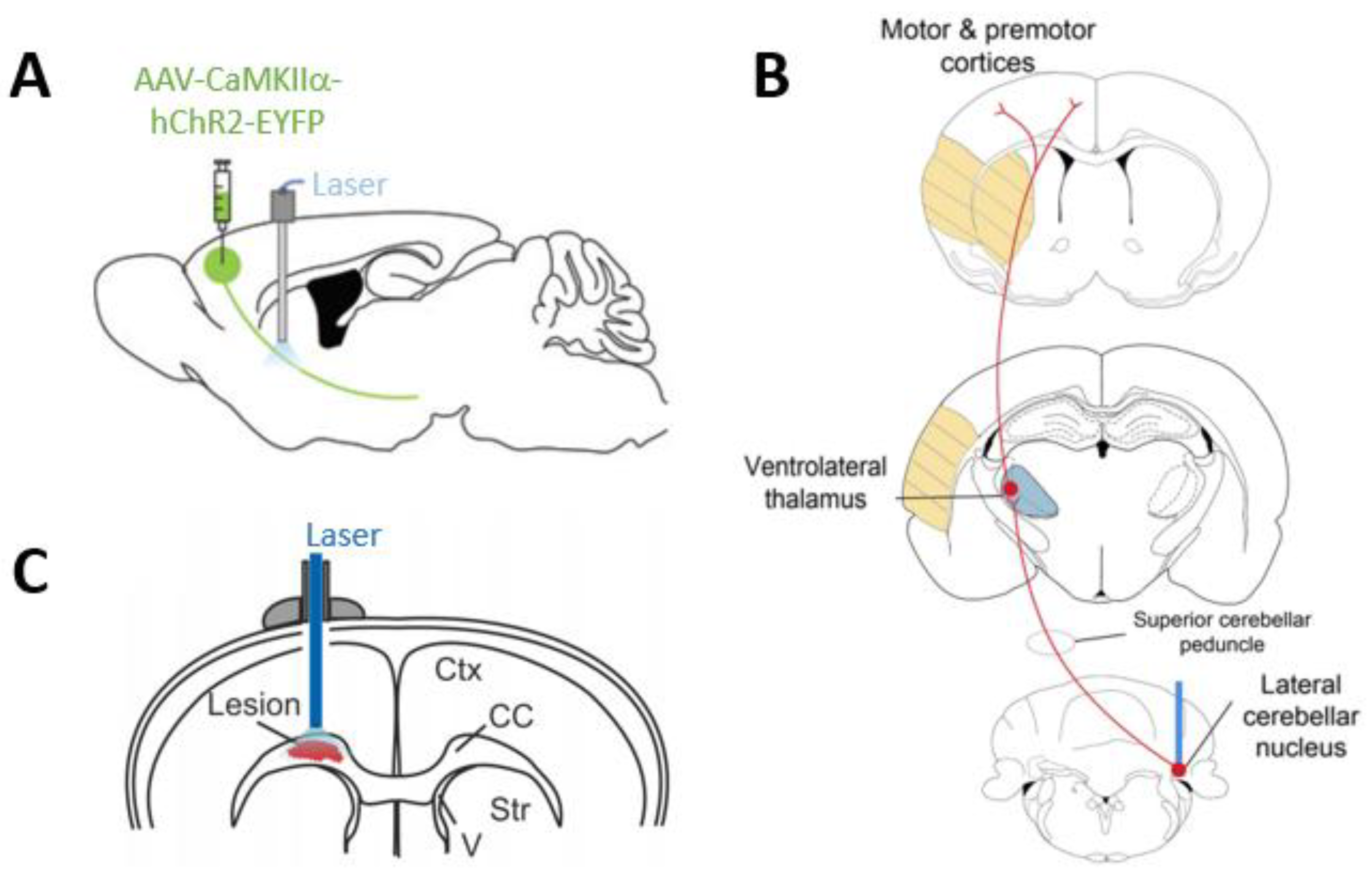
- Shah, A.M.; Ishizaka, S.; Cheng, M.Y.; Wang, E.H.; Bautista, A.R.; Levy, S.; Smerin, D.; Sun, G.; Steinberg, G.K. Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery after stroke. Sci. Rep. 2017, 7, 46612. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.C.; Habermacher, C.; Graciarena, M.; Houry, P.Y.; Nishiyama, A.; Nait-Oumesmar, B.; Angulo, M.C. Neuronal activity in vivo enhances functional myelin repair. JCI Insight 2019, 5, 123434. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson’s disease: Influence of antiparkinsonian treatment and cortical stimulation. Clin. Neurophysiol. 2005, 116, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bordia, T.; Perez, X.A.; Heiss, J.; Zhang, D.; Quik, M. Optogenetic activation of striatal cholinergic interneurons regulates L-dopa-induced dyskinesias. Neurobiol. Dis. 2016, 91, 47–58. [Google Scholar] [CrossRef]
- Jiang, L.; Li, W.; Mamtilahun, M.; Song, Y.; Ma, Y.; Qu, M.; Lu, Y.; He, X.; Zheng, J.; Fu, Z.; et al. Optogenetic Inhibition of Striatal GABAergic Neuronal Activity Improves Outcomes After Ischemic Brain Injury. Stroke 2017, 48, 3375–3383. [Google Scholar] [CrossRef]
- He, X.; Lu, Y.; Lin, X.; Jiang, L.; Tang, Y.; Tang, G.; Chen, X.; Zhang, Z.; Wang, Y.; Yang, G.Y. Optical inhibition of striatal neurons promotes focal neurogenesis and neurobehavioral recovery in mice after middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2017, 37, 837–847. [Google Scholar] [CrossRef]
- Song, M.; Yu, S.P.; Mohamad, O.; Cao, W.; Wei, Z.Z.; Gu, X.; Jiang, M.Q.; Wei, L. Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice. Neurobiol. Dis. 2017, 98, 9–24. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, L.; Li, W.; Qu, M.; Song, Y.; He, X.; Zhang, Z.; Yang, G.Y.; Wang, Y. Optogenetic Inhibition of Striatal Neuronal Activity Improves the Survival of Transplanted Neural Stem Cells and Neurological Outcomes after Ischemic Stroke in Mice. Stem Cells Int. 2017, 2017, 4364302. [Google Scholar] [CrossRef]
- Tennant, K.A.; Taylor, S.L.; White, E.R.; Brown, C.E. Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain. Nat. Commun. 2017, 8, 15879. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Y.; Wang, E.H.; Woodson, W.J.; Wang, S.; Sun, G.; Lee, A.G.; Arac, A.; Fenno, L.E.; Deisseroth, K.; Steinberg, G.K. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 12913–12918. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wu, X.; Ma, H.; Wang, Q.; Wang, Y.; Luo, Y.; Li, C.; Xu, H. Optogenetic Stimulation Enhanced Neuronal Plasticities in Motor Recovery after Ischemic Stroke. Neural Plast. 2019, 2019, 5271573. [Google Scholar] [CrossRef] [PubMed]
- Paz, J.T.; Davidson, T.J.; Frechette, E.S.; Delord, B.; Parada, I.; Peng, K.; Deisseroth, K.; Huguenard, J.R. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 2013, 16, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Covington, H.E., 3rd; Lobo, M.K.; Maze, I.; Vialou, V.; Hyman, J.M.; Zaman, S.; LaPlant, Q.; Mouzon, E.; Ghose, S.; Tamminga, C.A.; et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 2010, 30, 16082–16090. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.D.; Shinohara, R.; Liu, R.J.; Pothula, S.; DiLeone, R.J.; Duman, R.S. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat. Commun. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C.; et al. Adenovirus-Associated Virus Vector–Mediated Gene Transfer in Hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Jaski, B.E.; Jessup, M.L.; Mancini, D.M.; Cappola, T.P.; Pauly, D.F.; Greenberg, B.; Borow, K.; Dittrich, H.; Zsebo, K.M.; Hajjar, R.J.; et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 2009, 15, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J.; et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2011, 124, 304–313. [Google Scholar] [CrossRef]
- Schmieder, F.; Klapper, S.; Koukourakis, N.; Busskamp, V.; Czarske, J. Optogenetic Stimulation of Human Neural Networks Using Fast Ferroelectric Spatial Light Modulator—Based Holographic Illumination. Appl. Sci. 2018, 8, 1180. [Google Scholar] [CrossRef]
- Andersson, M.; Avaliani, N.; Svensson, A.; Wickham, J.; Pinborg, L.H.; Jespersen, B.; Christiansen, S.H.; Bengzon, J.; Woldbye, D.P.; Kokaia, M. Optogenetic control of human neurons in organotypic brain cultures. Sci. Rep. 2016, 6, 24818. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Denison, T. From Optogenetic Technologies to Neuromodulation Therapies. Sci. Transl. Med. 2013, 5, 177ps6. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S. Optogenetics and the future of neuroscience. Nat. Neurosci. 2015, 18, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.Y.; Boyden, E.S. Optogenetics and Translational Medicine. Sci. Transl. Med. 2013, 5, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Picaud, S.; Sahel, J.A.; Roska, B. Optogenetic therapy for retinitis pigmentosa. Gene Ther. 2012, 19, 169–175. [Google Scholar] [CrossRef]
- Steinberg, E.E.; Keiflin, R.; Boivin, J.R.; Witten, I.B.; Deisseroth, K.; Janak, P.H. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 2013, 16, 966–973. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagni, E.; Resta, F.; Mascaro, A.L.A.; Pavone, F.S. Optogenetics in Brain Research: From a Strategy to Investigate Physiological Function to a Therapeutic Tool. Photonics 2019, 6, 92. https://doi.org/10.3390/photonics6030092
Montagni E, Resta F, Mascaro ALA, Pavone FS. Optogenetics in Brain Research: From a Strategy to Investigate Physiological Function to a Therapeutic Tool. Photonics. 2019; 6(3):92. https://doi.org/10.3390/photonics6030092
Chicago/Turabian StyleMontagni, Elena, Francesco Resta, Anna Letizia Allegra Mascaro, and Francesco Saverio Pavone. 2019. "Optogenetics in Brain Research: From a Strategy to Investigate Physiological Function to a Therapeutic Tool" Photonics 6, no. 3: 92. https://doi.org/10.3390/photonics6030092
APA StyleMontagni, E., Resta, F., Mascaro, A. L. A., & Pavone, F. S. (2019). Optogenetics in Brain Research: From a Strategy to Investigate Physiological Function to a Therapeutic Tool. Photonics, 6(3), 92. https://doi.org/10.3390/photonics6030092




