Quantification of Cardiomyocyte Beating Frequency Using Fourier Transform Analysis
Abstract
1. Introduction
2. Materials and Methods
- where F is intensity and t is time. F0, the local minimum intensity, is found by
- where n = 0, 1, 2,… etc.
- whereAnd and
- where n = 0, 1, 2, … etc. and a = max(t)
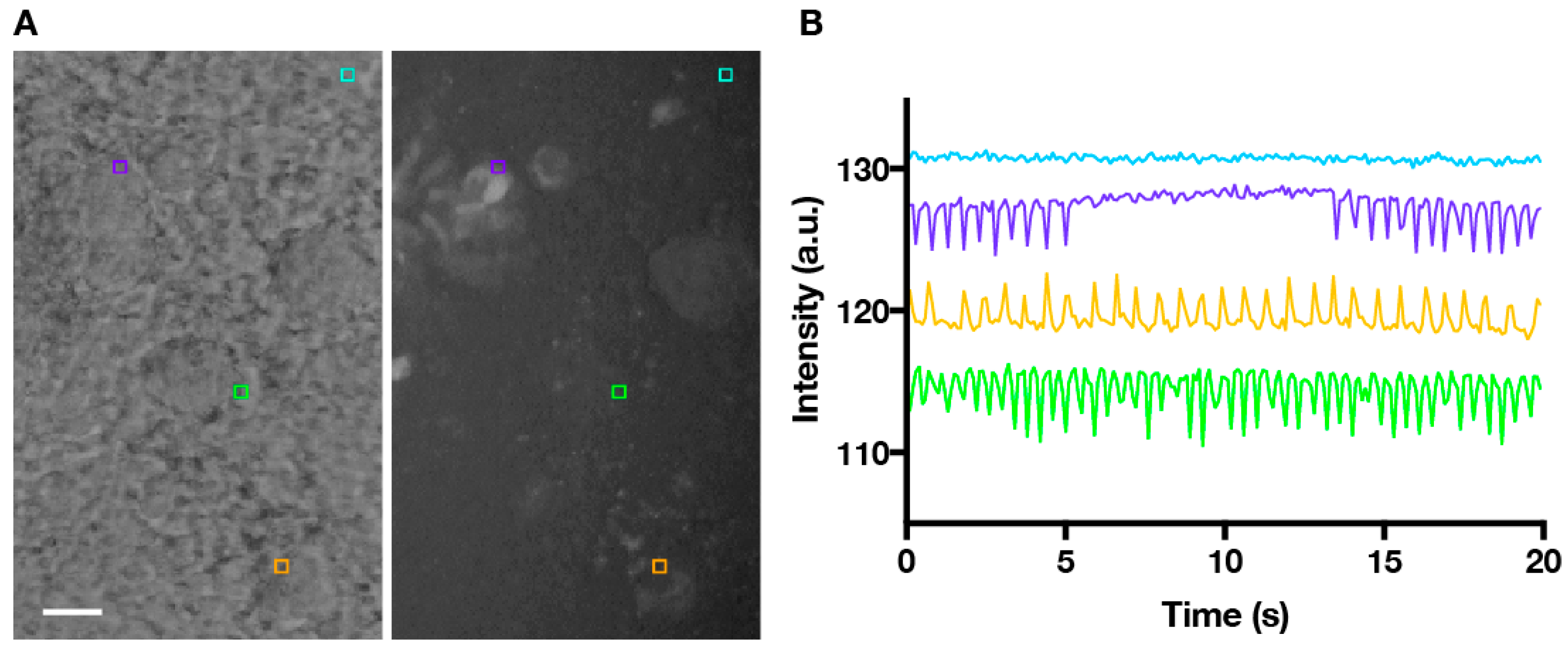
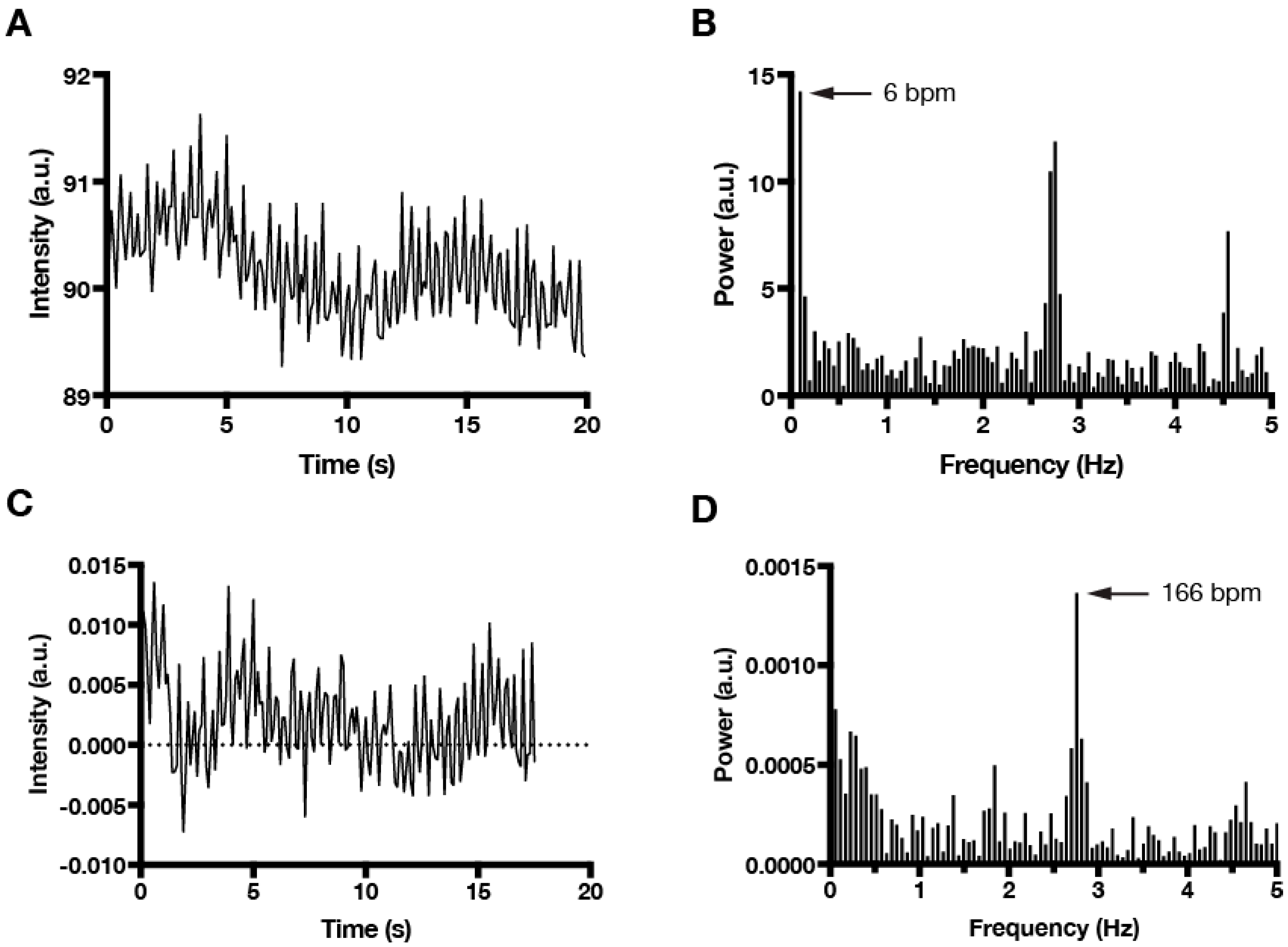
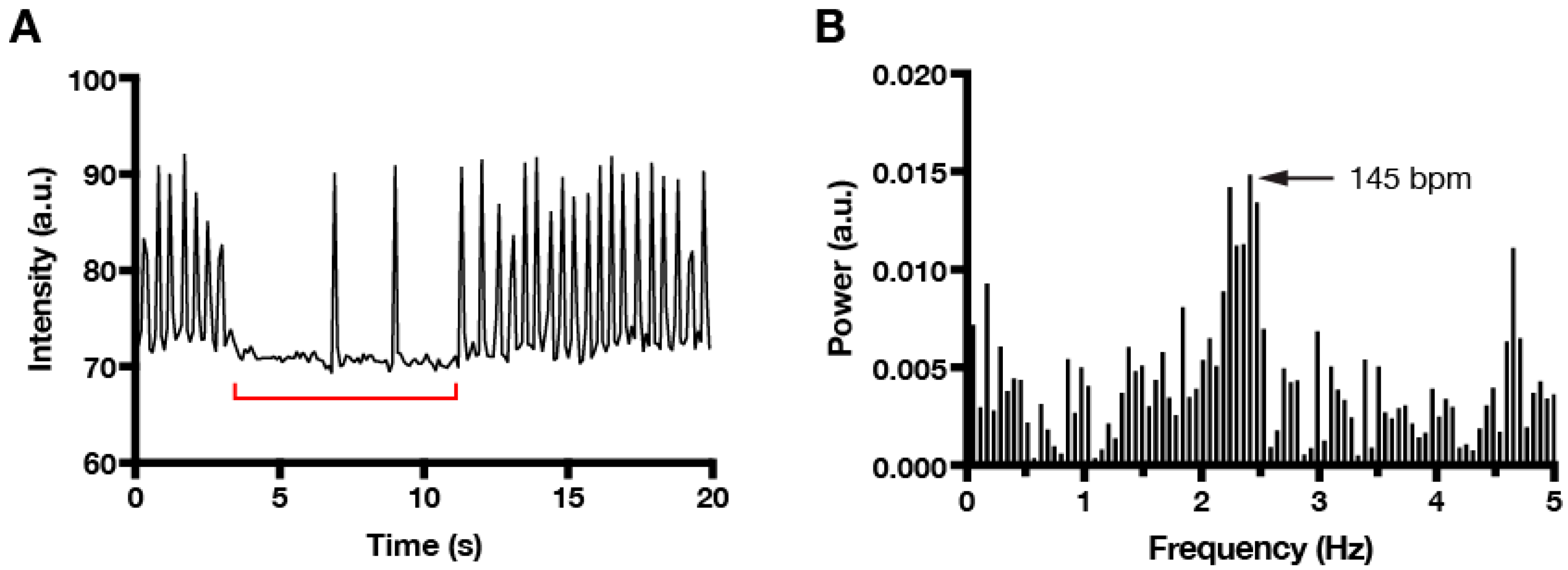
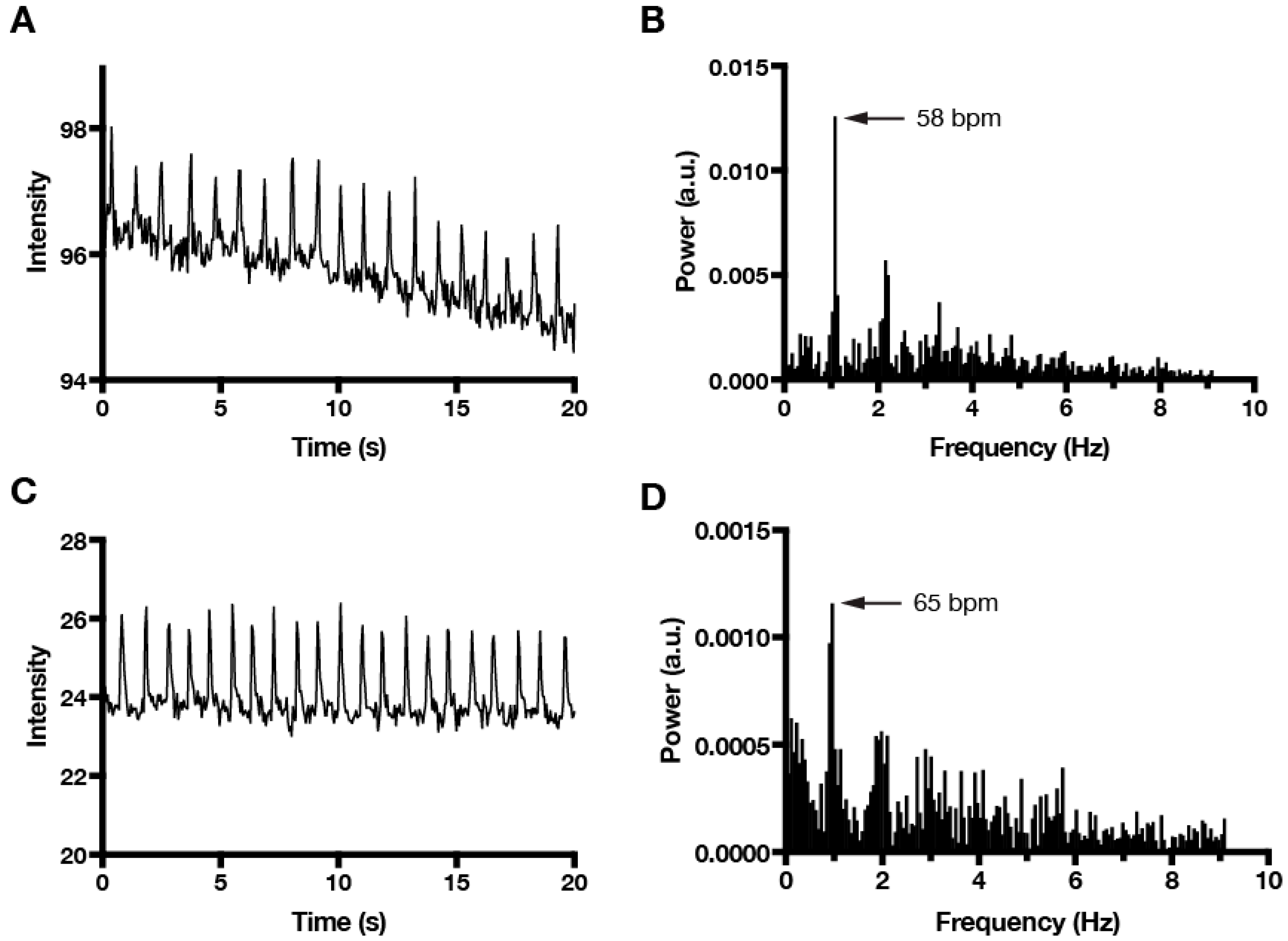
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, J.Q.; Ma, Y.; Lee, Y.; Thomson, J.A.; Kamp, T.J. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ. Res. 2003, 93, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, V.A.; Wobus, A.M.; Rohwedel, J.; Bader, M.; Hescheler, J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ. Res. 1994, 75, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Wobus, A.M.; Kaomei, G.; Shan, J.; Wellner, M.C.; Rohwedel, J.; Ji, G.; Fleischmann, B.; Katus, H.A.; Hescheler, J.; Franz, W.M. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 1997, 29, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Soonpaa, M.H.; Klug, M.G.; Pride, H.P.; Cooper, B.J.; Zipes, D.P.; Field, L.J. Stable fetal cardiomyocyte grafts in the hearts of dystrophic mice and dogs. J. Clin. Investig. 1995, 96, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Plotnikov, A.N.; Danilo, P., Jr.; Shlapakova, I.; Cohen, I.S.; Robinson, R.B.; Rosen, M.R. Expression and function of a biological pacemaker in canine heart. Circulation 2003, 107, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.N.; Sosunov, E.A.; Qu, J.; Shlapakova, I.N.; Anyukhovsky, E.P.; Liu, L.; Janse, M.J.; Brink, P.R.; Cohen, I.S.; Robinson, R.B.; et al. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation 2004, 109, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, A.; Plotnikov, A.N.; Shlapakova, I.; Danilo, P., Jr.; Kryukova, Y.; Qu, J.; Lu, Z.; Liu, H.; Pan, Z.; Potapova, I.; et al. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation 2006, 114, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.N.; Shlapakova, I.; Szabolcs, M.J.; Danilo, P., Jr.; Lorell, B.H.; Potapova, I.A.; Lu, Z.; Rosen, A.B.; Mathias, R.T.; Brink, P.R.; et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation 2007, 116, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Shlapakova, I.N.; Nearing, B.D.; Lau, D.H.; Boink, G.J.; Danilo, P., Jr.; Kryukova, Y.; Robinson, R.B.; Cohen, I.S.; Rosen, M.R.; Verrier, R.L. Biological pacemakers in canines exhibit positive chronotropic response to emotional arousal. Heart Rhythm 2010, 7, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Zander, J.; Meyers, K.; France, D.; Levine, R.; Porter, G.; Rivkees, S.A.; Morley, G.E.; Fishman, G.I. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc. Natl. Acad. Sci. USA 2002, 99, 10464–10469. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Nikolova, T.; Zahanich, I.; Sulzbacher, S.; Fuchs, J.; Yamanaka, S.; Graf, E.; Ravens, U.; Boheler, K.R.; Wobus, A.M. Differentiation induction of mouse embryonic stem cells into sinus node-like cells by suramin. Int. J. Cardiol. 2011, 147, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Legros, S.; Ortega, F.A.; Dai, Y.; Doss, M.X.; Christini, D.J.; Robinson, R.B.; Foley, A.C. Overexpression of Map3k7 activates sinoatrial node-like differentiation in mouse ES-derived cardiomyocytes. PLoS ONE 2017, 12, e0189818. [Google Scholar] [CrossRef] [PubMed]
- Devarasetty, M.; Forsythe, S.; Shupe, T.D.; Soker, S.; Bishop, C.E.; Atala, A.; Skardal, A. Optical Tracking and Digital Quantification of Beating Behavior in Bioengineered Human Cardiac Organoids. Biosensors 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Maddah, M.; Heidmann, J.D.; Mandegar, M.A.; Walker, C.D.; Bolouki, S.; Conklin, B.R.; Loewke, K.E. A non-invasive platform for functional characterization of stem-cell-derived cardiomyocytes with applications in cardiotoxicity testing. Stem Cell Rep. 2015, 4, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Lord, B.; Schulze, P.C.; Fryer, R.M.; Sarang, S.S.; Gullans, S.R.; Lee, R.T. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation 2003, 107, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Legros, S.; Artus, J.; Doss, M.X.; Khanin, R.; Hadjantonakis, A.K.; Foley, A. A comparative analysis of extra-embryonic endoderm cell lines. PLoS ONE 2010, 5, e12016. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Rochefort, N.L.; Chen, X.; Konnerth, A. In vivo two-photon imaging of sensory-evoked dendritic calcium signals in cortical neurons. Nat. Protoc. 2011, 6, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M. (Ed.) Fundamentals of Light Microscopy; Cambridge University Press: Cambridge, UK, 1982; Volume 6, p. 3. [Google Scholar]
- Nussbaumer, H.J. Fast Fourier Transform and Convolution Algorithms; Springer: Berlin/Heidelberg, Germany, 1982; Volume 2. [Google Scholar]
- Allessie, M.A.; Boyden, P.A.; Camm, A.J.; Kleber, A.G.; Lab, M.J.; Legato, M.J.; Rosen, M.R.; Schwartz, P.J.; Spooner, P.M.; Van Wagoner, D.R.; et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001, 103, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bracewell, R.N.; Bracewell, R. The Fourier Transform and Its Applications; McGraw Hill: New York, NY, USA, 2000. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reno, A.; Hunter, A.W.; Li, Y.; Ye, T.; Foley, A.C. Quantification of Cardiomyocyte Beating Frequency Using Fourier Transform Analysis. Photonics 2018, 5, 39. https://doi.org/10.3390/photonics5040039
Reno A, Hunter AW, Li Y, Ye T, Foley AC. Quantification of Cardiomyocyte Beating Frequency Using Fourier Transform Analysis. Photonics. 2018; 5(4):39. https://doi.org/10.3390/photonics5040039
Chicago/Turabian StyleReno, Allison, Andrew W. Hunter, Yang Li, Tong Ye, and Ann C. Foley. 2018. "Quantification of Cardiomyocyte Beating Frequency Using Fourier Transform Analysis" Photonics 5, no. 4: 39. https://doi.org/10.3390/photonics5040039
APA StyleReno, A., Hunter, A. W., Li, Y., Ye, T., & Foley, A. C. (2018). Quantification of Cardiomyocyte Beating Frequency Using Fourier Transform Analysis. Photonics, 5(4), 39. https://doi.org/10.3390/photonics5040039





