Abstract
By selecting the core materials and grafted-hair polymers, hairy particles with polymer brushes can create various types of functional materials. In recent years, polydopamine (PDA) particles that are obtained by polymerizing dopamine, which is an amino acid derivative, have attracted attention for various applications. Herein, we present a novel approach for creating photonic and magnetic materials from hairy PDA particles. By grafting a hydrophilic hair polymer, we have succeeded in producing photonic materials capable of structural color changes. Furthermore, we have demonstrated the preparation of magnetic materials by immobilizing holmium, which is one of the lanthanide elements, by electrostatic interactions onto a cationic hair polymer. These results demonstrate the possibility of hairy PDA particles for a wide range of applications, such as for photonic and magnetic materials.
1. Introduction
The development of photonic materials is an attractive topic in materials science due to the numerous applications and scientific importance of these materials [1,2,3]. Structural color materials are types of photonic materials and are based on the optical interaction between light and submicron-sized structures [4,5,6]. There are several types of structures that produce structural colors, for example, reflection, diffraction, interference, and diffusion. Many studies are currently underway examining structural color using diffusion by the assembly of colloidal particles [7]. While silica particles or polymer particles, such as polystyrene particles, are usually used as components, these particles exhibit light scattering, giving rise to milky white colors. To overcome this drawback, studies have been reported in which the scattered light is suppressed so as to produce a bright structural color by adding light absorbing black materials (e.g., carbon black) [8,9,10].
In contrast, we have developed methods for the preparation of structural color materials using artificial melanin particles with light absorbing abilities as a single component [11,12,13,14,15]. While melanin is well known as an ultraviolet absorber, it is also an important material in the structural color of organisms. A well-known example of a melanin-based structural color includes the color that is produced by male peacocks’ feathers [16,17]. In the case of peacock feathers, iridescent structural color is produced by arrays of rod-like melanin granules [16,17]. We have successfully demonstrated the preparation of monodisperse polydopamine (PDA) particles (artificial melanin particles), which mimic melanin granules, as well as their use as a component of bright structural color materials [11].
In recent years, there has been enhanced interest in hairy particles, which have unique properties, by observing these types of core materials and polymer brushes. Hairy particles are particles containing grafted polymers on their surface, and they are usually obtained by “grafting from” or “grafting onto” processes, using functional hair polymers [18,19,20]. While PDA particles are useful as photonic materials (vide supra), to our knowledge, there is no report on the synthesis of hairy particles with PDA particles being used as the core.
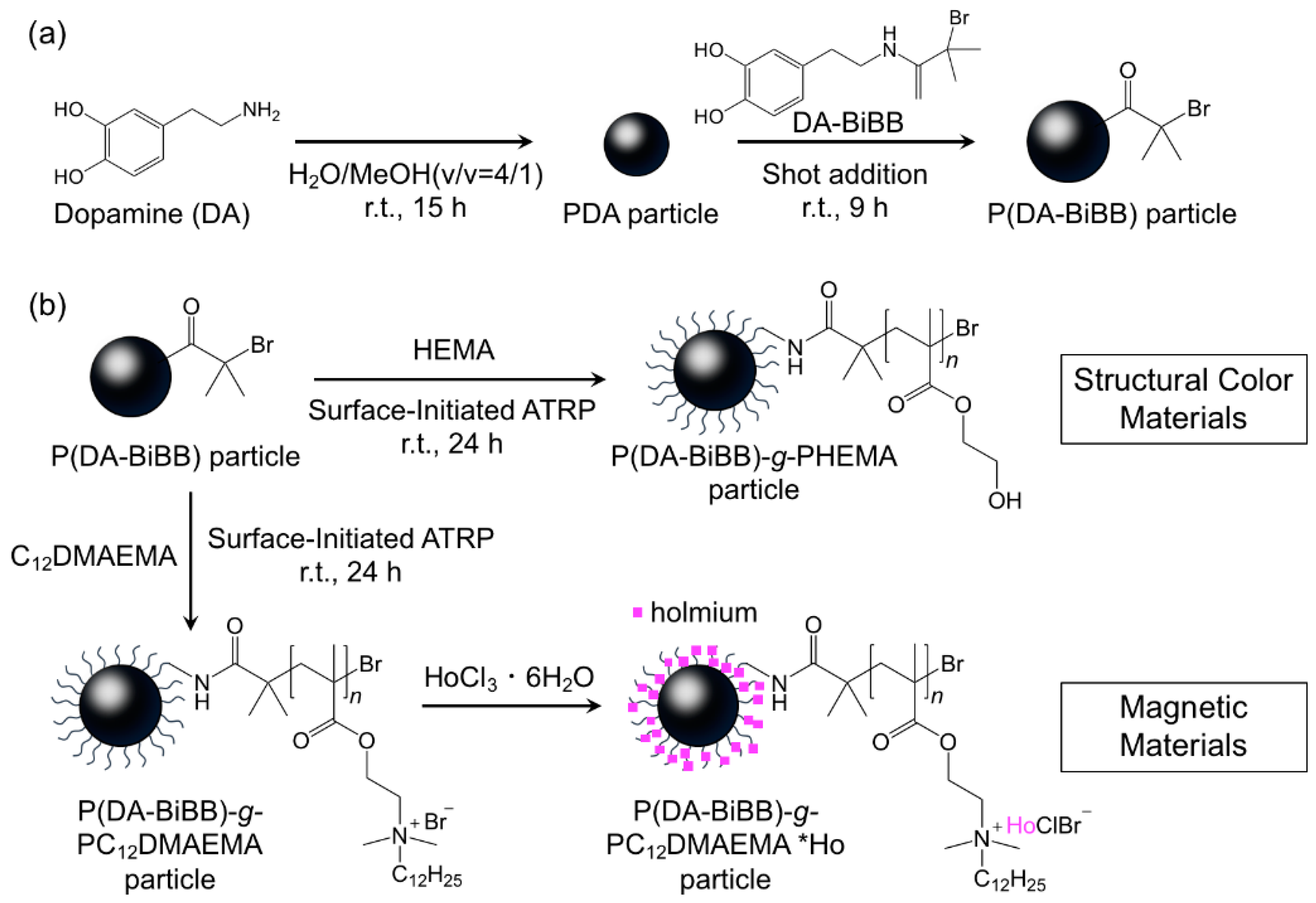
In this paper, we describe the preparation of hairy PDA particles in order to create functional photonic and magnetic materials. The core PDA particles with atom transfer radical polymerization (ATRP) initiating groups, called P(DA-BiBB) particles, were prepared by the self-oxidative polymerization of dopamine (DA) and ATRP initiator bearing-DA. The monomer (i.e., 2-hydroxyethyl methacrylate (HEMA) or N,N-dimethyl-N-n-dodecyl-N-2-methacryloyloxyethyl ammonium bromide (C12DMAEMA)) was then polymerized onto the P(DA-BiBB) particles using surface-initiated ATRP. The structural colors from the P(DA-BiBB) particles and PHEMA-bearing particles, such as the P(DA-BiBB)-g-PHEMA particles, were investigated. We also demonstrated the preparation of magnetic materials by immobilizing holmium (Ho) [21,22], which exhibits a high magnetic moment, onto the C12DMAEMA-bearing particles, called P(DA-BiBB)-g-PC12DMAEMA particles, using electrostatic interactions. The results show the usability of hairy PDA particles as synthetic platforms for photonic and magnetic materials.
2. Materials and Methods
2.1. Materials
Dopamine hydrochloride (DA), tris(hydroxymethyl)aminomethane (Tris), holmium (III) chloride hexahydrate (HoCl3·6H2O), 2-bromoisobutyryl bromide (BiBB), N,N-dimethylformamide (DMF), 2-hydroxyethyl methacrylate (HEMA), triethylamine (TEA), copper (II) bromide (CuBr2), sodium l-ascorbate, and methanol were obtained from Kanto Chemical Co., Inc., Tokyo, Japan. Deionized water with a resistance of 18.2 MΩ·cm was obtained by passing water through a Millipore Simplicity UV system. Tris[2-(dimethylamino) ethyl] amine (Me6TREN) [23] and N,N-dimethyl-N-n-dodecyl-N-2-methacryloyloxyethyl ammonium bromide (C12DMAEMA) [24] were synthesized according to methods reported in the literature. All of the other chemicals and solvents were of reagent grade and were used as received.
2.2. Measurements
The Fourier transform infrared (FTIR) spectra were measured using an IR spectrophotometer (FTIR-420; JASCO Co., Tokyo, Japan). The hydrodynamic diameter (Dh) of the particles in water was measured using dynamic light scattering (DLS) (ELSZ; Otsuka Electronics Co., Ltd., Osaka, Japan). Scanning electron microscopy (SEM) micrographs were obtained using a scanning electron microscope (JSM-6510A; JEOL Ltd., Tokyo, Japan) operated at 15–20 kV, with an energy-dispersive spectroscopy (EDS, EX-94300S4L1Q; JEOL Ltd., Tokyo, Japan). A reflection spectroscopy was performed using a spectrophotometer (V-650; JASCO Co., Tokyo, Japan) equipped with a reflection spectroscopy unit (ARSV-732; JASCO Co., Tokyo, Japan). The thermogravimetric (TG) analysis was performed in air at a heating rate of 10 °C min−1 using a thermogravimetry/differential thermal analyzer (TG8120; Rigaku Co., Tokyo, Japan).
2.3. Synthesis of DA-BiBB Solution
ATRP initiator bearing-DA, DA-BiBB, was obtained according to our paper [25,26]. Briefly, DA (1.60 g, 8.44 mmol), BiBB (2.08 mL), and TEA (2.35 mL) in DMF (80 mL) were stirred for 2 h at room temperature with nitrogen, producing the DA-BiBB solution.
2.4. Preparation of ATRP Initiator-Bearing PDA Particles
The DA (1.80 g, 9.49 mmol), Tris (14.4 g, 119 mmol), and water/methanol (4/1) solution (1170 mL) were placed in a 2 L flask, and the mixture was stirred at 30 °C. After 15 h, the DA-BiBB solution (60 mL) was added to the mixture, and the mixture was further stirred at 30 °C for 9 h. The ATRP initiator-bearing PDA particles were separated and purified repeatedly by centrifugation (14,500 rpm for 20 min) and were redispersed in deionized water, yielding the P(DA-BiBB) particles.
2.5. Preparation of Hairy PDA Particles
Monomer (HEMA (0.13 g, 1.0 mmol) or C12DMAEMA (0.55 g, 0.35 mmol)), CuBr2 (26.8 mg, 0.12 mmol), Me6TREN (27.6 mg, 0.12 mmol), and P(DA-BiBB) particles (40 mg) dispersed in deionized water (33.4 mL) were placed in a glass tube. The mixture was deoxygenated by purging with nitrogen for 30 min, and was placed in a water bath at room temperature. A nitrogen-purged aqueous solution of sodium l-ascorbate (1.20 mL, 0.12 mmol) was added to the mixture. After 24 h, the polymerization was stopped by purging with oxygen, and the particles were separated and purified repeatedly by centrifugation (14,500 rpm for 20 min) and redispersion, yielding the hairy PDA particles (i.e., the P(DA-BiBB)-g-PHEMA particles and P(DA-BiBB)-g-PC12DMAEMA particles.).
2.6. Preparation of Magnetic Hairy PDA Particles
P(DA-BiBB)-g-PC12DMAEMA particles (30 mg) and HoCl3·6H2O (0.51 g, 1.34 mmol) in methanol (25 mL) were stirred at room temperature. After 6 h, the particles were separated and purified repeatedly by centrifugation (14,500 rpm for 20 min) and redispersion with water, yielding Ho-bearing particles. The synthesized product is designated as the P(DA-BiBB)-g-PC12DMAEMA*Ho particles.
2.7. Production of Structural Colors from the Assembly of Particles
P(DA-BiBB) or P(DA-BiBB)-g-PHEMA particles (10 wt%) in quartz cells were directly centrifuged at 1500 rpm for 12 h so as to check for the appearance of structural colors.
3. Results and Discussion
3.1. Preparation of ATRP Initiator-Bearing PDA Particles
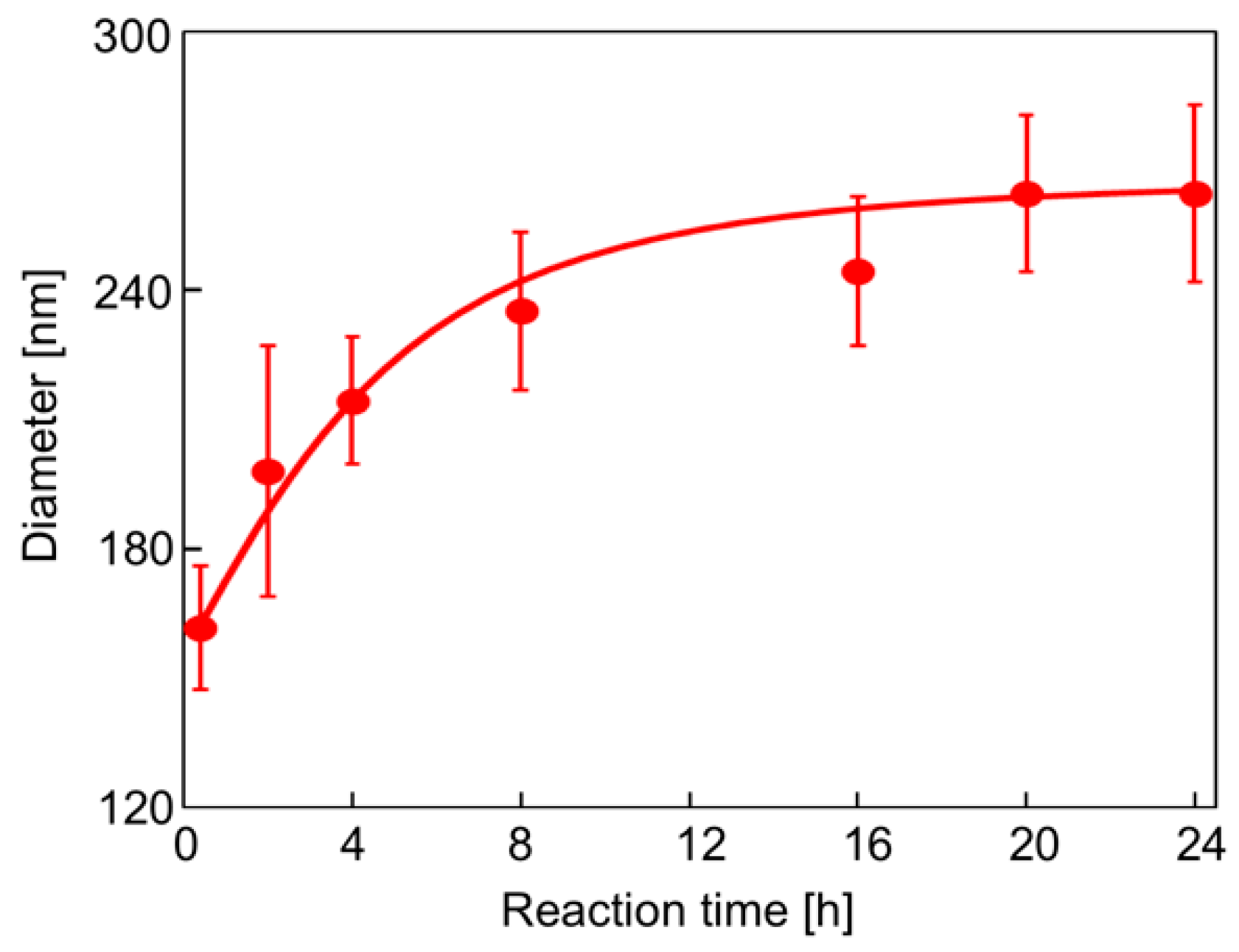
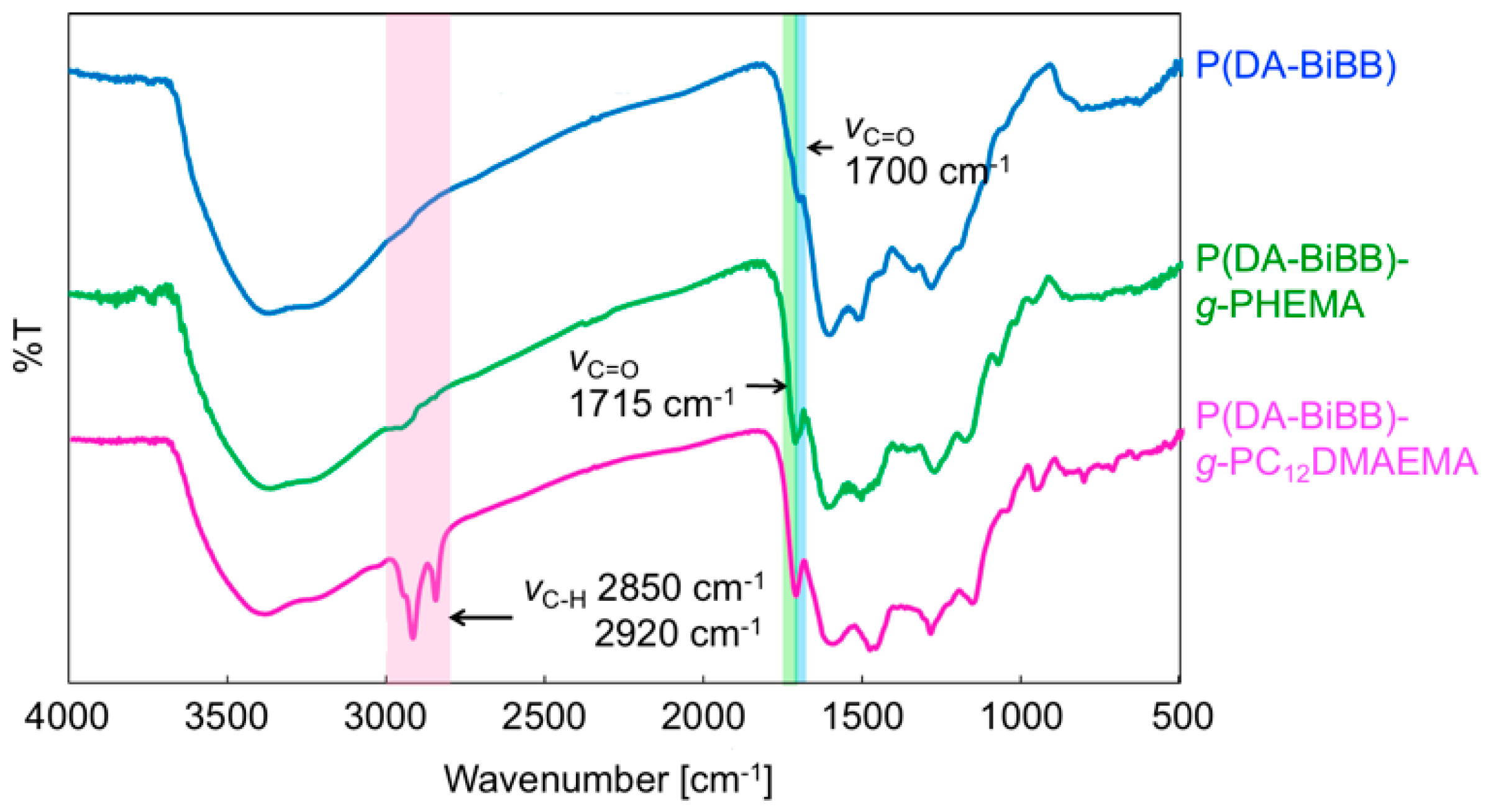
Figure 1 shows a synthetic route for the hairy PDA particles. Firstly, ATRP initiator-bearing PDA particles, P(DA-BiBB) particles, were prepared by DA polymerization. To selectively introduce a polymerization initiating group to the particle surface, it is useful to add DA-BiBB at later stages of polymerization. We measured the particle diameter change in the PDA particles over time. As has already been reported, the polymerization of DA was carried out in a mixed solvent of water/methanol to produce monodisperse PDA particles [11]. The diameter of the PDA particles was gradually increased while increasing the reaction time, and reached a plateau after approximately 12 h (Figure 2). Then, DA-BiBB was added by the shot method 15 h after the start of polymerization. The introduction of the initiating groups onto the surface of the PDA particles was confirmed using IR spectroscopy (Figure 3). A characteristic signal of 1700 cm−1, corresponding to the C=O vibration mode of the BiBB part (peak marked with an arrow with a blue line in Figure 3) was obtained, and the introduction of the polymerization initiating group was confirmed.
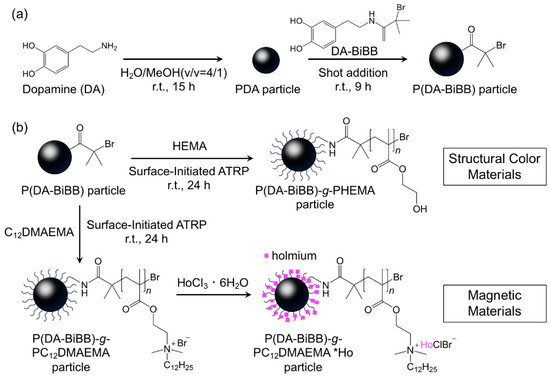
Figure 1.
Schematic representation of the preparation of (a) P(DA-BiBB) particles and (b) hairy polydopamine (PDA) particles.
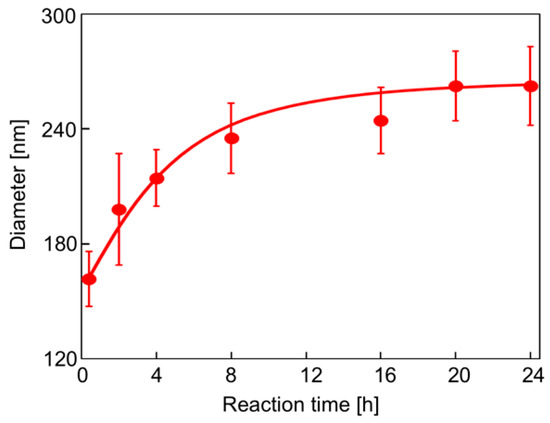
Figure 2.
Particle diameter of PDA particles with different reaction times.
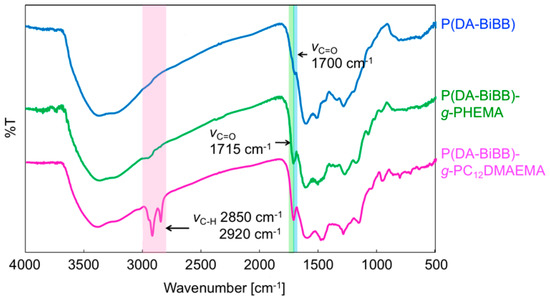
Figure 3.
IR spectra of P(DA-BiBB) particles, P(DA-BiBB)-g-PHEMA particles, and P(DA-BiBB)-g-PC12DMAEMA particles.
3.2. Control of Structural Colors
The P(DA-BiBB) particles were used as substrates for SI-ATRP in order to generate hairy PDA particles. HEMA was selected as a monomer for the structural color materials. The P(DA-BiBB) particles were dispersed in a HEMA solution, and SI-ATRP was conducted for 24 h at room temperature. After polymerization, the particles were separated by centrifugation. The major peaks at 1715 cm−1 (C=O stretching vibration), which are presented in Figure 3 with a green line, indicated the presence of PHEMA on the PDA particles.
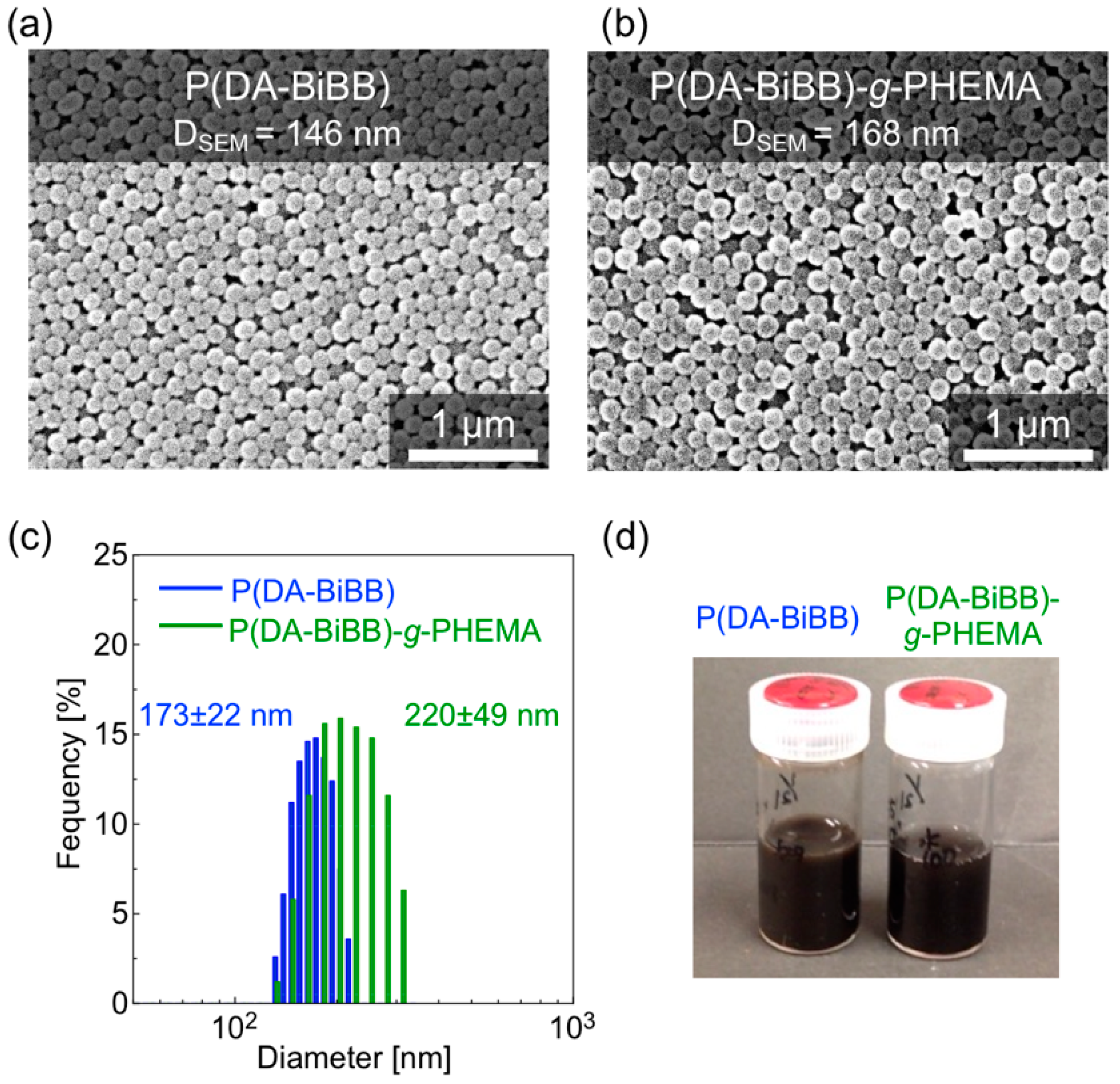
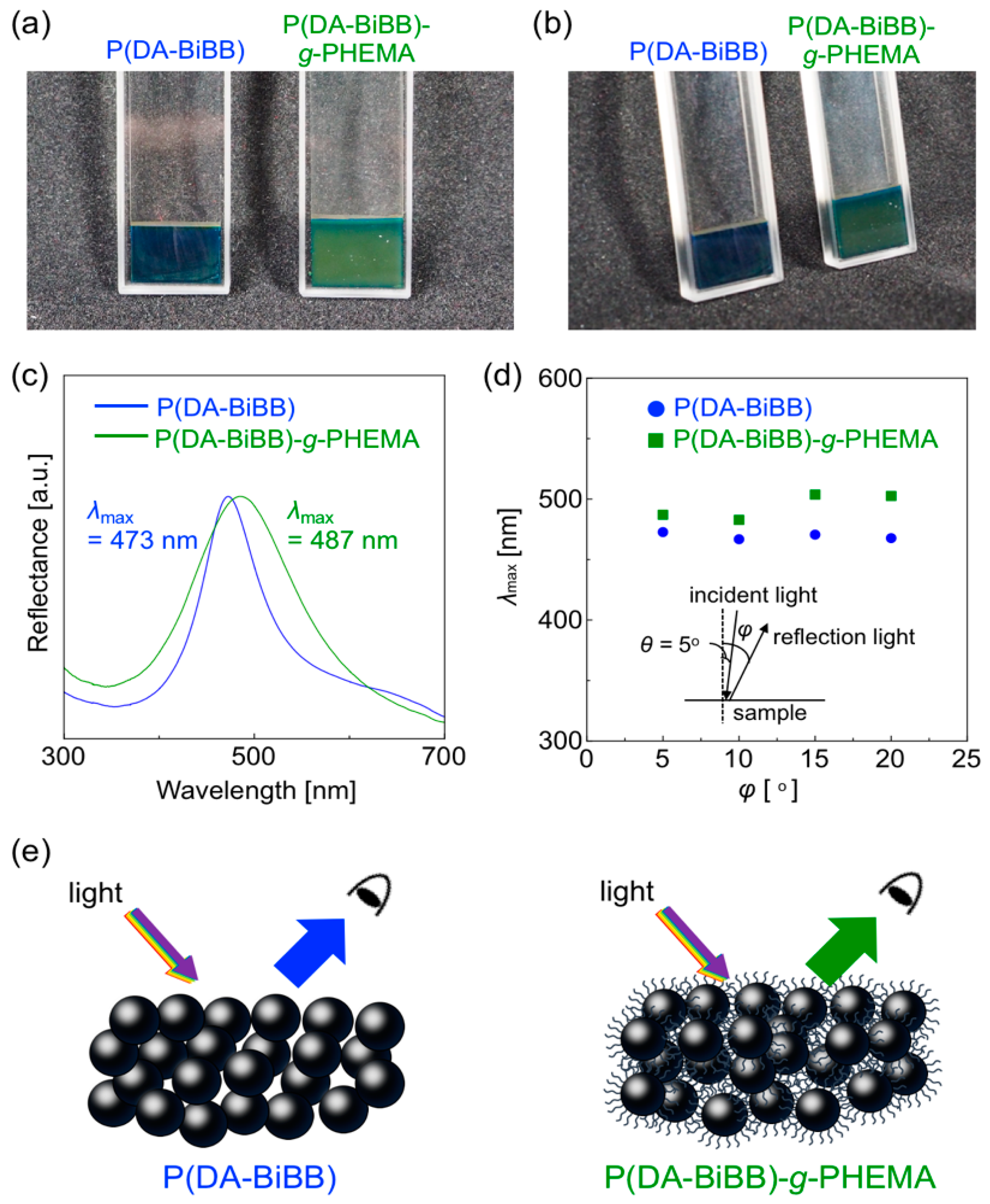
Figure 4a,b shows the SEM images of the resulting particles. Monodispersed P(DA-BiBB) and P(DA-BiBB)-g-PHEMA particles were obtained with an average particle size of ca. 146 and 168 nm, respectively. As the SEM image of the particles indicates, the diameter distribution (coefficient of variation (CV)) of the particles was ca. 7%. After grafting the PHEMA, the dried hairy PDA particles have coarser surfaces than the P(DA-BiBB) particles, but they apparently maintain monodispersity. The volume-average diameters of the P(DA-BiBB)-g-PHEMA particles in water, as measured by DLS, are ca. 220 nm, which are larger than the ca. 173 nm of the core P(DA-BiBB) particles, indicating that the P(DA-BiBB)-g-PHEMA particles have hydrated PHEMA layers of ca. 24 nm in thickness (Figure 4c). The particles obtained were well dispersed in the water. As shown in Figure 4d, the water dispersion of the P(DA-BiBB) and P(DA-BiBB)-g-PHEMA particles showed black colors, which is a typical absorption color of PDA.
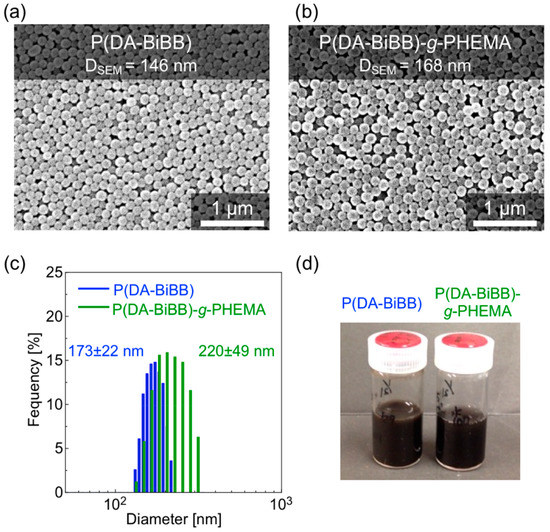
Figure 4.
(a,b) SEM images, (c) size distributions measured by dynamic light scattering (DLS), and (d) photographs of a dispersed solution of particles (0.5 wt% in water).
In accordance with our previous report [11], the P(DA-BiBB) and P(DA-BiBB)-g-PHEMA particles (10 wt% in water) in the quartz cells were centrifuged at 1500 rpm for 12 h. As shown on the left side of Figure 5a, a bright blue structural color was obtained after the centrifugation of the samples using P(DA-BiBB) particles (solid concentration: ca. 50 wt% in water). In contrast, the assembly of P(DA-BiBB)-g-PHEMA particles showed green structural colors, suggesting a redshift of the reflectance spectrum (Figure 5a, right side). The photographs of the same samples viewed from another angle are shown in Figure 5b. It was suggested that a non-iridescent color was obtained, as neither of the structural colors changed color. Figure 5c shows the normalized reflection spectra of the samples. The spectrum of the blue sample prepared from the P(DA-BiBB) particles had a reflection peak at 473 nm. The reflection peaks for the green sample were redshifted to 487 nm, suggesting an increase in the distance of the particles due to the PHEMA hairs. With the naked eye, the structural color did not depend on the angle (vide supra). The reflection spectra were measured at different angles by fixing the light source and sample, and by rotating the detector. Figure 5d is a plot of λmax of the reflection spectrum as a function of the detection angles. The reflection spectra that were obtained at several different angles for each sample were relatively the same, clearly indicating the formation of non-iridescent structural colors.
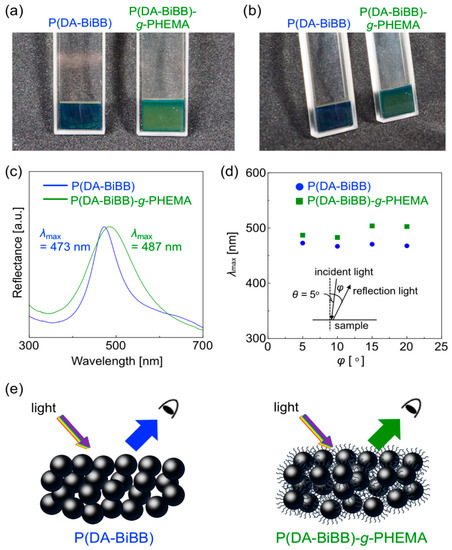
Figure 5.
(a,b) Digital camera images of concentrated dispersions of P(DA-BiBB) and P(DA-BiBB)-g-PHEMA particles (approximately 50 wt% in water). (c) Reflection spectra of the samples shown in (a). (d) Plots of λmax for the reflection spectra of the samples as a function of the incident angle (φ). (e) Schematic illustration of the non-iridescent structural colors by the assembly of P(DA-BiBB) and P(DA-BiBB)-g-PHEMA particles.
In the structural color from the assembly of particles, the peak value is estimated based on Bragg’s law, Equation (1) [27].
where m is the order of diffraction; λ is the wavelength of light; ni and Vi are the refractive index and volume fraction of each component, respectively; d is the center-to-center distance between the nearest particles; and θ is the angle between the incident light and the diffraction crystal planes. The d value was calculated using Equation (1), assuming that Bragg’s law holds (m = 1, θ = 90, and λ = 473 or 487 nm). The refractive index (n) is the effective refractive index of the particle/H2O composite. The packing factor is 0.74, as in the close-packed face-centered cubic (FCC) structure. Thus, = 0.74 × np2 + 0.26 × ns2, where np and ns are the refractive indices of the particles and solvent, respectively (H2O: ns = 1.33). The refractive index of the P(DA-BiBB) and P(DA-BiBB)-g-PHEMA particles (np) was set to 1.76, as we have previously reported that the refractive index of PDA is ca. 1.76 [12]. The center-to-center distance between the particles for P(DA-BiBB) and P(DA-BiBB)-g-PHEMA was estimated to be ca. 207 nm and 213 nm, respectively, which is relatively comparable to the actual particle diameters measured by DLS (vide supra). As shown in the schematic diagram of Figure 5e, the apparent particle size increases with the presence of PHEMA hair, and, as a result, the structural color was redshifted.
3.3. Control of Magnetic Behaviors
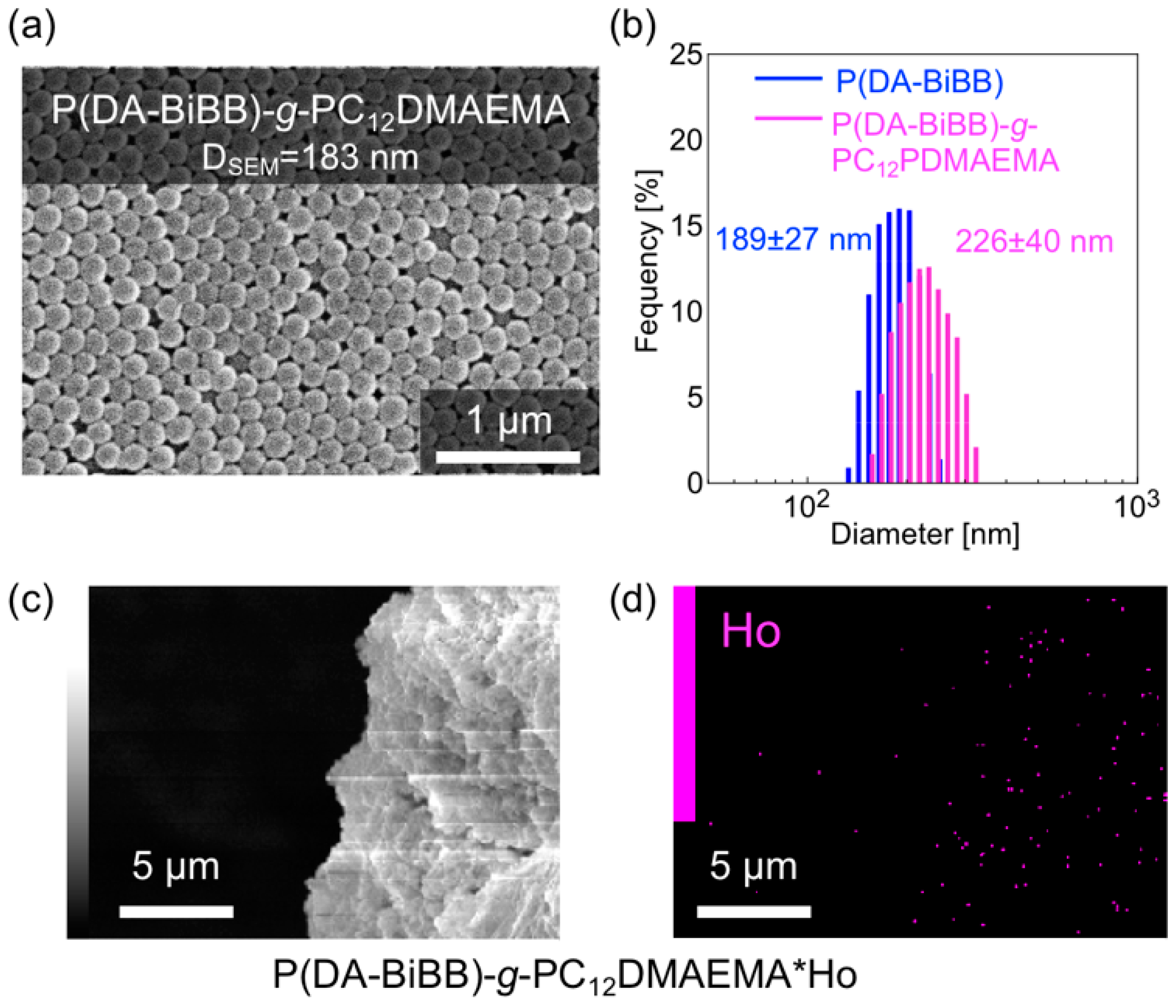
Magnetically responsive hair was formed on the surface of the P(DA-BiBB) particles, using C12DMAEMA as a monomer. A comparison of the IR spectra suggests that PC12DMAEMA is initiated by P(DA-BiBB) particles by SI-ATRP (pink line in Figure 3). As shown in Figure 6a, spherical P(DA-BiBB)-g-PC12DMAEMA particles with diameters of ca. 183 nm were obtained. From the DLS measurement of the particles in the water-dispersed state, the particle size of the P(DA-BiBB)-g-PC12DMAEMA particles increased to 226 nm, suggesting the formation of PC12DMAEMA hydrated hairs of 19 nm (Figure 6b). With reference to the method for preparing a Ho-supported surfactant, reported previously by us [21], HoCl3·6H2O was added to a methanol dispersion of the particles. As C12DMAEMA has quaternized nitrogen and is positively charged, we believe that it binds to HoCl3 by electrostatic interactions. After stirring for 6 h, the particles were repeatedly purified by centrifugation, until the supernatant changed from a pink color to being colorless, which is a typical color of Ho. Figure 6c,d shows the SEM image of the P(DA-BiBB)-g-PC12DMAEMA*Ho particles together with the energy dispersive X-ray spectrometry (EDS) mapping data of Ho. The SEM images and Ho mappings overlapped, suggesting the formation of Ho-carrying particles. The amount of Ho carried on the particles was calculated using TG analysis. Under this condition, Ho is considered present, as Ho2O3. Assuming that the residue after the measurement was attributable to Ho, it was calculated that ca. 4.14 mmol of Ho was introduced per 1 g of particles.
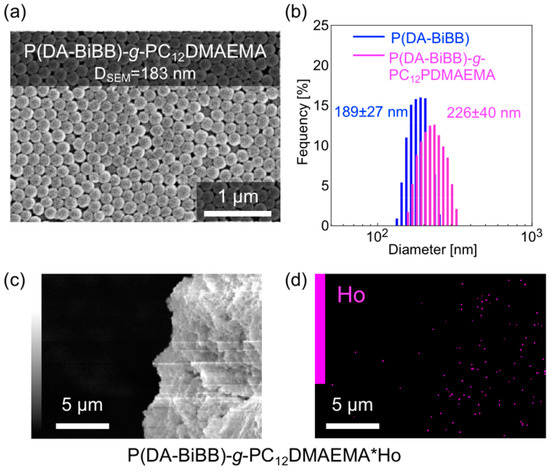
Figure 6.
(a) SEM image; (b) size distributions measured by DLS of P(DA-BiBB)-g-PC12DMAEMA particles. (c) SEM image and (d) SEM-energy dispersive X-ray spectrometry (EDS) mapping data of P(DA-BiBB)-g-PC12DMAEMA*Ho particles.
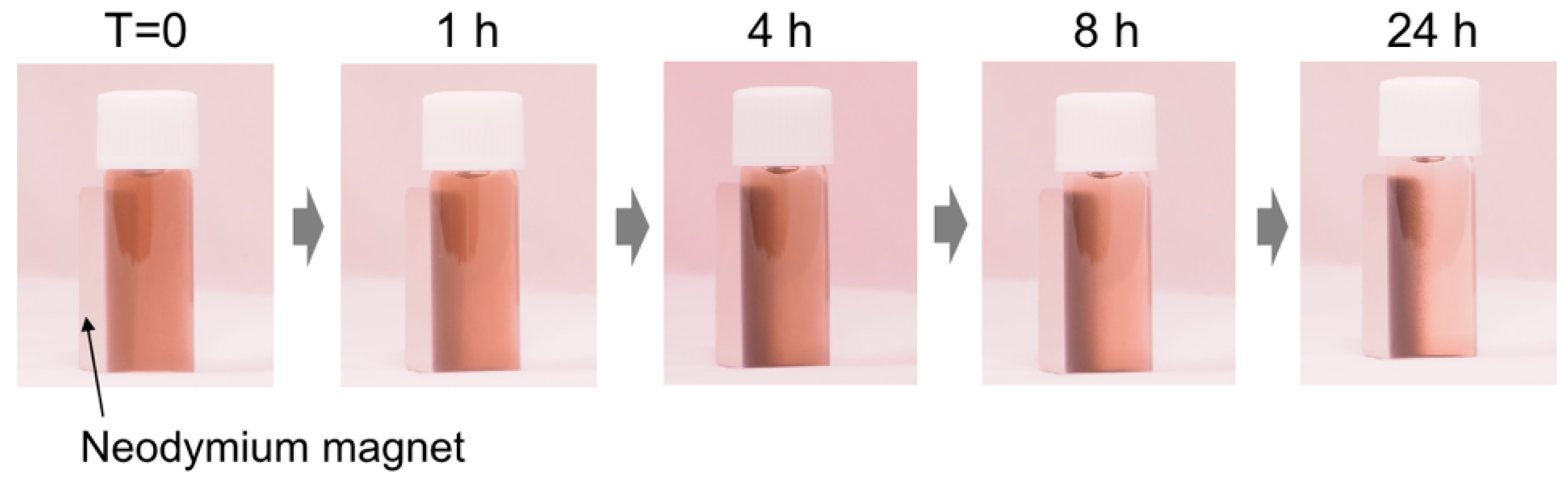
The structural color tuning by the magnetic assembly is an important subject for device application. Several researchers have reported the development of structural colors by magnetic assemblies using Fe2O3 magnetic nanoparticles-based materials as a component [28,29,30,31,32,33,34,35,36,37]. Thus, the magnetic response of the P(DA-BiBB)-g-PC12DMAEMA*Ho particles was investigated toward structural color tuning. The synthesized P(DA-BiBB)-g-PC12DMAEMA*Ho particles were well dispersed in H2O, and the dispersion exhibited a blackish brown color similar to that of PDA. A neodymium magnet (500 mT) was placed on the side of the glass bottle containing the particle dispersion (0.5 wt%) and was left to stand (Figure 7). Over time, the particles were gradually attracted to the magnet. After 24 h, most of the particles were found to be attracted to the magnet, suggesting that the particles with magnetic polymer hair responded to the magnet. Unfortunately, the structural color due to the particle assembly could not be confirmed in the present experiment. This is probably due to the weak magnetism of the fabricated Ho-bearing particles compared with the conventional Fe2O3 magnetic nanoparticles. Although further experiments for the preparation of particles with strong magnetism are required, including the control of the PC12DMAEMA hair lengths and the optimization of the loading amount of Ho, its application to the manufacturing of various materials in the future can be expected, as Ho can be easily introduced by electrostatic interactions.
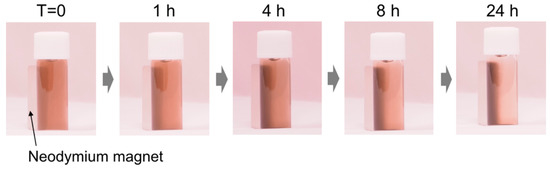
Figure 7.
Photographs when a magnet is placed side of a glass bottle containing a water dispersion of P(DA-BiBB)-g-PC12DMAEMA*Ho particles.
4. Conclusions
In conclusion, we prepared structural color materials from the assembly of P(DA-BiBB) particles or P(DA-BiBB)-g-PHEMA particles. The photonic materials prepared from both of the particles showed non-iridescent bright structural colors. The reflection peaks for the samples of the P(DA-BiBB)-g-PHEMA particles were redshifted to 487 nm from the 473 nm reflection peak of the samples of the P(DA-BiBB) particles. These structural color changes were caused by the increase in the distance of the particles as a result of the PHEMA hairs, suggesting that the structural color can be easily changed by the length of the water-soluble hair polymer. We also demonstrated the preparation of magnetic materials by introducing Ho by electrostatic interactions on PDA particles with cationic hair, PC12DMAEMA. The particles that were obtained were well dispersed in water and were collected by a neodymium magnet. These findings demonstrate the utility of hairy PDA particles for the production of various functional materials.
Author Contributions
M.K. designed the experiments, performed the data analysis, supervised the project, and wrote the manuscript. K.U., Y.N., and A.K. carried out the experiments with respect to the preparation and characterization of hairy particles, and photonic and magnetic materials. T.T. and K.K. were involved in the data analysis and interpretation of the experimental results. All of the authors discussed the results and edited the manuscript.
Funding
This research was funded by JSPS KAKENHI (grant numbers 15H01593 and 17H03110), the Iketani Science and Technology Foundation, and the Chiba University Venture Business Laboratory Project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paquet, C.; Kumacheva, E. Nanostructured polymers for photonics. Mater. Today 2008, 11, 48–56. [Google Scholar] [CrossRef]
- Wuttig, M.; Bhaskaran, H.; Taubner, T. Phase-change materials for non-volatile photonic applications. Nat. Photonics 2017, 11, 465–476. [Google Scholar] [CrossRef]
- Staude, I.; Schilling, J. Metamaterial-inspired silicon nanophotonics. Nat. Photonics 2017, 11, 274–284. [Google Scholar] [CrossRef]
- Kolle, M.; Lee, S. Progress and opportunities in soft photonics and biologically inspired optics. Adv. Mater. 2018, 30, 1702669. [Google Scholar] [CrossRef] [PubMed]
- Isapour, G.; Lattuada, M. Bioinspired stimuli-responsive color-changing systems. Adv. Mater. 2018, 30, 1707069. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz Hedayati, M.; Elbahri, M. Review of metasurface plasmonic structural color. Plasmonics 2017, 12, 1463. [Google Scholar] [CrossRef]
- Goerlitzer, E.S.A.; Taylor, R.N.K.; Vogel, N. Bioinspired photonic pigments from colloidal self-assembly. Adv. Mater. 2018, 30, 1706654. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.D.; Noh, H.; Liew, S.F.; Saranathan, V.; Schreck, C.F.; Yang, L.; Park, J.G.; Prum, R.O.; Mochrie, S.G.J.; O’Hern, C.S.; et al. Biomimetic isotropic nanostructures for structural coloration. Adv. Mater. 2010, 22, 2939–2944. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Yoshioka, S.; Takano, A.; Arai, S.; Nueangnoraj, K.; Nishihara, H.; Teshima, M.; Ohtsuka, Y.; Seki, T. Production of colored pigments with amorphous arrays of black and white colloidal particles. Angew. Chem. Int. Ed. 2013, 52, 7261–7265. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y. Environment and human friendly colored materials prepared using black and white components. Chem. Commun. 2018, 54, 4905–4914. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Biomimetic non-iridescent structural color materials from polydopamine black particles that mimic melanin granules. J. Mater. Chem. C 2015, 3, 720–724. [Google Scholar]
- Kawamura, A.; Kohri, M.; Morimoto, G.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Full-color biomimetic photonic materials with iridescent and non-iridescent structural colors. Sci. Rep. 2016, 6, 33984. [Google Scholar] [PubMed]
- Kawamura, A.; Kohri, M.; Yoshioka, S.; Taniguchi, T.; Kishikawa, K. Structural color tuning: Mixing melanin-like particles with different diameters to create neutral colors. Langmuir 2017, 33, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Yamazaki, S.; Kawamura, A.; Taniguchi, T.; Kishikawa, K. Bright structural color films independent of background prepared by the dip-coating of biomimetic melanin-like particles having polydopamine shell layers. Colloids Surf. A 2017, 532, 564–569. [Google Scholar] [CrossRef]
- Kohri, M.; Yanagimoto, K.; Kawamura, A.; Hamada, K.; Imai, Y.; Watanabe, T.; Ono, T.; Taniguchi, T.; Kishikawa, K. Polydopamine-based 3D structural color materials: Spherical photonic balls and fibers from melanin-like particles having polydopamine shell layers. ACS Appl. Mater. Interfaces 2018, 10, 7640–7648. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Kinoshita, S. Effect of macroscopic structure in iridescent color of the peacock feathers. Forma 2002, 17, 169–181. [Google Scholar]
- Zi, J.; Yu, X.; Li, Y.; Hu, X.; Xu, C.; Wang, X.; Liu, X.; Fu, R. Coloration strategies in peacock feathers. Proc. Natl. Acad. Sci. USA 2003, 100, 12576–12578. [Google Scholar] [PubMed]
- Kawaguchi, H. Functional polymer microspheres. Prog. Polym. Sci. 2000, 25, 1171–1210. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Chevigny, P.C.; Dalmas, F.; Cola, E.D.; Gigmes, D.; Bertin, D.; Boué, F.; Jestin, J. Polymer-grafted-nanoparticles nanocomposites: Dispersion, grafted chain conformation, and rheological behavior. Macromolecules 2011, 44, 122–133. [Google Scholar] [CrossRef]
- Kawamura, A.; Kohri, M.; Taniguchi, T.; Kishikawa, K. Surface modification of polydopamine particles via magnetically-responsive surfactants. Trans. Mater. Res. Soc. Jpn. 2016, 41, 301–304. [Google Scholar] [CrossRef]
- Kohri, M.; Yanagimoto, K.; Kohaku, K.; Shiomoto, S.; Kobayashi, M.; Imai, A.; Shiba, F.; Taniguchi, T.; Kishikawa, K. Magnetically responsive polymer network constructed by poly(acrylic acid) and holmium. Macromolecules 2018, 51, 6740–6745. [Google Scholar] [CrossRef]
- Kohri, M.; Sato, M.; Abo, F.; Inada, T.; Kasuya, M.; Taniguchi, T.; Nakahira, T. Preparation and lectin binding specificity of polystyrene particles grafted with glycopolymers bearing S-linked carbohydrates. Eur. Polym. J. 2011, 47, 2351–2360. [Google Scholar] [CrossRef]
- Kohri, M.; Kobayashi, A.; Fukushima, H.; Kojima, T.; Taniguchi, T.; Saito, K.; Nakahira, T. Enzymatic miniemulsion polymerization of styrene with a polymerizable surfactant. Polym. Chem. 2012, 3, 900–906. [Google Scholar] [CrossRef]
- Kohri, M.; Kohma, H.; Shinoda, Y.; Yamauchi, M.; Yagai, S.; Kojima, T.; Taniguchi, T.; Kishikawa, K. A colorless functional polydopamine thin layer as a basis for polymer capsules. Polym. Chem. 2013, 4, 2696–2702. [Google Scholar] [CrossRef]
- Kohri, M.; Shinoda, Y.; Kohma, H.; Nannichi, Y.; Yamauchi, M.; Yagai, S.; Kojima, T.; Taniguchi, T.; Kishikawa, K. Facile synthesis of free-standing polymer brush films based on a colorless polydopamine thin layer. Macromol. Rapid Commun. 2013, 34, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Hu, Y.; Yin, Y. Highly tunable superparamagnetic colloidal photonic crystals. Angew. Chem. Int. Ed. 2007, 46, 7428–7431. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Hu, Y.; Zhang, T.; Huynh, T.; Yin, Y. Self-assembly and field-responsive optical diffractions of superparamagnetic colloids. Langmuir 2008, 24, 3671–3680. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yin, Y. Magnetically tunable colloidal photonic structures in alkanol solutions. Adv. Mater. 2008, 20, 3485–3491. [Google Scholar] [CrossRef]
- Kim, H.; Ge, J.; Kim, J.; Choi, S.; Lee, H.; Lee, H.; Park, W.; Yin, Y.; Kwon, S. Structural colour printing using a magnetically tunable and lithographically fixable photonic crystal. Nat. Photon. 2009, 3, 534–540. [Google Scholar] [CrossRef]
- Ge, J.; He, L.; Goebl, J.; Yin, Y. Assembly of magnetically tunable photonic crystals in nonpolar solvents. J. Am. Chem. Soc. 2009, 131, 3484–3486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, H.; Xie, Z.; Shum, H.C.; Wang, B.; Gu, Z. Bioinspired multifunctional Janus particles for droplet manipulation. J. Am. Chem. Soc. 2013, 135, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Zhao, Y.; Zhong, H.; Liang, J.; Zhou, J.; Shen, H. Hydrophilic magnetochromatic nanoparticles with controllable sizes and super-high magnetization for visualization of magnetic field intensity. Sci. Rep. 2015, 23, 17063. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 6233. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Zhang, Q.; Wang, H.; Li, Y. Fabrication of magnetic field induced structural colored films with tunable colors and its application on security materials. J Colloid Interface Sci. 2017, 485, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Teshima, M.; Seki, T.; Takeoka, Y. Simple preparation of magnetic field-responsive structural colored Janus particles. Chem. Commun. 2018, 54, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).