FMTPen: A Miniaturized Handheld Fluorescence Molecular Tomography Probe for Image-Guided Cancer Surgery
Abstract
:1. Introduction
2. Materials and Methods
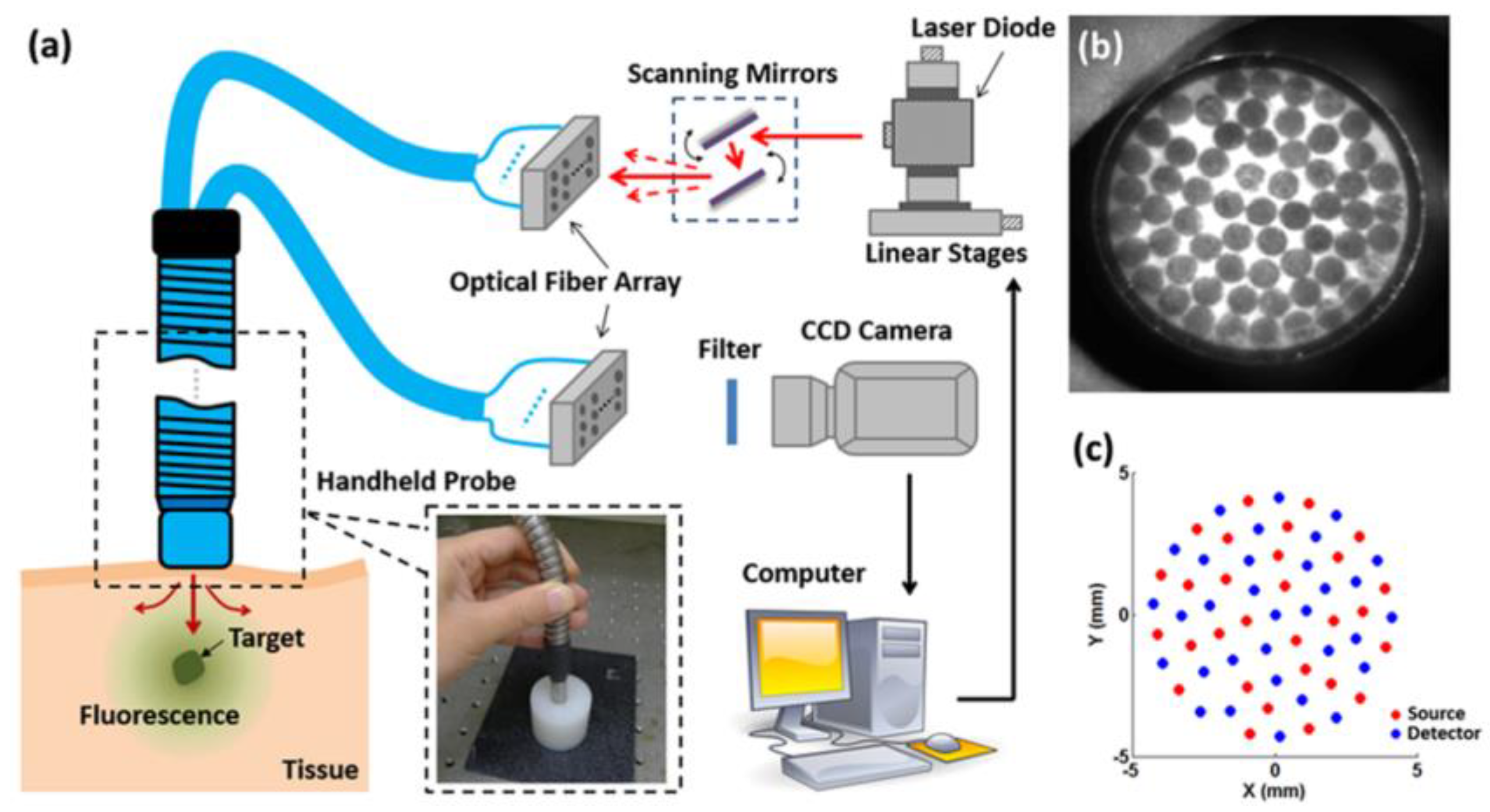
3. Results and Discussion
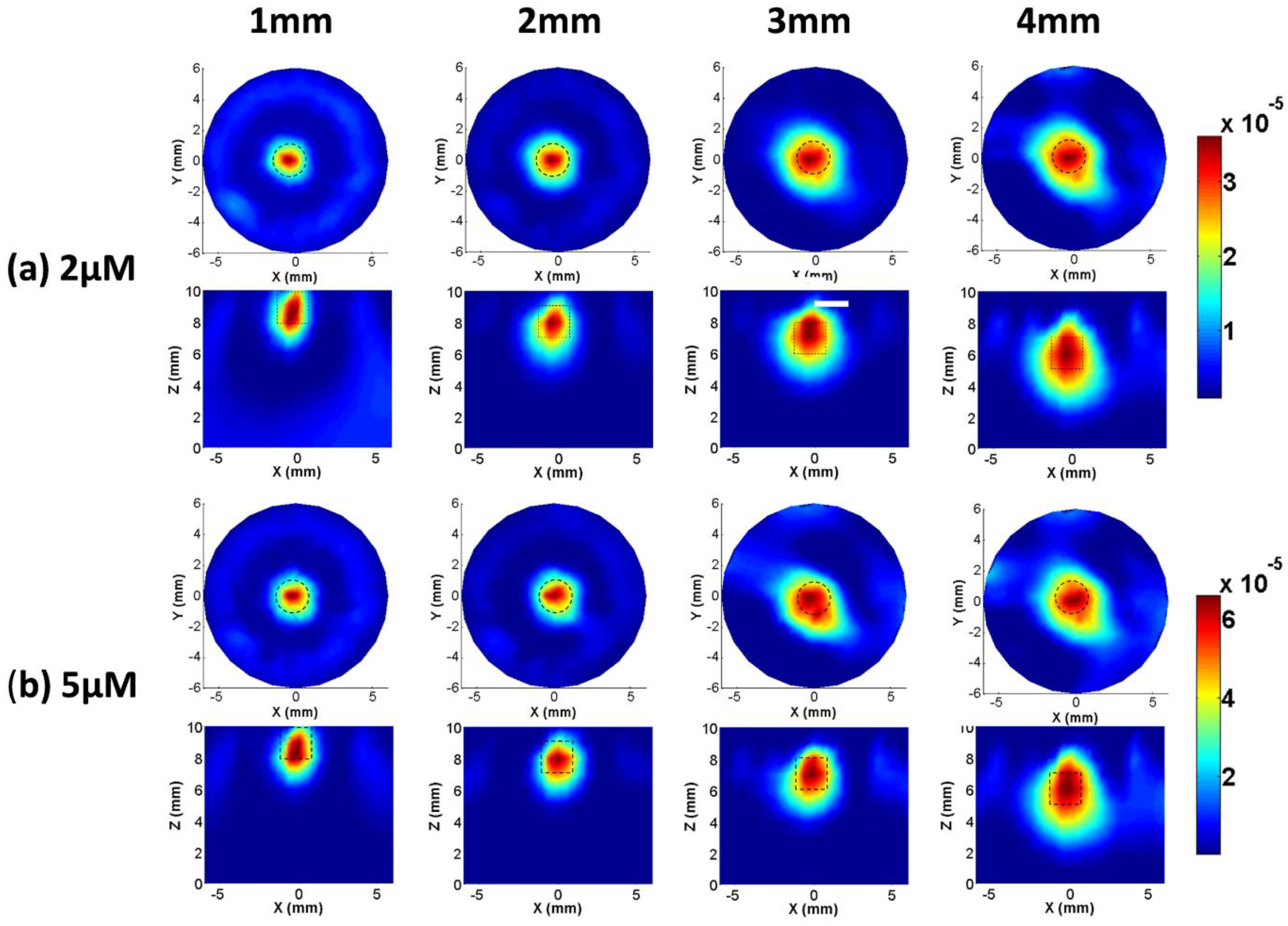
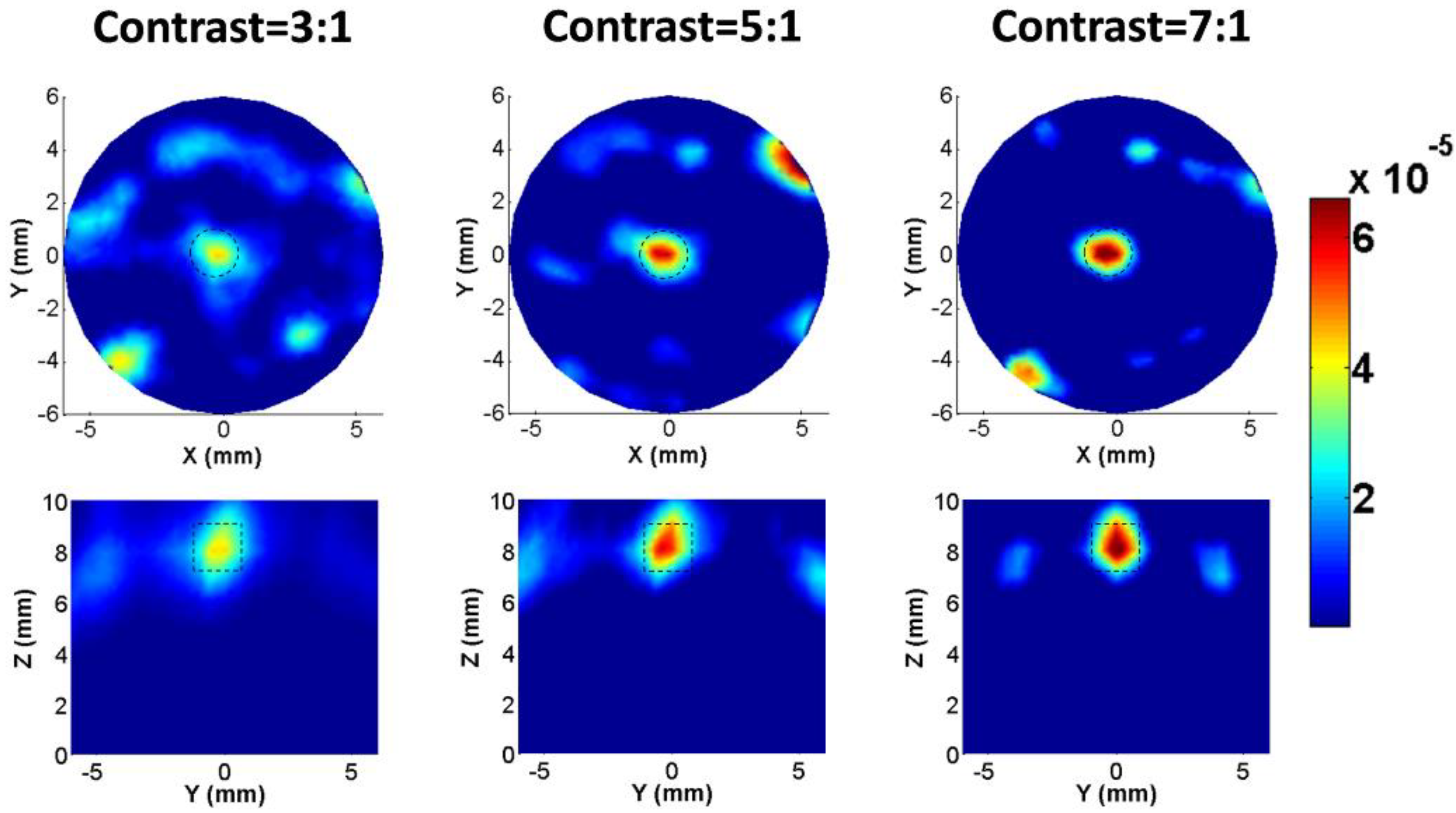
3.1. Phantom Experiments
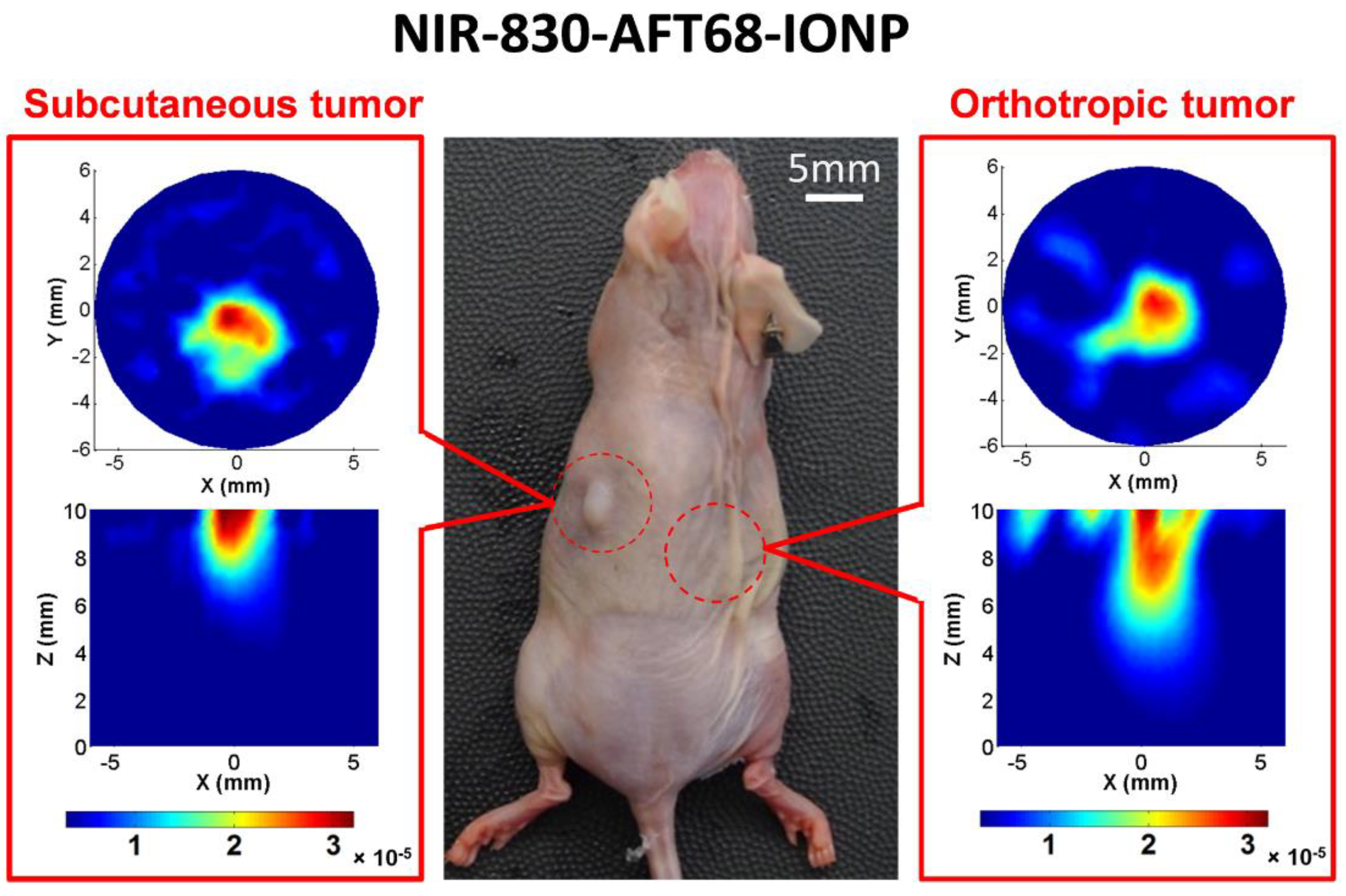
3.2. Animal Experiments

4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Leroux, P.D.; Winter, T.C.; Berger, M.S.; Mack, L.A.; Wang, K.; Elliott, J.P. A comparison between preoperative magnetic resonance and intraoperative ultrasound tumor volumes and margins. J. Clin. Ultrasound 1994, 22, 29–36. [Google Scholar]
- Trimble, E.L. The nih consensus conference on ovarian cancer: Screening, treatment, and follow-up. Gynecol. Oncol. 1994, 55, S1–S3. [Google Scholar]
- Tammela, J.; Lele, S. New modalities in detection of recurrent ovarian cancer. Curr. Opin. Obstet. Gynecol. 2004, 16, 5–9. [Google Scholar]
- Daisne, J.-F.; Sibomana, M.; Bol, A.; Cosnard, G.; Lonneux, M.; Grégoire, V. Evaluation of a multimodality image (ct, mri and pet) coregistration procedure on phantom and head and neck cancer patients: Accuracy, reproducibility and consistency. Radiot. Oncol. 2003, 69, 237–245. [Google Scholar]
- Wagnieres, G.A.; Star, W.M.; Wilson, B.C. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem. Photobiol. 1998, 68, 603–632. [Google Scholar]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Ann. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar]
- Valdés, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K. Quantitative fluorescence in intracranial tumor: Implications for ala-induced ppix as an intraoperative biomarker. J. Neurosurg. 2011, 115, 11. [Google Scholar]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar]
- Yang, L.; Mao, H.; Cao, Z.; Wang, Y.A.; Peng, X.; Wang, X.; Sajja, H.K.; Wang, L.; Duan, H.; Ni, C. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology 2009, 136, 1514–1525. [Google Scholar]
- Waldeck, J.; Häger, F.; Höltke, C.; Lanckohr, C.; von Wallbrunn, A.; Torsello, G.; Heindel, W.; Theilmeier, G.; Schäfers, M.; Bremer, C. Fluorescence reflectance imaging of macrophage-rich atherosclerotic plaques using an αvβ3 integrin–targeted fluorochrome. J. Nucl. Med. 2008, 49, 1845–1851. [Google Scholar]
- Josserand, V.; Texier-Nogues, I.; Huber, P.; Favrot, M.C.; Coll, J.-L. Non-invasive in vivo optical imaging of the lacz and luc gene expression in mice. Gene Therapy 2007, 14, 1587–1593. [Google Scholar]
- Kircher, M.F.; Mahmood, U.; King, R.S.; Weissleder, R.; Josephson, L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Can. Res. 2003, 63, 8122–8125. [Google Scholar]
- Parungo, C.P.; Ohnishi, S.; Kim, S.-W.; Kim, S.; Laurence, R.G.; Soltesz, E.G.; Chen, F.Y.; Colson, Y.L.; Cohn, L.H.; Bawendi, M.G. Intraoperative identification of esophageal sentinel lymph nodes with near-infrared fluorescence imaging. J. Thorac. Cardiovasc. Surg. 2005, 129, 844–850. [Google Scholar]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar]
- Nguyen, Q.T.; Olson, E.S.; Aguilera, T.A.; Jiang, T.; Scadeng, M.; Ellies, L.G.; Tsien, R.Y. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc. Nat. Acad. Sci. 2010, 107, 4317–4322. [Google Scholar] [CrossRef] [PubMed]
- Themelis, G.; Yoo, J.S.; Soh, K.-S.; Schulz, R.; Ntziachristos, V. Real-time intraoperative fluorescence imaging system using light-absorption correction. J. Biomed. Opt. 2009. [Google Scholar] [CrossRef]
- He, B.; Xi, L.; Samuelson, S.R.; Xie, H.; Yang, L.; Jiang, H. Microelectromechanical systems scanning-mirror-based handheld probe for fluorescence molecular tomography. Appl. Opt. 2012, 51, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jiang, H.; Cao, Z.; Yang, L.; Mao, H.; Lipowska, M. A handheld fluorescence molecular tomography system for intraoperative optical imaging of tumor margins. Med. Phys. 2011, 38, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part Fibre Toxicol 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Satpathy, M.; Zhao, Q.; Qian, W.; Yang, L.; Jiang, H. Her-2/neu targeted delivery of a nanoprobe enables dual photoacoustic and fluorescence tomography of ovarian cancer. Nanomed.: Nanotechnol. Biol. Med. 2014, 10, 669–677. [Google Scholar] [CrossRef]
- Kossodo, S.; Pickarski, M.; Lin, S.-A.; Gleason, A.; Gaspar, R.; Buono, C.; Ho, G.; Blusztajn, A.; Cuneo, G.; Zhang, J. Dual in vivo quantification of integrin-targeted and protease-activated agents in cancer using fluorescence molecular tomography (fmt). Molecul. Imag. Biol. 2010, 12, 488–499. [Google Scholar] [CrossRef]
- Rudin, M.; Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Dis. 2003, 2, 123–131. [Google Scholar] [CrossRef]
- Jiang, H. Frequency-domain fluorescent diffusion tomography: A finite-element-based algorithm and simulations. Appl. Opt. 1998, 37, 5337–5343. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Barnhill, H.; Liang, X.; Wang, Q.; Jiang, H. A new probe using hybrid virus-dye nanoparticles for near-infrared fluorescence tomography. Opt. Commun. 2005, 255, 366–374. [Google Scholar] [CrossRef]
- Tan, Y.; Jiang, H. Diffuse optical tomography guided quantitative fluorescence molecular tomography. Appl. Opt. 2008, 47, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Diffuse Optical Tomography: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Yang, L.; Peng, X.-H.; Wang, Y.A.; Wang, X.; Cao, Z.; Ni, C.; Karna, P.; Zhang, X.; Wood, W.C.; Gao, X. Receptor-targeted nanoparticles for in vivo imaging of breast cancer. Clin. Can. Res. 2009, 15, 4722–4732. [Google Scholar] [CrossRef]
- Yang, H.; Xi, L.; Samuelson, S.; Xie, H.; Yang, L.; Jiang, H. Handheld miniature probe integrating diffuse optical tomography with photoacoustic imaging through a mems scanning mirror. Biomed. Opt. Exp. 2013, 4, 427–432. [Google Scholar] [CrossRef]
- Satpathy, M.; Wang, L.; Zielinski, R.; Qian, W.; Lipowska, M.; Capala, J.; Lee, G.Y.; Xu, H.; Wang, Y.A.; Mao, H. Active targeting using her‐2‐affibody‐conjugated nanoparticles enabled sensitive and specific imaging of orthotopic her‐2 positive ovarian tumors. Small 2014, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, J.; Carney, P.R.; Jiang, H. Towards real-time detection of seizures in awake rats with gpu-accelerated diffuse optical tomography. J. Neurosci. Meth. 2015, 240, 28–36. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; He, B.; Dai, X.; Satpathy, M.; Yang, L.; Jiang, H. FMTPen: A Miniaturized Handheld Fluorescence Molecular Tomography Probe for Image-Guided Cancer Surgery. Photonics 2015, 2, 279-287. https://doi.org/10.3390/photonics2010279
Yang H, He B, Dai X, Satpathy M, Yang L, Jiang H. FMTPen: A Miniaturized Handheld Fluorescence Molecular Tomography Probe for Image-Guided Cancer Surgery. Photonics. 2015; 2(1):279-287. https://doi.org/10.3390/photonics2010279
Chicago/Turabian StyleYang, Hao, Bin He, Xianjin Dai, Minati Satpathy, Lily Yang, and Huabei Jiang. 2015. "FMTPen: A Miniaturized Handheld Fluorescence Molecular Tomography Probe for Image-Guided Cancer Surgery" Photonics 2, no. 1: 279-287. https://doi.org/10.3390/photonics2010279
APA StyleYang, H., He, B., Dai, X., Satpathy, M., Yang, L., & Jiang, H. (2015). FMTPen: A Miniaturized Handheld Fluorescence Molecular Tomography Probe for Image-Guided Cancer Surgery. Photonics, 2(1), 279-287. https://doi.org/10.3390/photonics2010279




