Impact of Corneal-Hydration-Induced Changes in Ablation Efficiency During Refractive Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Changes of Corneal Hydration with Time
2.2. Change in Ablation Efficiency Due to Variations in Corneal Hydration
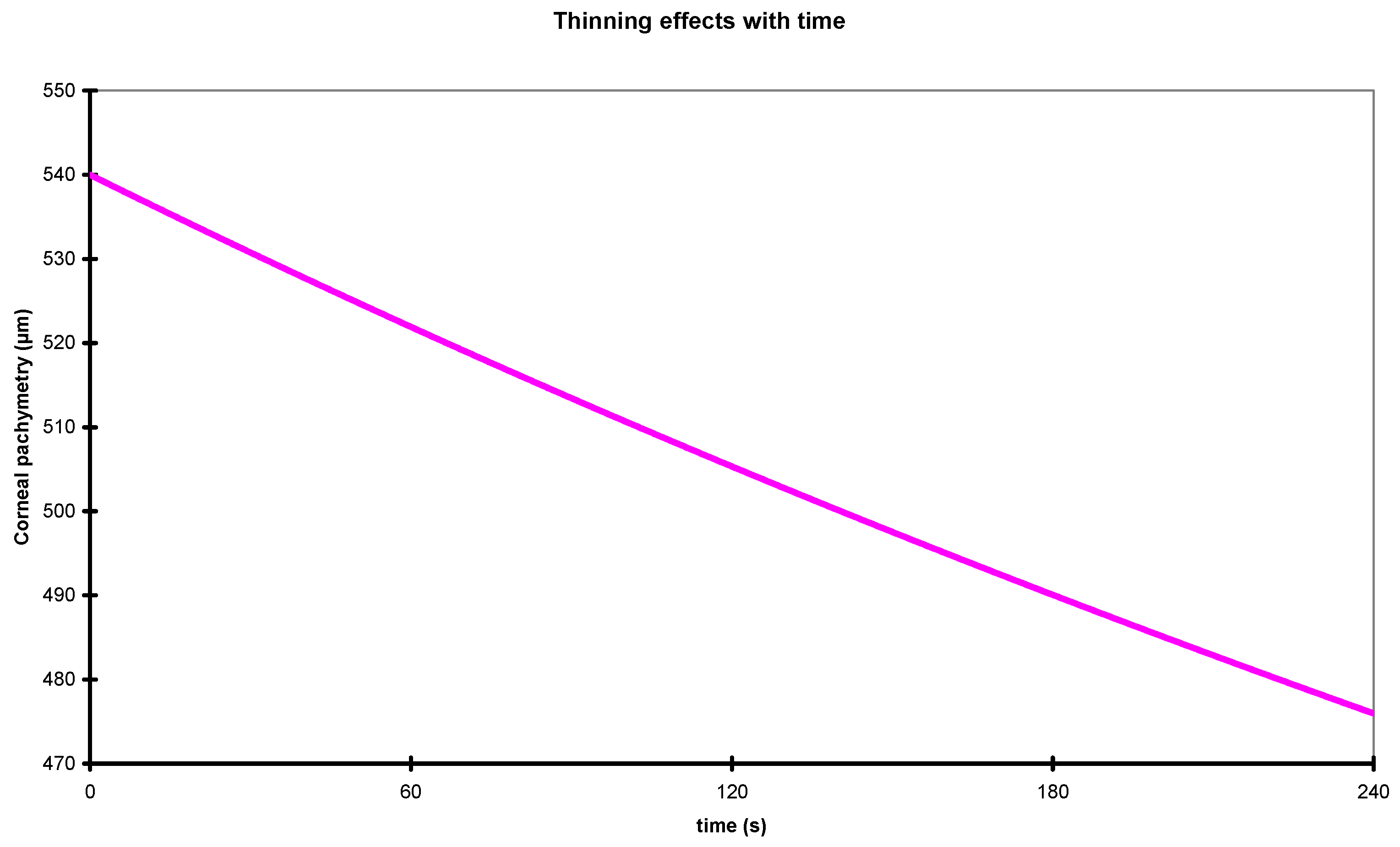
2.3. Corneal Thinning with Time
2.4. Change in Ablation Efficiency Due to Variations in Corneal Hydration and Thickness
2.5. Analysis of the Impact of the Change in Ablation Efficiency Due to Variations in Corneal Hydration and Thickness in Refractive Surgery Treatments
3. Results
3.1. Change in Ablation Efficiency Due to Variations in Corneal Hydration and Thickness
3.2. Analysis of the Impact of the Change in Ablation Efficiency Due to Variations in Corneal Hydration and Thickness in Refractive Surgery Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krueger, R.R.; Trokel, S.L. Quantitation of corneal ablation by ultraviolet laser light. Arch. Ophthalmol. 1985, 103, 1741–1742. [Google Scholar] [CrossRef] [PubMed]
- Manns, F.; Shen, J.H.; Söderberg, P.; Matsui, T.; Parel, J.M. Development of an algorithm for corneal reshaping with a scanning laser beam. Appl. Opt. 1995, 34, 4600–4608. [Google Scholar] [CrossRef] [PubMed]
- Mrochen, M.; Kaemmerer, M.; Seiler, T. Wavefront-guided laser in situ keratomileusis: Early results in three eyes. J. Refract. Surg. 2000, 16, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Barriuso, E.; Lloves, J.M.; Marcos, S.; Navarro, R.; Llorente, L.; Barbero, S. Ocular Aberrations before and after Myopic Corneal Refractive Surgery: LASIK-Induced Changes Measured with Laser Ray Tracing. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1396–1403. [Google Scholar]
- Dougherty, P.J.; Wellish, K.L.; Maloney, R.K. Excimer laser ablation rate and corneal hydration. Am. J. Ophthalmol. 1994, 118, 169–176. [Google Scholar] [CrossRef]
- Oshika, T.; Klyce, S.D.; Smolek, M.K.; McDonald, M.B. Corneal hydration and central islands after excimer laser photorefractive keratectomy. J. Cataract. Refract. Surg. 1998, 24, 1575–1580. [Google Scholar] [CrossRef]
- Stein, H.A.; Salim, A.G.; Stein, R.M.; Cheskes, A. Corneal cooling and rehydration during photorefractive keratectomy to reduce postoperative corneal haze. J. Refract. Surg. 1999, 15, S232–S233. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Jo, J.M. Corneal hydration affects ablation during laser in situ keratomileusis surgery. Cornea 2001, 20, 394–397. [Google Scholar] [CrossRef]
- Patel, S.; Alió, J.L.; Artola, A. Changes in the refractive index of the human corneal stroma during laser in situ keratomileusis. Effects of exposure time and method used to create the flap. J. Cataract. Refract. Surg. 2008, 34, 1077–1082. [Google Scholar] [CrossRef]
- Fisher, B.T.; Hahn, D.W. Development and numerical solution of a mechanistic model for corneal tissue ablation with the 193 nm argon fluoride excimer laser. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007, 24, 265–277. [Google Scholar] [CrossRef]
- Patel, S.; Alió, J.L.; Javaloy, J.; Perez-Santonja, J.J.; Artola, A.; Rodriguez-Prats, J. Human cornea before and after refractive surgery using a new device: VCH-1. Cornea 2008, 27, 1042–1049. [Google Scholar] [CrossRef]
- Müller, L.J.; Pels, E.; Vrensen, G.F. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br. J. Ophthalmol. 2001, 85, 437–443. [Google Scholar] [CrossRef]
- Neuhann, I.M.; Lege, B.A.; Bauer, M.; Hassel, J.M.; Hilger, A.; Neuhann, T.F. Online optical coherence pachymetry as a safety measure for laser in situ keratomileusis treatment in 1859 cases. J. Cataract. Refract. Surg. 2008, 34, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Pfaeffl, W.A. Safety limits of online optical coherence pachymetry. J. Cataract. Refract. Surg. 2009, 35, 610–611, Author Reply 611–612. [Google Scholar] [CrossRef] [PubMed]
- Pfaeffl, W.A.; Kunze, M.; Zenk, U.; Pfaeffl, M.B.; Schuster, T.; Lohmann, C. Predictive factors of femtosecond laser flap thickness measured by online optical coherence pachymetry subtraction in sub-Bowman keratomileusis. J. Cataract. Refract. Surg. 2008, 34, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Pallikaris, I.G.; Siganos, D.S. Excimer laser in situ keratomileusis and photorefractive keratectomy for correction of high myopia. J. Refract. Corneal Surg. 1994, 10, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Ditzen, K.; Huschka, H.; Pieger, S. Laser in situ keratomileusis for hyperopia. J. Cataract. Refract. Surg. 1998, 24, 42–47. [Google Scholar] [CrossRef]
- El Danasoury, M.A.; Waring, G.O.; el Maghraby, A.; Mehrez, K. Excimer laser in situ keratomileusis to correct compound myopic astigmatism. J. Refract. Surg. 1997, 13, 511–520. [Google Scholar] [CrossRef]
- O’Donnell, C.B.; Kemner, J.; O’Donnell, F.E. Ablation smoothness as a function of excimer laser delivery system. J. Cataract. Refract. Surg. 1996, 22, 682–685. [Google Scholar] [CrossRef]
- Müller, B.; Boeck, T.; Hartmann, C. Effect of excimer laser beam delivery and beam shaping on corneal sphericity in photorefractive keratectomy. J. Cataract. Refract. Surg. 2004, 30, 464–470. [Google Scholar] [CrossRef]
- Mrochen, M.; Kaemmerer, M.; Mierdel, P.; Seiler, T. Increased higher-order optical aberrations after laser refractive surgery: A problem of subclinical decentration. J. Cataract. Refract. Surg. 2001, 27, 362–369. [Google Scholar] [CrossRef]
- Mrochen, M.; Krueger, R.R.; Bueeler, M.; Seiler, T. Aberration-sensing and wavefront-guided laser in situ keratomileusis: Management of decentered ablation. J. Refract. Surg. 2002, 18, 418–429. [Google Scholar] [CrossRef]
- Taylor, N.M.; Eikelboom, R.H.; van Sarloos, P.P.; Reid, P.G. Determining the accuracy of an eye tracking system for laser refractive surgery. J. Refract. Surg. 2000, 16, S643–S646. [Google Scholar] [CrossRef]
- Effect of Spot Size, Ablation Depth, and Eye-Tracker Latency on the Optical Outcome of Corneal Laser Surgery with a Scanning Spot Laser|Request PDF. ResearchGate. Available online: https://www.researchgate.net/publication/252375283_Effect_of_spot_size_ablation_depth_and_eye-tracker_latency_on_the_optical_outcome_of_corneal_laser_surgery_with_a_scanning_spot_laser (accessed on 12 May 2025).
- Zadok, D.; Carrillo, C.; Missiroli, F.; Litwak, S.; Robledo, N.; Chayet, A.S. The effect of corneal flap on optical aberrations. Am. J. Ophthalmol. 2004, 138, 190–193. [Google Scholar] [CrossRef]
- Mrochen, M.; Seiler, T. Influence of corneal curvature on calculation of ablation patterns used in photorefractive laser surgery. J. Refract. Surg. 2001, 17, S584–S587. [Google Scholar] [CrossRef] [PubMed]
- Hersh, P.S.; Fry, K.; Blaker, J.W. Spherical aberration after laser in situ keratomileusis and photorefractive keratectomy. Clinical results and theoretical models of etiology. J. Cataract. Refract. Surg. 2003, 29, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.R.; Anera, R.G.; Jiménez del Barco, L.; Hita, E. Effect on laser-ablation algorithms of reflection losses and nonnormal incidence on the anterior cornea. Appl. Phys. Lett. 2002, 81, 1521–1523. [Google Scholar] [CrossRef]
- Anera, R.G.; Jiménez, J.R.; Jiménez del Barco, L.; Hita, E. Changes in corneal asphericity after laser refractive surgery, including reflection losses and nonnormal incidence upon the anterior cornea. Opt. Lett. 2003, 28, 417–419. [Google Scholar] [CrossRef]
- Cano, D.; Barbero, S.; Marcos, S. Comparison of real and computer-simulated outcomes of LASIK refractive surgery. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2004, 21, 926–936. [Google Scholar] [CrossRef]
- Jiménez, J.R.; Rodríguez-Marín, F.; Anera, R.G.; Jiménez Del Barco, L. Deviations of Lambert-Beer’s law affect corneal refractive parameters after refractive surgery. Opt. Express 2006, 14, 5411–5417. [Google Scholar] [CrossRef]
- Dorronsoro, C.; Cano, D.; Merayo-Lloves, J.; Marcos, S. Experiments on PMMA models to predict the impact of corneal refractive surgery on corneal shape. Opt. Express 2006, 14, 6142–6156. [Google Scholar] [CrossRef]
- Roberts, C. Biomechanics of the cornea and wavefront-guided laser refractive surgery. J. Refract. Surg. 2002, 18, S589–S592. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tang, M.; Shekhar, R. Mathematical model of corneal surface smoothing after laser refractive surgery. Am. J. Ophthalmol. 2003, 135, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C. Biomechanical customization: The next generation of laser refractive surgery. J. Cataract. Refract. Surg. 2005, 31, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Macrae, S.; Williams, D.R.; Cox, I.G. Causes of spherical aberration induced by laser refractive surgery. J. Cataract. Refract. Surg. 2005, 31, 127–135. [Google Scholar] [CrossRef]
- Heat and Mass Transfer—E. R. G. Eckert and R. M. Drake PDF|PDF|Heat Transfer|Chemical Engineering. Scribd. Available online: https://www.scribd.com/doc/257702159/Heat-And-Mass-Transfer-E-R-G-Eckert-And-R-M-Drake-pdf (accessed on 12 May 2025).
- Mikhailov, M.; Ozisik, M. Unified Analysis and Solutions of Heat and Mass Diffusion; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1984; ISBN 978-0-471-89830-6. [Google Scholar]
- Crank, J.; Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1979; ISBN 978-0-19-853411-2. [Google Scholar]
- Polse, K.A.; Brand, R.; Mandell, R.; Vastine, D.; Demartini, D.; Flom, R. Age differences in corneal hydration control. Investig. Ophthalmol. Vis. Sci. 1989, 30, 392–399. [Google Scholar]
- Mandell, R.B.; Polse, K.A.; Brand, R.J.; Vastine, D.; Demartini, D.; Flom, R. Corneal hydration control in Fuchs’ dystrophy. Investig. Ophthalmol. Vis. Sci. 1989, 30, 845–852. [Google Scholar]
- Herse, P.R. Corneal hydration control in normal and alloxan-induced diabetic rabbits. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2205–2213. [Google Scholar]
- Weston, B.C.; Bourne, W.M.; Polse, K.A.; Hodge, D.O. Corneal hydration control in diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 1995, 36, 586–595. [Google Scholar]
- Matsuura, T.; Gorti, S.; Tanaka, T.; Hara, Y.; Saishin, M. Determination of corneal gel dynamics. Eur. Biophys. J. EBJ 1999, 28, 357–368. [Google Scholar] [CrossRef]
- Fuchs, H.U. The Dynamics of Heat; Springer Science & Business Media: New York, NY, USA, 1996; ISBN 978-0-387-94603-0. [Google Scholar]
- Maurice, D.M. The structure and transparency of the cornea. J. Physiol. 1957, 136, 263–286.1. [Google Scholar] [CrossRef]
- Aurich, H.; Wirbelauer, C.; Jaroszewski, J.; Hartmann, C.; Pham, D.T. Continuous measurement of corneal dehydration with online optical coherence pachymetry. Cornea 2006, 25, 182–184. [Google Scholar] [CrossRef]
- Arbelaez, M.C.; Vidal, C.; Arba Mosquera, S. Central ablation depth and postoperative refraction in excimer laser myopic correction measured with ultrasound, Scheimpflug, and optical coherence pachymetry. J. Refract. Surg. 2009, 25, 699–708. [Google Scholar] [CrossRef]
- Qi, P.; Sun, L.; Ma, J.; Yao, J.; Lin, L.; Zhang, L.; Wang, Y.; Liu, W. Ex vivo quantitative analysis of human corneal stroma dehydration by near-infrared absorption spectroscopy. J. Biophotonics 2019, 12, e201800472. [Google Scholar] [CrossRef]
- Shraiki, M.; Arba-Mosquera, S. Simulation of the Impact of Refractive Surgery Ablative Laser Pulses with a Flying-Spot Laser Beam on Intrasurgery Corneal Temperature. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3713–3722. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Zhang, Z.; Yu, X. Corneal stromal dehydration and optimal stromal exposure time during corneal refractive surgery measured using a three-dimensional optical profiler. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von. Graefes Arch. Klin. Exp. Ophthalmol. 2022, 260, 4005–4013. [Google Scholar] [CrossRef]



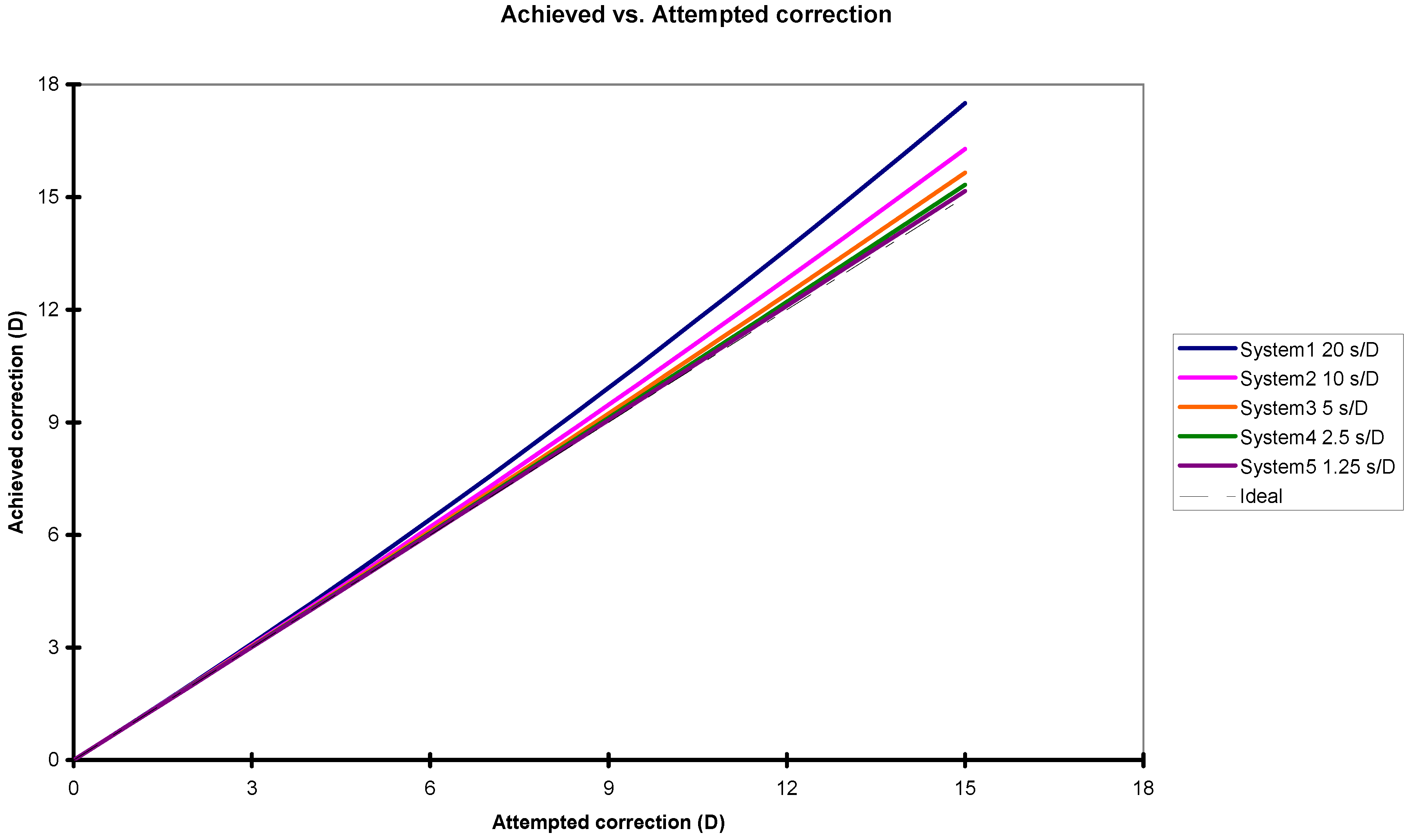
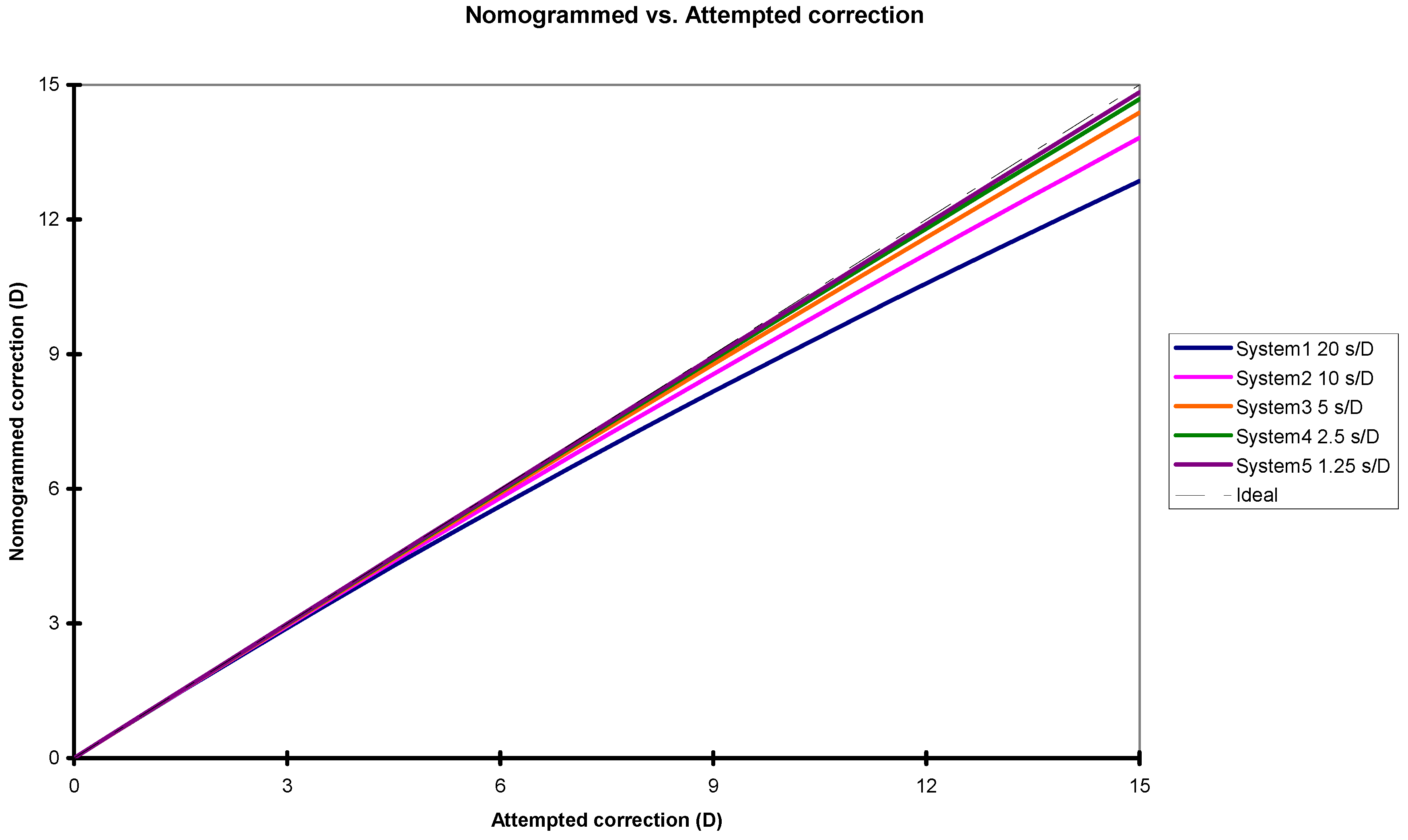
| Attempted Correction (Diopter) | System Correction Speeds: 20 s/D | System Correction Speeds: 10 s/D | System Correction Speeds: 5 s/D | System Correction Speeds: 2.5 s/D | System Correction Speeds: 1.25 s/D | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Achieved Correction in D | Nomogram Factor in Percentage | Achieved Correction in D | Nomogram Factor in Percentage | Achieved Correction in D | Nomogram Factor in Percentage | Achieved Correction in D | Nomogram Factor in Percentage | Achieved Correction in D | Nomogram Factor in Percentage | |
| 1.00 | 1.01 | −1.1% | 1.01 | −0.6% | 1.00 | −0.3% | 1.00 | −0.1% | 1.00 | −0.1% |
| 2.00 | 2.05 | −2.3% | 2.02 | −1.1% | 2.01 | −0.6% | 2.01 | −0.1% | 2.00 | −0.1% |
| 3.00 | 3.10 | −3.3% | 3.05 | −1.7% | 3.03 | −0.9% | 3.01 | −0.2% | 3.01 | −0.2% |
| 4.00 | 4.18 | −4.4% | 4.09 | −2.3% | 4.05 | −1.1% | 4.02 | −0.3% | 4.01 | −0.3% |
| 5.00 | 5.29 | −5.4% | 5.14 | −2.8% | 5.07 | −1.4% | 5.04 | −0.4% | 5.02 | −0.4% |
| 6.00 | 6.41 | −6.4% | 6.21 | −3.3% | 6.10 | −1.7% | 6.05 | −0.4% | 6.03 | −0.4% |
| 7.00 | 7.56 | −7.4% | 7.28 | −3.9% | 7.14 | −2.0% | 7.07 | −0.5% | 7.04 | −0.5% |
| 8.00 | 8.73 | −8.3% | 8.37 | −4.4% | 8.19 | −2.3% | 8.09 | −0.6% | 8.05 | −0.6% |
| 9.00 | 9.92 | −9.2% | 9.47 | −4.9% | 9.23 | −2.5% | 9.12 | −0.7% | 9.06 | −0.7% |
| 10.00 | 11.13 | −10.1% | 10.57 | −5.4% | 10.29 | −2.8% | 10.15 | −0.7% | 10.07 | −0.7% |
| 11.00 | 12.36 | −11.0% | 11.69 | −5.9% | 11.35 | −3.1% | 11.18 | −0.8% | 11.09 | −0.8% |
| 12.00 | 13.61 | −11.9% | 12.82 | −6.4% | 12.42 | −3.3% | 12.21 | −0.9% | 12.10 | −0.9% |
| 13.00 | 14.89 | −12.7% | 13.96 | −6.9% | 13.49 | −3.6% | 13.25 | −0.9% | 13.12 | −0.9% |
| 14.00 | 16.18 | −13.5% | 15.12 | −7.4% | 14.57 | −3.9% | 14.28 | −1.0% | 14.14 | −1.0% |
| 15.00 | 17.50 | −14.3% | 16.28 | −7.9% | 15.65 | −4.1% | 15.33 | −1.1% | 15.16 | −1.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosquera, S.A.; Verma, S. Impact of Corneal-Hydration-Induced Changes in Ablation Efficiency During Refractive Surgery. Photonics 2025, 12, 769. https://doi.org/10.3390/photonics12080769
Mosquera SA, Verma S. Impact of Corneal-Hydration-Induced Changes in Ablation Efficiency During Refractive Surgery. Photonics. 2025; 12(8):769. https://doi.org/10.3390/photonics12080769
Chicago/Turabian StyleMosquera, Samuel Arba, and Shwetabh Verma. 2025. "Impact of Corneal-Hydration-Induced Changes in Ablation Efficiency During Refractive Surgery" Photonics 12, no. 8: 769. https://doi.org/10.3390/photonics12080769
APA StyleMosquera, S. A., & Verma, S. (2025). Impact of Corneal-Hydration-Induced Changes in Ablation Efficiency During Refractive Surgery. Photonics, 12(8), 769. https://doi.org/10.3390/photonics12080769





