Research Progress on Immunological Biochips Based on Surface Plasmon Resonance
Abstract
1. Introduction
2. Surface Plasmon Resonance
2.1. Basic Principles and Characteristics of Surface Plasmon Resonance
2.2. Excitation Mode of SPR
2.2.1. Prism Coupling
2.2.2. Grating Excitation
2.3. The Properties of Nanoparticles and LSPR
3. Detection Method and Principle
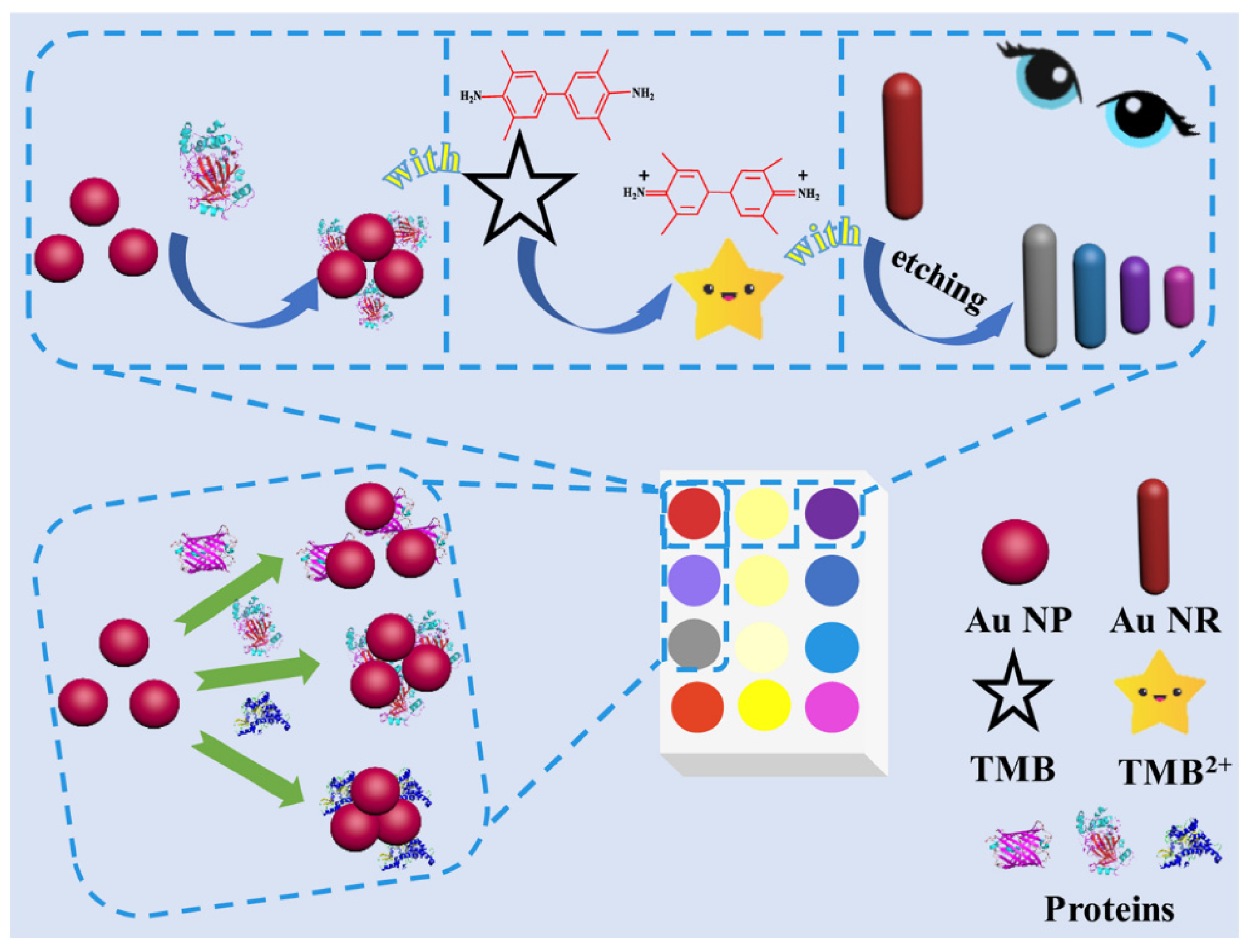
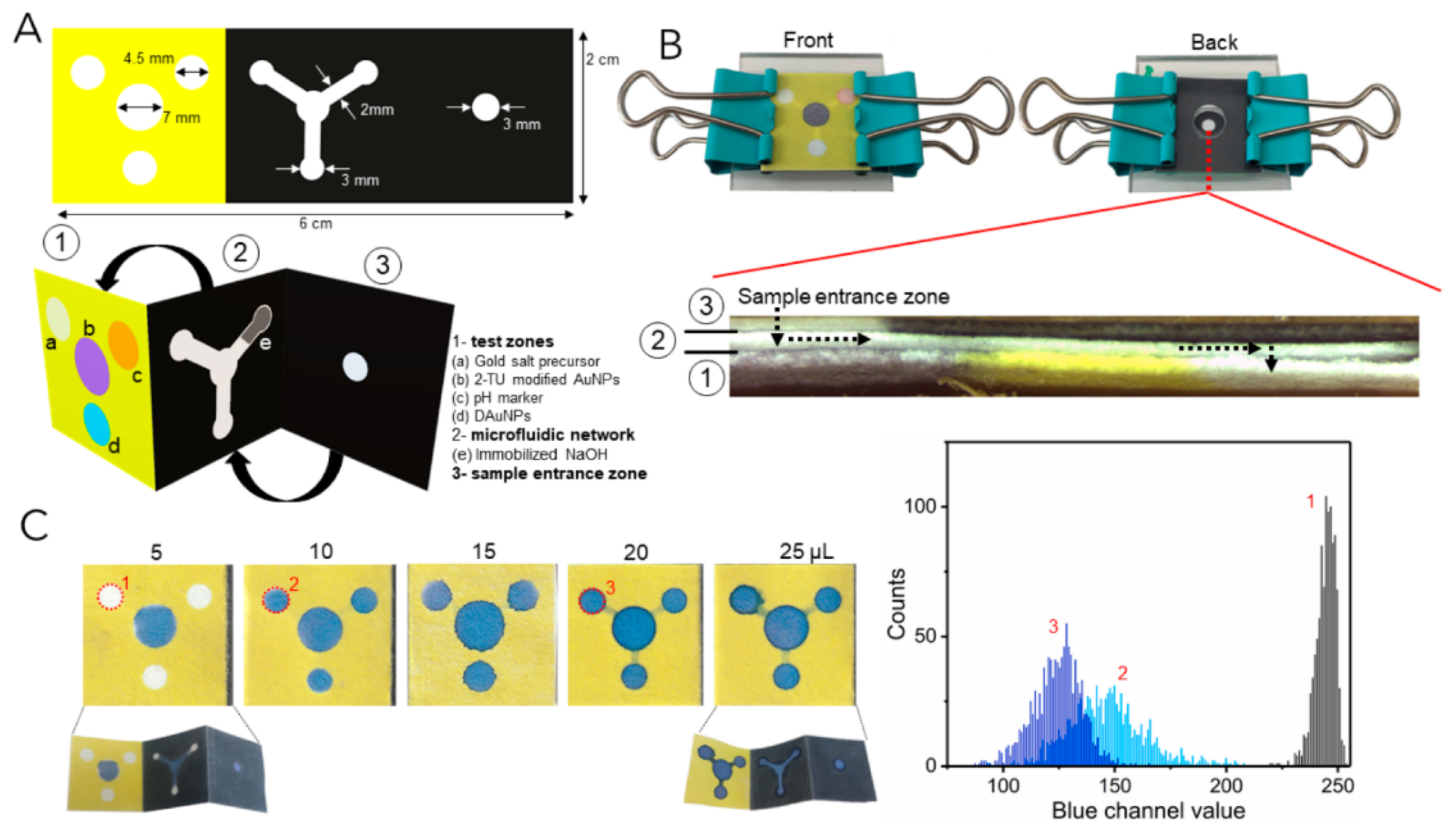
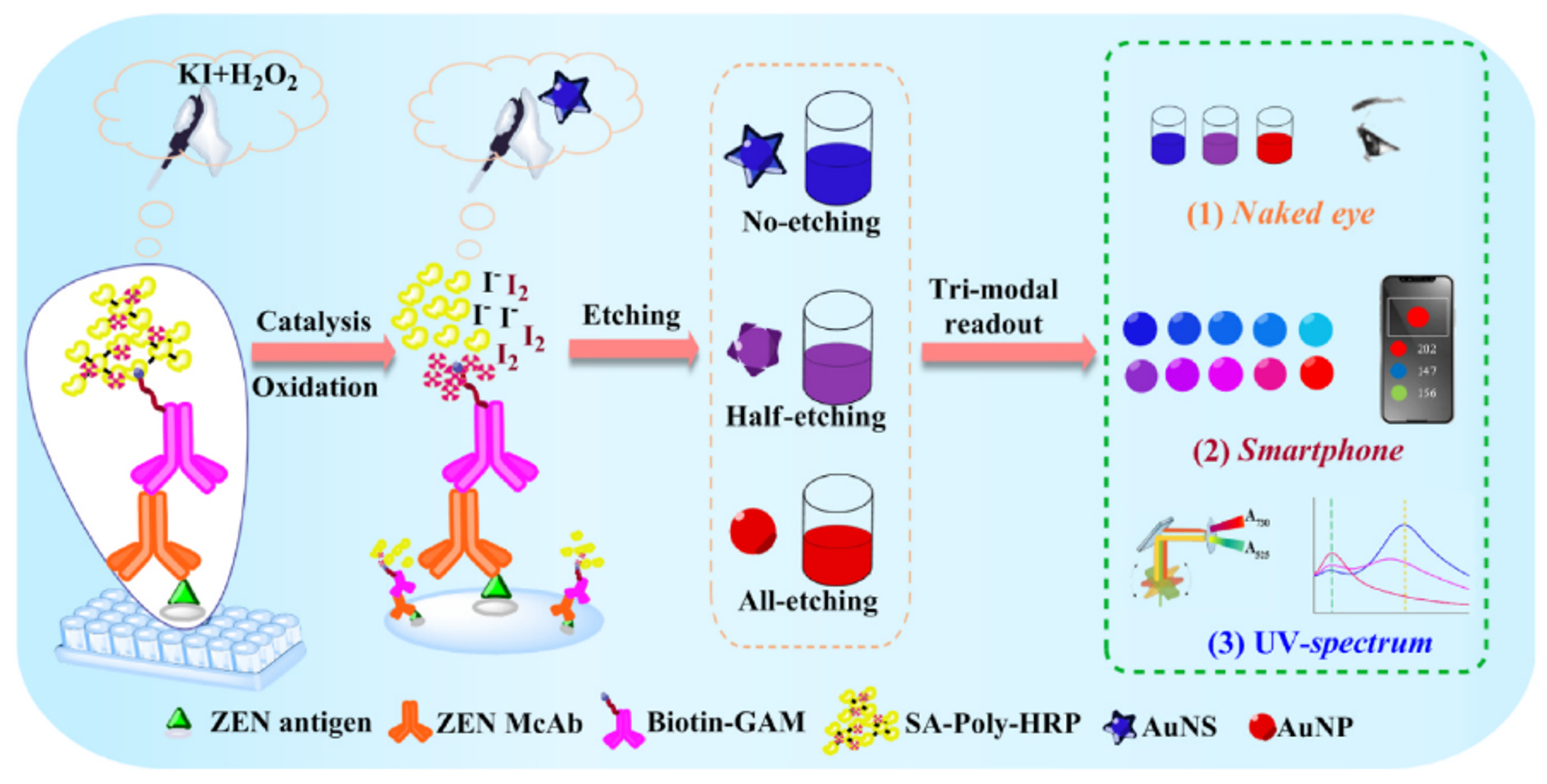
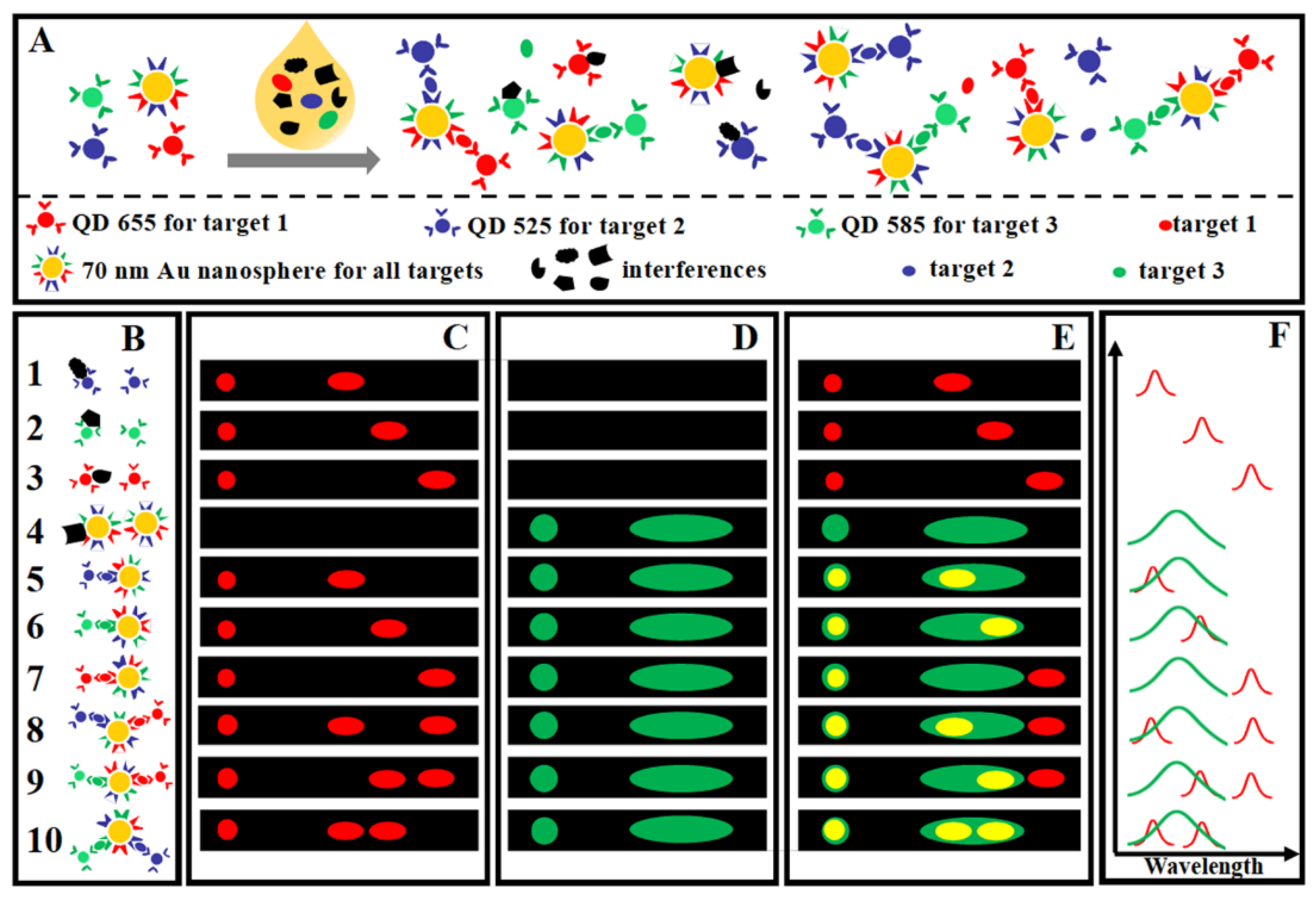
3.1. Colorimetric Sensing
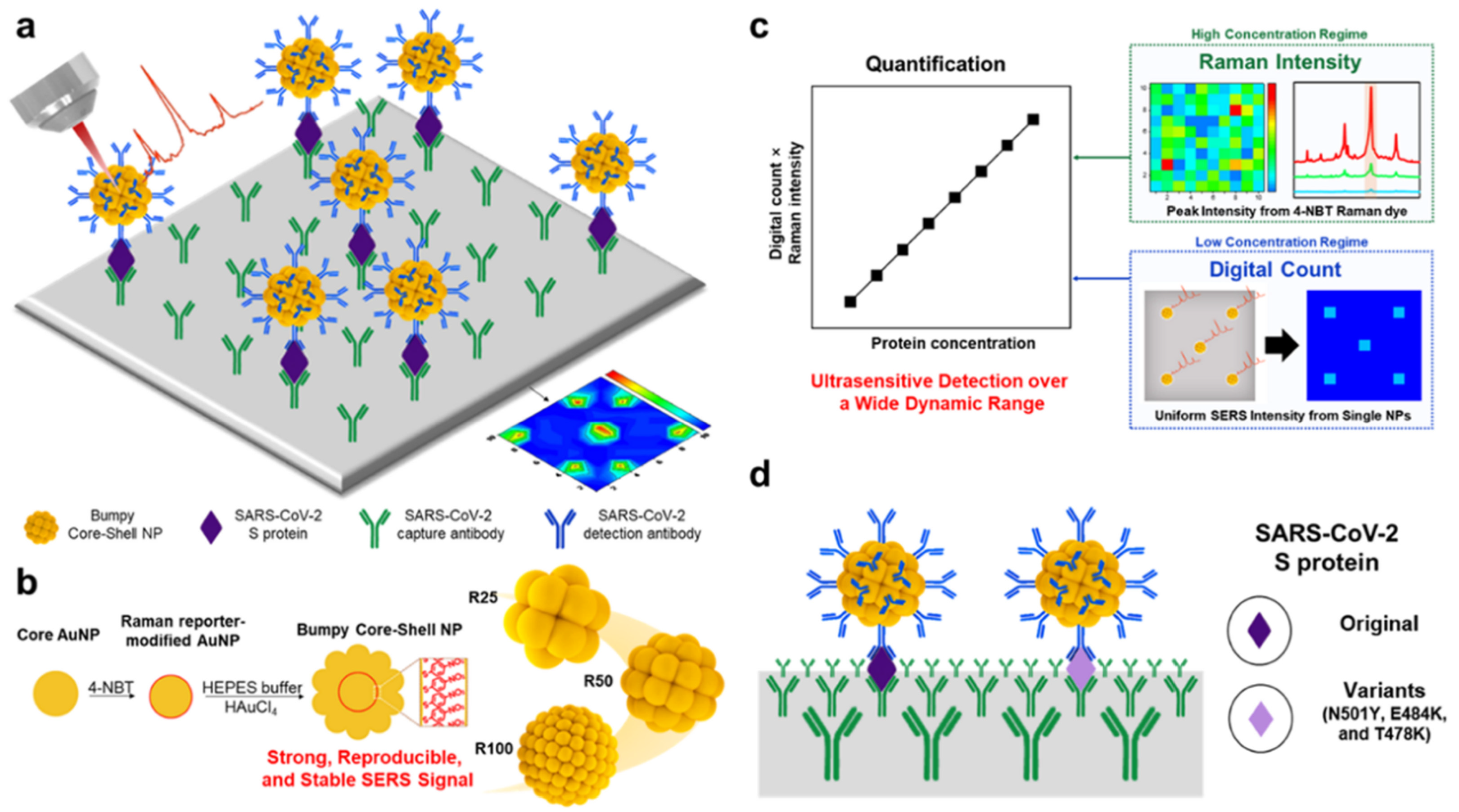
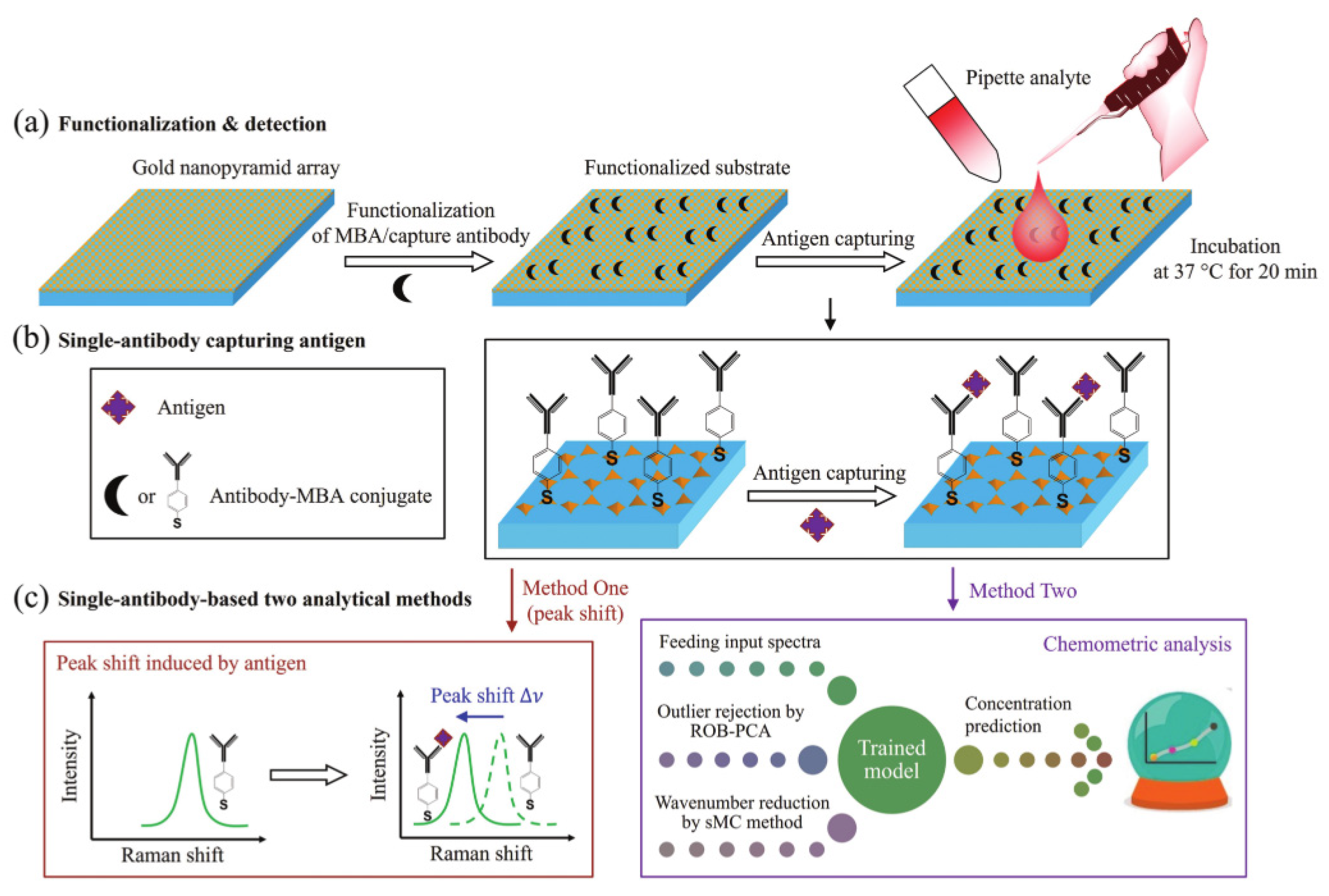
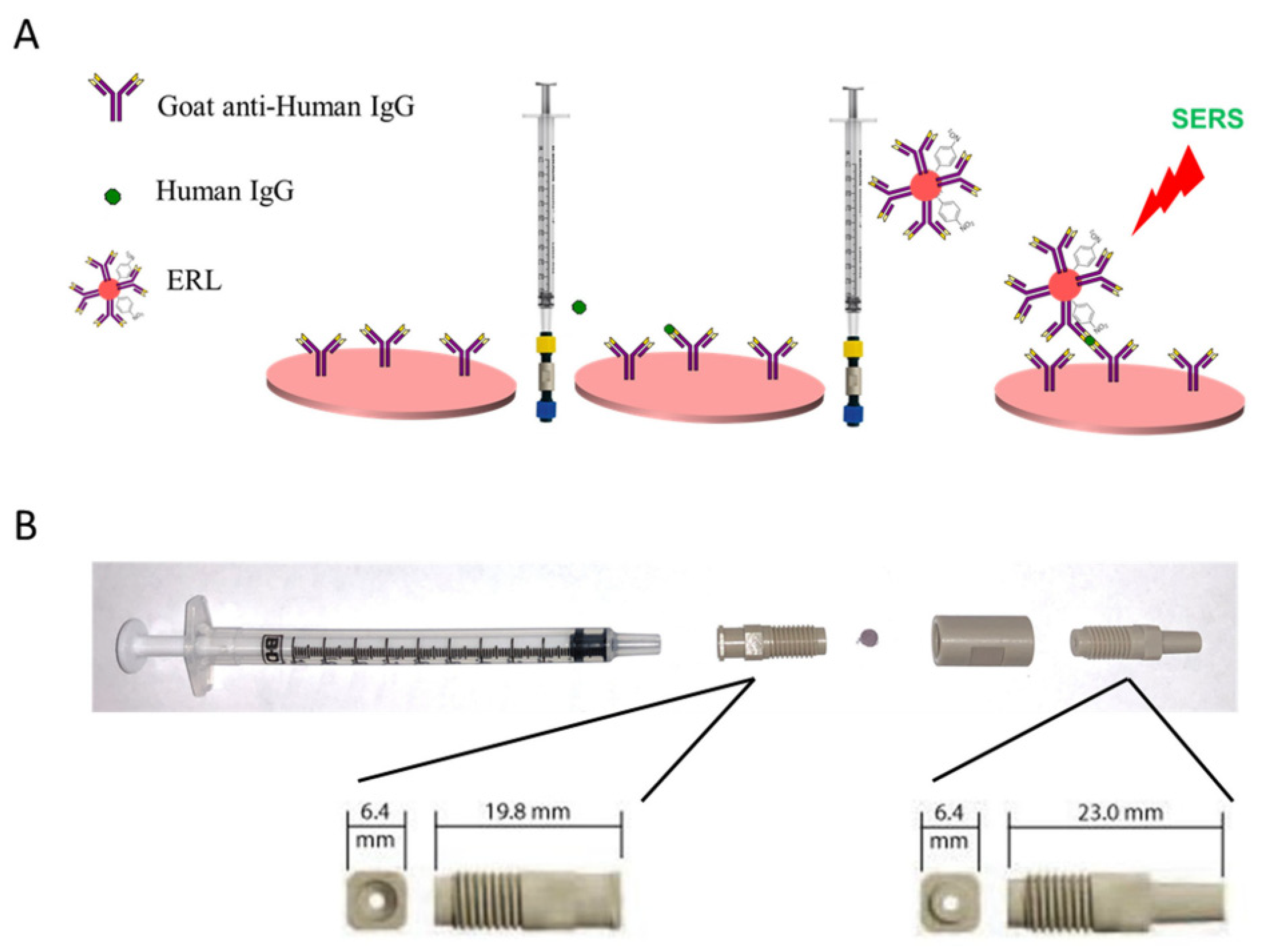
3.2. Surface Enhanced Raman Scattering
3.3. SPR Enhanced Fluorescence
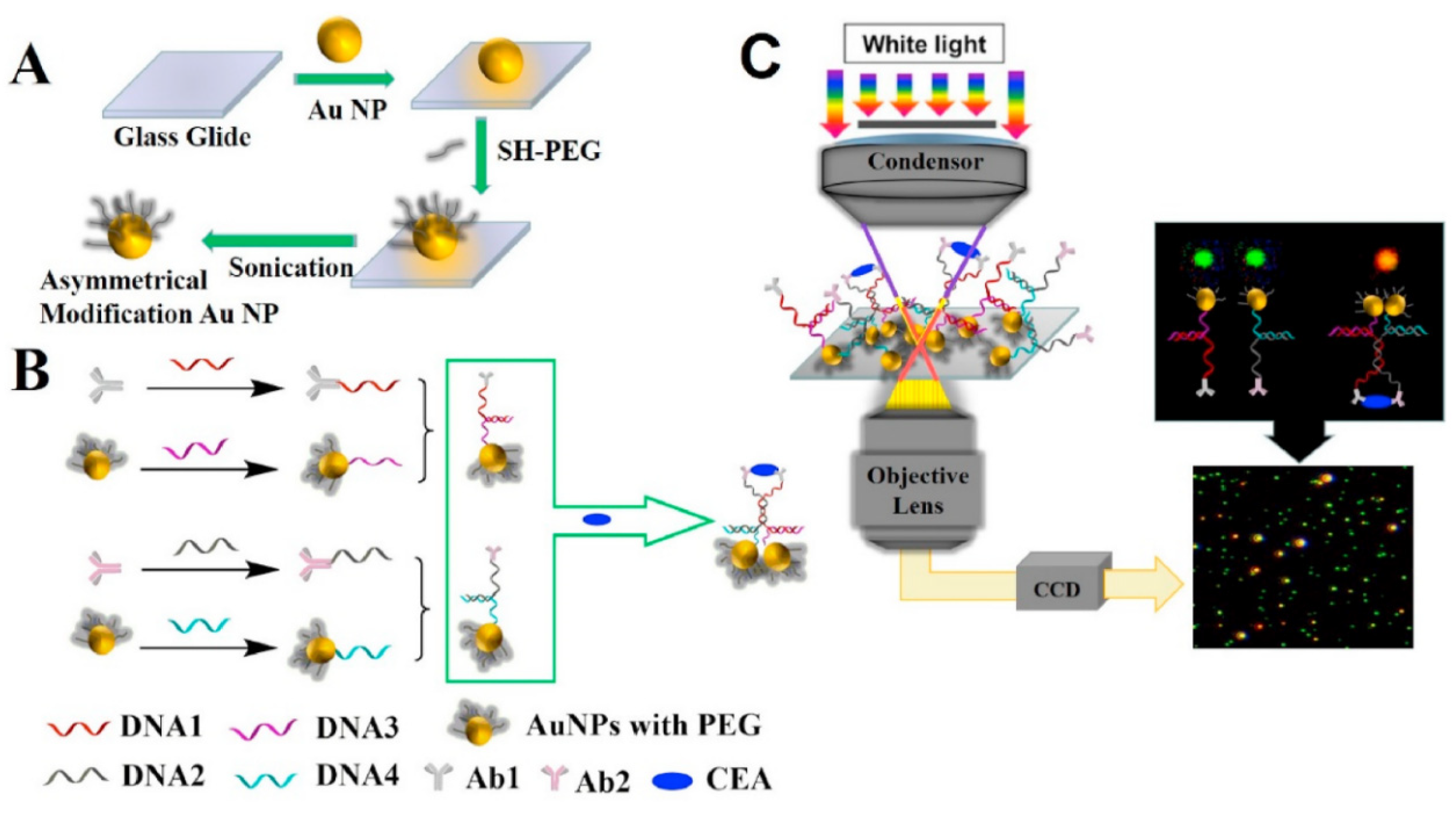
3.4. Dark Field Detection
3.5. Other Plasmonic Sensors
4. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| SPR | Surface plasmon resonance |
| SPPs | Surface plasmon polariton |
| LSP | Local surface plasmon |
| SPR | Surface plasmon resonance |
| LSPR | Local surface plasmon resonance |
| POCT | Point of care testing |
| SERS | Surface enhanced Raman scattering |
| DFM | Dark-Field Microscopy |
| NP | Nanoparticle |
| NR | Nanorod |
| NDKA | Nucleoside diphosphate kinase A |
| LFA | Lateral flow assay |
| PSPR | Propagating surface plasmon resonance |
| GF | Gold film |
References
- Seth, A.; Liu, Y.; Gupta, R.; Wang, Z.; Mittal, E.; Kolla, S.; Rathi, P.; Gupta, P.; Parikh, B.A.; Genin, G.M.; et al. Plasmon-Enhanced Digital Fluoroimmunoassay for Subfemtomolar Detection of Protein Biomarkers. Nano Lett. 2024, 24, 229–237. [Google Scholar] [CrossRef]
- Salehnezhad, Z.; Soroosh, M.; Mondal, H. A Highly Sensitive Plasmonic Graphene-Based Structure for Deoxyribonucleic Acid Detection. Photonics 2024, 11, 549. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, K.; Mi, H.; Qiu, Y.; Son, J.; Park, H.J.; Nam, J.-M.; Lee, J.-H. A rapid and sensitive fluorescence biosensor based on plasmonic PCR. Nanoscale 2021, 13, 7348–7354. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xiong, M.; Li, L.; Yan, Y.; Li, J.; Feng, Z.; Li, Y.; Zhao, J.; Dong, Y.; Li, X.; et al. One-Step Purification of IgE Epitope-Specific Antibody Using Immunomagnetic Beads and Highly Sensitive Detection of Bovine β-Lactoglobulin for the Prediction of Milk Allergenicity in Foods. J. Agric. Food Chem. 2023, 71, 14068–14078. [Google Scholar] [CrossRef]
- Tran, N.H.T.; Trinh, K.T.L.; Lee, J.H.; Yoon, W.J.; Ju, H. Reproducible Enhancement of Fluorescence by Bimetal Mediated Surface Plasmon Coupled Emission for Highly Sensitive Quantitative Diagnosis of Double-Stranded DNA. Small 2018, 14, e1801385. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Ali, M.M.; Su, H.M.; Filipe, C.D.M.; Didar, T.F. Sentinel Wraps: Real-Time Monitoring of Food Contamination by Printing DNAzyme Probes on Food Packaging. ACS Nano 2018, 12, 3287–3294. [Google Scholar] [CrossRef]
- Xiong, Y.; Fu, T.; Zhang, D.; Zhang, S.; Xu, H. Superradiative plasmonic nanoantenna biosensors enable sensitive immunoassay using the naked eye. Nanoscale 2021, 13, 2429–2435. [Google Scholar] [CrossRef]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Yan, R.; Wang, T.; Yue, X.; Wang, H.; Zhang, Y.-H.; Xu, P.; Wang, L.; Wang, Y.; Zhang, J. Highly sensitive plasmonic nanorod hyperbolic metamaterial biosensor. Photonics Res. 2022, 10, 84. [Google Scholar] [CrossRef]
- Arcadio, F.; Zeni, L.; Montemurro, D.; Eramo, C.; Di Ronza, S.; Perri, C.; D’Agostino, G.; Chiaretti, G.; Porto, G.; Cennamo, N. Biochemical sensing exploiting plasmonic sensors based on gold nanogratings and polymer optical fibers. Photonics Res. 2021, 9, 1397. [Google Scholar] [CrossRef]
- Gramotnev, D.K.; Bozhevolnyi, S.I. Plasmonics beyond the diffraction limit. Nat. Photonics 2010, 4, 83–91. [Google Scholar] [CrossRef]
- Ozbay, E. Plasmonics: Merging Photonics and Electronics at Nanoscale Dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Brzeska, S.; Makowski, M. Exploring the interactions of biologically active compounds (including drugs) with biomolecules: Utilizing Surface Plasmon Resonance and SwitchSense techniques. TrAC Trends Anal. Chem. 2024, 176, 117764. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Khodaie, A.; Heidarzadeh, H. Design and analysis of a multi-modal refractive index plasmonic biosensor based on split ring resonator for detection of the various cancer cells. Opt. Quantum Electron. 2024, 56, 1439. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Li, P.; Nie, A.; Zhai, K.; Xiang, J.; Mu, C.; Wen, F.; Wang, B.; Xue, T.; et al. Ferroelectric Bi2O2Te-Based Plasmonic Biosensor for Ultrasensitive Biomolecular Detection. Small 2024, 20, 2312175. [Google Scholar] [CrossRef]
- Jin, C.; Wu, Z.; Molinski, J.H.; Zhou, J.; Ren, Y.; Zhang, J.X.J. Plasmonic nanosensors for point-of-care biomarker detection. Mater. Today Bio 2022, 14, 100263. [Google Scholar] [CrossRef]
- Sönnichsen, C.; Franzl, T.; Wilk, T.; von Plessen, G.; Feldmann, J.; Wilson, O.; Mulvaney, P. Drastic reduction of plasmon damping in gold nanorods. Phys. Rev. Lett. 2002, 88, 077402. [Google Scholar] [CrossRef]
- Ropers, C.; Neacsu, C.C.; Elsaesser, T.; Albrecht, M.; Raschke, M.B.; Lienau, C. Grating-coupling of surface plasmons onto metallic tips: A nanoconfined light source. Nano Lett. 2007, 7, 2784–2788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Kravtsov, V.; Wang, Z.; Zhou, Z.; Dou, L.; Huang, D.; Wang, Z.; Cheng, X.; Raschke, M.B.; Jiang, T. Applications of ultrafast nano-spectroscopy and nano-imaging with tip-based microscopy. eLight 2025, 5, 1. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, H.; Deng, Q.; Zhang, S.; Xu, H. Temperature-dependent dark-field scattering of single plasmonic nanocavity. Nanophotonics 2020, 9, 3347–3356. [Google Scholar] [CrossRef]
- Novo, C.; Gomez, D.; Perez-Juste, J.; Zhang, Z.; Petrova, H.; Reismann, M.; Mulvaney, P.; Hartland, G.V. Contributions from radiation damping and surface scattering to the linewidth of the longitudinal plasmon band of gold nanorods: A single particle study. Phys. Chem. Chem. Phys. 2006, 8, 3540–3546. [Google Scholar] [CrossRef]
- Ramasamy, M.; Ha, J.W. Single-Particle Spectroelectrochemistry: Revealing the Electrochemical Tuning Mechanism of Chemical Interface Damping in 1,2-Benzenedithiol-Adsorbed Single Gold Nanorods. Anal. Chem. 2024, 96, 18043–18051. [Google Scholar] [CrossRef]
- Lee, S.Y.; Tsalu, P.V.; Kim, G.W.; Seo, M.J.; Hong, J.W.; Ha, J.W. Tuning Chemical Interface Damping: Interfacial Electronic Effects of Adsorbate Molecules and Sharp Tips of Single Gold Bipyramids. Nano Lett. 2019, 19, 2568–2574. [Google Scholar] [CrossRef] [PubMed]
- Foerster, B.; Spata, V.A.; Carter, E.A.; Sönnichsen, C.; Link, S. Plasmon damping depends on the chemical nature of the nanoparticle interface. Sci. Adv. 2019, 5, eaav0704. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pelton, M.; Guyot-Sionnest, P. Reduced damping of surface plasmons at low temperatures. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 79, 035418. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Bondarchuk, I.S.; Losytskyy, M.Y.; Alexeenko, A.A. Temperature Dependence of Photoluminescence from Silver Nanoparticles. Plasmonics 2014, 9, 93–101. [Google Scholar] [CrossRef]
- Konrad, A.; Wackenhut, F.; Hussels, M.; Meixner, A.J.; Brecht, M. Temperature Dependent Luminescence and Dephasing of Gold Nanorods. J. Phys. Chem. C 2013, 117, 21476–21482. [Google Scholar] [CrossRef]
- Arbouet, A.; Voisin, C.; Christofilos, D.; Langot, P.; Fatti, N.D.; Vallée, F.; Lermé, J.; Celep, G.; Cottancin, E.; Gaudry, M.; et al. Electron-phonon scattering in metal clusters. Phys. Rev. Lett. 2003, 90, 177401. [Google Scholar] [CrossRef]
- Voisin, C.; Christofilos, D.; Loukakos, P.; Del Fatti, N.; Vallée, F.; Lermé, J.; Gaudry, M.; Cottancin, E.; Pellarin, M.; Broyer, M. Ultrafast electron-electron scattering and energy exchanges in noble-metal nanoparticles. Phys. Rev. B 2004, 69, 195416. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Notizen: Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. Naturforschung A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Phys. 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Wark, A.W.; Lee, H.J.; Corn, R.M. Long-range surface plasmon resonance imaging for bioaffinity sensors. Anal. Chem. 2005, 77, 3904–3907. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; El-Sayed, M.A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, T.; Jiang, J.; Liu, K.; Wang, S.; Zhang, Z.; Jing, J. Progress in Sensitivity Enhancement for Optical Fibre Surface Plasmon Resonance Sensing. Chin. J. Lasers 2021, 48, 1906002. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Mukhtar, W.M.; Halim, R.M.; Hassan, H.P. Optimization of SPR signals: Monitoring the physical structures and refractive indices of prisms. EPJ Web Conf. 2017, 162, 01001. [Google Scholar] [CrossRef]
- Ekimov, A.I.; Efros, A.L.; Onushchenko, A.A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- Gleiter, H. Nanocrystalline Material. Prog. Mater. Sci. 1989, 33, 223–315. [Google Scholar] [CrossRef]
- Brus, L.E. Electron—Electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Ashley, J.; D’Aurelio, R.; Piekarska, M.; Temblay, J.; Pleasants, M.; Trinh, L.; Rodgers, T.L.; Tothill, I.E. Development of a β-Lactoglobulin Sensor Based on SPR for Milk Allergens Detection. Biosensors 2018, 8, 32. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Meng, Z.; Han, X.X.; Cai, L.; Xu, B.; Liu, R.; Song, L.; He, C.; Cheng, Z.; et al. Flexible SERS Biosensor Based on Core-Shell Nanotags for Sensitive and Multiple Detection of T1DM Biomarkers. Anal. Chem. 2023, 95, 14203–14208. [Google Scholar] [CrossRef]
- Jia, P.; Kong, D.; Ebendorff-Heidepriem, H. Flexible Plasmonic Tapes with Nanohole and Nanoparticle Arrays for Refractometric and Strain Sensing. ACS Appl. Nano Mater. 2020, 3, 8242–8246. [Google Scholar] [CrossRef]
- Tian, L.; Cao, M.; Cheng, H.; Wang, Y.; He, C.; Shi, X.; Li, T.; Li, Z. Plasmon-Stimulated Colorimetry Biosensor Array for the Identification of Multiple Metabolites. ACS Appl. Mater. Interfaces 2024, 16, 6849–6858. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, L.; Liu, Y.; Liu, P.; Wang, W.; Luo, X. A morphology-based ultrasensitive multicolor colorimetric assay for detection of blood glucose by enzymatic etching of plasmonic gold nanobipyramids. Anal. Chim. Acta 2019, 1071, 53–58. [Google Scholar] [CrossRef]
- Wang, F.; Na, N.; Ouyang, J. A catalytic—Regulated gold nanorods etching process as a receptor with multiple readouts for protein detection. Sens. Actuators B Chem. 2020, 318, 128215. [Google Scholar] [CrossRef]
- Toma, M.; Namihara, S.; Kajikawa, K. Direct detection of neuron-specific enolase using a spectrometer-free colorimetric plasmonic biosensor. Anal. Methods 2023, 15, 2755–2760. [Google Scholar] [CrossRef]
- Russell, S.M.; de la Rica, R. Paper transducers to detect plasmon variations in colorimetric nanoparticle biosensors. Sens. Actuators B Chem. 2018, 270, 327–332. [Google Scholar] [CrossRef]
- Pinheiro, T.; Marques, A.C.; Carvalho, P.; Martins, R.; Fortunato, E. Paper Microfluidics and Tailored Gold Nanoparticles for Nonenzymatic, Colorimetric Multiplex Biomarker Detection. ACS Appl. Mater. Interfaces 2021, 13, 3576–3590. [Google Scholar] [CrossRef]
- Lu, W.; Tian, Y.; Teng, W.; Qiu, X.; Li, M. Plasmonic colorimetric immunosensor based on Poly-HRP and AuNS etching for tri-modal readout of small molecule. Talanta 2023, 265, 124883. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar]
- Otto, A. Surface-Enhanced Raman Scattering: “Classical” and “Chemical” Origins. In Light Scattering in Solids IV: Electronics Scattering, Spin Effects, SERS, and Morphic Effects; Springer: Berlin/Heidelberg, Germany, 1984; p. 289. [Google Scholar] [CrossRef]
- Schatz, G.C.; Van Duyne, R.P. Electromagnetic Mechanism of Surface-Enhanced Spectroscopy. In Handbook of Vibrational Spectroscopy; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Eremina, O.E.; Czaja, A.T.; Fernando, A.; Aron, A.; Eremin, D.B.; Zavaleta, C. Expanding the Multiplexing Capabilities of Raman Imaging to Reveal Highly Specific Molecular Expression and Enable Spatial Profiling. ACS Nano 2022, 16, 10341–10353. [Google Scholar] [CrossRef]
- Almehmadi, L.M.; Curley, S.M.; Tokranova, N.A.; Tenenbaum, S.A.; Lednev, I.K. Surface Enhanced Raman Spectroscopy for Single Molecule Protein Detection. Sci. Rep. 2019, 9, 12356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-N.; Register, J.K.; Fales, A.M.; Gandra, N.; Cho, E.H.; Boico, A.; Palmer, G.M.; Klitzman, B.; Vo-Dinh, T. Surface-enhanced Raman scattering nanosensors for in vivo detection of nucleic acid targets in a large animal model. Nano Res. 2018, 11, 4005–4016. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, H.; Zhang, T.; Xu, Y.; Gao, F.; Chen, W.; Zheng, G.; Zhang, S.; Xu, H. Quantitative and sensitive detection of alpha fetoprotein in serum by a plasmonic sensor. Nanophotonics 2022, 11, 4821–4829. [Google Scholar] [CrossRef]
- Lyu, N.; Pedersen, B.; Shklovskaya, E.; Rizos, H.; Molloy, M.P.; Wang, Y. SERS characterization of colorectal cancer cell surface markers upon anti-EGFR treatment. Exploration 2022, 2, 20210176. [Google Scholar] [CrossRef]
- Guo, H.; Qian, K.; Cai, A.; Tang, J.; Liu, J. Ordered gold nanoparticle arrays on the tip of silver wrinkled structures for single molecule detection. Sens. Actuators B Chem. 2019, 300, 126846. [Google Scholar] [CrossRef]
- Chandra, K.; Kumar, V.; Werner, S.E.; Odom, T.W. Separation of Stabilized MOPS Gold Nanostars by Density Gradient Centrifugation. ACS Omega 2017, 2, 4878–4884. [Google Scholar] [CrossRef]
- Kim, S.; Joo, J.-H.; Kim, W.; Bang, A.; Choi, H.W.; Moon, S.W.; Choi, S. A facile, portable surface-enhanced Raman spectroscopy sensing platform for on-site chemometrics of toxic chemicals. Sens. Actuators B Chem. 2021, 343, 130102. [Google Scholar] [CrossRef]
- Kim, J.; Song, X.; Ji, F.; Luo, B.; Ice, N.F.; Liu, Q.; Zhang, Q.; Chen, Q. Polymorphic Assembly from Beveled Gold Triangular Nanoprisms. Nano Lett. 2017, 17, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Bang, A.; Kim, S.; Lee, G.-J.; Kim, Y.-H.; Choi, S. Adiponectin-targeted SERS immunoassay biosensing platform for early detection of gestational diabetes mellitus. Biosens. Bioelectron. 2022, 213, 114488. [Google Scholar] [CrossRef]
- Zong, C.; Premasiri, R.; Lin, H.; Huang, Y.; Zhang, C.; Yang, C.; Ren, B.; Ziegler, L.D.; Cheng, J.X. Plasmon-enhanced stimulated Raman scattering microscopy with single-molecule detection sensitivity. Nat. Commun. 2019, 10, 5318. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Li, K.; Liu, H.; Xiao, R.; Wang, W.; Wang, C.; Wang, S. Fe3O4@Au SERS tags-based lateral flow assay for simultaneous detection of serum amyloid A and C-reactive protein in unprocessed blood sample. Sens. Actuators B Chem. 2020, 320, 128350. [Google Scholar] [CrossRef]
- Shim, J.-E.; Kim, Y.J.; Choe, J.-H.; Lee, T.G.; You, E.-A. Single-Nanoparticle-Based Digital SERS Sensing Platform for the Accurate Quantitative Detection of SARS-CoV-2. ACS Appl. Mater. Interfaces 2022, 14, 38459–38470. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Mishra, S.M.; Roy, A.; Roy, A.; Senapati, D.; Satpati, B. Multiple Gold Nanoparticle Cores within a Single SiO2 Shell for Preservable Solid-State Surface-Enhanced Raman Scattering and Catalytic Sensing. ACS Appl. Nano Mater. 2023, 6, 15606–15619. [Google Scholar] [CrossRef]
- Ge, S.; Wang, M.; Zhu, S.; Wu, H.; Li, J.; Liu, D.; Huang, Q.; Li, S.; Sun, X. Hypersensitive detection of CYFRA21-1 by SERS dual antibody sandwich method. Sens. Actuators Rep. 2024, 7, 100198. [Google Scholar] [CrossRef]
- Zheng, P.; Raj, P.; Wu, L.; Szabo, M.; Hanson, W.A.; Mizutani, T.; Barman, I. Leveraging Nanomechanical Perturbations in Raman Spectro-Immunoassays to Design a Versatile Serum Biomarker Detection Platform. Small 2022, 18, 2204541. [Google Scholar] [CrossRef]
- Xiang, Y.; Zheng, Y.; Liu, S.; Liu, G.; Li, Z.; Dong, W. Comparison of the sensitivity of Western blotting between PVDF and NC membranes. Sci. Rep. 2021, 11, 12022. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, S.; Liu, Q.; Xu, T.; Zhang, X. Microfluidic-based plasmonic microneedle biosensor for uric acid ultrasensitive monitoring. Sens. Actuators B Chem. 2024, 398, 134685. [Google Scholar] [CrossRef]
- Tuckmantel Bido, A.; Brolo, A.G. Digital SERS Protocol Using Au Nanoparticle-Based Extrinsic Raman Labels for the Determination of SARS-CoV-2 Spike Protein in Saliva Samples. ACS Appl. Nano Mater. 2023, 6, 15426–15436. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, T.; Chen, P.; Zong, S.; Wu, L.; Cui, Y.; Wang, Z. Long-lived SERS Matrix for Real-Time Biochemical Detection Using “Frozen” Transition State. ACS Sens. 2023, 8, 3360–3369. [Google Scholar] [CrossRef]
- Atta, S.; Zhao, Y.; Li, J.Q.; Vo-Dinh, T. Dual-Modal Colorimetric and Surface-Enhanced Raman Scattering (SERS)-Based Lateral Flow Immunoassay for Ultrasensitive Detection of SARS-CoV-2 Using a Plasmonic Gold Nanocrown. Anal. Chem. 2024, 96, 4783–4790. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, X.; He, Z.; Wu, L.; Xie, X.; Sun, Z.; Zhang, S.; Cao, H.; Hammock, B.D.; Liu, X. Colorimetric and surface-enhanced Raman scattering dual-mode lateral flow immunosensor using phage-displayed shark nanobody for the detection of crustacean allergen tropomyosin. J. Hazard. Mater. 2024, 468, 133821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, K.; Su, Y.; Hu, S.; Liang, X.; Luo, Q.; Luo, H. Colorimetric and surface-enhanced Raman scattering dual-mode magnetic immunosensor for ultrasensitive detection of blood phosphorylated tau in Alzheimer’s disease. Biosens. Bioelectron. 2023, 222, 114935. [Google Scholar] [CrossRef]
- Liu, X.; Su, X.; Chen, M.; Xie, Y.; Li, M. Self-calibrating surface-enhanced Raman scattering-lateral flow immunoassay for determination of amyloid-β biomarker of Alzheimer’s disease. Biosens. Bioelectron. 2024, 245, 115840. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Wang, X.; Lei, M.; Shi, Z.; Liu, L.; Xu, C. Visual and quantitative lateral flow immunoassay based on Au@PS SERS tags for multiplex cardiac biomarkers. Talanta 2024, 274, 126040. [Google Scholar] [CrossRef]
- Clarke, O.J.R.; Goodall, B.L.; Hui, H.P.; Vats, N.; Brosseau, C.L. Development of a SERS-Based Rapid Vertical Flow Assay for Point-of-Care Diagnostics. Anal. Chem. 2017, 89, 1405–1410. [Google Scholar] [CrossRef]
- Chen, R.; Liu, B.; Ni, H.; Chang, N.; Luan, C.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assays based on core–shell SERS nanotags for multiplex prostate cancer biomarker detection. Analyst 2019, 144, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Ebbah, E.; Amissah, A.; Kim, J.-H.; Driskell, J.D. SERS-based immunoassay on a plasmonic syringe filter for improved sampling and labeling efficiency of biomarkers. Analyst 2024, 149, 221–230. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, T.; Xu, Z.; Zhang, H.; Wang, D.; Zhao, X.; Huang, Y.; Liu, Q.; Fu, B.; Dai, Z.; et al. Immune-like sandwich multiple hotspots SERS biosensor for ultrasensitive detection of NDKA biomarker in serum. Talanta 2024, 271, 125630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, Y.; Li, G.; Zou, S.; Ling, L. Chemical–Chemical Redox Cycle Signal Amplification Strategy Combined with Dual Ratiometric Immunoassay for Surface-Enhanced Raman Spectroscopic Detection of Cardiac Troponin I. Anal. Chem. 2023, 95, 16677–16682. [Google Scholar] [CrossRef]
- Pazin, W.M.; Furini, L.N.; Braz, D.C.; Popolin-Neto, M.; Fernandes, J.D.; Leopoldo Constantino, C.J.; Oliveira, O.N., Jr. Explainable Machine Learning to Unveil Detection Mechanisms with Au Nanoisland-Based Surface-Enhanced Raman Scattering for SARS-CoV-2 Antigen Detection. ACS Appl. Nano Mater. 2024, 7, 2335–2342. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2023, 35, e2107986. [Google Scholar] [CrossRef]
- Minopoli, A.; Della Ventura, B.; Lenyk, B.; Gentile, F.; Tanner, J.A.; Offenhäusser, A.; Mayer, D.; Velotta, R. Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nat. Commun. 2020, 11, 6134. [Google Scholar] [CrossRef]
- Kar, A.; Praneeth, N.V.S.; Khatua, S.; Datta, B. Use of Single-Molecule Plasmon-Enhanced Fluorescence to Investigate Ligand Binding to G-Quadruplex DNA. J. Phys. Chem. Lett. 2023, 14, 6321–6327. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Duan, S.; Yi, J.; Wang, C.; Radjenovic, P.M.; Tian, Z.Q.; Li, J.F. Real-time detection of single-molecule reaction by plasmon-enhanced spectroscopy. Sci. Adv. 2020, 6, eaba6012. [Google Scholar] [CrossRef]
- Tran, V.T.; Ju, H. Fluorescence Based on Surface Plasmon Coupled Emission for Ultrahigh Sensitivity Immunoassay of Cardiac Troponin I. Biomedicines 2021, 9, 448. [Google Scholar] [CrossRef]
- Lai, X.; Zhang, G.; Zhang, G.; Su, L.; Liu, C.; He, W.; Ang, E.H.; Lai, W.; Deng, S. Polydopamine-modulated anisotropic co-growth plasmonic blackbody for efficient ultra-broad-spectrum quenching to establish multicolor fluorescent immunoassay. Chem. Eng. J. 2024, 495, 153083. [Google Scholar] [CrossRef]
- Deng, K.; Yu, Z.L.; Hu, X.; Liu, J.; Hong, X.; Zi, G.G.L.; Zhang, Z.; Tian, Z.Q. NIR-II fluorescent Ag(2)Se polystyrene beads in a lateral flow immunoassay to detect biomarkers for breast cancer. Mikrochim. Acta 2023, 190, 462. [Google Scholar] [CrossRef]
- Amrhein, K.; Taylor, M.L.; Wilson, R.; Gallops, C.E.; Annamer, A.; Vinduska, V.; Kwizera, E.A.; Zhang, H.; Wang, Y.; Hoang, T.B.; et al. Dual Imaging Single Vesicle Surface Protein Profiling and Early Cancer Detection. ACS Appl. Mater. Interfaces 2023, 15, 2679–2692. [Google Scholar] [CrossRef] [PubMed]
- Qavi, A.J.; Jiang, Q.; Aman, M.J.; Vu, H.; Zetlin, L.; Dye, J.M.; Froude, J.W.; Leung, D.W.; Holtsberg, F.; Crick, S.L.; et al. A Flexible, Quantitative Plasmonic-Fluor Lateral Flow Assay for the Rapid Detection of Orthoebolavirus zairense and Orthoebolavirus sudanense. ACS Infect. Dis. 2024, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.; Barbic, M.; Smith, D.; Schultz, D.; Schultz, S.J.J.J.; Mock, M.; Barbic, D.R.; Smith, D.A.; Schultz, S. Schultz: Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Wu, X.; Li, T.; Tao, G.; Lin, R.; Pei, X.; Liu, F.; Li, N. A universal and enzyme-free immunoassay platform for biomarker detection based on gold nanoparticle enumeration with a dark-field microscope. Analyst 2017, 142, 4201–4205. [Google Scholar] [CrossRef]
- Xu, S.; Guo, L.; Chen, L.; Luo, F.; Qiu, B.; Lin, Z. Dark field microscope-based single nanoparticle identification coupled with statistical analysis for ultrasensitive biotoxin detection in complex sample matrix. Microchim. Acta 2020, 187, 413. [Google Scholar] [CrossRef]
- Qian, X.; Shen, Y.; Yuan, J.; Yang, C.-T.; Zhou, X. Visual and Ultrasensitive Detection of a Coronavirus Using a Gold Nanorod Probe under Dark Field. Biosensors 2022, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wen, Y.; Ren, W.; Duan, H.; Lin, J.; Irudayaraj, J. Hyperspectral dark-field microscopy for pathogen detection based on spectral angle mapping. Sens. Actuators B Chem. 2022, 367, 132042. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, Q.; Wang, H.; Zhu, W.; Liu, J.; Wang, Z. A general scattering proximity immunoassay with the formation of dimer of gold nanoparticle. Talanta 2021, 233, 122515. [Google Scholar] [CrossRef]
- Jin, Z.-Y.; He, C.-H.; Xi, C.-Y.; Wang, Y.; Abdalla, E.; Chen, B.-B.; Li, D.-W. Ultrasensitive detection of tyrosinase with click reaction-combined dark-field imaging platform. Talanta 2024, 273, 125931. [Google Scholar] [CrossRef]
- Yoshimura, K.; Patmawati; Maeda, M.; Kamiya, N.; Zako, T. Protein-Functionalized Gold Nanoparticles for Antibody Detection Using the Darkfield Microscopic Observation of Nanoparticle Aggregation. Anal. Sci. 2021, 37, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Huang, T.; Lou, D.; Peng, Z.; He, Y.; Zhang, X.; Gu, N.; Zhang, Y. Artificial Intelligence-Aided Multiple Tumor Detection Method Based on Immunohistochemistry-Enhanced Dark-Field Imaging. Anal. Chem. 2022, 94, 1037–1045. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Pan, X.; Gai, H. Multiplexed Homogeneous Immunoassay Based on Counting Single Immunocomplexes together with Dark-Field and Fluorescence Microscopy. Anal. Chem. 2022, 94, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, N.; Li, P.; Zhang, Y.; Sun, S.; Mao, J.; Liu, S.; Wei, W. Detection of monoamine oxidase B using dark-field light scattering imaging and colorimetry. Chem. Commun. 2022, 58, 12329–12332. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, X.; Chen, L.; Wu, J.; Jiang, Z.; Shi, X. Smartphone analysis and dark-field microscopy observation for aflatoxin B1 detection based on the disintegration of core-shell Ag@Au nanoparticles. Food Biosci. 2024, 60, 104406. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, L.; Miao, H.; Jing, X. “Add on” Dual-Modal Optical Immunoassay by Plasmonic Metal NP-Semiconductor Composites. Anal. Chem. 2021, 93, 3250–3257. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. Gold nanoring core-shell satellites with abundant built-in hotspots and great analyte penetration: An immunoassay platform for the SERS/fluorescence-based detection of carcinoembryonic antigen. Chem. Eng. J. 2021, 409, 128173. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.U.; Jeon, M.J.; Kim, S.-K.; Hwang, S.-H.; Hong, M.E.; Sim, S.J. Bio-conjugated nanoarchitectonics with dual-labeled nanoparticles for a colorimetric and fluorescent dual-mode serological lateral flow immunoassay sensor in detection of SARS-CoV-2 in clinical samples. RSC Adv. 2023, 13, 27225–27232. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Zhong, S.; Zhang, J.; Yan, R.; Xu, P.; Zhang, Y.-h.; Yue, X.; Wang, L.; Wang, Y.; et al. Sensitivity investigation of a biosensor with resonant coupling of propagating surface plasmons to localized surface plasmons in the near infrared region. Nanoscale 2023, 15, 10826–10833. [Google Scholar] [CrossRef]
- Mittal, S.; Saharia, A.; Ismail, Y.; Petruccione, F.; Bourdine, A.V.; Morozov, O.G.; Demidov, V.V.; Yin, J.; Singh, G.; Tiwari, M. Spiral Shaped Photonic Crystal Fiber-Based Surface Plasmon Resonance Biosensor for Cancer Cell Detection. Photonics 2023, 10, 230. [Google Scholar] [CrossRef]
- Allison, G.; Sana, A.K.; Ogawa, Y.; Kato, H.; Ueno, K.; Misawa, H.; Hayashi, K.; Suzuki, H. A Fabry-Pérot cavity coupled surface plasmon photodiode for electrical biomolecular sensing. Nat. Commun. 2021, 12, 6483. [Google Scholar] [CrossRef] [PubMed]
- Ehiabhili, J.; Prabhu, R.; Kannan, S. Highly Sensitive D-SPR Sensors with Optimized Metallic Thin Films for Bio-Analyte Detection. Photonics 2024, 11, 764. [Google Scholar] [CrossRef]
- Hu, S.; Sun, C.; Wu, X.; Peng, Y. Polarization-Independent Terahertz Surface Plasmon Resonance Biosensor for Species Identification of Panax and Paeonia. Photonics 2023, 10, 250. [Google Scholar] [CrossRef]
- Clabassi, E.; Balestra, G.; Siciliano, G.; Polimeno, L.; Tarantini, I.; Primiceri, E.; Tobaldi, D.M.; Cuscunà, M.; Quaranta, F.; Passaseo, A.; et al. Hybrid Plasmonic Symmetry-Protected Bound state in the Continuum Entering the Zeptomolar Biodetection Range. Small 2025, 21, e2411827. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, X.; Liang, H.; Zheng, Y. Photonic-Metamaterial-Based, Near-Field-Enhanced Biosensing Approach for Early Detection of Lung and Ovarian Cancer. Photonics 2024, 11, 1020. [Google Scholar] [CrossRef]





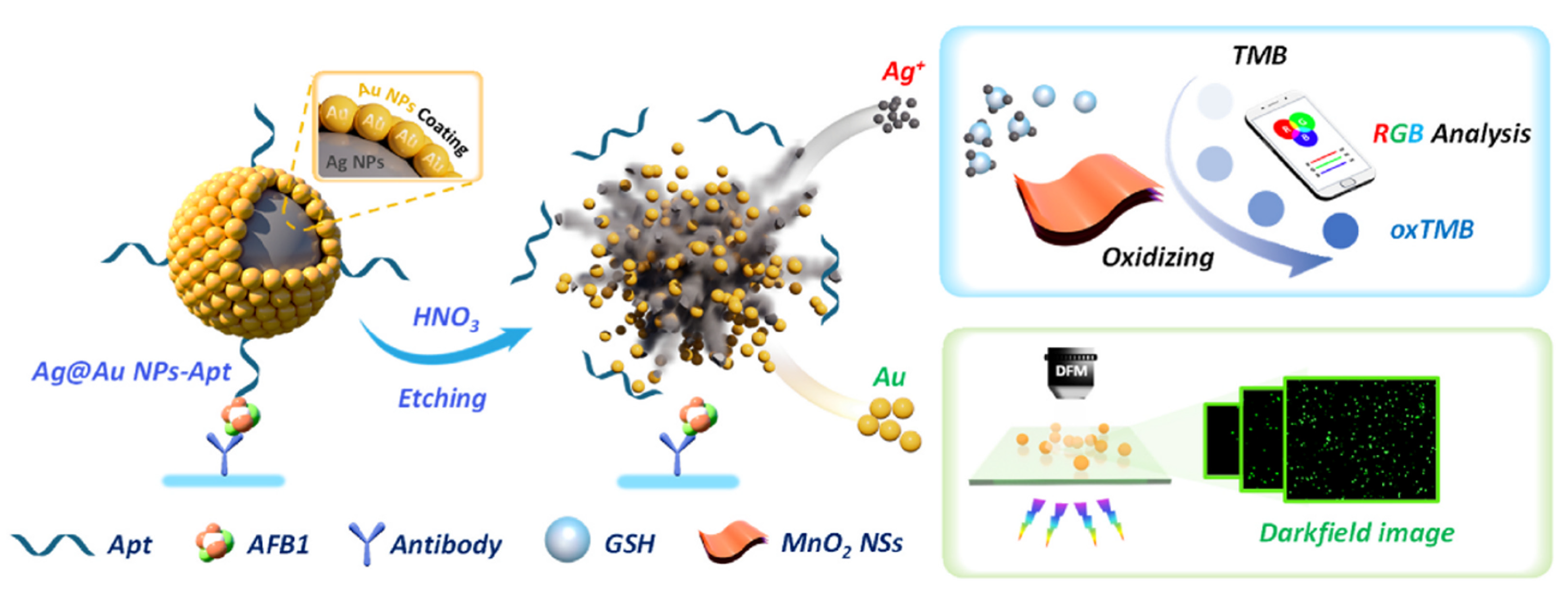
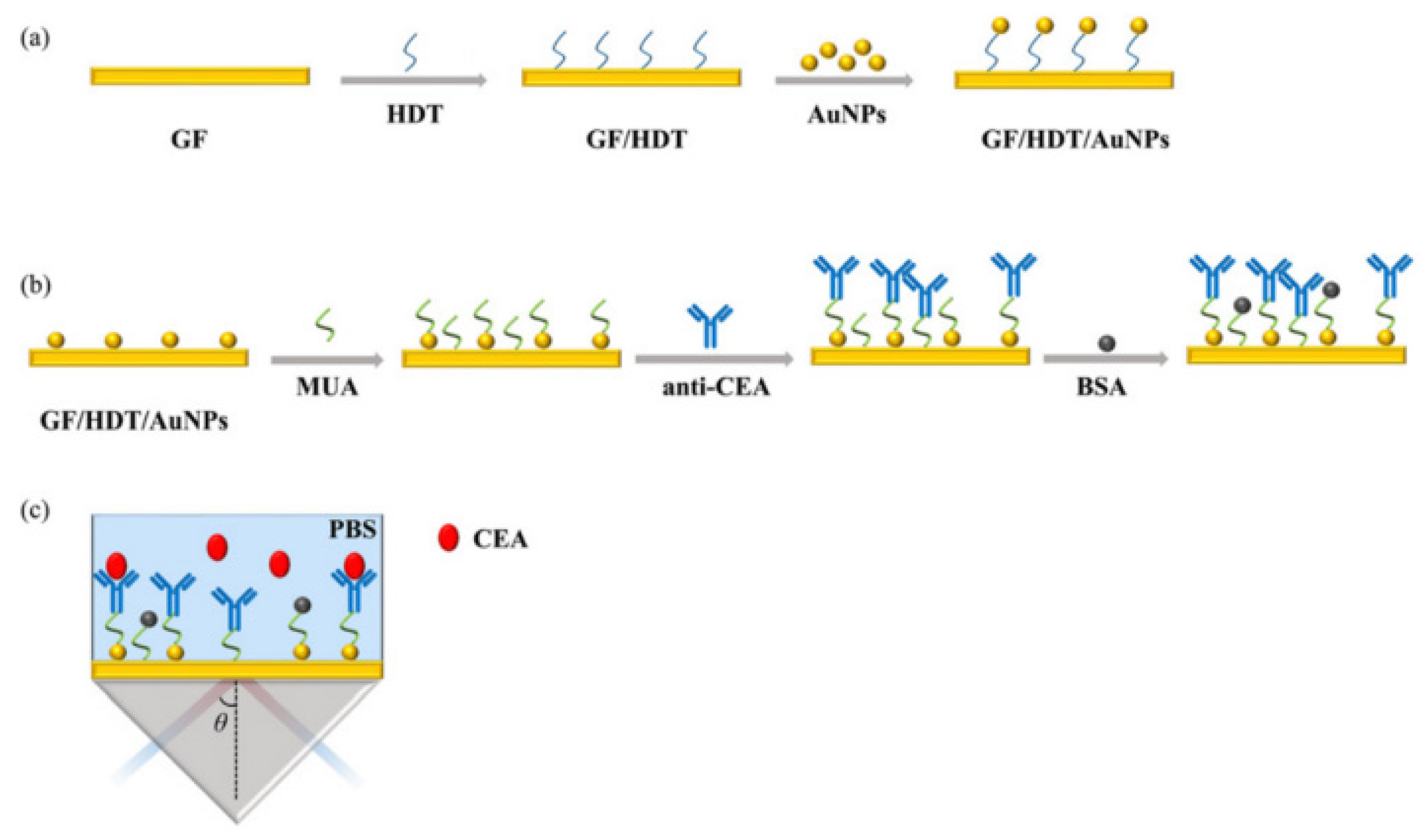

| Method | Analyte | Sensitivity | Detection Range | References |
|---|---|---|---|---|
| Colorimetric sensing | Horseradish peroxidase | 0.02 mM | 0.05–90 μM | [34] |
| C reactive protein | 3 × 10−8 g/mL | 10−8–10−5 g/mL | [37] | |
| Zeralenone | 0.10 ng/mL | 0.10–2.0 ng/mL | [39] | |
| Glucose, uric acid, cholesterol | 1.25 Mm, 71 μM, 81 μM | 1.25–20 mM, 0–1 mM, 0–325 μM | [38] | |
| Surface enhanced Raman scattering | Adiponectin | 3.0 × 10−16 g/mL | 10−15–10−6 g/mL | [53] |
| SARS-CoV-2 spike protein | 7.1 × 10 −16 M | 3.7 × 10−15–3.7 × 10−8 M | [56] | |
| Starch like protein-β1-42 | 12.1 pM | 0.1–50 nM | [68] | |
| Cardiac troponin I | 0.33 pg/mL | 0.001–50.0 ng/mL | [44] | |
| Plasmon fluorescence | Chloramphenicol, sulfamethoxazole | 0.0045 ng/mL, 0.038 ng/mL | 0.005–500 ng/mL, 0.0005–50 ng/mL | [80] |
| Cardiac troponin I | 21.2 ag mL−1 | 0–0.5 pg/mL | [79] | |
| DNA target | 3.3 copies/μL | 10−1–10−6 copies/uL | [3] | |
| IL-6 | 283 ag/mL | 0.81 ag/mL–5000 pg/mL | [1] | |
| Dark-field detection | E. coli | 61 CFU/mL | 6.1 × 101–6.1 × 106 CFU/mL | [88] |
| Tyrosinase | 0.003 U/L | 0.01–0.8 U/L | [90] | |
| Monoamine oxidase | 0.4 ng/mL | 0.5–20.0 ng/mL | [94] | |
| Aflatoxin B1 | 3.67 fg/mL | 10−12–10−8 g/mL | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Hu, Y.; Zhang, W.; Zhang, T.; He, X. Research Progress on Immunological Biochips Based on Surface Plasmon Resonance. Photonics 2025, 12, 294. https://doi.org/10.3390/photonics12040294
Wang M, Hu Y, Zhang W, Zhang T, He X. Research Progress on Immunological Biochips Based on Surface Plasmon Resonance. Photonics. 2025; 12(4):294. https://doi.org/10.3390/photonics12040294
Chicago/Turabian StyleWang, Mengyao, Yangming Hu, Wenjun Zhang, Tianzhu Zhang, and Xiaobo He. 2025. "Research Progress on Immunological Biochips Based on Surface Plasmon Resonance" Photonics 12, no. 4: 294. https://doi.org/10.3390/photonics12040294
APA StyleWang, M., Hu, Y., Zhang, W., Zhang, T., & He, X. (2025). Research Progress on Immunological Biochips Based on Surface Plasmon Resonance. Photonics, 12(4), 294. https://doi.org/10.3390/photonics12040294




