Abstract
Optical sensing technologies are revolutionizing global food safety surveillance through exceptional sensitivity, rapid response, and high portability. This review systematically evaluates five major platforms, revealing unprecedented detection capabilities from sub-picomolar to single-cell resolution. Surface plasmon resonance achieves detection limits for veterinary drugs with superior molecular recognition. Quantum dot fluorescence sensors reach sensitivity for pesticides, enabling rapid on-site screening. Surface-enhanced Raman scattering attains sensitivity for heavy metals, ideal for trace contaminants. Laser-induced breakdown spectroscopy delivers multi-elemental analysis within seconds at detection limits. Colorimetric assays provide cost-effective preliminary screening in resource-limited settings. We propose a stratified detection framework that strategically allocates differentiated sensing technologies across food supply chain nodes, addressing heterogeneous demands while eliminating resource inefficiencies from deploying high-precision instruments for routine screening. Integration of microfluidics, artificial intelligence, and mobile platforms accelerates evolution toward multimodal fusion and decentralized deployment. Despite advances, critical challenges persist: matrix interference, environmental robustness, and standardized protocols. Future breakthroughs require interdisciplinary innovation in materials science, intelligent data processing, and system integration, transforming laboratory prototypes into intelligent early warning networks spanning the entire food supply chain.
1. Introduction
Food safety remains a formidable global public health challenge. The complexity of risk management is significantly amplified by the diverse nature and insidious hazards posed by various contaminants. Data from the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) reveal that in 2019, approximately 600 million people globally suffered from foodborne illnesses, resulting in an estimated 420,000 deaths, with children accounting for nearly 40% of these fatalities [1]. This sobering reality underscores the urgent imperative to establish efficient, sensitive, and broadly deployable detection systems for food contaminants.
Given the wide-ranging sources and disparate properties of food contaminants, this review systematically categorizes and evaluates two critical classes of risk factors: chemical contaminants, specifically heavy metal ions and pesticide/veterinary drug residues, and biological contaminants, including Salmonella and other pathogenic microorganisms. Heavy metal ions are of paramount concern due to their environmental persistence, bioaccumulation potential, and the neurotoxic, developmental, and carcinogenic risks they pose even at low-dose exposures [2,3,4]. Monitoring reports from the European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration (FDA) consistently highlight their widespread presence and exceedance of regulatory limits in seafood and infant formulas, with the WHO emphasizing their contribution to millions of foodborne illnesses annually worldwide [5,6,7]. Pesticide and veterinary drug residues are extensively used in areas of intensive agriculture, and their metabolites present risks of neurotoxicity, carcinogenicity, and endocrine disruption, further contributing to the spread of antimicrobial resistance. Monitoring data from EFSA and the U.S. Department of Agriculture (USDA) continuously report issues of excessive residues in fruits, vegetables, grains, and animal-derived foods [8,9,10,11]. Pathogenic bacteria are the predominant causative agents of foodborne diseases. The U.S. Centers for Disease Control and Prevention (CDC) reports approximately 48 million cases of foodborne illness annually in the United States, while European surveillance reports identify Salmonella as one of the most common pathogens. Raw dairy products, aquatic products, and ready-to-eat foods pose high risks due to the inherent difficulties in microbial control [12,13,14]. These three categories of contaminants, characterized by their high insidiousness, cumulativity, and potential for synergistic contamination effects, collectively present a core challenge to current food safety regulatory frameworks.
The increasing prevalence of concealed, cumulative, and composite pollutants in food systems demands significant advancements in detection technologies—particularly in sensitivity, specificity, speed, and field applicability. Conventional analytical platforms, such as chromatography–mass spectrometry (GC-MS and LC-MS) and atomic absorption spectroscopy (AAS), offer accurate qualitative and quantitative assessments and continue to serve as foundational reference methods for food safety. In parallel, molecular diagnostic techniques, including polymerase chain reaction (PCR), provide reliable identification of pathogenic microorganisms and remain indispensable for microbial risk assessment [15,16,17,18]. Optical sensors, however, offer transformative solutions to these challenges. Their ultrahigh sensitivity enables detection at picomolar to femtomolar levels; rapid response times support on-site screening within minutes; unique optical signal transduction mechanisms facilitate selective identification of diverse pollutant types; and their portability and integrability provide a viable pathway for constructing real-time monitoring networks from farm to fork.
Building on the distinct performance profiles of various optical sensing modalities, we propose a stratified detection framework tailored to specific contaminant categories and supply chain nodes. This approach enables differentiated deployment strategies that optimize detection efficiency and cost-effectiveness while minimizing resource waste. This review systematically evaluates the latest advances in optical sensing technologies for food safety, with a focus on representative contaminants including heavy metal ions, veterinary and pesticide residues, and pathogenic microorganisms. We analyze sensing principles, performance enhancement strategies, and real-world applications (Figure 1), integrating multidisciplinary insights to clarify the strengths and application domains of each technique. By providing a theoretical foundation and strategic perspective, this work aims to accelerate the practical implementation of highly sensitive, rapid, portable, and intelligent optical sensing systems in food safety monitoring, contributing to global efforts in safeguarding public health.
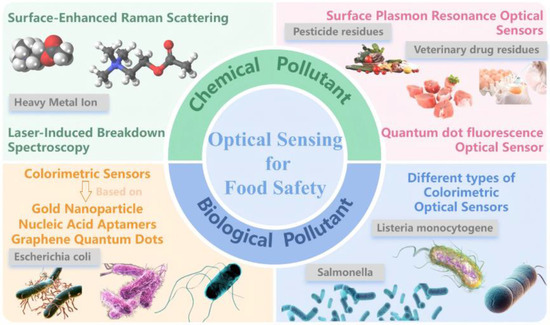
Figure 1.
Overview of optical sensor detection for two major contaminants in food safety.
2. Optical Sensing for Chemical Contaminant Detection
Given the prominent role of heavy metal ions (due to their persistent toxicity and bioaccumulation) and pesticide and veterinary drug residues (due to their structural diversity and trace-level risks in food safety), this section focuses on optical sensing strategies for these two critical classes of chemical contaminants. For pesticide and veterinary drug residues, surface plasmon resonance (SPR) sensors offer label-free, minute-level specific identification of veterinary antibiotics by real-time monitoring of refractive index changes induced by molecular binding. This makes them particularly suitable for high-throughput screening of trace residues in meat products [19]. Meanwhile, quantum dot (QD) fluorescence sensors leverage their size-tunable optical properties and photostability to achieve picogram-level high-sensitivity detection of organophosphorus/chlorine pesticides, effectively overcoming matrix interference and enabling visual tracking of residues in fruits and vegetables [20]. For heavy metal ions, surface-enhanced Raman scattering (SERS) technology capitalizes on the electromagnetic field enhancement generated by nanostructures to specifically capture fingerprint spectra of trace ions within complex food matrices, demonstrating excellent anti-interference capabilities [21]. Laser-induced breakdown spectroscopy (LIBS), on the other hand, enables simultaneous, rapid, and quantitative multi-element analysis through characteristic elemental emission spectra, offering a unique advantage for high-throughput on-site pre-warning [22,23,24]. The following sections will systematically dissect the performance breakthrough mechanisms and optimization pathways of these technologies for both types of contaminants.
2.1. Pesticide and Veterinary Drug Residues Detection
2.1.1. Application of SPR Sensors for Veterinary Drug Residue Detection
In the 1990s, Sternesjö and colleagues pioneered the application of SPR technology for the detection of sulfamethoxazole in milk [25]. Leveraging its unique real-time monitoring capabilities and high specificity, SPR quickly emerged as a crucial tool for drug residue analysis [26,27,28]. As depicted in Figure 2, this sensing technology offers an efficient solution for establishing a “farm-to-fork” comprehensive monitoring system, demonstrating significant value in the precise management and control of chemical contaminants throughout the food supply chain.
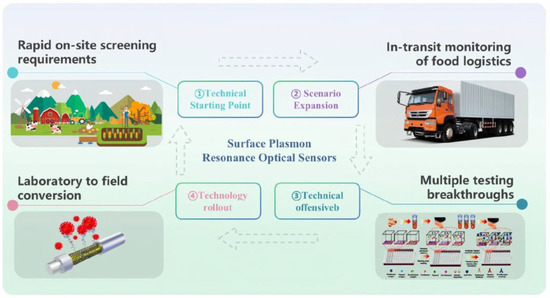
Figure 2.
Overview of the classification of SPR sensors targeting veterinary drug residues for applications throughout the supply chain.
Addressing the need for rapid on-site screening at farms, a 2023 development introduced a molecularly imprinted polymer/graphene oxide-enhanced SPR sensor. This platform achieved a remarkably low detection limit of 0.021 for phenoxymethylpenicillin, with a relative standard deviation of less than 1% across four replicate experiments, enabling highly sensitive and stable on-site precision detection [29]. Leveraging SPR’s inherent advantage in real-time monitoring of molecular interactions, this technology has been further extended to facilitate screening during food logistics [30]. Innovations in nanomaterials are pushing the detection limits of SPR platforms. For instance, Tan et al. [31] developed a two-dimensional material-enhanced SPR sensor employing an indirect competitive immunoassay strategy, which reduced the detection limit for sulfonamides by nearly 12-fold compared to a bare gold SPR system. This provides a robust technical solution for rapid, ultra-trace residue screening in logistical scenarios. Beyond conventional immunorecognition-based strategies, the incorporation of aptamers as novel biorecognition elements has reinvigorated SPR sensing. A recent study developed a label-free and regenerable SPR biosensor by constructing a reduced graphene oxide matrix on a gold SPR film via electrophoretic deposition. Leveraging stacking interactions, anti-lysozyme DNA aptamers were immobilized with high stability, achieving a detection limit of 0.5 nM for lysozyme. This work highlights the synergistic advantages of combining two-dimensional materials with aptamer-based recognition, particularly in enhancing selectivity and sensor reusability [32]. Building on this foundation, emerging metal–dielectric–graphene hybrid heterostructures have demonstrated significant performance enhancements through precise interfacial engineering. Studies show that mixed configurations based on complementary metal oxide semiconductor-compatible media—such as Cu/Al2O3 and Ag/Si3N4—can achieve SPR spectral sensitivities exceeding 30,000, with phase sensitivity improved by more than an order of magnitude compared to conventional gold film platforms [33]. These enhancements stem from the concentrated electromagnetic energy of plasmonic waves at the dielectric/graphene interface and the formation of stationary surface dipoles that generate intense localized electric fields. Such mechanisms open new avenues for the development of ultrasensitive, label-free biosensing platforms.
Addressing the formidable challenge of multiplexed detection for structurally complex veterinary drug residues during transport, Fan et al. have achieved a significant technological breakthrough utilizing a gold–platinum nanoflower-coupled plasmonic metasurface sensor. As illustrated in the left panel of Figure 3, the sensor’s metasurface plasmon resonance chip strip device boasts a unique fabrication process, enabling the multiplexed detection of small molecules. The right panel of Figure 3 further elucidates the sensor’s performance and the process of multiplexed detection for florfenicol, flubendazole, and enrofloxacin in whole-egg liquid. In practical applications, under simulated transport conditions, the APNMSPR demonstrated remarkably low detection limits of 0.81, 1.12, and 1.74 ppt for the aforementioned three veterinary drugs, respectively. This represents a three-order-of-magnitude increase in sensitivity and dramatically reduces analysis time to just 10 min, effectively resolving critical bottlenecks in high-throughput screening [34].
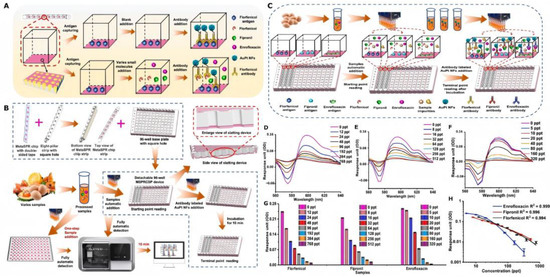
Figure 3.
(A) Schematic of the Meta-SPR chip–based competitive immunoassay for small-molecule detection. (B) Configuration of the detachable high-throughput MSPRCSP platform and its integration for multiplexed quantitative analysis. (C) Workflow of simultaneous detection of florfenicol, fipronil, and enrofloxacin in real samples. Full-spectrum response unit variations for (D) florfenicol (0–768 ppt), (E) fipronil (0–512 ppt), and (F) enrofloxacin (0–320 ppt). (G) Decrease in OD differential values (OD600–OD575) with increasing concentrations of small molecules in whole-egg samples during multiplex analysis. (H) Calibration curves for multiplexed quantification of florfenicol, fipronil, and enrofloxacin in real matrices.
The development of a portable FO-SPR handheld device modified with graphene accelerates the transition of SPR detection from laboratory-based platforms to field-deployable rapid diagnostics. This integrated system incorporates a sensing probe, a signal acquisition and processing module, and an embedded power management unit, enabling complete analysis within 20 min. Notably, it maintains 77.2–93.2% reproducibility after storage at 4 °C for eight days, demonstrating exceptional field applicability and operational stability [35].
Future advancements in SPR sensors will expand their application in real-time veterinary drug residue monitoring through further miniaturization and system integration. Innovations in sensor architecture and application-driven research are poised to enable SPR optical platforms to deliver increasingly efficient and precise food safety assurance across global supply chains.
2.1.2. Application of Quantum Dot Fluorescence Sensors in Pesticide Residue Detection
Quantum dot-based sensors face critical challenges in photostability and chemical robustness during practical deployment. Experimental evidence indicates that CdTe quantum dots exhibit significant fluorescence quenching, with signal intensity declining by up to 40% under continuous illumination for one hour. In contrast, carbon quantum dots demonstrate superior photostability, with signal attenuation remaining below 10% over a 24 h period. This comparative performance underscores the potential of carbon-based nanomaterials for long-term, reliable sensing applications in complex environments. Quantum dot-based fluorescent sensors have emerged as highly efficient tools for pesticide residue detection, with numerous studies demonstrating their remarkable capabilities. These sensors routinely achieve detection limits ranging from sub-nanomolar to femtomolar concentrations and offer rapid response times without demanding intricate sample pretreatment, thereby enabling promising avenues for on-site screening applications [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Most of the current research directions are focused on several aspects, as shown in Figure 4: sensitivity, resistance to matrix interference, and portable miniaturization.
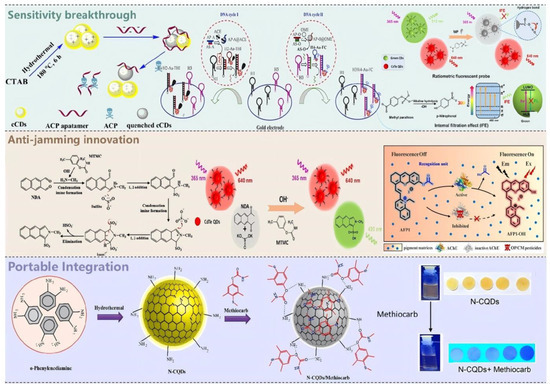
Figure 4.
Overview of Quantum Dot Fluorescent Sensors for Pesticide Residue Detection in Three Directions of Breakthrough.
Both carbon dots and graphene quantum dots have exhibited exceptional analytical sensitivity in this domain. For instance, Saberi et al. engineered a fluorescence quenching-recovery aptasensor by leveraging electrostatic adsorption between cationic carbon dots and anionic aptamers, enabling ultrasensitive detection of acetamiprid with a limit of detection as low as [52]. Building upon this foundation, the team led by Li Ruiyi developed a hybrid electrochemical sensing platform integrating gold nanoparticles with graphene quantum dots, coupled with a dual-target-induced DNA recycling amplification strategy. This system achieves an ultralow detection limit of 16.7 fM (1.67 × 10−14 M) for omethoate, representing a dramatic enhancement in sensitivity over conventional electrochemical methods [53]. As detection demands evolve toward real-time, on-site monitoring, the team led by Jiang Changlong pioneered a ratiometric probe based on CdTe quantum dots and green-emitting carbon dots. Leveraging the inner filter effect (IFE)-mediated selective quenching of green fluorescence, this system generates a pronounced ratiometric signal response. Coupled with smartphone-based colorimetric readout, it seamlessly integrates ultrasensitive detection with field-deployable portability, offering an innovative, practical solution for in situ environmental pollutant monitoring [54].
The detection of pesticides in complex matrices presents a significant analytical challenge, yet researchers are continually pushing the boundaries of detection through innovative probe designs. To mitigate signal interference induced by strong native pigment absorption in highly pigmented food matrices, the Tea Research Institute of the Chinese Academy of Agricultural Sciences developed a near-infrared (NIR)-excitable acetylcholinesterase (AChE) inhibition-based fluorescent probe. By circumventing inner filter effects, this probe enables accurate detection of organophosphorus pesticides, including dichlorvos, in complex food systems such as beetroot and blueberry. It achieves a limit of detection (LOD) of 0.0186 for dichlorvos, with sensitivity rivaling that of UPLC-MS/MS in key performance metrics, thereby substantially enhancing analytical feasibility under pigment-rich conditions [55]. Another study leverages CdTe quantum dots as a stable background fluorophore and employs an in situ Strecker-type reaction to generate green-emitting isoindoles, thereby constructing a dual-emission ratiometric fluorescent probe for the visual and quantitative detection of carbamate pesticides. Integrated with smartphone-based imaging and paper-based microfluidics, this platform enables rapid analysis in complex real-world matrices, including water, apple, and cabbage samples, with a limit of detection as low as , demonstrating robust performance even under challenging matrix conditions [56]. This design effectively corrects for environmental variations, leading to a reduced detection limit for carbamates in real cabbage extracts. This represents a significant step towards more stable and precise detection in challenging, complex matrices.
Looking ahead, the integration of quantum dot sensors with portable readout systems represents a crucial direction for the advancement of detection technologies [57,58]. Sanyukta Patel and collaborators devised a smartphone-integrated, nitrogen-doped carbon quantum dot sensor for the rapid and effective detection of methomyl pesticide in vegetables. Real-time analysis was achieved by recording sensor output via a smartphone and processing the data with ImageJ software (Redmi Note 7 Pro) [59]. This accomplishment not only validated the feasibility of integrated detection systems but also provided a practical paradigm for future technological developments. Such advancements are vital for meeting the demand for rapid detection across diverse scenarios, driving the evolution of analytical techniques towards enhanced efficiency and convenience.
2.2. Heavy Metal Ions Detection
2.2.1. Application of SERS in Food Products
In practical applications of SERS, critical experimental parameters, such as laser wavelength (typically 532 nm or 785 nm), laser power (ranging from 1 to 100 mW), and focal spot size (1–10 μm), play a decisive role in determining the efficiency of plasmonic “hot spot” formation and the resulting signal enhancement. Precise control over these variables is essential for optimizing electromagnetic field localization and achieving high-sensitivity molecular detection. Pioneering work by R. Mayer’s team, through systematic experimental and theoretical investigations, first elucidated the enhancement mechanisms on rough noble metal surfaces (e.g., Au, Ag, and Cu), laying a crucial theoretical foundation for SERS technology [60]. With innovations in nanomaterials and characterization techniques, SERS has since achieved single-molecule detection capabilities, boasting enhancement factors reaching an astounding 1014, thereby pushing analytical sensitivity to unprecedented levels [61]. Despite its exceptional sensitivity, the practical deployment of SERS technology continues to face significant reproducibility challenges. Substrates fabricated across different batches often exhibit signal variation coefficients exceeding 15%, undermining consistency in analytical performance. Moreover, long-term stability remains inadequate in complex food matrices, with signal attenuation frequently surpassing 30% within 24 h. These limitations highlight the urgent need for standardized fabrication protocols and enhanced substrate robustness to enable reliable, real-world SERS-based food safety monitoring. Building upon these theoretical breakthroughs and technological advantages, SERS has evolved into an indispensable analytical tool across diverse fields, including chemistry, biology, and food science [62,63,64,65,66]. Notably, in the realm of food safety monitoring, SERS technology has demonstrated exceptional applicability, achieving significant breakthroughs particularly in the critical area of heavy metal ion detection [67,68,69,70,71,72], offering transformative solutions for the development of novel food safety surveillance systems.
- 1.
- Breakthroughs in Detection Performance: Synergistic Enhancement of Sensitivity and Selectivity;
Innovations in substrate material design have continually pushed the physical limits of Surface-Enhanced Raman Scattering detection, enabling a synergistic enhancement of both sensitivity and selectivity.
Early research in this area saw the development of ZnO submicron “petal” structures as SERS substrates. By optimizing the ZnO band structure to enhance interfacial charge transfer and integrating a free DNA walker amplification strategy, researchers successfully reduced the detection limit for Pb2+ to an impressive 3.55 pM [73].
Building upon this, researchers have developed a SERS substrate based on silver phosphate (Ag3PO4) microcubes functionalized with organic bipyridine (BPy) ligands. This platform captures target analytes via ligand coordination and leverages the unique microcubic architecture to generate densely distributed electromagnetic “hot spots.” As a result, the system enables ultrasensitive detection of Hg2+ and Pb2+ ions, achieving detection limits as low as 10−15 M (femtomolar level) and analytical enhancement factors (AEFs) up to 1010. In addition to its exceptional sensitivity, the substrate exhibits robust stability and reusability for at least four consecutive cycles, underscoring its strong potential for heavy metal ion monitoring in complex environmental matrices. As illustrated in Figure 5, the linear relationship between SERS intensity and metal ion concentration, along with signal comparisons before and after adsorption, elucidated the enhancement mechanism of this system [74]. With advances in materials science, researchers have successfully synthesized Ag@ZrO2@Ag core–shell composite nanospheres with tunable SERS activity by integrating the sol–gel method with in situ reduction deposition. This substrate enables highly selective SERS detection of Cr (VI), achieving a detection limit as low as . It exhibits excellent linearity (R2 = 0.97) within concentration ranges below the EPA safety threshold and has been successfully applied to quantitative analysis of real industrial wastewater [75]. Recently, the construction of three-dimensional nano-hotspot architectures has driven a leap forward in SERS sensitivity. As depicted in Figure 5A,B, these highly controllable 3D substrates enable the detection of Hg2+ within minutes, achieving an exceptionally low detection limit of 0.2 pM [76].
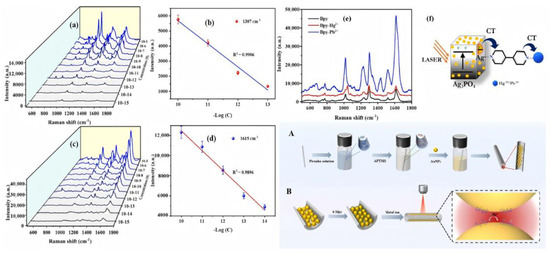
Figure 5.
SERS detection of Hg2+ and Pb2+ ions on Ag3PO4. (a,c) Concentration-dependent SERS spectra of Hg2+ (10−5 M–10−15 M) and Pb2+ (10−5 M–10−15 M), respectively. (b,d) Corresponding calibration curves at 1307 cm−1 (Hg2+) and 1615 cm−1 Pb2+). (e) Comparative SERS spectra of Bpy alone and in complex with Hg2+ or Pb2+ on Ag3PO4. (f) Schematic of the SERS mechanism: (A) fabrication of 3D hotspot nanostructures in a capillary, and (B) Hg2+ adsorption and detection within the capillary.
The progression from two-dimensional structures to three-dimensional architectures, and from single materials to composite systems, underscores the continuous improvement in SERS substrate materials and signal enhancement strategies. This evolution has positioned SERS technology to demonstrate its full potential across various applications in heavy metal ion detection, ranging from trace analysis to rapid on-site screening. This provides a highly sensitive detection paradigm crucial for food safety and environmental monitoring.
- 2.
- Expansion of Detection Scenarios: From Laboratory to On-Site Instant Analysis;
To meet the demands of practical applications, Surface-Enhanced Raman Scattering technology is rapidly evolving towards portability and real-time analysis. Compared to conventional laboratory instruments, portable SERS devices offer a compact form factor, rapid detection, and immediate data output, making them a crucial technology for on-site screening of heavy metal ions in food matrices [77].
Early efforts in this field centered on substrate engineering and platform miniaturization. To address the challenge of multiplex detection in complex matrices, researchers developed a low-cost, facilely fabricated paper chromatography-SERS (PC-SERS) platform based on gold sputtering, as illustrated in Figure 6. This platform employs a 4-MBA-modified “sandwich” architecture to selectively capture heavy metal ions, enabling the simultaneous detection of Cd2+, Cu2+, and Ni2+ in ground rice samples with a detection limit of . In addition to its multiplexing capability, the PC-SERS system exhibits excellent signal uniformity (RSD = 10.12%) and high fabrication accessibility [15]. Building on this foundation, researchers further introduced the “Place & Play SERS” concept, utilizing ultrathin, flexible polyvinyl alcohol-supported gold–silver nanonetworks as active substrates. This innovation enabled instantaneous, non-destructive detection without the need for sample pretreatment [78]. With the iterative upgrade of portable platforms, a lateral-flow immunoassay-coupled SERS device emerged. This platform integrates gold–silver core–shell nanoparticles as SERS probes, reducing the Hg2+ detection limit to , representing a 20-fold increase in sensitivity compared to standard visual detection methods [4]. Concurrently, breakthroughs in probe engineering have further enhanced on-site detection capabilities. DNA-functionalized metal and metal oxide nanoparticles have been introduced as SERS reporters. Leveraging the high selectivity of DNA recognition elements, these probes achieve precise target identification while simultaneously lowering detection limits [79].
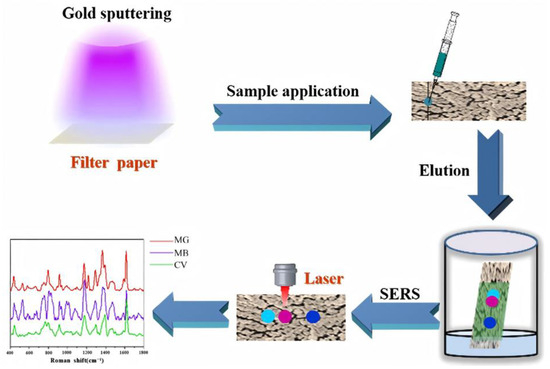
Figure 6.
Schematic Illustration of the Fabrication of AuPC Strips through Sputtering Au on a Filter Paper and the Following Combination of Paper Chromatography Separation and SERS Detection for Multicomponent Separation and Analysis.
Through multidimensional optimization, ranging from innovations in substrate materials to the construction of portable platforms, SERS technology demonstrates significant advantages in the portable and real-time on-site analysis of heavy metal ions. This provides an efficient and cost-effective solution for robust food safety monitoring.
- 3.
- Empowerment by Intelligent Algorithms: From Data Acquisition to Precise Analysis;
As spectral data complexity escalates, the integration of machine learning with Surface-Enhanced Raman Scattering has emerged as a pivotal breakthrough for enhancing heavy metal detection efficacy. The introduction of advanced algorithms fundamentally redefines the traditional paradigm of spectral analysis.
In initial explorations, researchers directly applied ML classifiers to raw SERS spectra to address fundamental challenges such as noise interference, baseline drift, and non-linear signal characteristics. For instance, in the detection of Pb(NO3)2, an algorithm-trained model achieved a balanced accuracy of 84.6% in independent batch tests [80]. This significantly bolstered the reliability of analyses in real-world water and food matrices, proving highly valuable for detecting heavy metals in seafood and drinking water, thereby facilitating rapid contaminant screening during food distribution. To overcome the limitations of single-element detection, Jin et al. innovatively fused SERS with fluorescence spectroscopy. They developed a joint data processing framework utilizing convolutional neural networks [81]. This method demonstrated exceptional multiplex quantitative capabilities in Tricholoma matsutake mushroom samples, achieving detection limits of 0.0712 for Pb2+ and 0.0795 for co-contaminants, with a coefficient of determination (R2) as high as 0.9963. This effectively enabled precise identification of trace substances in complex matrices.
This interdisciplinary technological fusion has fundamentally transformed heavy metal analysis from relying on human–empirical spectral interpretation to a data-driven intelligent workflow. Its value extends beyond the continuous reduction in detection limits, significantly improving analytical speed, selectivity, and operational robustness. This lays the technical foundation for constructing a real-time, on-site food safety assurance system.
2.2.2. Applications of LIBS in Food Analysis
Laser-Induced Breakdown Spectroscopy has achieved significant research milestones across diverse fields, including agriculture and industry [82,83,84,85]. This is largely due to its unique advantages: rapid analysis, minimal sample pretreatment requirements, and simultaneous multi-element detection capabilities. These inherent characteristics underscore its substantial potential for highly efficient heavy metal ion detection within the critical domain of food safety.
- 1.
- Enhancement of Sensitivity;
Breakthroughs in signal enhancement strategies are continuously driving improvements in the detection sensitivity of Laser-Induced Breakdown Spectroscopy. Early research in this area focused on Surface-Enhanced LIBS, which achieved significant signal amplification for heavy metals in water by utilizing elemental substrates such as Zn, Mg, Ni, and Si. As shown in Figure 7, the Zn substrate exhibited the strongest enhancement, leading to remarkably low detection limits of 0.0011 mg/L for Cr and 0.004 for Pb [86]. Building upon these findings, researchers further enhanced sensitivity through nanoparticle preconcentration techniques. By modifying sample surfaces with Al2O3 nanoparticles, pre-enrichment of Ni, Cr, and Cd was achieved under 532 nm laser excitation, reducing their detection limits to 9.61 , 8.49 , and 71.6 respectively [87]. With further technological advancements, optimizing the addition of conductive enhancers like graphite enabled the detection limit for Pb to be lowered to 26.142 , with a prediction correlation coefficient (Rp) of 0.994, demonstrating precise quantitative analysis capabilities [88].
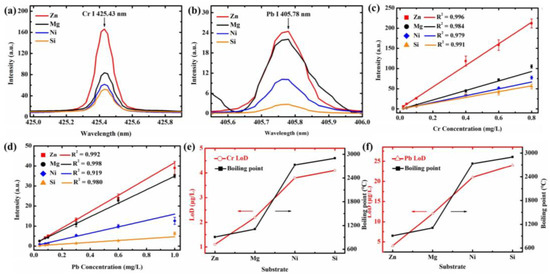
Figure 7.
Emission spectral intensity of Cr I 425.43 nm (a), 0.6 Cr of CrCl3) and 405.78 nm (b), 0.6 Pb of Pb(NO3)2 prepared on Zn, Mg, Ni, and Si substrates. Calibration curves of Cr I 425.43 nm (c) and Pb I 405.78 nm (d) for Zn, Mg, Ni, and Si substrates. The relationship between the LoDs of Cr I 425.43 nm (e) and Pb I 405.78 nm (f) obtained on different substrates and the boiling point (Arrows indicate the trend of increasing LoD with increasing boiling point of the substrate).
The progressive integration of SENLIBS, nanoparticle enrichment, and conductive enhancement techniques, from elemental substrate enhancement to nanoparticle preconcentration and finally to conductive material optimization, has not only led to a sustained reduction in LIBS detection limits but also significantly improved the accuracy of quantitative heavy metal analysis. This underscores the unique technical advantages and application value of LIBS optical sensors in heavy metal ion detection.
- 2.
- Enhancement of Quantitative Analysis Capability;
The quantitative analytical capabilities of LIBS have undergone a stepwise enhancement through systematic methodological innovations, establishing a multidimensional technological breakthrough system in heavy metal detection, particularly for the challenging interferences posed by complex food matrices.
In early investigations, research teams applied multivariate linear regression combined with an internal standard calibration strategy for heavy metal analysis in municipal solid waste incineration fly ash [23]. By specifically correcting for matrix effects, they successfully established quantitative models for Cd, Cr, Cu, Pb, and Zn, achieving detection limits of 11.13, 44.87, 36.18, 10.83, and 12.27 , respectively, with average relative errors controlled within 6.8–20.3%. The versatility of this calibration framework facilitated its direct transfer to food safety detection scenarios, laying the methodological foundation for subsequent technical optimizations.
At the spectral interpretation level, the introduction of Fourier Self-Deconvolution technology resolved the issue of overlapping lines in LIBS spectra of water samples [22]. By narrowing line widths to optimize spectral resolution, the detection limit for Pb was reduced to 79.66 , significantly enhancing the practical utility for on-site monitoring of water pollution in food processing. Addressing the unique characteristics of plant matrices, LIBS–Near-Infrared Spectroscopy data fusion technology achieved cross-modal information complementarity. As shown in Figure 8, NIR provides molecular fingerprint information, while LIBS contributes elemental emission data. Their synergy resulted in R2 values of 0.9858, 0.9811, and 0.9460 for quantitative models of Zn, Cu, and Pb in lily tissues, respectively, with the Root Mean Square Error of Prediction decreasing to 4.30–8.39 mg/kg [89]. With the increasing penetration of artificial intelligence, the integration of Convolutional Neural Networks with AI algorithms has pushed the detection accuracy for Cd and Zn in hyperaccumulator plants to new heights [90]. CNN-based models achieved an R2 of 0.9887 and a Residual Predictive Deviation of 9.46 for Cd detection, and R2 and RPD values of 0.9887 and 15.21, respectively, for Zn detection. This provides highly accurate analytical tools for tracking metal uptake and assessing crop safety.
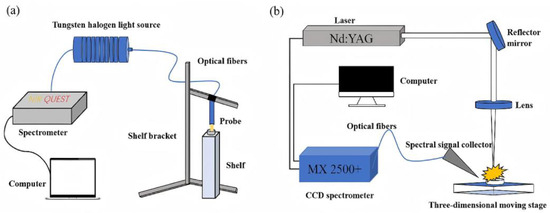
Figure 8.
(a) Schematic diagram of the NIR device. (b) Schematic diagram of the LIBS device.
The synergistic advancements, from matrix correction to cross-modal fusion and AI modeling, along with the optimization afforded by multivariate regression and FSD, have concurrently enhanced the sensitivity and accuracy of LIBS in complex matrices. This establishes a complete detection chain extending from the laboratory to on-site applications. Furthermore, the technical synergy between LIBS and SERS, through spectral complementarity, offers comprehensive technical support for full-chain food safety monitoring.
3. Application of Optical Sensing Technologies in Biological Contaminant Detection
Salmonella poses a primary global threat in foodborne illnesses due to its rapid proliferation, highly insidious transmission, and severe pathogenic outcomes. Concurrently, other pathogens such as Escherichia coli O157:H7 and Listeria monocytogenes also constitute significant public health risks. Confronted with the urgent need for rapid detection of these pathogens, optical sensing technologies have emerged as crucial tools in food safety monitoring due to their superior analytical performance [91,92,93,94]. Among these, colorimetric sensing technology demonstrates notable advantages in the detection of Salmonella and other foodborne pathogens, attributed to its operational simplicity, naked-eye readability, and rapid response characteristics [95,96,97,98,99,100,101]. This technology facilitates intuitive screening by leveraging visible color changes induced by nanoparticle aggregation. Furthermore, its diverse signal transduction mechanisms allow for adaptation to various detection scenarios, offering a range of technical solutions for the rapid and sensitive detection of Salmonella and other foodborne pathogens, thereby continuously advancing detection technologies toward greater efficiency and convenience.
3.1. Application of Different Types of Colorimetric Sensors for Salmonella Detection
3.1.1. Gold Nanoparticle-Based Colorimetric Sensors for Detecting Salmonella
Gold nanoparticles are ideal core materials for colorimetric sensors due to their unique optical properties and excellent biocompatibility. In the context of Salmonella detection, AuNPs, when conjugated with antibodies, can specifically recognize bacteria, leading to particle aggregation. This aggregation results in a distinct color change, enabling rapid, on-site screening without the need for complex instrumentation. Compared to traditional culture or molecular detection methods, these sensors simplify sample preparation and eliminate the need for expensive equipment, offering a highly attractive solution for routine food safety inspections [102,103,104].
Significant performance improvements have been achieved in AuNP-based colorimetric sensors [105,106]. A colorimetric sensing platform, constructed by hybridizing long-stranded single-stranded DNA (547 nt) with unmodified gold nanoparticles (AuNPs), enables highly specific detection of Salmonella Typhimurium. The method exploits the stabilizing effect of long-chain DNA on AuNPs to establish a linear correlation between colorimetric response and bacterial concentration, achieving a limit of detection (LOD) of 2.56 CFU/mL in pure culture media. Notably, it demonstrates robust recovery rates in real food matrices, underscoring its strong potential for practical deployment in food safety surveillance [107]. Another advanced technique utilizes unfunctionalized AuNPs in a highly acidic environment to construct a pH-driven colorimetric detection system. Acid-induced red-shifting of the AuNP plasmon resonance produces a visible color change from red to purple, blue, and ultimately colorless within five minutes upon bacterial interaction. This method enables rapid visual detection of multiple foodborne pathogens with detection limits ranging from 105 to 107 CFU/mL, and the intensity of color change allows for semi-quantitative differentiation of bacterial concentrations without the need for surface functionalization or complex sample pretreatment [108].
These next-generation AuNP sensors exhibit robust performance in complex food matrices and hold promising prospects for portable commercial platform applications. Extensive research published in leading international journals provides a solid theoretical and experimental foundation for advancing AuNP-based colorimetric technology within food safety monitoring systems [109], thoroughly validating its technical potential and research value.
3.1.2. Colorimetric Sensors Based on Nucleic Acid Aptamers for Salmonella Detection
The evolution of molecular recognition techniques has led to critical breakthroughs in Salmonella detection through the development of nucleic acid aptamer-based colorimetric sensors. Aptamers, with their high affinity and specificity for target pathogens, not only simplify the detection process but also significantly enhance both sensitivity and selectivity. Compared to traditional antibody-based detection, aptamers offer superior synthesis reproducibility, enhanced stability, and robust performance in complex matrices, positioning them as a core technological pathway for next-generation pathogen diagnostics.
Continuous reductions in detection thresholds have been achieved through technological innovations employing diverse signal transduction mechanisms. Researchers developed a SERS platform based on an aptamer–cDNA competitive binding mechanism, employing p-aminothiophenol (PATP) as a Raman reporter to enable ultrasensitive identification of Salmonella Typhimurium. Under optimized conditions, the system exhibits excellent linearity across a dynamic range of 56 to , with a limit of detection (LOD) as low as . Its practical feasibility has been validated in spiked milk samples, demonstrating robust performance in complex biological matrices [110]. Furthermore, by precisely assembling multivalent aptamers onto a DNA triangular nanostructure and co-loading horseradish peroxidase (HRP) with SYBR Green I, the study engineered a dual-mode colorimetric/fluorescent sensing platform. In fluorescence mode, it achieves a detection limit of for Salmonella while demonstrating robust applicability across complex food matrices, including milk, egg white, and poultry meat, underscoring its potential for real-world pathogen surveillance [111]. By coupling programmable DNA release with enzyme-mimicking metal nanoclusters and triplex-triggered nano-catalysis, this work delivers an ultrasensitive, wide-dynamic-range biosensor for Salmonella Typhimurium in dairy products, achieving quantification from 26 CFU/mL to 2.6 million CFU/mL, where the LOD is 2.6 CFU/mL, even under minimal sample preparation. This represents a transformative advance toward point-of-use pathogen diagnostics in complex food systems [112].
These achievements demonstrate that aptamer-driven colorimetric sensors are rapidly advancing toward higher sensitivity, enhanced matrix adaptability, and simplified operation. Their unique recognition and amplification strategies not only propel the development of rapid on-site pathogen screening technologies but also provide innovative early warning tools for food safety management, showcasing clear application value and broad prospects in the field of food inspection.
3.1.3. Colorimetric Sensors Based on Graphene Quantum Dots for Salmonella Detection
Graphene quantum dot-based colorimetric sensors enable precise quantitative detection of Salmonella through an aptamer-mediated fluorescence quenching mechanism triggered by specific binding to target bacteria. These sensors offer high sensitivity and rapid response times compared to traditional techniques, providing a highly efficient solution for Salmonella detection [113].
Significant breakthroughs have been achieved in optimizing the detection performance of these sensors. Notably, a fluorescent immunosensor constructed with iron porphyrin bio-mimicked graphene quantum dots, as depicted in Figure 9a, achieved a detection limit of for Salmonella Typhi Vi antigen in human serum, demonstrating excellent sensitivity and biocompatibility through a fluorescence resonance energy transfer mechanism [114]. Furthermore, a peroxidase-mimicking platform based on Cu(II)-modified reduced graphene oxide combined with PCR facilitated rapid Salmonella detection in milk, reaching a low detection limit of 0.51 with good recovery rates and remarkable specificity [115]. An advanced quantum dot-labeled phage receptor binding protein nanoprobe was also developed, as shown in Figure 9b. This nanoprobe, utilizing immunomagnetic separation to form a sandwich structure, achieved a detection limit as low as within 2 h and has been successfully validated in real food samples [116].
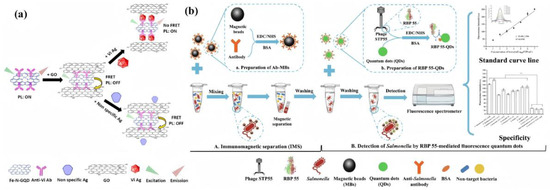
Figure 9.
(a) The FRET mechanism for GO as an acceptor and Fe-N-GQDs as a donor. In the first step, GO comes into the proximity of Fe-N-GQDs/Ab and quenches its PL. In the upward process, in the presence of the target (Vi Ag), due to the specific antibody–antigen interaction, the distance between Fe-N-GQDs and GO effectively increases, and PL is recovered. However, in the absence of the target, PL remains quenched (the downward process). (b) Schematic illustration for fluorescent detection of Salmonella based on nanoprobes RBP 55-QD.
These advancements collectively demonstrate the exceptional performance of GQD-based colorimetric sensors in Salmonella detection. Their attributes of speed, sensitivity, and user-friendliness present a highly promising technological pathway for food safety monitoring, indicating broad application prospects.
3.2. Colorimetric Sensors for Detection of Other Foodborne Pathogens
Beyond Salmonella detection, colorimetric sensors have demonstrated remarkable efficacy in the effective detection of a wide array of other foodborne pathogens. The synergistic combination of nanomaterials with biorecognition elements has significantly expanded their applicability, enabling the identification of common pathogens such as Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus in critical food processing environments like those for meat, dairy, and fresh produce. While conventional methods such as bacterial culture, PCR, and immunoassay offer accuracy, they are often characterized by cumbersome procedures, extended turnaround times, and the necessity for specialized equipment. In stark contrast, colorimetric sensors present compelling advantages, including instrument-free operation, visual readouts, high sensitivity, and rapid response times. These attributes position them as an ideal choice for on-site food safety monitoring. Numerous research findings consistently underscore the superior detection performance of colorimetric sensors across various foodborne pathogens [117,118,119].
The development of highly sensitive and specific biosensors is crucial for combating foodborne pathogens. For instance, Kang et al. designed a dual-mode sensor targeting Listeria monocytogenes that integrates exponential amplification and strand displacement reactions with platinum nanoparticle-mediated signal enhancement. This innovative platform achieved detection limits of 38 CFU/mL in colorimetric mode and 10 CFU/mL in fluorescence mode and has been successfully validated in milk samples. The synergistic combination of exponential amplification and strand displacement reactions not only boosted detection accuracy but also streamlined the operational workflow [120].
To address Escherichia coli O157:H7, a pathogen implicated in hemorrhagic colitis and hemolytic uremic syndrome, Ali et al. engineered an aptamer-functionalized Au@Fe3O4 nanozyme-based colorimetric sensor. Leveraging the intrinsic peroxidase-mimicking activity of the nanozyme, the system catalyzes the oxidation of TMB to generate a chromogenic signal; target bacterial binding to the aptamer suppresses this catalytic reaction, enabling quantitative detection. The sensor exhibits a linear dynamic range from 101 to 108 CFU/mL and achieves an ultralow limit of detection (LOD) of 3 CFU/mL, positioning it among the most sensitive label-free platforms for foodborne pathogen surveillance [121]. Further advancing this field, Guo et al. developed a CuSe-based nanozyme sensor that integrates magnetic separation, colorimetric nanozyme activity, and infrared bacterial inactivation [122]. Employing a sandwich capture format, this sensor completed detection within 80 min, achieving E. coli detection limits of 35 CFU/mL in milk and mineral water.
In a more systematic approach, a colorimetric sensor array leveraging the oxidase-mimicking Ag/Mn3O4 nanozyme was engineered to discriminate six key foodborne pathogens, including E. coli, S. aureus, S. enterica, and L. monocytogenes, through pathogen-specific modulation of TMB chromogenic kinetics. Combined with PCA, this system enables accurate identification and quantification of single or mixed bacterial populations at low concentrations. Notably, the same nanozyme catalyzes superoxide radical generation, achieving broad-spectrum bactericidal efficacy. This tri-functional platform, unifying sensitive detection, precise classification, and on-demand disinfection, represents a transformative strategy for next-generation food safety diagnostics [123].
The aforementioned studies unequivocally demonstrate the multifunctionality and high sensitivity of colorimetric sensors in foodborne pathogen detection. Their inherent characteristics—rapid response, operational simplicity, and high sensitivity—position them as ideal tools for early warning systems and risk management in food safety.
While optical sensing technologies have indeed matured into rapid and intuitive detection methods, significant challenges remain. Foremost among these are the need to effectively mitigate high background interference from complex food matrices and to enhance their multiplex detection capabilities. Addressing these limitations will critically inform future research directions, guiding both technological optimization and the broader application of these powerful sensing platforms.
4. Comparative Performance of Optical Techniques for Detecting Chemical and Biological Contaminants
Table 1 summarizes representative performance benchmarks of five mainstream optical sensing platforms in comparison with conventional analytical methods for food safety monitoring. The data reveal clear differentiation in capability: SERS and quantum dot-based fluorescence exhibit outstanding sensitivity. In multiple studies, SERS has achieved detection limits in the picomolar or even sub-picromolar range, while quantum dot probes have reached nanomolar or lower responses for certain pesticides, making both highly suitable for trace analysis and molecular fingerprint identification. SPR excels in label-free, real-time monitoring and quantitative analysis of molecular interactions, aligning well with rapid detection scenarios. LIBS offers the unique advantage of simultaneous multi-element detection without complex sample pretreatment, enabling high-throughput on-site screening. Colorimetric assays stand out for their low cost and portability, making them ideal for preliminary field screening and rapid alerts.

Table 1.
Benchmarking typical performance of major optical sensing platforms versus conventional techniques for food safety detection.
A cross-platform comparison also exposes shared limitations. High-sensitivity techniques such as SERS and SPR continue to face challenges in substrate uniformity, cost, and scalable manufacturing. Although LIBS is operationally convenient, its performance can be affected by matrix interference in complex food systems. Meanwhile, the sensitivity and quantitative accuracy of colorimetric assays remain insufficient for detecting contaminants at extremely low concentrations. Based on the comparative insights in Table 1, future research priorities should include improving the reproducible fabrication of high-sensitivity substrates, reducing system-level costs, and advancing multimodal integration to simultaneously achieve high analytical performance and robust field deployability.
5. Future Trends and Challenges
Table 1 demonstrates that optical sensing platforms offer clear advantages in detection sensitivity and portability, yet challenges persist in cost control and large-scale deployment. Although these technologies hold transformative potential for food safety monitoring, their transition from laboratory research to real-world implementation remains hindered by critical barriers. Their current strengths lie in high sensitivity, rapid response, and suitability for on-site detection. However, issues such as matrix interference, limited environmental stability, and the absence of standardized protocols constitute the primary technological bottlenecks restricting practical adoption. Overcoming these challenges necessitates multidisciplinary collaborative innovation: developing anti-interference sensing interfaces at the material level to stably address matrix variations, constructing miniaturized multimodal integrated platforms at the system level for comprehensive chain-wide monitoring, and optimizing decision-making timeliness through data-driven intelligent algorithms (Figure 10). Here, we systematically dissect the breakthrough pathways and unresolved issues in the technological evolution and industrial translation of optical sensors, providing a scientific blueprint for building the next generation of intelligent monitoring systems.
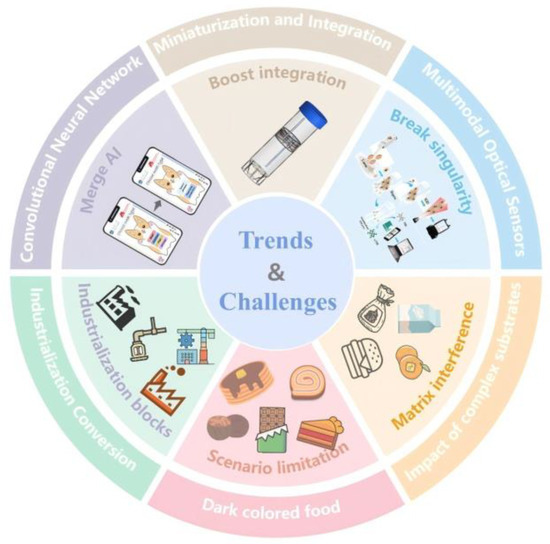
Figure 10.
Future Trends and Challenges in Optical Sensor Food Detection.
Future development is unfolding along three converging trajectories. First, multimodal optical sensors that integrate orthogonal signals, such as SERS, colorimetry, and fluorescence, are overcoming the limitations of single-channel detection in complex sample matrices and substantially enhancing analytical reliability [62,124]. Existing studies indicate that dual-modality data fusion can compensate for the weaknesses of individual channels and improve quantitative accuracy; for example, hybrid SERS/colorimetric devices have demonstrated up to a 20-fold increase in sensitivity [125]. Accordingly, future efforts should prioritize advances in signal co-registration, fusion algorithms, and portable implementation strategies. The integration of microfluidic chips with smartphones drives device portability [126,127,128,129,130]. Examples include self-powered colorimetric sensors enabling full Salmonella detection within 2 h [131] and smartphone-assisted platforms completing E. coli quantification in 30 min [132]. Artificial intelligence (AI) algorithms are overcoming data analysis bottlenecks; convolutional neural networks, for example, achieve a 99.83% identification accuracy in complex spectral analysis, reducing errors by 50% [69,133,134]. These advancements lay the groundwork for establishing a comprehensive chain-wide monitoring system, yet several challenges remain.
The core challenges are concentrated in three key bottlenecks: Complex food matrix interference leads to non-specific adsorption signal drift during SPR detection of veterinary drug residues and competitive component masking of characteristic peaks during SERS analysis of heavy metals. Environmental fluctuations also cause quantum dot fluorescence quenching and performance degradation of LIBS components. Limitations in multi-scenario adaptability are primarily evident in the interference of dark food backgrounds with colorimetric readings, difficulties in suppressing flow noise in microfluidic platforms, portable devices having detection limits 1–2 orders of magnitude inferior to laboratory instruments, and insufficient stability of nanoprobes in extreme environments. The industrialization process faces multiple bottlenecks, including increased production costs due to the lack of scalable manufacturing processes for nanomaterials, technical specification confusion caused by the lack of a standardized validation framework, and cross-matrix prediction errors in AI models resulting from insufficient training data, all of which significantly hinder technology translation and commercial deployment.
In conclusion, the further development of optical sensor technology necessitates synergistic breakthroughs across materials science, microfluidics, and data science. Developing mechanically durable flexible substrates requires innovation in nanocomposite interfaces, constructing truly multimodal platforms depends on miniaturized optical integration techniques, and intelligent sensing networks demand solutions for wireless power supply and cloud security issues, among others.
6. Conclusions
This review provides a systematic evaluation of recent advances in five major optical sensing technologies for food safety monitoring, namely SPR, quantum dot fluorescence, SERS, LIBS, and colorimetric sensing. Particular attention is given to breakthroughs in detecting representative contaminants such as heavy metal ions, pesticide and veterinary drug residues, and pathogens like Salmonella. SPR has achieved ppt-level identification of veterinary drug residues, while quantum dot-based platforms have reached femtomolar detection limits for pesticides. SERS has demonstrated sub-picomolar sensitivity for heavy metal ions. LIBS enables multi-element, synchronous analysis within seconds, and colorimetric assays now approach single-cell detection capability. Collectively, these advances lay the technological groundwork for real-time, end-to-end surveillance systems spanning the entire food supply chain—from production and processing to distribution and consumption.
To address persistent bottlenecks—including matrix interference, limited environmental robustness, and the absence of standardized regulatory frameworks—this review proposes an innovative “tiered detection architecture.” This framework strategically aligns the most appropriate sensing technologies with the distinct requirements of each stage in the food supply chain, thereby optimizing resource allocation and analytical efficiency. Looking ahead, interdisciplinary convergence across materials science, microfluidic engineering, and intelligent algorithms will be essential to drive the transition of optical sensors from laboratory prototypes to industrial-scale platforms characterized by multimodal integration, miniaturization, and embedded intelligence. Such advancements will enable a paradigm shift in food safety oversight—from passive response to proactive early warning—providing critical technological support for global food security.
Author Contributions
F.F.: Writing—original draft, Literature search, Conceptualization. Z.L.: Literature search, Formal analysis, Data curation. Z.H.: Formal analysis, Data curation, Conceptualization. Y.S.: Visualization, Validation, Literature search. K.H.: Validation, Formal analysis, Data curation. Y.T. (Corresponding Author): Writing—review and editing, Supervision, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the projects supported by the National Natural Science Foundation of China (51975261), the Natural Science Foundation of Jiangsu Province (BK20200912), the China Postdoctoral Science Foundation (2023M741435), the Aeronautical Science Foundation of China (2023Z045160001), and the Jiangsu Province Postgraduate Research and Practice Innovation Program (SJCX24—2400). This research is also supported by the Zhenjiang Key Laboratory of Advanced Manufacturing of Aerospace Components (SS2023009).
Data Availability Statement
No new data were created.
Acknowledgments
During the preparation of this manuscript, the author used DeepSeek V3 for the purposes of literature information organization. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hoejskov, P. Capacity building and support from international organizations. Eur. J. Public Health 2020, 30 (Suppl. S5), V351. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Zhang, L.; Chen, Z.; Zhang, W.; Ren, M.; Shi, J.; Xu, X.; Yang, Y. Surface-enhanced Raman scattering based on noble metal nanoassemblies for detecting harmful substances in food. Crit. Rev. Food Sci. Nutr. 2024, 65, 5431–5452. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Song, P.; Xia, L. Examples in the detection of heavy metal ions based on surface-enhanced Raman scattering spectroscopy. Nanophotonics 2021, 10, 4419–4445. [Google Scholar] [CrossRef]
- Yao, L.; Chen, Y.; Wang, R.; Yan, C.; Xu, J.; Yao, B.; Cheng, J.; Chen, W. Rapid and sensitive detection of Hg2+ with a SERS-enhanced lateral flow strip. Analyst 2022, 147, 4337–4347. [Google Scholar] [CrossRef]
- Cabrera, L.C.; Di Piazza, G.; Dujardin, B.; Pastor, P.M. The 2021 European Union report on pesticide residues in food. Efsa J. 2023, 21, 7939. [Google Scholar] [CrossRef]
- Hoffman-Pennesi, D.; Winfield, S.; Gavelek, A.; Farakos, S.M.S.; Spungen, J. Infants’ and young children’s dietary exposures to lead and cadmium: FDA total diet study 2018–2020. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2024, 41, 1454–1479. [Google Scholar] [CrossRef]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Cabrera, L.C.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Pastor, P.M. The 2022 European Union report on pesticide residues in food. Efsa J. 2024, 22, 8753. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Faas, J.; Krska, R.; Khiaosa-ard, R.; Zebeli, Q. Residues of pesticides and veterinary drugs in diets of dairy cattle from conventional and organic farms in Austria. Environ. Pollut. 2023, 316, 120626. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, J.; Zhou, Q. Improved Detection of Veterinary Drug Residues: Advancing Analytical Techniques to Ensure Food Safety. Curr. Pharm. Anal. 2023, 19, 745–758. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Michlig, N.; Lightfield, A.R.; Domesle, A.; Wiggins, S.; Duverna, R.; Weyrauch, K.; Green, J.E.; Zipperer, L. Antibiotic Residues in Cattle Reported to Be Raised Without Antibiotics. J. Agric. Food Chem. 2025, 73, 847–860. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses report. Efsa J. 2024, 22, 9106. [Google Scholar] [CrossRef]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.J.S.; Cui, Z.; Tierney, R.; Griffin, P.M.; Hoekstra, R.M.; Payne, D.C.; Rose, E.B.; Devine, C.; Namwase, A.S.; Mirza, S.A.; et al. Foodborne Illness Acquired in the United States—Major Pathogens, 2019. Emerg. Infect. Dis. 2025, 31, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, Z.; Fang, H.; Zhang, Q.; Zhou, Q.; Chen, Z.; Yang, H.; Wang, F. Au Sputtered Paper Chromatography Tandem Raman Platform for Sensitive Detection of Heavy Metal Ions. ACS Sens. 2020, 5, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Du, H.; Li, Z.; Wang, Y.; Yang, Q.; Wu, W. Nanomaterial-based Optical Biosensors for the Detection of Foodborne Bacteria. Food Rev. Int. 2022, 38, 655–684. [Google Scholar] [CrossRef]
- Guo, X.; Tian, H.; Yang, F.; Fan, S.; Zhang, J.; Ma, J.; Ai, L.; Zhang, Y. Rapid determination of 103 common veterinary drug residues in milk and dairy products by ultra performance liquid chromatography tandem mass spectrometry. Front. Nutr. 2022, 9, 879518. [Google Scholar] [CrossRef]
- An, N.; Li, K.; Zhang, Y.; Wen, T.; Liu, W.; Liu, G.; Li, L.; Jin, W. A multiplex and regenerable surface plasmon resonance (MR-SPR) biosensor for DNA detection of genetically modified organisms. Talanta 2021, 231, 122361. [Google Scholar] [CrossRef]
- Xie, X.; Huang, T.; Huang, M. Research Progress in the Application of Carbon Dot-Based Fluorescent Aptamer Sensors in Food Safety Detection. Food Sci. 2024, 45, 361–372. [Google Scholar]
- Xu, M.-L.; Gao, Y.; Han, X.-X.; Zhao, B. Innovative Application of SERS in Food Quality and Safety: A Brief Review of Recent Trends. Foods 2022, 11, 2097. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, Y.; Wu, F.; Cao, Y.; Yu, Y.; Wang, X. Research on LIBS Quantitative Analysis of Heavy Metal Concentration in Polluted Water-Based on Fourier Self-Deconvolution Method. In Proceedings of the Annual Conference of Chinese-Society-of-Optical-Engineering (CSOE) on Optical Spectroscopy and Imaging (AOPC), Beijing, China, 7–9 July 2019. [Google Scholar]
- Yao, S.; Zhang, L.; Zhu, Y.; Wu, J.; Lu, Z.; Lu, J. Evaluation of heavy metal element detection in municipal solid waste incineration fly ash based on LIBS sensor. Waste Manag. 2020, 102, 492–498. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Du, M.; Zhao, Y.; Yu, K. Enhanced Laser-Induced Breakdown Spectroscopy for Heavy Metal Detection in Agriculture: A Review. Sensors 2022, 22, 5679. [Google Scholar] [CrossRef] [PubMed]
- Sternesjo, A.; Mellgren, C.; Bjorck, L. Determination of sulfamethazine residues in milk by a surface plasmon resonance-based biosensor assay. Anal. Biochem. 1995, 226, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Xu, Y.; Zareef, M.; Li, H.; Rong, Y.; Chen, Q. Recent advances of nanomaterial-based optical sensor for the detection of benzimidazole fungicides in food: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 2851–2872. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef]
- Hassan, M.M.; Li, H.; Ahmad, W.; Zareef, M.; Wang, J.; Xie, S.; Wang, P.; Ouyang, Q.; Wang, S.; Chen, Q. Au@Ag nanostructure based SERS substrate for simultaneous determination of pesticides residue in tea via solid phase extraction coupled multivariate calibration. LWT-Food Sci. Technol. 2019, 105, 290–297. [Google Scholar] [CrossRef]
- Celik, O.; Saylan, Y.; Gokturk, I.; Yilmaz, F.; Denizli, A. A surface plasmon resonance sensor with synthetic receptors decorated on graphene oxide for selective detection of benzylpenicillin. Talanta 2023, 253, 123939. [Google Scholar] [CrossRef]
- Butt, M.A. Surface Plasmon Resonance-Based Biodetection Systems: Principles, Progress and Applications—A Comprehensive Review. Biosensors 2025, 15, 35. [Google Scholar] [CrossRef]
- Tan, J.; Chen, Y.; He, J.; Occhipinti, L.G.; Wang, Z.; Zhou, X. Two-dimensional material-enhanced surface plasmon resonance for antibiotic sensing. J. Hazard. Mater. 2023, 455, 131644. [Google Scholar] [CrossRef]
- Subramanian, P.; Lesniewski, A.; Kaminska, I.; Vlandas, A.; Vasilescu, A.; Niedziolka-Jonsson, J.; Pichonat, E.; Happy, H.; Boukherroub, R.; Szunerits, S. Lysozyme detection on aptamer functionalized graphene-coated SPR interfaces. Biosens. Bioelectron. 2013, 50, 239–243. [Google Scholar] [CrossRef]
- Kravets, V.G.; Wu, F.; Yu, T.C.; Grigorenko, A.N. Metal-Dielectric-Graphene Hybrid Heterostructures with Enhanced Surface Plasmon Resonance Sensitivity Based on Amplitude and Phase Measurements. Plasmonics 2022, 17, 973–987. [Google Scholar] [CrossRef]
- Fan, H.; Li, R.; Chen, Y.; Zhang, H.; Zeng, S.; Ji, W.; Hu, W.; Yin, S.; Li, Y.; Liu, G.L.; et al. Flexible nanoplasmonic sensor for multiplexed and rapid quantitative food safety analysis with a thousand-times sensitivity improvement. Biosens. Bioelectron. 2024, 248, 115974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Qian, S.; Zhu, S.; Lu, J.; Hu, Y.; Zhang, C.; Geng, Y.; Chen, X.; Guo, Y.; Chen, Z.; et al. A Point-of-Care Testing Device Utilizing Graphene-Enhanced Fiber Optic SPR Sensor for Real-Time Detection of Infectious Pathogens. Biosensors 2023, 13, 1029. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J.; et al. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef]
- Song, W.; Zhai, X.; Shi, J.; Zou, X.; Xue, Y.; Sun, Y.; Sun, W.; Zhang, J.; Huang, X.; Li, Z.; et al. A ratiometric fluorescence amine sensor based on carbon quantum dot-loaded electrospun polyvinylidene fluoride film for visual monitoring of food freshness. Food Chem. 2024, 434, 137423. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Wang, Y.; Guo, Z.; Huang, X.; Li, Z.; Zhang, X.; Zhang, J.; Xiao, J.; et al. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Li, M.; Zou, X.; Shi, J. Efficient preparation of dual-emission ratiometric fluorescence sensor system based on aptamer-composite and detection of bis(2-ethylhexyl) phthalate in pork. Food Chem. 2021, 352, 129352. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Li, Q.; Shi, Y.; Zhai, X.; Xu, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J.; et al. Copper nanoclusters @ nitrogen-doped carbon quantum dots-based ratiometric fluorescence probe for lead (II) ions detection in porphyra. Food Chem. 2020, 320, 126623. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Qiu, Y.; Li, P.; Liu, B.; Yang, L.; Barnych, B.; Hammock, B.D.; Zhang, C. Highly Specific Monoclonal Antibody and Sensitive Quantum Dot Beads-Based Fluorescence Immunochromatographic Test Strip for Tebuconazole Assay in Agricultural Products. J. Agric. Food Chem. 2019, 67, 9096–9103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Han, Y.; Ling, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cao, L.; Cai, H.; Yang, W.; Lu, H.; Adila, A.; Zhang, B.; Cao, Y.; Huang, W.; Xu, W.; et al. A rapid microfluidic paper-based chip sensor using ratiometric fluorescence and molecularly imprinted polymers for visual detection of sulfadiazine in actual samples. J. Food Compos. Anal. 2025, 139, 107108. [Google Scholar] [CrossRef]
- Marimuthu, M.; Xu, K.; Song, W.; Chen, Q.; Wen, H. Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 2025, 463, 141288. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Wang, S.; Yan, Y. Mesoporous silica-based molecularly imprinted fluorescence sensor for the ultrafast and sensitive recognition of oxytetracycline. J. Food Compos. Anal. 2022, 108, 104427. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.; Shi, X.; Han, Y.; Guo, Z.; Liu, Y. Development of Carbon Quantum Dot-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine in Hot Pot Soup Base. Food Anal. Methods 2020, 13, 1042–1049. [Google Scholar] [CrossRef]
- Sharma, A.S.; Ali, S.; Sabarinathan, D.; Murugavelu, M.; Li, H.; Chen, Q. Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5765–5801. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Sun, L.; Kong, D.; Sheng, J.; Wang, K.; Dong, C. A Green, Simple, and Rapid Detection for Amaranth in Candy Samples Based on the Fluorescence Quenching of Nitrogen-Doped Graphene Quantum Dots. Food Anal. Methods 2019, 12, 1658–1665. [Google Scholar] [CrossRef]
- Saberi, Z.; Rezaei, B.; Ensafi, A.A. Fluorometric label-free aptasensor for detection of the pesticideacetamiprid by using cationic carbon dots prepared with cetrimonium bromide. Microchim. Acta 2019, 186, 273. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, N.; Xu, D.; Li, Z. Electrochemical detection of omethoate and acetamiprid in vegetable and fruit with high sensitivity and selectivity based on pomegranate-like gold nanoparticle and double target-induced DNA cycle signal amplification. Sens. Actuators B-Chem. 2022, 359, 131597. [Google Scholar] [CrossRef]
- Qin, L.; Guo, Y.; Li, L.; Lin, D.; Li, Y.; Xu, S.; Jiang, C. Ratiometric Fluorescent Sensor Based on Hydrogen-Bond Triggering the Internal Filter Effect for Enzyme-Free and Visual Monitoring Pesticide Residues. ACS Sustain. Chem. Eng. 2023, 11, 11032–11040. [Google Scholar] [CrossRef]
- Wu, Z.; Hao, Z.; Chai, Y.; Li, A.; Wang, C.; Zhang, X.; Chen, H.; Lu, C. Near-infrared-excitable acetylcholinesterase-activated fluorescent probe for sensitive and anti-interference detection of pesticides in colored food. Biosens. Bioelectron. 2023, 233, 115341. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Cao, J.; Lin, D.; Xu, S.; Li, Y.; Jiang, C. A Strecker-like reaction triggering fluorescent sensing platform for enzyme-free and visual quantitative monitoring of carbamates. Chem. Eng. J. 2023, 464, 142550. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Shi, Y.; Zou, X.; Arslan, M.; Zhang, W.; Huang, X.; Li, Z.; Xu, Y. Use of a smartphone for visual detection of melamine in milk based on Au@Carbon quantum dots nanocomposites. Food Chem. 2019, 272, 58–65. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Li, Y.; Shi, J.; Huang, X.; Li, Z.; Zhang, J.; Li, W.; Xu, Y.; Zou, X. Easy-to-Use Visual Sensing System for Milk Freshness, Sensitized with Acidity-Responsive N-Doped Carbon Quantum Dots. Foods 2022, 11, 1855. [Google Scholar] [CrossRef]
- Patel, S.; Shrivas, K.; Sinha, D.; Karbhal, I.; Patle, T.K.; Monisha; Tikeshwari. A portable smartphone-assisted digital image fluorimetry for analysis of methiocarb pesticide in vegetables: Nitrogen-doped carbon quantum dots as a sensing probe. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 299, 122824. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Duyne, R.P.V. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Patel, S.; Shrivas, K.; Sinha, D.; Karbhal, I.; Patle, T.K.; Monisha; Tikeshwari. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Hassan, M.M.; Ali, S.; Chen, Q. SERS based artificial peroxidase enzyme regulated multiple signal amplified system for quantitative detection of foodborne pathogens. Food Control 2021, 123, 107733. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zareef, M.; Xu, Y.; Li, H.; Chen, Q. SERS based sensor for mycotoxins detection: Challenges and improvements. Food Chem. 2021, 344, 128652. [Google Scholar] [CrossRef]
- Perales-Rondon, J.V.; Colina, A.; Gonzalez, M.C.; Escarpa, A. Roughened silver microtubes for reproducible and quantitative SERS using a template-assisted electrosynthesis approach. Appl. Mater. Today 2020, 20, 100710. [Google Scholar] [CrossRef]
- Li, H.; Hu, W.; Hassan, M.M.; Zhang, Z.; Chen, Q. A facile and sensitive SERS-based biosensor for colormetric detection of acetamiprid in green tea based on unmodified gold nanoparticles. J. Food Meas. Charact. 2019, 13, 259–268. [Google Scholar] [CrossRef]
- Zhu, J.; Agyekum, A.A.; Kutsanedzie, F.Y.H.; Li, H.; Chen, Q.; Ouyang, Q.; Jiang, H. Qualitative and quantitative analysis of chlorpyrifos residues in tea by surface-enhanced Raman spectroscopy (SERS) combined with chemometric models. LWT-Food Sci. Technol. 2018, 97, 760–769. [Google Scholar] [CrossRef]
- Zhai, W.L.; You, T.Y.; Ouyang, X.H.; Wang, M. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1887–1909. [Google Scholar] [CrossRef]
- Lin, D.Y.; Yu, C.Y.; Ku, C.A.; Chung, C.K. Design, Fabrication, and Applications of SERS Substrates for Food Safety Detection: Review. Micromachines 2023, 14, 1343. [Google Scholar] [CrossRef]
- Li, H.H.; Geng, W.H.; Hassan, M.M.; Zuo, M.; Wei, W.Y.; Wu, X.Y.; Ouyang, Q.; Chen, Q.S. Rapid detection of chloramphenicol in food using SERS flexible sensor coupled artificial intelligent tools. Food Control 2021, 128, 108186. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zareef, M.; Jiao, T.H.; Liu, S.S.; Xu, Y.; Viswadevarayalu, A.; Li, H.H.; Chen, Q.S. Signal optimized rough silver nanoparticle for rapid SERS sensing of pesticide residues in tea. Food Chem. 2021, 338, 127796. [Google Scholar] [CrossRef]
- Hassan, M.M.; He, P.H.; Zareef, M.; Li, H.H.; Chen, Q.S. Rapid detection and prediction of chloramphenicol in food employing label-free HAu/Ag NFs-SERS sensor coupled multivariate calibration. Food Chem. 2022, 374, 131765. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Sun, Y.; Shi, J.Y.; Zhang, W.; Zhang, X.A.; Huang, X.W.; Zou, X.B.; Li, Z.H.; Wei, R.C. Facile synthesis of Au@Ag core-shell nanorod with bimetallic synergistic effect for SERS detection of thiabendazole in fruit juice. Food Chem. 2022, 370, 131276. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Yang, X.; Yuan, R.; Chai, Y. A SERS biosensor constructed by calcined ZnO substrate with high-efficiency charge transfer for sensitive detection of Pb2+. Sens. Actuators B-Chem. 2021, 343, 130142. [Google Scholar] [CrossRef]
- Kamal, S.; Yang, T.C.-K. Silver enriched silver phosphate microcubes as an efficient recyclable SERS substrate for the detection of heavy metal ions. J. Colloid Interface Sci. 2022, 605, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ben, Z.; Ma, G.; Xu, F. UIO-66/Ag/TiO2 Nanocomposites as Highly Active SERS Substrates for Quantitative Detection of Hexavalent Chromium. Chemosensors 2023, 11, 315. [Google Scholar] [CrossRef]
- Li, J.; Peng, W.; Wang, A.; Wan, M.; Zhou, Y.; Zhang, X.-G.; Jin, S.; Zhang, F.-L. Highly sensitive and selective SERS substrates with 3D hot spot buildings for rapid mercury ion detection. Analyst 2023, 148, 4044–4052. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Ahmad, W.; Zareef, M.; Rong, Y.; Xu, Y.; Jiao, T.; He, P.; Li, H.; Chen, Q. Rapid detection of mercury in food via rhodamine 6G signal using surface-enhanced Raman scattering coupled multivariate calibration. Food Chem. 2021, 358, 129844. [Google Scholar] [CrossRef]
- Kitahama, Y.; Pancorbo, P.M.; Segawa, H.; Marumi, M.; Xiao, T.-H.; Hiramatsu, K.; Yang, W.; Goda, K. Place & Play SERS: Sample collection and preparation-free surface-enhanced Raman spectroscopy. Anal. Methods 2023, 15, 1028–1036. [Google Scholar] [CrossRef]
- Zhou, X.; Pu, H.; Sun, D.-W. DNA functionalized metal and metal oxide nanoparticles: Principles and recent advances in food safety detection. Crit. Rev. Food Sci. Nutr. 2021, 61, 2277–2296. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Khan, S.; Wahab, A.; Kim, M. Machine Learning-Based Heavy Metal Ion Detection Using Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 596. [Google Scholar] [CrossRef]
- Jin, Y.; Li, C.; Huang, Z.; Jiang, L. Simultaneous Quantitative Determination of Low-Concentration Preservatives and Heavy Metals in Tricholoma Matsutakes Based on SERS and FLU Spectral Data Fusion. Foods 2023, 12, 4267. [Google Scholar] [CrossRef]
- Wang, L.; Tolok, G.; Fu, Y.X.; Xu, L.; Li, L.; Gao, H.; Zhou, Y. Application and Research Progress of Laser-Induced Breakdown Spectroscopy in Agricultural Product Inspection. ACS Omega 2024, 9, 24203–24218. [Google Scholar] [CrossRef] [PubMed]
- Stefas, D.; Gyftokostas, N.; Kourelias, P.; Nanou, E.; Tananaki, C.; Kanelis, D.; Liolios, V.; Kokkinos, V.; Bouras, C.; Couris, S. Honey discrimination based on the bee feeding by Laser Induced Breakdown Spectroscopy. Food Control 2022, 134, 108770. [Google Scholar] [CrossRef]
- Singh, V.K.; Sharma, N.; Verma, O.N.; Singh, V.K.; Tripathi, D.K.; Lee, Y.; Kumar, S.; Rai, P.K.; Gondal, M.A. Review: Application of LIBS to Elemental Analysis and Mapping of Plant Samples. Atom. Spectrosc. 2021, 42, 99–113. [Google Scholar] [CrossRef]
- Chen, X.; Ali, S.; Yuan, L.M.; Guo, F.Y.; Huang, G.Z.; Shi, W.; Chen, X.J. Characterization and source analysis of heavy metals contamination in microplastics by Laser-Induced Breakdown Spectroscopy. Chemosphere 2022, 287, 132172. [Google Scholar] [CrossRef]
- Ma, S.; Tang, Y.; Ma, Y.; Chu, Y.; Chen, F.; Hu, Z.; Zhu, Z.; Guo, L.; Zeng, X.; Lu, Y. Determination of trace heavy metal elements in aqueous solution using surface-enhanced laser-induced breakdown spectroscopy. Opt. Express 2019, 27, 15091–15099. [Google Scholar] [CrossRef]
- Niu, S.; Zheng, L.; Khan, A.Q.; Zeng, H. Laser-Induced Breakdown Spectroscopic (LIBS) Analysis of Trace Heavy Metals Enriched by Al2O3 Nanoparticles. Appl. Spectrosc. 2019, 73, 380–386. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Q.; Liu, X.; Liu, P.; Yu, L. Quantitative Analysis of Pb in Soil Using Laser-Induced Breakdown Spectroscopy Based on Signal Enhancement of Conductive Materials. Molecules 2024, 29, 3699. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, Y.; Hao, N.; Miao, P.; Li, X.; Liu, C.; Li, Z. Data fusion of Laser-induced breakdown spectroscopy and Near-infrared spectroscopy to quantitatively detect heavy metals in lily. Microchem. J. 2023, 190, 108670. [Google Scholar] [CrossRef]
- Lu, Y.; Tao, Z.; Nie, L.; Guo, X.; Pan, T.; Chen, R.; Li, T.; Kong, W.; Liu, F. Quantitative elemental mapping of heavy metals translocation and accumulation in hyperaccumulator plant using laser-induced breakdown spectroscopy with interpretable deep learning. Comput. Electron. Agric. 2025, 230, 109907. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, F.; Huang, X.Y.; Aheto, J.H.; Yu, S.S.; Wang, Y.; Zhang, X.R.; Ren, Y. Fast monitoring the dynamic change of total acids during apple vinegar fermentation process using a colorimetric IDA sensor array. Food Chem. 2022, 387, 132867. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, H.; Lin, J.J.; Adade, S.; Chen, Q.S.; Xue, Z.L.; Chan, C.M. Non-destructive detection of multi-component heavy metals in corn oil using nano-modified colorimetric sensor combined with near-infrared spectroscopy. Food Control 2022, 133, 108640. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.Y.; Zhang, H.; Wang, Z.P.; Wu, S.J. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wu, A.; Xu, L.; Xu, C.; Liu, L.; Kuang, H.; Xu, X. Recent progress on lateral flow immunoassays in foodborne pathogen detection. Food Biosci. 2023, 52, 102475. [Google Scholar] [CrossRef]
- Lv, R.Q.; Huang, X.Y.; Ye, W.T.; Aheto, J.H.; Xu, H.X.; Dai, C.X.; Tian, X.Y. Research on the reaction mechanism of colorimetric sensor array with characteristic volatile gases-TMA during fish storage. J. Food Process Eng. 2019, 42, e12952. [Google Scholar] [CrossRef]
- Lv, R.Q.; Huang, X.Y.; Aheto, J.H.; Mu, L.J.; Tian, X.Y. Analysis of fish spoilage by gas chromatography-mass spectrometry and electronic olfaction bionic system. J. Food Saf. 2018, 38, e12557. [Google Scholar] [CrossRef]
- Lin, H.; Man, Z.X.; Kang, W.C.; Guan, B.B.; Chen, Q.S.; Xue, Z.L. A novel colorimetric sensor array based on boron-dipyrromethene dyes for monitoring the storage time of rice. Food Chem. 2018, 268, 300–306. [Google Scholar] [CrossRef]
- Li, L.Q.; Xie, S.M.; Zhu, F.Y.; Ning, J.M.; Chen, Q.S.; Zhang, Z.Z. Colorimetric sensor array-based artificial olfactory system for sensing Chinese green tea’s quality: A method of fabrication. Int. J. Food Prop. 2017, 20, 1762–1773. [Google Scholar] [CrossRef]
- Huang, X.Y.; Lv, R.Q.; Wang, S.; Aheto, J.H.; Dai, C.X. Integration of computer vision and colorimetric sensor array for nondestructive detection of mango quality. J. Food Process Eng. 2018, 41, e12873. [Google Scholar] [CrossRef]
- Huang, X.W.; Zou, X.B.; Shi, J.Y.; Li, Z.H.; Zhao, J.W. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Han, F.K.; Huang, X.Y.; Teye, E. Novel prediction of heavy metal residues in fish using a low-cost optical electronic tongue system based on colorimetric sensors array. J. Food Process Eng. 2019, 42, e12983. [Google Scholar] [CrossRef]
- Zhai, X.D.; Li, Z.H.; Shi, J.Y.; Huang, X.W.; Sun, Z.B.; Zhang, D.; Zou, X.B.; Sun, Y.; Zhang, J.J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-W.; Li, Z.-H.; Zou, X.-B.; Shi, J.-Y.; Mao, H.-P.; Zhao, J.-W.; Hao, L.-M.; Holmes, M. Detection of meat-borne trimethylamine based on nanoporous colorimetric sensor arrays. Food Chem. 2016, 197, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Zou, X.B.; Huang, X.W.; Shi, J.Y.; Mariod, A.A. Discrimination of honeys using colorimetric sensor arrays, sensory analysis and gas chromatography techniques. Food Chem. 2016, 206, 37–43. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, X.; Xu, Y.; Liu, C.; Zou, X.; Li, Z.; Shi, J.; Huang, X. Facile fabrication of three-dimensional gold nanodendrites decorated by silver nanoparticles as hybrid SERS-active substrate for the detection of food contaminants. Food Control 2021, 122, 107772. [Google Scholar] [CrossRef]
- Kutsanedzie, F.Y.H.; Agyekum, A.A.; Annavaram, V.; Chen, Q. Signal-enhanced SERS-sensors of CAR-PLS and GA-PLS coupled AgNPs for ochratoxin A and aflatoxin B1 detection. Food Chem. 2020, 315, 126231. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Hu, H.; Huang, Y.; Yang, X.; Wang, Q.; Chen, X. Improving the detection limit of Salmonella colorimetry using long ssDNA of asymmetric-PCR and non-functionalized AuNPs. Anal. Biochem. 2021, 626, 114229. [Google Scholar] [CrossRef]
- Du, J.; Yu, Z.; Hu, Z.; Chen, J.; Zhao, J.; Bai, Y. A low pH-based rapid and direct colorimetric sensing of bacteria using unmodified gold nanoparticles. J. Microbiol. Methods 2021, 180, 106110. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric aptasensor for the detection of Salmonella enterica serovar typhimurium using ZnFe2O4-reduced graphene oxide nanostructures as an effective peroxidase mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Ouyang, Q.; Zhao, J. Fabricating a Novel Raman Spectroscopy-Based Aptasensor for Rapidly Sensing Salmonella typhimurium. Food Anal. Methods 2017, 10, 3032–3041. [Google Scholar] [CrossRef]
- Ma, N.; Sun, M.; Shi, H.; Xue, L.; Zhang, M.; Yang, W.; Dang, Y.; Qiao, Z. A Colorimetric/Fluorescent Dual-Mode Aptasensor for Salmonella Based on the Magnetic Separation of Aptamers and a DNA-Nanotriangle Programmed Multivalent Aptamer. Foods 2023, 12, 3853. [Google Scholar] [CrossRef]
- Pang, L.; Li, S.; Liu, B.; Su, Q.; Qu, B.; Zhang, W.; Yang, X.; Jiang, Y. Colorimetric biosensor based on aptamer recognition-induced multi-DNA release and peroxidase-mimicking three-way junction DNA-Ag/PtNCs for the detection of Salmonella typhimurium. Talanta 2024, 274, 125930. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Shi, Y.; Zou, X.; Tahir, H.E.; Holmes, M.; Zhang, W.; Huang, X.; Li, Z.; Xu, Y. A dual-mode sensor for colorimetric and fluorescent detection of nitrite in hams based on carbon dots-neutral red system. Meat Sci. 2019, 147, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kamal, Z.; Ghobadi, M.Z.; Mohseni, S.M.; Ghourchian, H. High-performance porphyrin-like graphene quantum dots for immuno-sensing of Salmonella typhi. Biosens. Bioelectron. 2021, 188, 113334. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liao, T.; Zhou, H.; Huang, Y.; Chen, P.; Yang, X.; Chen, X. Colorimetric method for Salmonella spp. detection based on peroxidase-like activity of Cu(II)-rGO nanoparticles and PCR. Anal. Biochem. 2021, 615, 114068. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhu, W.; Huang, C.; Zhang, Y.; Wang, J.; Wang, X. Quantum dot-labeled phage-encoded RBP 55 as a fluorescent nanoprobe for sensitive and specific detection of Salmonella in food matrices. Food Chem. 2023, 428, 136724. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Sun, H.; Wang, M.X.; Chen, Q.S.; Guo, Z.M.; Wu, J.Z. Rapid Pseudomonas Species Identification from Chicken by Integrating Colorimetric Sensors with Near-Infrared Spectroscopy. Food Anal. Methods 2018, 11, 1199–1208. [Google Scholar] [CrossRef]
- Li, H.H.; Kutsanedzie, F.; Zhao, J.W.; Chen, Q.S. Quantifying Total Viable Count in Pork Meat Using Combined Hyperspectral Imaging and Artificial Olfaction Techniques. Food Anal. Methods 2016, 9, 3015–3024. [Google Scholar] [CrossRef]
- Chen, Q.S.; Hu, W.W.; Su, J.; Li, H.H.; Ouyang, Q.; Zhao, J.W. Nondestructively sensing of total viable count (TVC) in chicken using an artificial olfaction system based colorimetric sensor array. J. Food Eng. 2016, 168, 259–266. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, S.; Ma, C.; Guo, R.; Yu, X.; Lin, T.; Pang, W.; Liu, Y.; Jiao, J.; Xu, M.; et al. A Dual-Mode Colorimetric and Fluorescence Biosensor Based on a Nucleic Acid Multiplexing Platform for the Detection of Listeria monocytogenes. Anal. Chem. 2025, 97, 1853–1860. [Google Scholar] [CrossRef]
- Ali, R.; Alattar, A.; Alshaman, R.; Ghabban, A.; Alanazi, S.; Al-Brahimi, H.; Alatwi, M.; Jlawi, A.; Albalawi, A.; Alatawi, A.M.A.; et al. Sensing the invisible: Ultrasensitive and selective colorimetric detection of E. coli O157:H7 based on masking the peroxidase-mimetic activity of aptamer-modified Au/Fe3O4. Food Chem. 2024, 443, 138564. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Ma, X.; Cai, M.; Liu, S.; Sun, C.; Chi, Y.; Xu, K. A colorimetric biosensor with infrared sterilization based on CuSe nanoparticles for the detection of E. coli O157:H7 in food samples. Microbiol. Spectr. 2024, 12, e03978-23. [Google Scholar] [CrossRef]
- Xu, X.; Yang, J.; Hao, G.; Wang, B.; Ma, T.; Zhu, S.; Gao, L.; Yang, Z.-q. Three in one: A multifunctional oxidase-mimicking Ag/Mn3O4 nanozyme for colorimetric determination, precise identification, and broad-spectrum inactivation of foodborne pathogenic bacteria. Food Chem. 2025, 464, 141620. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, H. Monitoring of Chlorpyrifos Residues in Corn Oil Based on Raman Spectral Deep-Learning Model. Foods 2023, 12, 2402. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, M.; Mi, F.; Zhang, Y.; Geng, P.; Zhang, S.; Song, H.; Chen, G. A SERS/colorimetric biosensor based on AuNSs@Ag core-shell Prussian blue nanozyme for non-interference and rapid detection of Staphylococcus aureus in milk. Microchim. Acta 2025, 192, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Bian, F.; Wang, Y.F.; Hu, L.; Yang, N.; Mao, H.P. A Method for Capture and Detection of Crop Airborne Disease Spores Based on Microfluidic Chips and Micro Raman Spectroscopy. Foods 2022, 11, 3462. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Cao, L.L.; Lu, H.J.; Huang, Y.; Yang, W.Q.; Cai, Y.Z.; Li, S.M.; Li, S.Q.; Zhao, J.W.; Xu, W.Z. Custom-printed microfluidic chips using simultaneous ratiometric fluorescence with “Green” carbon dots for detection of multiple antibiotic residues in pork and water samples. J. Food Sci. 2024, 89, 5980–5992. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hassan, M.M.; Sharma, A.S.; Li, H.H.; Chen, Q.S. Recent advancement in nano-optical strategies for detection of pathogenic bacteria and their metabolites in food safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 486–504. [Google Scholar] [CrossRef]
- Wang, Y.F.; Mao, H.P.; Zhang, X.D.; Liu, Y.; Du, X.X. A Rapid Detection Method for Tomato Gray Mold Spores in Greenhouse Based on Microfluidic Chip Enrichment and Lens-Less Diffraction Image Processing. Foods 2021, 10, 3011. [Google Scholar] [CrossRef]
- Jayan, H.; Yin, L.M.; Xue, S.S.; Zou, X.B.; Guo, Z.M. Raman spectroscopy-based microfluidic platforms: A promising tool for detection of foodborne pathogens in food products. Food Res. Int. 2024, 180, 114052. [Google Scholar] [CrossRef]
- Wang, L.; Rong, N.; Xi, X.; Wang, M.; Huo, X.; Yuan, J.; Qi, W.; Li, Y.; Lin, J. Power-free colorimetric biosensing of foodborne bacteria in centrifugal tube. Biosens. Bioelectron. 2023, 220, 114905. [Google Scholar] [CrossRef]
- Hong, B.; Wang, W.; Li, Y.; Ma, Y.; Wang, J. Specific separation and sensitive detection of foodborne pathogens by phage-derived bacterial-binding protein-nano magnetic beads coupled with smartphone-assisted paper sensor. Biosens. Bioelectron. 2024, 247, 115911. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, Y.; Gu, Y.L.; Xiang, T.Y.; Shen, H.; Wang, Y.; Hu, Z.Y.; Zheng, Z.; Yu, Z.L.; Wu, Q.; et al. Metal-polyphenol Multistage Competitive Coordination System for Colorimetric Monitoring Meat Freshness. Adv. Mater. 2025, 37, e2503246. [Google Scholar] [CrossRef]
- Bonah, E.; Huan, X.Y.; Yi, R.; Aheto, J.H.; Osae, R.; Golly, M. Electronic nose classification and differentiation of bacterial foodborne pathogens based on support vector machine optimized with particle swarm optimization algorithm. J. Food Process Eng. 2019, 42, e13236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).