Optical Fiber Sensing Technologies in Radiation Therapy
Abstract
1. Introduction
2. Optical Fiber Dosimetry
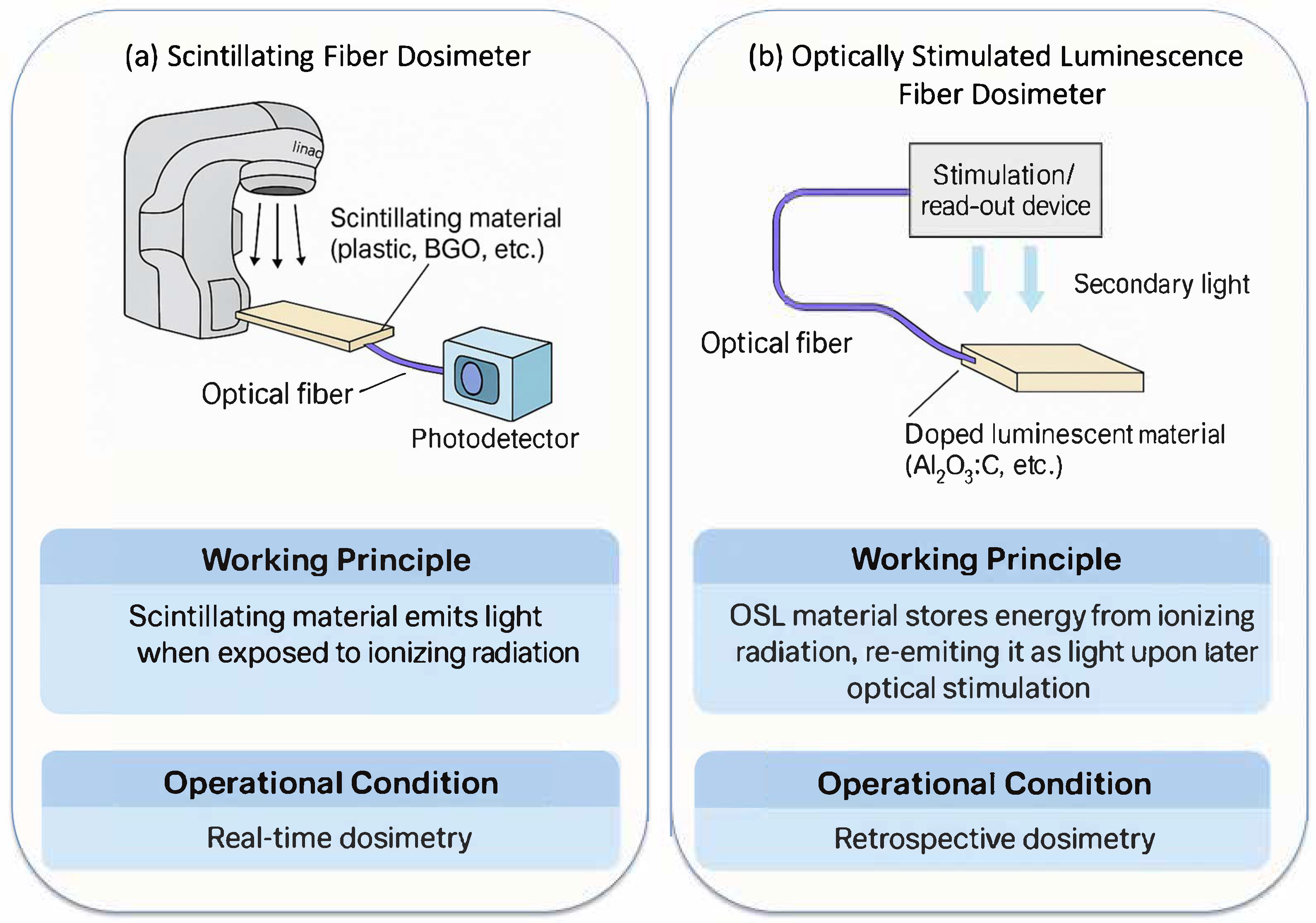
2.1. Scintillating Fiber Dosimeters
2.2. Optically Stimulated Luminescence Fiber Dosimeters
2.3. Other Types of Fiber Dosimeters
3. Optical Fiber Spectroscopy
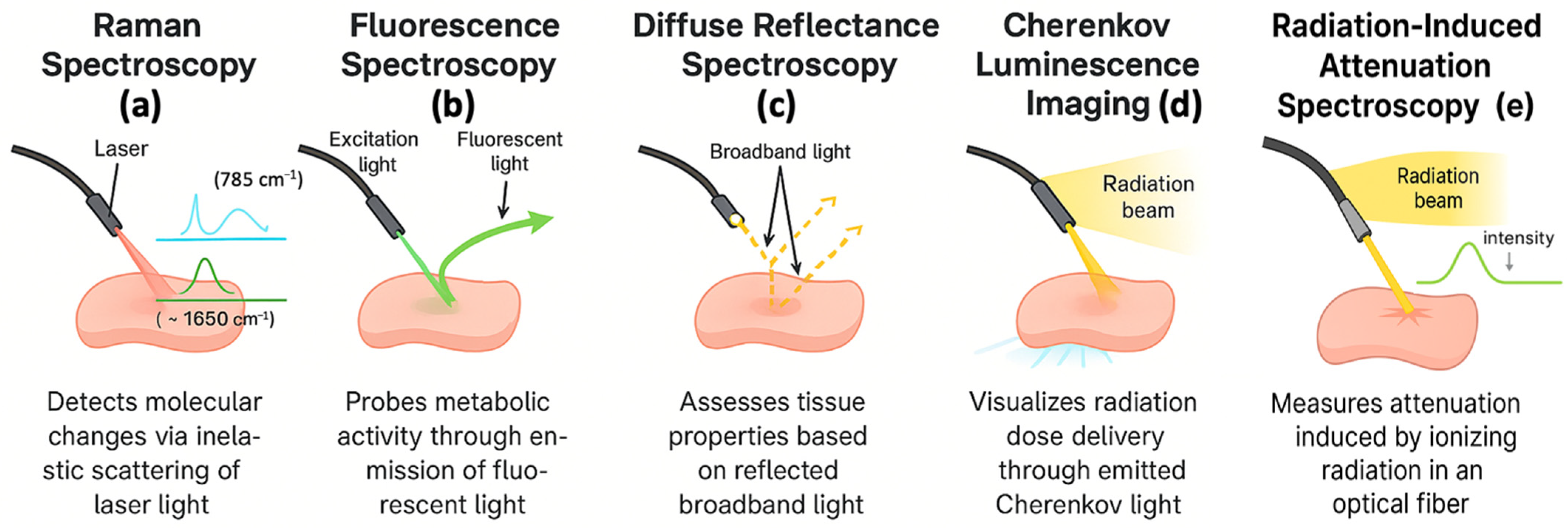
3.1. Raman Spectroscopy
3.2. Fluorescence Spectroscopy
3.3. Diffuse Reflectance Spectroscopy
3.4. Cherenkov Luminescence Imaging
3.5. Radiation-Induced Attenuation Spectroscopy
4. Applications of Optical Fiber Sensors in Radiation Therapy
4.1. Adaptive Radiation Therapy
4.2. Real-Time Monitoring and Imaging
4.3. Clinical Integration and Workflow Advantages
5. Challenges and Future Directions
5.1. Fiber-Optic Dosimetric Technical Challenges
5.2. Fiber-Optic Spectroscopic Technical Challenges
5.3. Clinical Translation and Adoption Challenges
5.4. Future Directions and Developments
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Cho, B. Intensity-Modulated Radiation Therapy: A Review with a Physics Perspective. Radiat. Oncol. J. 2018, 36, 1. [Google Scholar] [CrossRef]
- Folkert, M.R.; Timmerman, R.D. Stereotactic Ablative Body Radiosurgery (SABR) or Stereotactic Body Radiation Therapy (SBRT). Adv. Drug Deliv. Rev. 2017, 109, 3–14. [Google Scholar] [CrossRef]
- De Los Santos, J.; Popple, R.; Agazaryan, N.; Bayouth, J.E.; Bissonnette, J.-P.; Bucci, M.K.; Dieterich, S.; Dong, L.; Forster, K.M.; Indelicato, D. Image Guided Radiation Therapy (IGRT) Technologies for Radiation Therapy Localization and Delivery. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 33–45. [Google Scholar] [CrossRef]
- Mohan, R.; Grosshans, D. Proton Therapy–Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef]
- Schardt, D.; Elsässer, T.; Schulz-Ertner, D. Heavy-Ion Tumor Therapy: Physical and Radiobiological Benefits. Rev. Mod. Phys. 2010, 82, 383–425. [Google Scholar] [CrossRef]
- Chetty, I.J.; Cai, B.; Chuong, M.D.; Dawes, S.L.; Hall, W.A.; Helms, A.R.; Kirby, S.; Laugeman, E.; Mierzwa, M.; Pursley, J. Quality and Safety Considerations for Adaptive Radiation Therapy: An ASTRO White Paper. Int. J. Radiat. Oncol. Biol. Phys. 2025, 122, 838–864. [Google Scholar] [CrossRef] [PubMed]
- Beaton, L.; Bandula, S.; Gaze, M.N.; Sharma, R.A. How Rapid Advances in Imaging Are Defining the Future of Precision Radiation Oncology. Br. J. Cancer 2019, 120, 779–790. [Google Scholar] [CrossRef]
- Fiz, F.; Iori, M.; Fioroni, F.; Biroli, M.; D’Agostino, G.R.; Gelardi, F.; Erba, P.A.; Versari, A.; Chiti, A.; Sollini, M. Molecular Guidance for Planning External Beam Radiation Therapy in Oncology. In Nuclear Oncology; Springer: Cham, Switzerland, 2022; pp. 1–40. ISBN 3319260677. [Google Scholar]
- Cai, B.; Banks, T.I.; Shen, C.; Prasad, R.; Bal, G.; Lin, M.-H.; Godley, A.; Pompos, A.; Garant, A.; Westover, K. Strategies for Offline Adaptive Biology-Guided Radiotherapy (BgRT) on a PET-Linac Platform. Cancers 2025, 17, 2470. [Google Scholar] [CrossRef] [PubMed]
- Dona Lemus, O.M.; Cao, M.; Cai, B.; Cummings, M.; Zheng, D. Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers 2024, 16, 1206. [Google Scholar] [CrossRef]
- Grégoire, V.; Haustermans, K.; Geets, X.; Roels, S.; Lonneux, M. PET-Based Treatment Planning in Radiotherapy: A New Standard? J. Nucl. Med. 2007, 48, 68S–77S. [Google Scholar]
- Glide-Hurst, C.K.; Lee, P.; Yock, A.D.; Olsen, J.R.; Cao, M.; Siddiqui, F.; Parker, W.; Doemer, A.; Rong, Y.; Kishan, A.U. Adaptive Radiation Therapy (ART) Strategies and Technical Considerations: A State of the ART Review from NRG Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1054–1075. [Google Scholar] [CrossRef]
- Suarez, M.A.; Lim, T.; Robillot, L.; Maillot, V.; Lihoreau, T.; Bontemps, P.; Pazart, L.; Grosjean, T. Miniaturized Fiber Dosimeter of Medical Ionizing Radiations on a Narrow Optical Fiber. Opt. Express 2019, 27, 35588–35599. [Google Scholar] [CrossRef]
- Elsharkawi, A.S.A.; Elazab, H.A.; Askar, M.A.; Abdelrahman, I.Y.; Arafa, A.A.; Gomma, L.R.; Lo, Y.-L. Biocompatibility and Radiosensitivity of a Fiber Optical-Based Dosimeter: Biological Applications. Biomed. Opt. Express 2024, 15, 3492–3506. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, C.; Dai, Q.; Kong, L. Soft and Stretchable Polymeric Optical Waveguide-Based Sensors for Wearable and Biomedical Applications. Sensors 2019, 19, 3771. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.; Holloway, L.; Rosenfeld, A.; Li, E. Fibre-Optic Dosimetry for MRI-LINACs: A Mini-Review. Front. Phys. 2022, 10, 879624. [Google Scholar] [CrossRef]
- O’Keeffe, S.; McCarthy, D.; Woulfe, P.; Grattan, M.W.D.; Hounsell, A.R.; Sporea, D.; Mihai, L.; Vata, I.; Leen, G.; Lewis, E. A Review of Recent Advances in Optical Fibre Sensors for in Vivo Dosimetry during Radiotherapy. Br. J. Radiol. 2015, 88, 20140702. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Knorr, F.; Latka, I.; Vogt, M.; Hofmann, G.O.; Popp, J.; Schie, I.W. Real-Time Molecular Imaging of near-Surface Tissue Using Raman Spectroscopy. Light Sci. Appl. 2022, 11, 90. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Wu, C.; Zhang, X.; Huang, X. The Accuracy of Fiber-Optic Raman Spectroscopy in the Detection and Diagnosis of Head and Neck Neoplasm in Vivo: A Systematic Review and Meta-Analysis. PeerJ 2023, 11, e16536. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, L.; Wang, Y.; Xiong, Z. Current Trends of Raman Spectroscopy in Clinic Settings: Opportunities and Challenges. Adv. Sci. 2024, 11, 2300668. [Google Scholar] [CrossRef]
- Li, Z.; Lan, N.; Cheng, Z.; Jin, F.; Song, E.; Xu, Z.; Zhang, Y.; Feng, Y.-Z.; Cai, X.; Ran, Y. In Vivo Fiber-Optic Fluorescent Sensor for Real-Time PH Monitoring of Tumor Microenvironment. Chem. Eng. J. 2024, 493, 152495. [Google Scholar] [CrossRef]
- Xie, H.; Xie, Z.; Mousavi, M.; Bendsoe, N.; Brydegaard, M.; Axelsson, J.; Andersson-Engels, S. Design and Validation of a Fiber Optic Point Probe Instrument for Therapy Guidance and Monitoring. J. Biomed. Opt. 2014, 19, 71408. [Google Scholar] [CrossRef]
- Thomas, T.P.; Myaing, M.T.; Ye, J.Y.; Candido, K.; Kotlyar, A.; Beals, J.; Cao, P.; Keszler, B.; Patri, A.K.; Norris, T.B. Detection and Analysis of Tumor Fluorescence Using a Two-Photon Optical Fiber Probe. Biophys. J. 2004, 86, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.K.; Lukose, J.; Upadhya, R.; Pai, M.V.; Kartha, V.B.; Chidangil, S. In Vivo Spectroscopy: Optical Fiber Probes for Clinical Applications. Expert Rev. Med. Devices 2022, 19, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, Y.; Wang, C.; Chen, Y.; Tjin, S.C.; Wei, L. Recent Advances in Optical Fiber Enabled Radiation Sensors. Sensors 2022, 22, 1126. [Google Scholar] [CrossRef]
- Sporea, D. Optical Fiber Sensors in Ionizing Radiation Environments. In Handbook of Optical Fibers; Springer: Singapore, 2019; pp. 1913–1954. ISBN 9811070873. [Google Scholar]
- Ohta, T.; Nozawa, Y.; Ohira, S.; Nawa, K.; Yamashita, H.; Nakagawa, K. Characterization of a Practically Designed Plastic Scintillation Plate Dosimeter. Med. Phys. 2025, 52, e17904. [Google Scholar] [CrossRef]
- Bauer, C.J.; Schneider, F.; Göbel, I.D.; Oppitz, H.; Giordano, F.A.; Fleckenstein, J. Characterization of a Novel Plastic Scintillation Detector for in Vivo Electron Dosimetry. arXiv 2025, arXiv:2509.04933. [Google Scholar] [CrossRef]
- Ciarrocchi, E.; Ravera, E.; Cavalieri, A.; Celentano, M.; Del Sarto, D.; Di Martino, F.; Linsalata, S.; Massa, M.; Masturzo, L.; Moggi, A. Plastic Scintillator-Based Dosimeters for Ultra-High Dose Rate (UHDR) Electron Radiotherapy. Phys. Medica 2024, 121, 103360. [Google Scholar] [CrossRef]
- Fontbonne, J.M.; Iltis, G.; Ban, G.A.; Battala, A.; Vernhes, J.C.; Tillier, J.; Bellaize, N.; Le Brun, C.; Tamain, B.; Mercier, K. Scintillating Fiber Dosimeter for Radiation Therapy Accelerator. IEEE Trans. Nucl. Sci. 2002, 49, 2223–2227. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Xu, S.; Liu, H.; Li, X. Fiber Bragg Grating-Based Smart Garment for Monitoring Human Body Temperature. Sensors 2022, 22, 4252. [Google Scholar] [CrossRef]
- Kinet, D.; Mégret, P.; Goossen, K.W.; Qiu, L.; Heider, D.; Caucheteur, C. Fiber Bragg Grating Sensors toward Structural Health Monitoring in Composite Materials: Challenges and Solutions. Sensors 2014, 14, 7394–7419. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Marple, E.; Mahadevan-Jansen, A. Performance Assessment of Probe-Based Raman Spectroscopy Systems for Biomedical Analysis. Biomed. Opt. Express 2023, 14, 3597–3609. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Bradley, D.A.; Tarif, Z.H.; Khodaei, A.; Basaif, A.; Ibrahim, S.A.; Abdul-Rashid, H.A. Time-Resolved Optical Fiber Measurements: A Review of Scintillator Materials and Applications. Radiat. Detect. Technol. Methods 2025, 9, 1–16. [Google Scholar] [CrossRef]
- Archambault, L.; Briere, T.M.; Pönisch, F.; Beaulieu, L.; Kuban, D.A.; Lee, A.; Beddar, S. Toward a Real-Time in Vivo Dosimetry System Using Plastic Scintillation Detectors. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 280–287. [Google Scholar] [CrossRef]
- Kharzheev, Y.N. Radiation Hardness of Scintillation Detectors Based on Organic Plastic Scintillators and Optical Fibers. Phys. Part. Nucl. 2019, 50, 42–76. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Q.; Wang, Q.; Li, Y.; Perks, R.M.; Zhao, L. Advances on Inorganic Scintillator-Based Optic Fiber Dosimeters. EJNMMI Phys. 2020, 7, 60. [Google Scholar] [CrossRef]
- Shi, Z.; Lv, S.; Tang, G.; Tang, J.; Jiang, L.; Qian, Q.; Zhou, S.; Yang, Z. Multiphase Transition toward Colorless Bismuth–Germanate Scintillating Glass and Fiber for Radiation Detection. ACS Appl. Mater. Interfaces 2020, 12, 17752–17759. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, A.; Kim, R.; Moon, J.H. Evaluation of the Detection Efficiency of LYSO Scintillator in the Fiber-Optic Radiation Sensor. Sci. Technol. Nucl. Install. 2014, 2014, 248403. [Google Scholar] [CrossRef]
- Hu, Y.; Qin, Z.; Ma, Y.; Zhao, W.; Sun, W.; Zhang, D.; Chen, Z.; Wang, B.; Tian, H.; Lewis, E. Characterization of Fiber Radiation Dosimeters with Different Embedded Scintillator Materials for Radiotherapy Applications. Sens. Actuators A Phys. 2018, 269, 188–195. [Google Scholar] [CrossRef]
- Gierej, A.; Baghdasaryan, T.; Martyn, M.; Woulfe, P.; Mc Laughlin, O.; Prise, K.; Workman, G.; O’Keeffe, S.; Rochlitz, K.; Verlinski, S. Mass-Manufacturable Scintillation-Based Optical Fiber Dosimeters for Brachytherapy. Biosens. Bioelectron. 2024, 255, 116237. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L.; Ruda, H.E. In Vivo Dosimetry in Radiotherapy: Techniques, Applications, and Future Directions. Encyclopedia 2025, 5, 40. [Google Scholar] [CrossRef]
- Lee, B.; Jang, K.W.; Cho, D.H.; Yoo, W.J.; Shin, S.H.; Kim, H.S.; Yi, J.H.; Kim, S.; Cho, H.; Park, B.G. Measurement of Two-Dimensional Photon Beam Distributions Using a Fiber-Optic Radiation Sensor for Small Field Radiation Therapy. IEEE Trans. Nucl. Sci. 2008, 55, 2632–2636. [Google Scholar] [CrossRef]
- Terasawa, K.; Doke, T.; Hasebe, N.; Kikuchi, J.; Kudo, K.; Murakami, T.; Takeda, N.; Tamura, T.; Torii, S.; Yamashita, M. Scintillating Fiber Camera for Neutron Dosimetry in Spacecraft. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2001, 457, 499–508. [Google Scholar] [CrossRef]
- Klavsen, M.F.; Ankjærgaard, C.; Behrens, C.P.; Vogelius, I.R.; Boye, K.; Hansen, R.H.; Andersen, C.E. Time-Resolved Plastic Scintillator Dosimetry in MR Linear Accelerators without Image Distortion. Radiat. Meas. 2022, 154, 106759. [Google Scholar] [CrossRef]
- Darafsheh, A.; Goddu, S.M.; Williamson, J.; Zhang, T.; Sobotka, L.G. Radioluminescence Dosimetry in Modern Radiation Therapy. Adv. Photonics Res. 2024, 5, 2300350. [Google Scholar] [CrossRef]
- Veronese, I.; Andersen, C.E.; Li, E.; Madden, L.; Santos, A.M.C. Radioluminescence-Based Fibre Optic Dosimeters in Radiotherapy: A Review. Radiat. Meas. 2024, 174, 107125. [Google Scholar] [CrossRef]
- Frelin, A.; Fontbonne, J.; Ban, G.; Colin, J.; Labalme, M.; Batalla, A.; Isambert, A.; Vela, A.; Leroux, T. Spectral Discrimination of Čerenkov Radiation in Scintillating Dosimeters. Med. Phys. 2005, 32, 3000–3006. [Google Scholar] [CrossRef]
- Archer, J.; Madden, L.; Li, E.; Carolan, M.; Petasecca, M.; Metcalfe, P.; Rosenfeld, A. Temporally Separating Cherenkov Radiation in a Scintillator Probe Exposed to a Pulsed X-Ray Beam. Phys. Medica 2017, 42, 185–188. [Google Scholar] [CrossRef]
- Archer, J.; Madden, L.; Li, E.; Carolan, M.; Rosenfeld, A. A Comparison of Temporal Cherenkov Separation Techniques in Pulsed Signal Scintillator Dosimetry. Biomed. Phys. Eng. Express 2018, 4, 44003. [Google Scholar] [CrossRef]
- McKeever, S.W.S. Optically Stimulated Luminescence: A Brief Overview. Radiat. Meas. 2011, 46, 1336–1341. [Google Scholar] [CrossRef]
- McKeever, S.W.S.; Blair, M.W.; Bulur, E.; Gaza, R.; Gaza, R.; Kalchgruber, R.; Klein, D.M.; Yukihara, E.G. Recent Advances in Dosimetry Using the Optically Stimulated Luminescence of Al2O3: C. Radiat. Prot. Dosim. 2004, 109, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Lee, S.B.; Jin, G.H. Performance of Optically Stimulated Luminescence Al2O3 Dosimeter for Low Doses of Diagnostic Energy X-Rays. Appl. Radiat. Isot. 2011, 69, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Buranurak, S.; Andersen, C.E. Fiber-Coupled Al2O3: C Radioluminescence Dosimetry for Total Body Irradiations. Radiat. Meas. 2016, 93, 46–54. [Google Scholar] [CrossRef]
- McKeever, S.W.; Moscovitch, M. On the Advantages and Disadvantages of Optically Stimulated Luminescence Dosimetry and Thermoluminescence Dosimetry. Radiat. Prot. Dosim. 2003, 104, 263–270. [Google Scholar] [CrossRef]
- Magne, S.; Ferdinand, P. Fiber Optic Remote Gamma Dosimeters Based on Optically Stimulated Luminescence: State-of-the-Art at CEA. In Proceedings of the 11th International Congress of the International Radiation Protection Association (IRPA), Madrid, Spain, 23–28 May 2004. [Google Scholar]
- Cygler, J.E.; Yukihara, E.G. Optically Stimulated Luminescence (OSL) Dosimetry in Radiotherapy; Medical Physics Publishing: Madison, WI, USA, 2009. [Google Scholar]
- Andersen, C.E.; Edmund, J.M.; Damkjær, S.M.S. Precision of RL/OSL Medical Dosimetry with Fiber-Coupled Al2O3: C: Influence of Readout Delay and Temperature Variations. Radiat. Meas. 2010, 45, 653–657. [Google Scholar] [CrossRef]
- Santos, A.M.C.; Mohammadi, M.; Afshar V, S. Evaluation of a Real-time BeO Ceramic Fiber-coupled Luminescence Dosimetry System for Dose Verification of High Dose Rate Brachytherapy. Med. Phys. 2015, 42, 6349–6356. [Google Scholar] [CrossRef]
- Birajdar, S.; Zhang, W.; Santos, A.; Hickson, K.; Afshar Vahid, S. Real-Time in Vivo Dose Measurement Using Ruby-Based Fibre Optic Dosimetry during Internal Radiation Therapy. Phys. Eng. Sci. Med. 2023, 46, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawi, A.S.A.; Alazab, H.A.; Sayed, M.; Askar, M.A.; Abdelrahman, I.Y.; Arafa, A.A.; Saleh, H.I.; Gomaa, L.R.; Du, Y.-C. A Fiber-Optical Dosimetry Sensor for Gamma-Ray Irradiation Measurement in Biological Applications. Biosensors 2023, 13, 1010. [Google Scholar] [CrossRef]
- Woulfe, P.; Sullivan, F.J.; Kam, W.; O’Keeffe, S. Optical Fiber Dosimeter for Real-Time in-Vivo Dose Monitoring during LDR Brachytherapy. Biomed. Opt. Express 2020, 11, 4027–4036. [Google Scholar] [CrossRef]
- Woulfe, P.; Sullivan, F.J.; O’Keeffe, S. Optical Fibre Sensors: Their Role in in Vivo Dosimetry for Prostate Cancer Radiotherapy. Cancer Nanotechnol. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Fernández, S.D.S.; García-Salcedo, R.; Mendoza, J.G.; Sánchez-Guzmán, D.; Rodríguez, G.R.; Gaona, E.; Montalvo, T.R. Thermoluminescent Characteristics of LiF: Mg, Cu, P and CaSO4: Dy for Low Dose Measurement. Appl. Radiat. Isot. 2016, 111, 50–55. [Google Scholar] [CrossRef]
- Parks, A.; Hallett, J.; Niver, A.; Zhang, R.; Bruza, P.; Pogue, B.W. Review of Cherenkov Imaging Technology Advances in Radiotherapy: Single-Photon-Level Imaging in High Ambient Light and Radiation Backgrounds. Biophotonics Discov. 2024, 1, 20901. [Google Scholar] [CrossRef]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Williams, B.B.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission. Front. Phys. 2020, 8, 328. [Google Scholar] [CrossRef]
- Kim, J.A.; Wales, D.J.; Yang, G. Optical Spectroscopy for in Vivo Medical Diagnosis—A Review of the State of the Art and Future Perspectives. Prog. Biomed. Eng. 2020, 2, 042001. [Google Scholar] [CrossRef]
- Kouri, M.A.; Spyratou, E.; Karnachoriti, M.; Kalatzis, D.; Danias, N.; Arkadopoulos, N.; Seimenis, I.; Raptis, Y.S.; Kontos, A.G.; Efstathopoulos, E.P. Raman Spectroscopy: A Personalized Decision-Making Tool on Clinicians’ Hands for in Situ Cancer Diagnosis and Surgery Guidance. Cancers 2022, 14, 1144. [Google Scholar] [CrossRef]
- Sun, Y.; Hatami, N.; Yee, M.; Phipps, J.; Elson, D.S.; Gorin, F.; Schrot, R.J.; Marcu, L. Fluorescence Lifetime Imaging Microscopy for Brain Tumor Image-Guided Surgery. J. Biomed. Opt. 2010, 15, 56022. [Google Scholar] [CrossRef]
- Butte, P.V.; Mamelak, A.N.; Nuno, M.; Bannykh, S.I.; Black, K.L.; Marcu, L. Fluorescence Lifetime Spectroscopy for Guided Therapy of Brain Tumors. Neuroimage 2011, 54, S125–S135. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, K.; Chang, K.; Klein, D.; Deng, Y.F.; Chang, V.; Phelps, J.E.; Ramanujam, N. Portable, Fiber-Based, Diffuse Reflection Spectroscopy (DRS) Systems for Estimating Tissue Optical Properties. Appl. Spectrosc. 2010, 65, 206–215. [Google Scholar] [CrossRef]
- Ciarrocchi, E.; Belcari, N. Cerenkov Luminescence Imaging: Physics Principles and Potential Applications in Biomedical Sciences. EJNMMI Phys. 2017, 4, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman Spectroscopy in Cancer Diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef] [PubMed]
- Desroches, J.; Jermyn, M.; Pinto, M.; Picot, F.; Tremblay, M.-A.; Obaid, S.; Marple, E.; Urmey, K.; Trudel, D.; Soulez, G. A New Method Using Raman Spectroscopy for in Vivo Targeted Brain Cancer Tissue Biopsy. Sci. Rep. 2018, 8, 1792. [Google Scholar] [CrossRef]
- Monaghan, J.F.; Byrne, H.J.; Lyng, F.M.; Meade, A.D. Radiobiological Applications of Vibrational Spectroscopy: A Review of Analyses of Ionising Radiation Effects in Biology and Medicine. Radiation 2024, 4, 276–308. [Google Scholar] [CrossRef]
- Mahadevan-Jansen, A.; Richards-Kortum, R.R. Raman Spectroscopy for the Detection of Cancers and Precancers. J. Biomed. Opt. 1996, 1, 31–70. [Google Scholar] [CrossRef]
- Wang, H.-W.; Wei, Y.-H.; Guo, H.-W. Reduced Nicotinamide Adenine Dinucleotide (NADH) Fluorescence for the Detection of Cell Death. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2009, 9, 1012–1017. [Google Scholar] [CrossRef]
- Sibai, M.; Mehidine, H.; Devaux, B.; Abi Haidar, D. Characterization of a Bimodal Multi-Fibre Optic Clinical Probe for in Situ Tissue Diagnosis Based on Spectrally-and Temporally-Resolved Autofluorescence. Front. Phys. 2023, 11, 1120314. [Google Scholar] [CrossRef]
- Saha, A.; Barman, I.; Dingari, N.C.; McGee, S.; Volynskaya, Z.; Galindo, L.H.; Liu, W.; Plecha, D.; Klein, N.; Dasari, R.R. Raman Spectroscopy: A Real-Time Tool for Identifying Microcalcifications during Stereotactic Breast Core Needle Biopsies. Biomed. Opt. Express 2011, 2, 2792–2803. [Google Scholar] [CrossRef]
- Dadgar, S.; Rajaram, N. Optical Imaging Approaches to Investigating Radiation Resistance. Front. Oncol. 2019, 9, 1152. [Google Scholar] [CrossRef]
- Rickard, A.G.; Mikati, H.; Mansourati, A.; Stevenson, D.; Krieger, M.; Rocke, D.; Esclamado, R.; Dewhirst, M.W.; Ramanujam, N.; Lee, W.T. A Clinical Study to Assess Diffuse Reflectance Spectroscopy with an Auto-Calibrated, Pressure-Sensing Optical Probe in Head and Neck Cancer. Curr. Oncol. 2023, 30, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Perekatova, V.; Kostyuk, A.; Kirillin, M.; Sergeeva, E.; Kurakina, D.; Shemagina, O.; Orlova, A.; Khilov, A.; Turchin, I. VIS-NIR Diffuse Reflectance Spectroscopy System with Self-Calibrating Fiber-Optic Probe: Study of Perturbation Resistance. Diagnostics 2023, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Putt, M.E.; Emanuele, M.J.; Shin, D.B.; Glatstein, E.; Yodh, A.G.; Busch, T.M. Treatment-Induced Changes in Tumor Oxygenation Predict Photodynamic Therapy Outcome. Cancer Res. 2004, 64, 7553–7561. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, S.; Troncoso, J.R.; Siegel, E.R.; Curry, N.M.; Griffin, R.J.; Dings, R.P.M.; Rajaram, N. Spectroscopic Investigation of Radiation-Induced Reoxygenation in Radiation-Resistant Tumors. Neoplasia 2021, 23, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Li, J.; Wang, P.; Lang, J.; Yang, Y. Cherenkov Luminescence in Tumor Diagnosis and Treatment: A Review. Photonics 2022, 9, 390. [Google Scholar] [CrossRef]
- Tanha, K.; Pashazadeh, A.M.; Pogue, B.W. Review of Biomedical Čerenkov Luminescence Imaging Applications. Biomed. Opt. Express 2015, 6, 3053–3065. [Google Scholar] [CrossRef]
- De Michele, V.; Marcandella, C.; Vidalot, J.; Paillet, P.; Morana, A.; Cannas, M.; Boukenter, A.; Marin, E.; Ouerdane, Y.; Girard, S. Origins of Radiation-Induced Attenuation in Pure-Silica-Core and Ge-Doped Optical Fibers under Pulsed X-Ray Irradiation. J. Appl. Phys. 2020, 128, 103101. [Google Scholar] [CrossRef]
- Campanella, C.; De Michele, V.; Morana, A.; Guttilla, A.; Mady, F.; Benabdesselam, M.; Marin, E.; Boukenter, A.; Ouerdane, Y.; Girard, S. Temperature Dependence of Radiation Induced Attenuation of Aluminosilicate Optical Fiber. IEEE Trans. Nucl. Sci. 2022, 69, 1515–1520. [Google Scholar] [CrossRef]
- Di Francesca, D.; Vecchi, G.L.; Girard, S.; Alessi, A.; Reghioua, I.; Boukenter, A.; Ouerdane, Y.; Kadi, Y.; Brugger, M. Radiation-Induced Attenuation in Single-Mode Phosphosilicate Optical Fibers for Radiation Detection. IEEE Trans. Nucl. Sci. 2017, 65, 126–131. [Google Scholar] [CrossRef]
- Di Francesca, D.; Vecchi, G.L.; Girard, S.; Morana, A.; Reghioua, I.; Alessi, A.; Hoehr, C.; Robin, T.; Kadi, Y.; Brugger, M. Qualification and Calibration of Single-Mode Phosphosilicate Optical Fiber for Dosimetry at CERN. J. Light. Technol. 2019, 37, 4643–4649. [Google Scholar] [CrossRef]
- Vecchi, G.L.; Di Francesca, D.; Sabatier, C.; Girard, S.; Alessi, A.; Guttilla, A.; Robin, T.; Kadi, Y.; Brugger, M. Infrared Radiation Induced Attenuation of Radiation Sensitive Optical Fibers: Influence of Temperature and Modal Propagation. Opt. Fiber Technol. 2020, 55, 102166. [Google Scholar] [CrossRef]
- Venketeswaran, A.; Lalam, N.; Wuenschell, J.; Ohodnicki, P.R., Jr.; Badar, M.; Chen, K.P.; Lu, P.; Duan, Y.; Chorpening, B.; Buric, M. Recent Advances in Machine Learning for Fiber Optic Sensor Applications. Adv. Intell. Syst. 2022, 4, 2100067. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Yu, Q.; Ren, L.; Liu, Q.; Zhao, Y. Application of Machine Learning in Optical Fiber Sensors. Measurement 2024, 228, 114391. [Google Scholar] [CrossRef]
- Alhallak, K.; Jenkins, S.V.; Lee, D.E.; Greene, N.P.; Quinn, K.P.; Griffin, R.J.; Dings, R.P.M.; Rajaram, N. Optical Imaging of Radiation-Induced Metabolic Changes in Radiation-Sensitive and Resistant Cancer Cells. J. Biomed. Opt. 2017, 22, 60502. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.K.M.M.; Zubair, H.T.; Begum, M.; Abdul-Rashid, H.A.; Yusoff, Z.; Omar, N.Y.M.; Ung, N.M.; Mat-Sharif, K.A.; Bradley, D.A. Real-Time Dosimetry in Radiotherapy Using Tailored Optical Fibers. Radiat. Phys. Chem. 2016, 122, 43–47. [Google Scholar] [CrossRef]
- Paulides, M.M.; Verduijn, G.M.; Van Holthe, N. Status Quo and Directions in Deep Head and Neck Hyperthermia. Radiat. Oncol. 2016, 11, 21. [Google Scholar] [CrossRef]
- Gora, M.J.; Suter, M.J.; Tearney, G.J.; Li, X. Endoscopic Optical Coherence Tomography: Technologies and Clinical Applications. Biomed. Opt. Express 2017, 8, 2405–2444. [Google Scholar] [CrossRef]
- Zhou, J.; Jokerst, J.V. Photoacoustic Imaging with Fiber Optic Technology: A Review. Photoacoustics 2020, 20, 100211. [Google Scholar] [CrossRef]
- Mangraviti, A.; Volpin, F.; Cha, J.; Cunningham, S.I.; Raje, K.; Brooke, M.J.; Brem, H.; Olivi, A.; Huang, J.; Tyler, B.M. Intraoperative Laser Speckle Contrast Imaging for Real-Time Visualization of Cerebral Blood Flow in Cerebrovascular Surgery: Results from Pre-Clinical Studies. Sci. Rep. 2020, 10, 7614. [Google Scholar] [CrossRef]
- Colvill, E.; Booth, J.; Nill, S.; Fast, M.; Bedford, J.; Oelfke, U.; Nakamura, M.; Poulsen, P.; Worm, E.; Hansen, R. A Dosimetric Comparison of Real-Time Adaptive and Non-Adaptive Radiotherapy: A Multi-Institutional Study Encompassing Robotic, Gimbaled, Multileaf Collimator and Couch Tracking. Radiother. Oncol. 2016, 119, 159–165. [Google Scholar] [CrossRef]
- Penner, C.; Usherovich, S.; Andru, S.; Bélanger-Champagne, C.; Duzenli, C.; Stoeber, B.; Hoehr, C. A Multi-Point Optical Fibre Sensor for Proton Therapy. Electronics 2024, 13, 1118. [Google Scholar] [CrossRef]
- Suchowerska, N.; Jackson, M.; Lambert, J.; Yin, Y.B.; Hruby, G.; McKenzie, D.R. Clinical Trials of a Urethral Dose Measurement System in Brachytherapy Using Scintillation Detectors. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 609–615. [Google Scholar] [CrossRef]
- Noor, N.M.; Hussein, M.; Bradley, D.A.; Nisbet, A. Investigation of the Use of Ge-Doped Optical Fibre for in Vitro IMRT Prostate Dosimetry. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 652, 819–823. [Google Scholar] [CrossRef]
- Issa, F.; Hugtenburg, R.P.; Nisbet, A.; Bradley, D.A. Novel High Resolution 125I Brachytherapy Source Dosimetry Using Ge-Doped Optical Fibres. Radiat. Phys. Chem. 2013, 92, 48–53. [Google Scholar] [CrossRef]
- Andersen, C.E.; Nielsen, S.K.; Greilich, S.; Helt-Hansen, J.; Lindegaard, J.C.; Tanderup, K. Characterization of a Fiber-coupled Luminescence Dosimetry System for Online in Vivo Dose Verification during Brachytherapy. Med. Phys. 2009, 36, 708–718. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Bhushan, M.; Khurana, S.; Jha, R. Advancements in Optical Fibre Sensors Using Artificial Intelligence Technology. In Optical Fiber Sensors and AI: Exploring the Fusion; Springer: Singapore, 2025; pp. 15–31. [Google Scholar]
- Bujugundla, R.S.; Pradhan, H.S. Emerging Technologies for Fiber-Optic Based Sensors in Biomedical Domain: A Review and Recent Developments. IEEE Trans. Instrum. Meas. 2024, 73, 7010332. [Google Scholar] [CrossRef]
- Beaulieu, L.; Beddar, S. Review of Plastic and Liquid Scintillation Dosimetry for Photon, Electron, and Proton Therapy. Phys. Med. Biol. 2016, 61, R305. [Google Scholar] [CrossRef] [PubMed]
- Edmund, J.M.; Andersen, C.E. Temperature Dependence of the Al2O3: C Response in Medical Luminescence Dosimetry. Radiat. Meas. 2007, 42, 177–189. [Google Scholar] [CrossRef]
- Yuan, X.; Song, Y.; Song, Y.; Xu, J.; Wu, Y.; Glidle, A.; Cusack, M.; Ijaz, U.Z.; Cooper, J.M.; Huang, W.E. Effect of Laser Irradiation on Cell Function and Its Implications in Raman Spectroscopy. Appl. Environ. Microbiol. 2018, 84, e02508-17. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Bottiroli, G. Autofluorescence Spectroscopy and Imaging: A Tool for Biomedical Research and Diagnosis. Eur. J. Histochem. EJH 2014, 58, 2461. [Google Scholar]
- Boudreau, C.; Wee, T.-L.; Duh, Y.-R.; Couto, M.P.; Ardakani, K.H.; Brown, C.M. Excitation Light Dose Engineering to Reduce Photo-Bleaching and Photo-Toxicity. Sci. Rep. 2016, 6, 30892. [Google Scholar] [CrossRef]
- Lopci, E.; Grassi, I.; Chiti, A.; Nanni, C.; Cicoria, G.; Toschi, L.; Fonti, C.; Lodi, F.; Mattioli, S.; Fanti, S. PET Radiopharmaceuticals for Imaging of Tumor Hypoxia: A Review of the Evidence. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 365. [Google Scholar]
- Liu, H.; Carpenter, C.M.; Jiang, H.; Pratx, G.; Sun, C.; Buchin, M.P.; Gambhir, S.S.; Xing, L.; Cheng, Z. Intraoperative Imaging of Tumors Using Cerenkov Luminescence Endoscopy: A Feasibility Experimental Study. J. Nucl. Med. 2012, 53, 1579–1584. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Mitchell, G.S.; Cherry, S.R. Simultaneous PET and Multispectral 3-Dimensional Fluorescence Optical Tomography Imaging System. J. Nucl. Med. 2011, 52, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, K.; Huang, C.-S.; Boesel, L.F.; Hufenus, R.; Heuberger, M. Recent Advances in Photoluminescent Polymer Optical Fibers. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100912. [Google Scholar] [CrossRef]
- Pilz, S.; Najafi, H.; Ryser, M.; Romano, V. Granulated Silica Method for the Fiber Preform Production. Fibers 2017, 5, 24. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Gladstone, D.J.; Pogue, B.W. Optical Dosimetry of Radiotherapy Beams Using Cherenkov Radiation: The Relationship between Light Emission and Dose. Phys. Med. Biol. 2014, 59, 3789. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Zhang, R.; Gladstone, D.J.; Jiang, S.; Hitchcock, W.; Friedman, O.D.; Glaser, A.K.; Jermyn, M.; Pogue, B.W. Cherenkov Video Imaging Allows for the First Visualization of Radiation Therapy in Real Time. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 615–622. [Google Scholar] [CrossRef]
- Moradi, F.; Ung, N.M.; Mahdiraji, G.A.; Khandaker, M.U.; See, M.H.; Taib, N.A.; Bradley, D.A. Evaluation of Ge-Doped Silica Fibre TLDs for in Vivo Dosimetry during Intraoperative Radiotherapy. Phys. Med. Biol. 2019, 64, 08NT04. [Google Scholar] [CrossRef] [PubMed]
- Le Deroff, C.; Pérès, E.A.; Ledoux, X.; Toutain, J.; Frelin-Labalme, A. In Vivo Surface Dosimetry with a Scintillating Fiber Dosimeter in Preclinical Image-guided Radiotherapy. Med. Phys. 2020, 47, 234–241. [Google Scholar] [CrossRef]
- Rai, S.; Shreya; Phogat, P.; Jha, R.; Singh, S. Machine Learning for Real-Time Data Analysis in Fiber Optic Sensing. In Optical Fiber Sensors and AI: Exploring the Fusion; Springer: Singapore, 2025; pp. 77–91. [Google Scholar]
- Katyal, J. AI Techniques for Signal Processing in Optical Fiber Sensors. In Optical Fiber Sensors and AI: Exploring the Fusion; Springer: Singapore, 2025; pp. 57–75. [Google Scholar]
- Correia, R.; James, S.; Lee, S.W.; Morgan, S.P.; Korposh, S. Biomedical Application of Optical Fibre Sensors. J. Opt. 2018, 20, 73003. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, M.; Cai, J.; Xu, Z.; Jin, F.; Zhang, Y.; Wang, W.; Ran, Y.; Zhang, D.; Guan, B.-O. Sensitive and Efficient Fluorescent Fiber-Optic Sensor for in-Situ Hypoxia Detection in Solid Tumor. IEEE Sens. J. 2022, 22, 22646–22653. [Google Scholar] [CrossRef]
- De Vita, E.; De Landro, M.; Massaroni, C.; Iadicicco, A.; Saccomandi, P.; Schena, E.; Campopiano, S. Fiber Optic Sensors-Based Thermal Analysis of Perfusion-Mediated Tissue Cooling in Liver Undergoing Laser Ablation. IEEE Trans. Biomed. Eng. 2020, 68, 1066–1073. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.F.; Tam, H.-Y.; Tao, X. Multifunctional Smart Optical Fibers: Materials, Fabrication, and Sensing Applications. Photonics 2019, 6, 48. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical Smart Textiles Based on Fiber Optic Technology: An Overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef]
- Blake, N.; Gaifulina, R.; Griffin, L.D.; Bell, I.M.; Thomas, G.M.H. Machine Learning of Raman Spectroscopy Data for Classifying Cancers: A Review of the Recent Literature. Diagnostics 2022, 12, 1491. [Google Scholar] [CrossRef]
- Kothari, R.; Jones, V.; Mena, D.; Bermúdez Reyes, V.; Shon, Y.; Smith, J.P.; Schmolze, D.; Cha, P.D.; Lai, L.; Fong, Y. Raman Spectroscopy and Artificial Intelligence to Predict the Bayesian Probability of Breast Cancer. Sci. Rep. 2021, 11, 6482. [Google Scholar] [CrossRef]
- Huang, L.; Sun, H.; Sun, L.; Shi, K.; Chen, Y.; Ren, X.; Ge, Y.; Jiang, D.; Liu, X.; Knoll, W. Rapid, Label-Free Histopathological Diagnosis of Liver Cancer Based on Raman Spectroscopy and Deep Learning. Nat. Commun. 2023, 14, 48. [Google Scholar] [CrossRef]
- Mannam, V.; Zhang, Y.; Yuan, X.; Ravasio, C.; Howard, S.S. Machine Learning for Faster and Smarter Fluorescence Lifetime Imaging Microscopy. J. Phys. Photonics 2020, 2, 42005. [Google Scholar]
- Xue, X.; Sun, H.; Yang, M.; Liu, X.; Hu, H.-Y.; Deng, Y.; Wang, X. Advances in the Application of Artificial Intelligence-Based Spectral Data Interpretation: A Perspective. Anal. Chem. 2023, 95, 13733–13745. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Minzioni, P.; Hui, J.; Yun, S.; Yetisen, A.K. Fiber Optic Devices for Diagnostics and Therapy in Photomedicine. Adv. Opt. Mater. 2024, 12, 2400478. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhou, Y.; Zheng, W.; Sun, Y.; Ma, G.; Zhao, Y. Optical Fiber Optofluidic Bio-chemical Sensors: A Review. Laser Photon. Rev. 2021, 15, 2000526. [Google Scholar]
- Liu, X.; Miao, J.; Fan, Q.; Zhang, W.; Zuo, X.; Tian, M.; Zhu, S.; Zhang, X.; Qu, L. Recent Progress on Smart Fiber and Textile Based Wearable Strain Sensors: Materials, Fabrications and Applications. Adv. Fiber Mater. 2022, 4, 361–389. [Google Scholar]

| Main Feature | Scintillating Fiber Dosimeter | Optically Stimulated Luminescence Fiber Dosimeter | Thermoluminescent Dosimeter Fiber | Cherenkov Fiber Dosimeter |
|---|---|---|---|---|
| Detection Principle | Scintillation upon radiation exposure | Traps radiation energy, later released by optical stimulation | Traps radiation energy, released upon heating | Detects Cherenkov light emitted by high-energy charged particles |
| Real-time Readout | Yes | No (Delayed) | No (Delayed) | Yes |
| Radiation Type | Photons, electrons, protons, neutrons depending on scintillator | Mainly photons, some sensitivity to electrons | Wide spectrum depending on dopant | High-energy charged particles (e.g., electrons, photons) |
| Sensitivity | High (depending on material) | Moderate to high | High | Low to moderate |
| Key Materials | Plastic scintillator, LYSO, BGO, Gd2O2S | Al2O3:C, rare-earth doped materials | LiF, CaSO4:Dy | Optical fiber itself (e.g., PMMA or silica) |
| Advantages | Real-time, high sensitivity, flexible integration and geometries | Retrospective readout, reusable, simple structure | Established material base, thermal readout | Real-time, no added material needed, directly tied to dose delivery |
| Disadvantages | Cherenkov contamination (stem effect), fragility | Requires stimulation source, no real-time monitoring | Requires heating, mechanical setup, sensitivity to environmental noise | Low signal intensity, needs high-sensitivity detectors |
| Clinical Suitability | In vivo dosimetry, real-time monitoring during RT | QA, retrospective dose verification, environmental dosimetry | QA, treatment verification | Dose verification during LINAC, superficial monitoring |
| Technique | Principle | Target Information | Advantages | Limitations | Fiber Compatibility |
|---|---|---|---|---|---|
| Raman Spectroscopy | Inelastic scattering of monochromatic light reveals vibrational modes | Molecular composition (DNA, proteins, lipids) | Label-free; high chemical specificity; detects biochemical changes | Weak signal; fluorescence interference; slow acquisition | Excellent (single-mode fibers) |
| Fluorescence Spectroscopy | Emission from intrinsic or extrinsic fluorophores upon excitation | Metabolic activity (e.g., NADH, FAD); redox state | High sensitivity; real-time metabolic imaging | Requires fluorophores; photobleaching; limited depth | Excellent (multi-mode fibers) |
| Diffuse Reflectance Spectroscopy | Measured absorption and scattering of broadband light | Blood volume, oxygenation, scattering coefficients | Simple, low-cost; physiological parameters in real time | Indirect measurements; limited depth and resolution | Excellent |
| Cherenkov Luminescence Imaging | Light emitted by high-energy particles exceeding light speed in tissue | Dose deposition, real-time beam visualization | Direct correlation with radiation delivery; no added contrast needed | Shallow depth; weak signal; needs intensified/time-gated cameras | Moderate (collection via fiber) |
| Radiation-Induced Attenuation Spectroscopy | Ionizing radiation causing wavelength-dependent attenuation in fiber | Dose distribution, radiation field mapping | Passive; label-free; works in high-radiation fields; distributed sensing | Requires careful calibration; irreversible in some fiber types | Excellent (especially for distributed sensing) |
| Optical Coherence Tomography | Low-coherence interferometry for cross-sectional imaging | Tissue microstructure and morphology | High-resolution; depth-resolved structural imaging; non-contact | Limited penetration (~1–2 mm); less chemical specificity | Excellent (fiber bundles or probes) |
| Photoacoustic Imaging | Light-induced acoustic signal via thermoelastic expansion | Optical absorption contrast (e.g., hemoglobin) | Functional + structural imaging; deeper than optical-only methods | Requires laser source and ultrasound detector | Good (hybrid probes) |
| Laser Speckle Contrast Imaging | Analysis of dynamic speckle patterns from moving RBCs | Microvascular blood flow | Label-free; fast acquisition; sensitive to perfusion changes | Limited to surface vasculature; sensitive to motion artifacts | Good (endoscopic probes) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guang, Z.; He, C.; Bry, V.; Le, A.; DeMarco, J.; Chetty, I.J. Optical Fiber Sensing Technologies in Radiation Therapy. Photonics 2025, 12, 1058. https://doi.org/10.3390/photonics12111058
Guang Z, He C, Bry V, Le A, DeMarco J, Chetty IJ. Optical Fiber Sensing Technologies in Radiation Therapy. Photonics. 2025; 12(11):1058. https://doi.org/10.3390/photonics12111058
Chicago/Turabian StyleGuang, Zhe, Chuan He, Victoria Bry, Anh Le, John DeMarco, and Indrin J. Chetty. 2025. "Optical Fiber Sensing Technologies in Radiation Therapy" Photonics 12, no. 11: 1058. https://doi.org/10.3390/photonics12111058
APA StyleGuang, Z., He, C., Bry, V., Le, A., DeMarco, J., & Chetty, I. J. (2025). Optical Fiber Sensing Technologies in Radiation Therapy. Photonics, 12(11), 1058. https://doi.org/10.3390/photonics12111058





