Gold Nanoparticles at a Liquid Interface: Towards a Soft Nonlinear Metasurface
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemicals
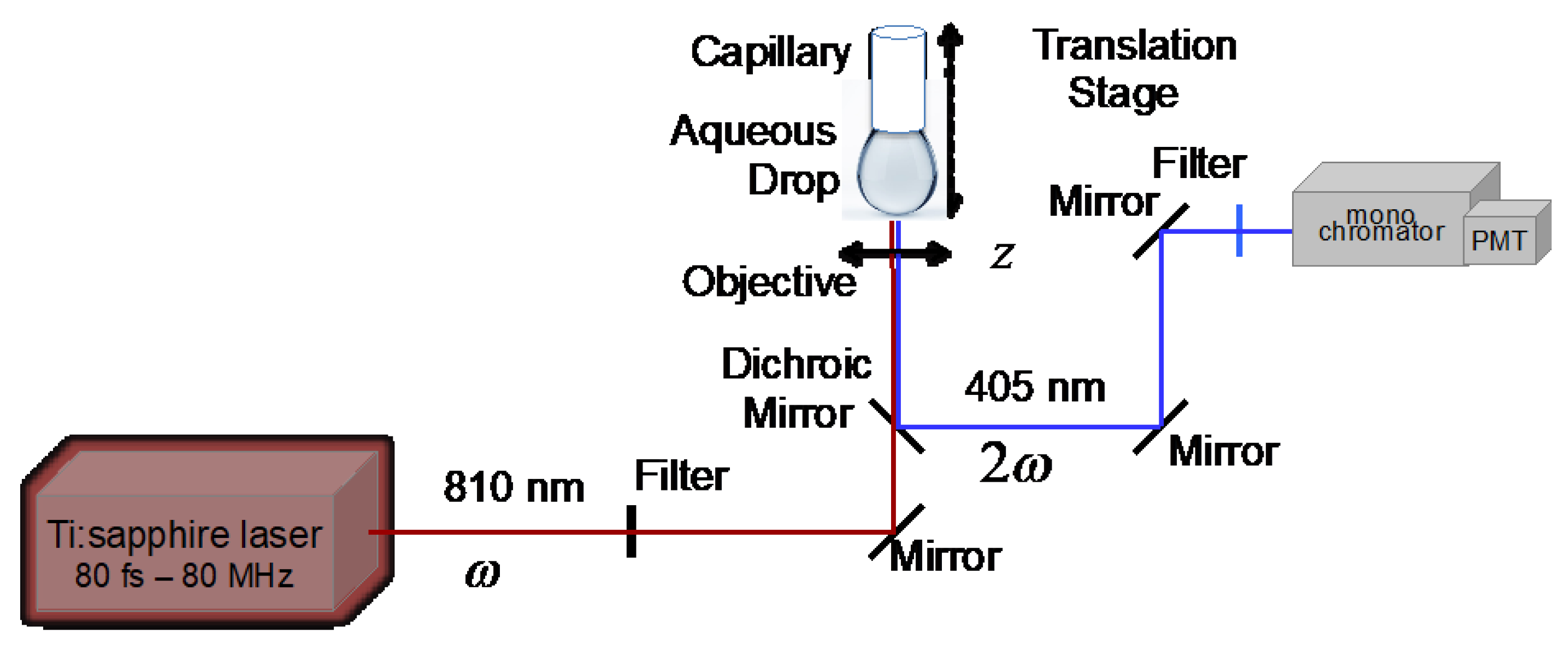
2.2. Acquisition of Longitudinal SHG Intensity Profiles at AuNP-Modified Aqueous Solution–Air Interfaces
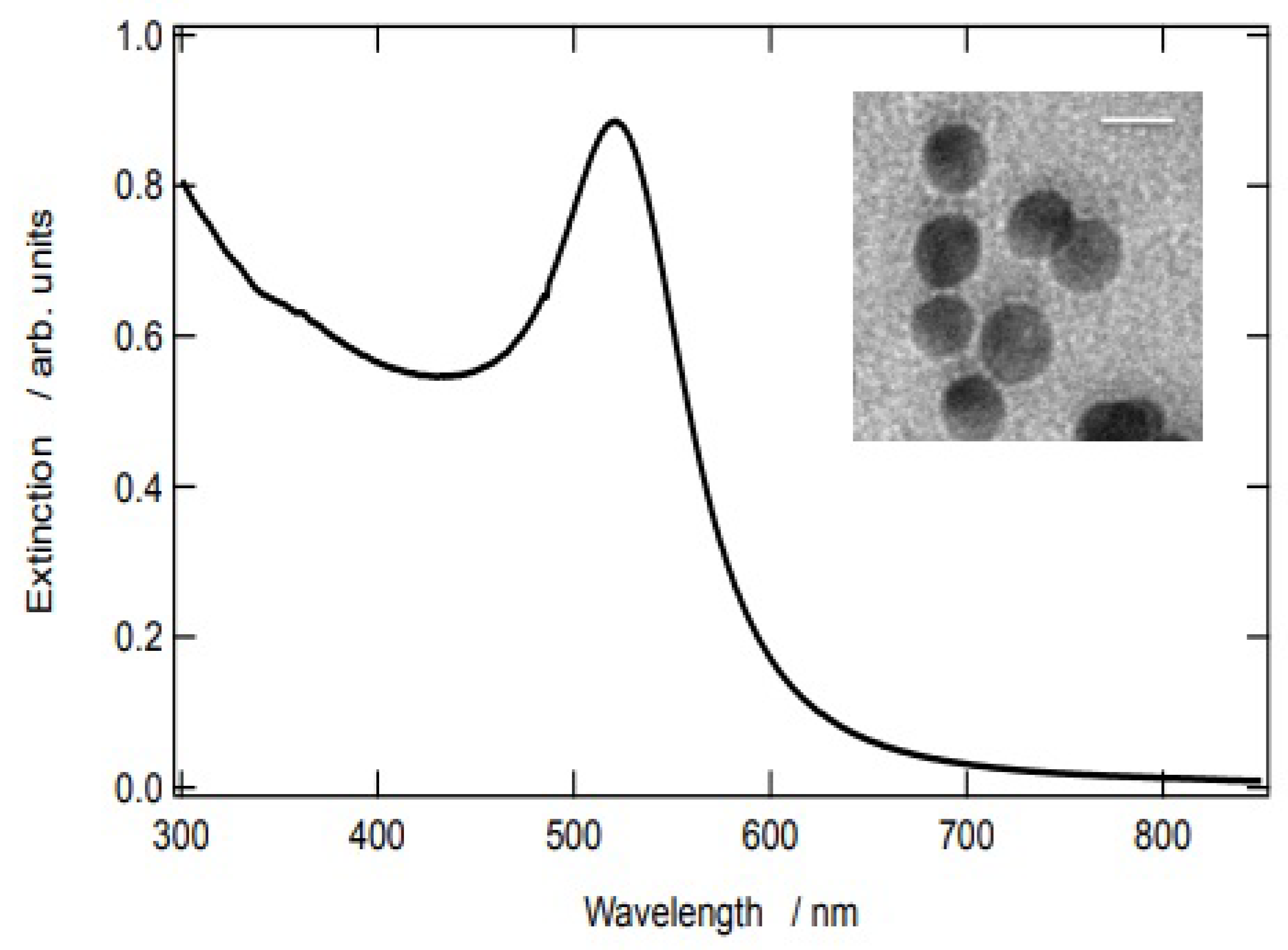
2.3. Synthesis and Characterization of AuNPs
3. Results and Discussion
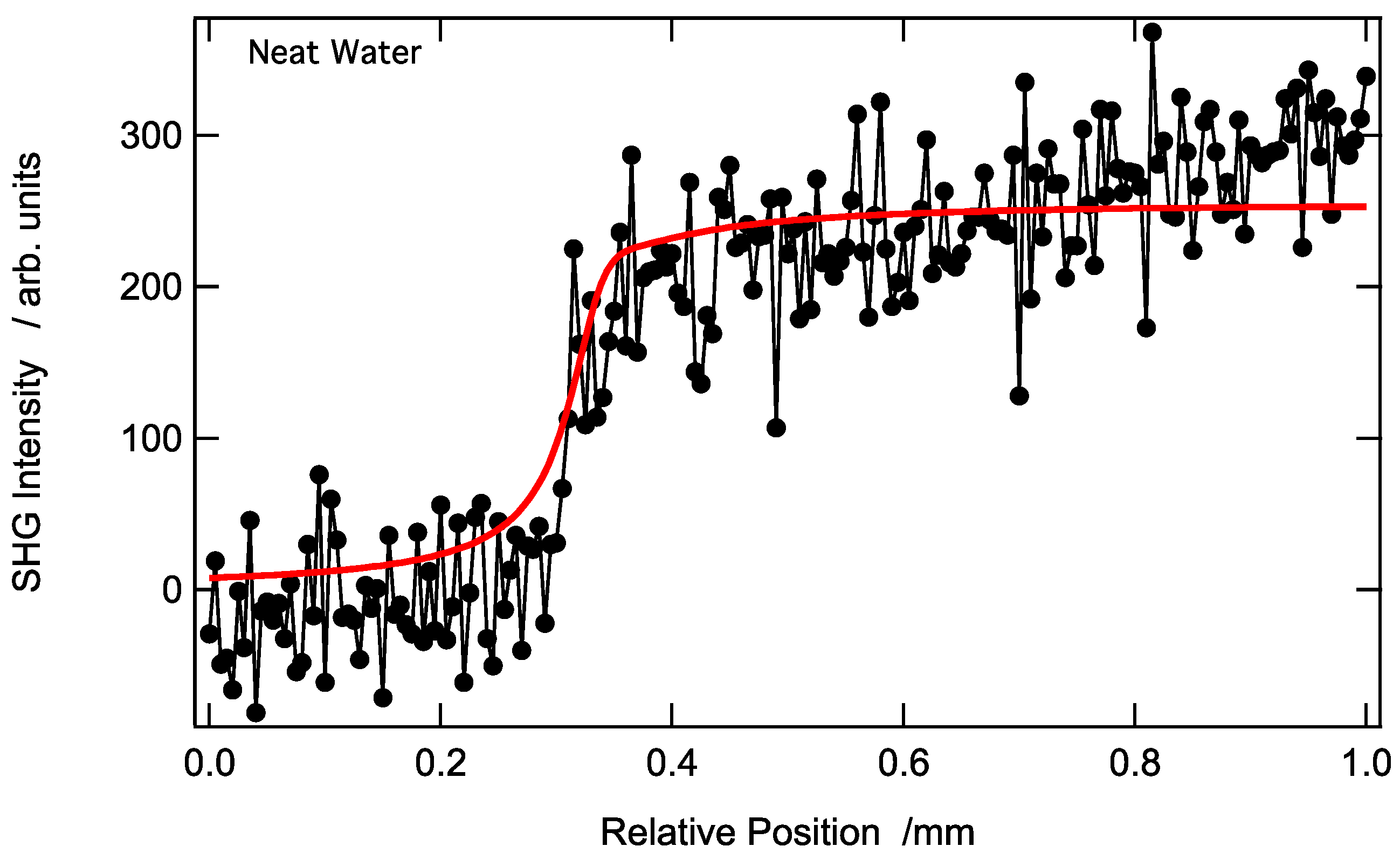
3.1. Longitudinal SHG Intensity Profile at a AuNP-Modified Aqueous Solution–Air Interface Prepared Using a Diluted Aqueous Solution of AuNPs
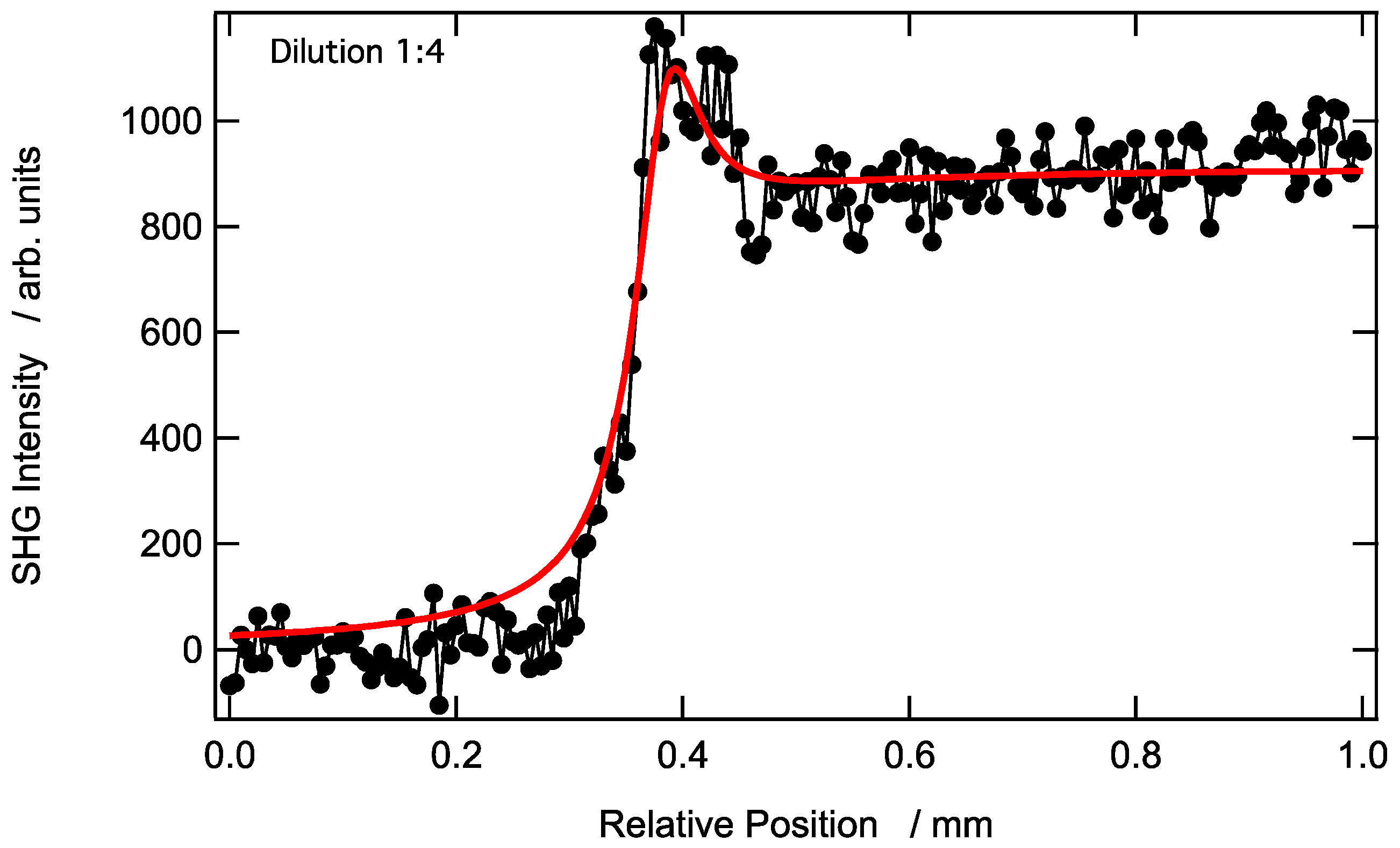
3.2. Longitudinal SHG Intensity Profile at a AuNP-Modified Aqueous Solution–Air Interface Prepared Using an Undiluted (Stock) Aqueous Solution of AuNPs
3.3. Theoretical Model to Describe SHG Intensity Generated at AuNP-Modified Aqueous Solution–Air Interfaces
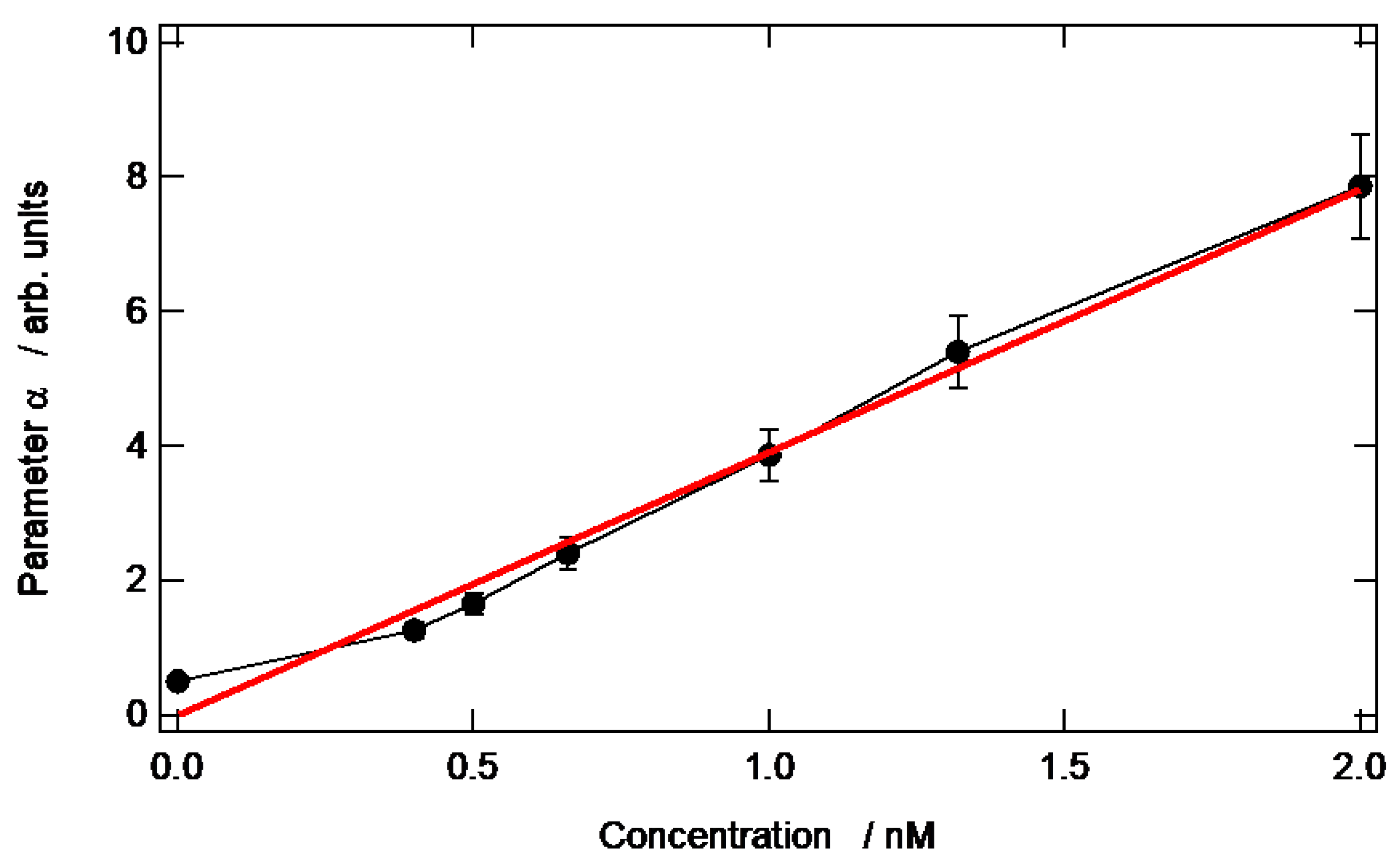
3.4. Evolution of the α Parameter as a Function of the AuNP Concentration in the Aqueous Droplet
3.5. The Limits of the Values Obtained from the Adjustment Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krasnok, A.; Tymchenko, M.; Alù, A. Nonlinear metasurfaces: A paradigm shift in nonlinear optics. Mater. Today 2018, 21, 8–21. [Google Scholar] [CrossRef]
- Walia, S.; Shah, C.; Gutruf, P.; Ahmadabadi, H.N.; Chowdhury, D.; Withayachumnnankul, W.; Bhaskaran, M.; Sriram, S. Flexible metasurfaces and metamaterials: A review of materials and fabrication processes at micro- and nano-scales. Appl. Phys. Rev. 2015, 2, 011303. [Google Scholar] [CrossRef]
- Samec, Z. Electrochemistry at the interface between two immiscible electrolyte solutions. Pure Appl. Chem. 2004, 76, 2147–2180. [Google Scholar] [CrossRef]
- Girault, H.H. Electroanalytical Chemistry, A Series of Advances; Bard, A.J., Zoski, C.G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; Volume 23, pp. 1–104, 5 Character 1. [Google Scholar]
- Higgins, D.A.; Corn, R.M. Second harmonic generation studies of adsorption at a liquid-liquid electrochemical interface. J. Phys. Chem. 1993, 97, 489–493. [Google Scholar] [CrossRef]
- Ruiz-Lopez, M.F.; Francisco, J.S.; Martins-Costa, M.T.C.; Anglada, J.M. Molecular reactions at aqueous interfaces. Nat. Rev. Chem. 2020, 4, 459. [Google Scholar] [CrossRef]
- Bera, M.K.; Chan, H.; Moyano, D.F.; Yu, H.; Tatur, S.; Amoanu, D.; Bu, W.; Rotello, V.M.; Meron, M.; Kral, P.; et al. Interfacial localization and voltage-tunable arrays of charged nanoparticles. Nano Lett. 2014, 14, 6816–6822. [Google Scholar] [CrossRef]
- Scanlon, M.D.; Smirnov, E.; Stockmann, T.J.; Peljo, P. Gold Nanofilms at Liquid−Liquid Interfaces: An Emerging Platform for Redox Electrocatalysis, Nanoplasmonic Sensors, and Electrovariable Optics. Chem. Rev. 2018, 118, 3722–3751. [Google Scholar] [CrossRef]
- Abid, J.-P.; Abid, M.; Bauer, C.; Girault, H.H.; Brevet, P.-F. Controlled Reversible Adsorption of Core-Shell Metallic Nanoparticles at the Polarized Water/1,2-Dichloroethane Interface Investigated by Optical Second-Harmonic Generation. J. Phys. Chem. C 2007, 111, 8849–8855. [Google Scholar] [CrossRef]
- Flatte, M.E.; Kornyshev, A.A.; Urbakh, M. Understanding voltage-induced localization of nanoparticles at a liquid–liquid interface. J. Phys. Condens. Matter 2008, 20, 073102. [Google Scholar] [CrossRef]
- Flatte, M.E.; Kornyshev, A.A.; Urbakh, M. Electrovariable Nanoplasmonics and Self-Assembling Smart Mirrors. J. Phys. Chem. C 2010, 114, 1735–1747. [Google Scholar] [CrossRef]
- Opallo, M.; Dusilo, K.; Warczak, M.; Kalisz, J. Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction at Polarizable Liquid|Liquid Interfaces. ChemElectroChem 2022, 9, e202200513. [Google Scholar] [CrossRef]
- Ozel, F.; Aslan, E.; Sarilmaz, A.; Patir, I.H. Hydrogen Evolution Catalyzed by Cu2WS4 at Liquid-Liquid Interfaces. ACS Appl. Mater. Interfaces 2016, 8, 25881–25887. [Google Scholar] [CrossRef] [PubMed]
- Olaya, A.J.; Schaming, D.; Brevet, P.-F.; Nagatani, H.; Zimmermann, T.; Vanicek, J.; Xu, H.-J.; Gros, C.P.; Barbe, J.-M.; Girault, H.H. Self-Assembled Molecular Rafts at Liquid|Liquid Interfaces for Four-Electron Oxygen Reduction. J. Am. Chem. Soc. 2012, 134, 498–506. [Google Scholar] [CrossRef]
- Aslan, E.; Patir, I.H. Catalysis of hydrogen evolution reaction by in situ electrodeposited amorphous molybdenum sulfide at soft interfaces. Mater. Today Energy 2021, 21, 100742. [Google Scholar] [CrossRef]
- Xuan, Y.; Huang, X.; Su, B. Biomimetic Oxygen Reduction Reaction Catalyzed by Microperoxidase-11 at Liquid/Liquid Interfaces. J. Phys. Chem. C 2015, 119, 11685–11693. [Google Scholar] [CrossRef]
- Huang, C.; Cui, M.; Sun, Z.; Liu, F.; Helms, B.A.; Russel, T.P. Self-Regulated Nanoparticle Assembly at Liquid/Liquid Interfaces: A Route to Adaptive Structuring of Liquids. Langmuir 2017, 33, 7994–8001. [Google Scholar] [CrossRef]
- Hojeij, M.; Younan, N.; Ribeaucourt, L.; Girault, H.H. Surface plasmon resonance of gold nanoparticles assemblies at liquid|liquid interfaces. Nanoscale 2010, 2, 1665–1669. [Google Scholar] [CrossRef][Green Version]
- Schaming, D.; Hojeij, M.; Younan, N.; Nagatani, H.; Lee, H.J.; Girault, H.H. Photocurrents at polarized liquid|liquid interfaces enhanced by a gold nanoparticle film. Phys. Chem. Chem. Phys. 2011, 13, 17704–17711. [Google Scholar] [CrossRef][Green Version]
- Zaera, F. Surface chemistry at the liquid/solid interface. Surf. Sci. 2011, 605, 1141–1145. [Google Scholar] [CrossRef]
- Eisenthal, K.B. Liquid Interfaces Probed by Second-Harmonic and Sum-Frequency Spectroscopy. Chem. Rev. 1996, 96, 1343–1360. [Google Scholar] [CrossRef]
- Tadjeddine, A.; Pluchery, O.; Le Rille, A.; Humbert, C.; Buck, M.; Peremans, A.; Zheng, W.Q. What can we learn from the non-linear optical investigation of the liquid|solid interface? J. Electroanal. Chem. 1999, 473, 25–33. [Google Scholar] [CrossRef]
- Liu, J.; Subir, M.; Nguyen, K.; Eisenthal, K.B. Second harmonic studies of ions crossing liposome membranes in real time. J. Phys. Chem. B 2008, 112, 15263–15266. [Google Scholar] [CrossRef]
- Roke, S. Nonlinear Optical Spectroscopy of Soft Matter Interfaces. Chem. Phys. Chem. 2009, 10, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Corn, R.M.; Higgins, D.A. Optical second harmonic generation as a probe of surface chemistry. Chem. Rev. 1994, 94, 107–125. [Google Scholar] [CrossRef]
- Brevet, P.F. Surface Second Harmonic Generation; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 1997. [Google Scholar]
- Clays, K.; Persoons, A. Hyper-Rayleigh scattering in solution. Phys. Rev. Lett. 1991, 66, 2980–2983. [Google Scholar] [CrossRef]
- Zyss, J.; Van, T.C.; Dhenaut, C.; Ledoux, I. Harmonic rayleigh scattering from nonlinear octupolar molecular media: The case of crystal violet. Chem. Phys. 1993, 177, 281–296. [Google Scholar] [CrossRef]
- Nagatani, H.; Piron, A.; Brevet, P.-F.; Fermín, D.J.; Girault, H.H. Surface Second Harmonic Generation of Cationic Water-Soluble Porphyrins at the Polarized Water/1,2-Dichloroethane Interface. Langmuir 2002, 18, 6647–6652. [Google Scholar] [CrossRef]
- Nagatani, H.; Samec, Z.; Brevet, P.-F.; Fermín, D.J.; Girault, H.H. Adsorption and Aggregation of meso-Tetrakis(4-carboxyphenyl)porphyrinato Zinc(II) at the Polarized Water|1,2-Dichloroethane Interface. J. Phys. Chem. B 2003, 107, 786–790. [Google Scholar] [CrossRef]
- Antoine, R.; Tamburello-Luca, A.A.; Hébert, P.; Brevet, P.F.; Girault, H.H. Picosecond dynamics of Eosin B at the air/water interface by time-resolved second harmonic generation: Orientational randomization and rotational relaxation. Chem. Phys. Lett. 1998, 288, 138–146. [Google Scholar] [CrossRef]
- Piron, A.; Brevet, P.F.; Girault, H.H. Surface second harmonic generation monitoring of the anion methyl orange during ion transfer reactions across a polarised water∣1,2-dichloroethane interface. J. Electroanal. Chem. 2000, 483, 29–36. [Google Scholar] [CrossRef]
- Rinuy, J.; Piron, A.; Brevet, P.F.; Blanchard-Desce, M.; Girault, H.H. Intramolecular Electron Density Redistribution Upon Hydrogen Bond Formation in the Anion Methyl Orange at the Water/1,2-Dichloroethane Interface Probed by Phase Interference Second Harmonic Generation. Chem. Eur. J. 2000, 6, 3434–3441. [Google Scholar] [CrossRef]
- Punzi, A.; Martin-Gassin, G.; Grilj, J.; Vauthey, E. Effect of Salt on the Excited-State Dynamics of Malachite Green in Bulk Aqueous Solutions and at Air/Water Interfaces: A Femtosecond Transient Absorption and Surface Second Harmonic Generation Study. J. Phys. Chem. C 2009, 113, 11822–11829. [Google Scholar] [CrossRef]
- Guyot-Sionnest, P.; Shen, Y.R. Local and nonlocal surface nonlinearities for surface optical second-harmonic generation. Phys. Rev. B 1987, 35, 4420–4426. [Google Scholar] [CrossRef] [PubMed]
- Dadap, J.I.; Shan, J.; Eisenthal, K.B.; Heinz, T.F. Second-Harmonic Rayleigh Scattering from a Sphere of Centrosymmetric Material. Phys. Rev. Lett. 1999, 83, 4045–4048. [Google Scholar] [CrossRef]
- Tsuboi, K.; Kajikawa, K. Arrays of microdots of gold nanoparticles immobilized above gold surface probed by optical second-harmonic microscopy. Appl. Phys. Lett. 2006, 88, 103102–103103. [Google Scholar] [CrossRef]
- Nappa, J.; Revillod, G.; Russier-Antoine, I.; Benichou, E.; Jonin, C.; Brevet, P.F. Electric dipole origin of the second harmonic generation of small metallic particles. Phys. Rev. B 2005, 71, 165407. [Google Scholar] [CrossRef]
- El Harfouch, Y.; Benichou, E.; Bertorelle, F.; Russier-Antoine, I.; Jonin, C.; Lascoux, N.; Brevet, P.-F. Effect of a thioalkane capping layer on the first hyperpolarizabilities of gold and silver nanoparticles. J. Phys. Condens. Matter 2012, 24, 124104. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J.J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Vance, F.W.; Lemon, B.I.; Hupp, J.T. Enormous Hyper-Rayleigh Scattering from Nanocrystalline Gold Particle Suspensions. J. Phys. Chem. B 1998, 102, 10091–10093. [Google Scholar] [CrossRef]
- Russier-Antoine, I.; Jonin, C.; Nappa, J.; Benichou, E.; Brevet, P.F. Wavelength dependence of the hyper Rayleigh scattering response from gold nanoparticles. J. Chem. Phys. 2004, 120, 10748–10752. [Google Scholar] [CrossRef]
- Boyd, R.W. Nonlinear Optics; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Behel, Z.; Mugnier, Y.; Le Dantec, R.; Chevolot, Y.; Monnier, V.; Brevet, P.F. Controlled Second Harmonic Generation with Optically Trapped Lithium Niobate Nanoparticles. Nano Lett. 2024, 24, 5699–5704. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, W.; Cheng, S.; Zhang, H.; Yi, Z.; Sun, T.; Wu, P.; Zeng, Q.; Raza, R. Tunable metamaterial absorption device based on Fabry–Perot resonance as temperature and refractive index sensing. Opt. Lasers Eng. 2024, 181, 108368. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaming, D.; Maurice, A.; Gumy, F.; Scanlon, M.D.; Jonin, C.; Girault, H.H.; Brevet, P.-F. Gold Nanoparticles at a Liquid Interface: Towards a Soft Nonlinear Metasurface. Photonics 2024, 11, 789. https://doi.org/10.3390/photonics11090789
Schaming D, Maurice A, Gumy F, Scanlon MD, Jonin C, Girault HH, Brevet P-F. Gold Nanoparticles at a Liquid Interface: Towards a Soft Nonlinear Metasurface. Photonics. 2024; 11(9):789. https://doi.org/10.3390/photonics11090789
Chicago/Turabian StyleSchaming, Delphine, Anthony Maurice, Frédéric Gumy, Micheál D. Scanlon, Christian Jonin, Hubert H. Girault, and Pierre-François Brevet. 2024. "Gold Nanoparticles at a Liquid Interface: Towards a Soft Nonlinear Metasurface" Photonics 11, no. 9: 789. https://doi.org/10.3390/photonics11090789
APA StyleSchaming, D., Maurice, A., Gumy, F., Scanlon, M. D., Jonin, C., Girault, H. H., & Brevet, P.-F. (2024). Gold Nanoparticles at a Liquid Interface: Towards a Soft Nonlinear Metasurface. Photonics, 11(9), 789. https://doi.org/10.3390/photonics11090789





