A Terahertz Point Source Meta-Sensor in Reflection Mode for Trace-Amount Bio-Sensing Applications
Abstract
1. Introduction
2. Materials and Methods
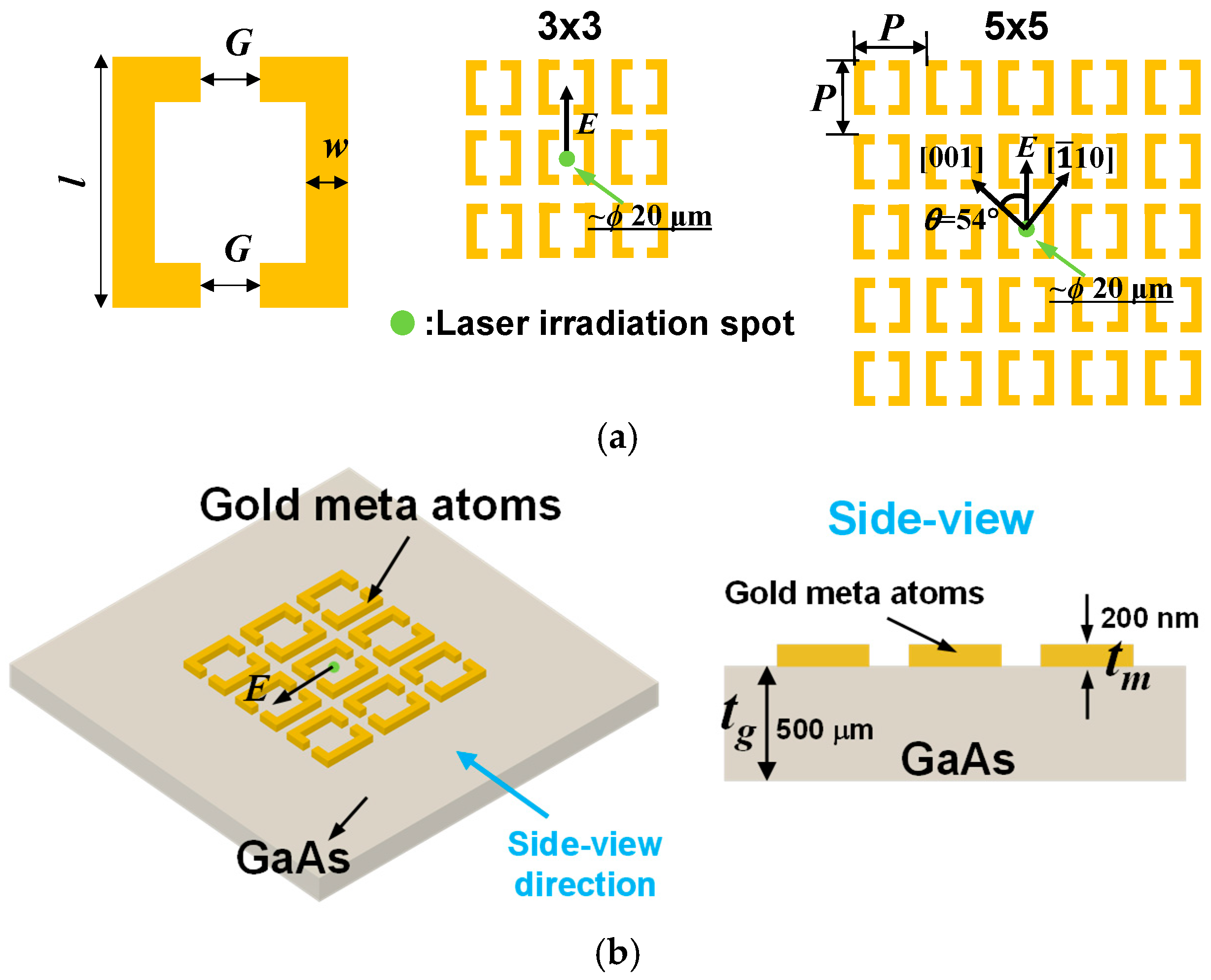
2.1. Meta-Atom Structure Arrangement and Fabrication
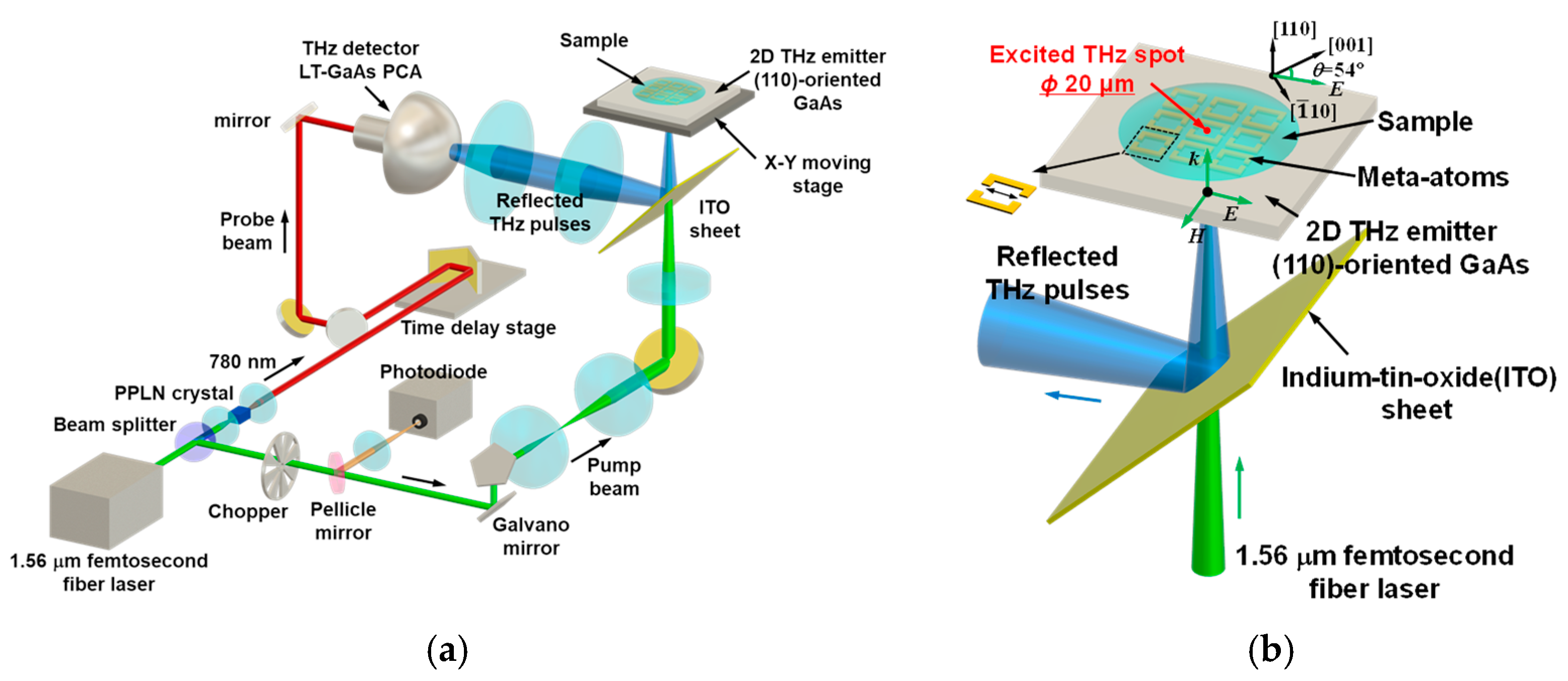
2.2. Experimental Setup
2.3. Sample Measurement
3. Results and Discussion
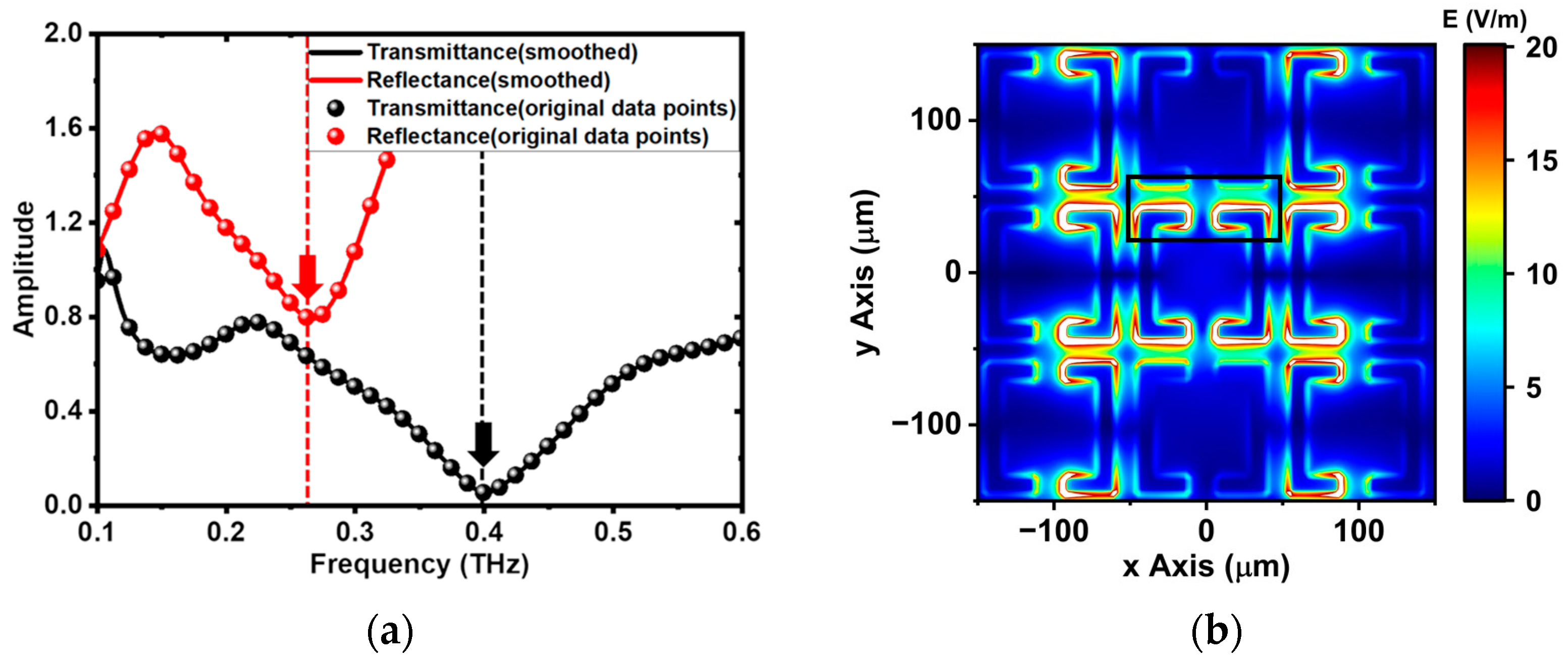
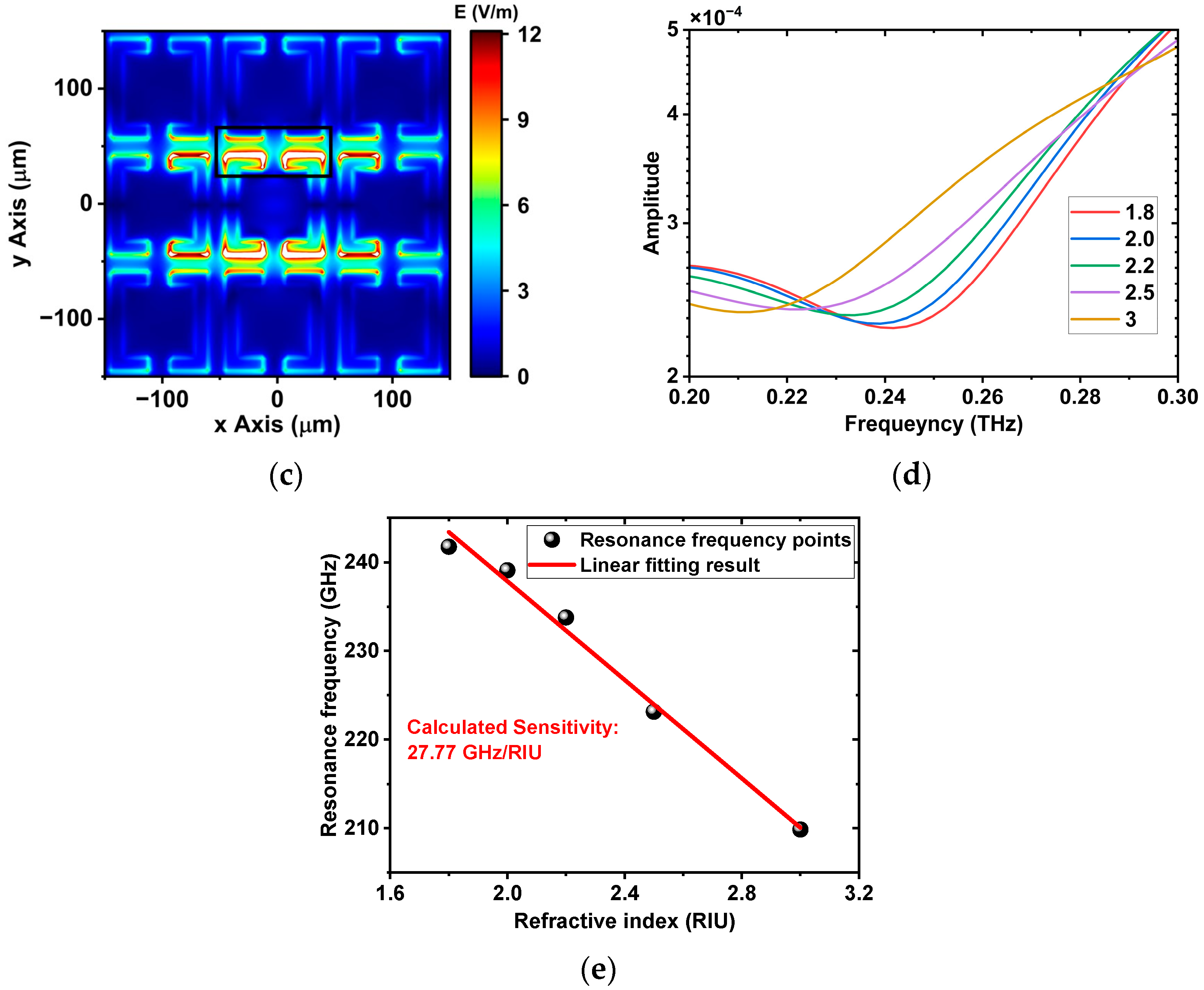
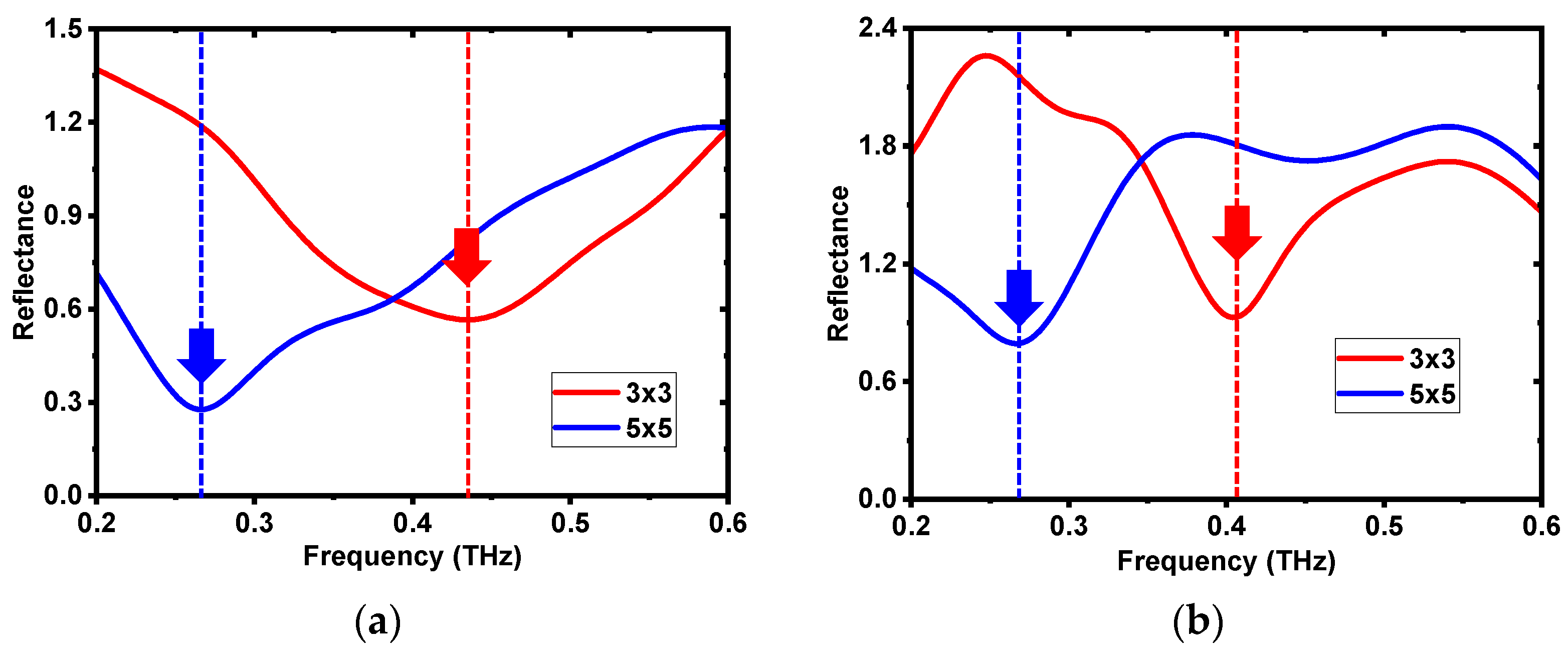
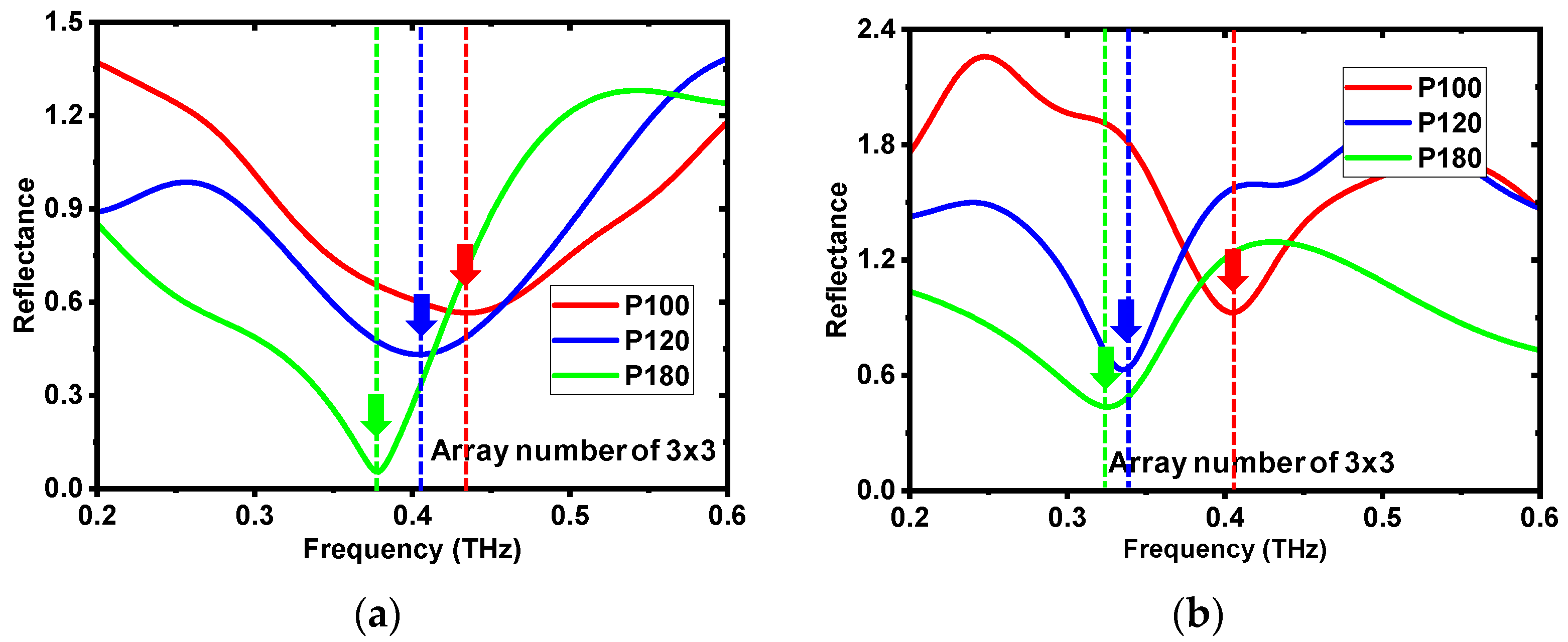
3.1. Comparison between Experiments and Simulation
3.2. Applications
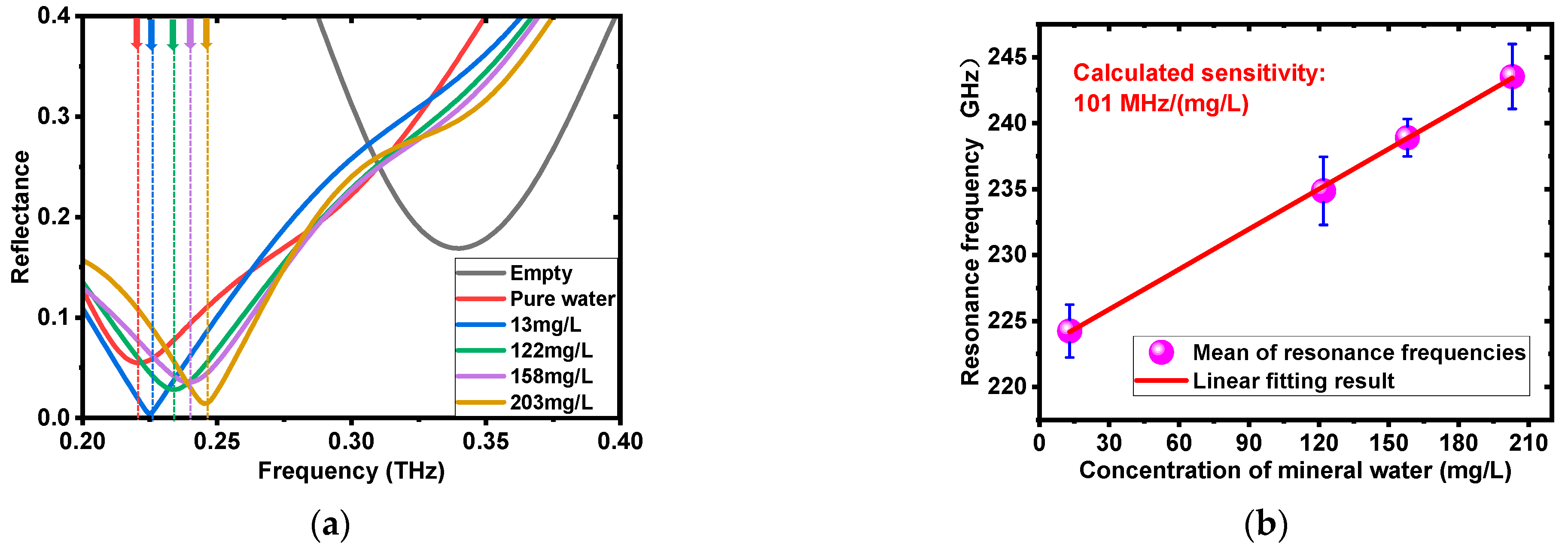
3.2.1. Mineral Water Measurement
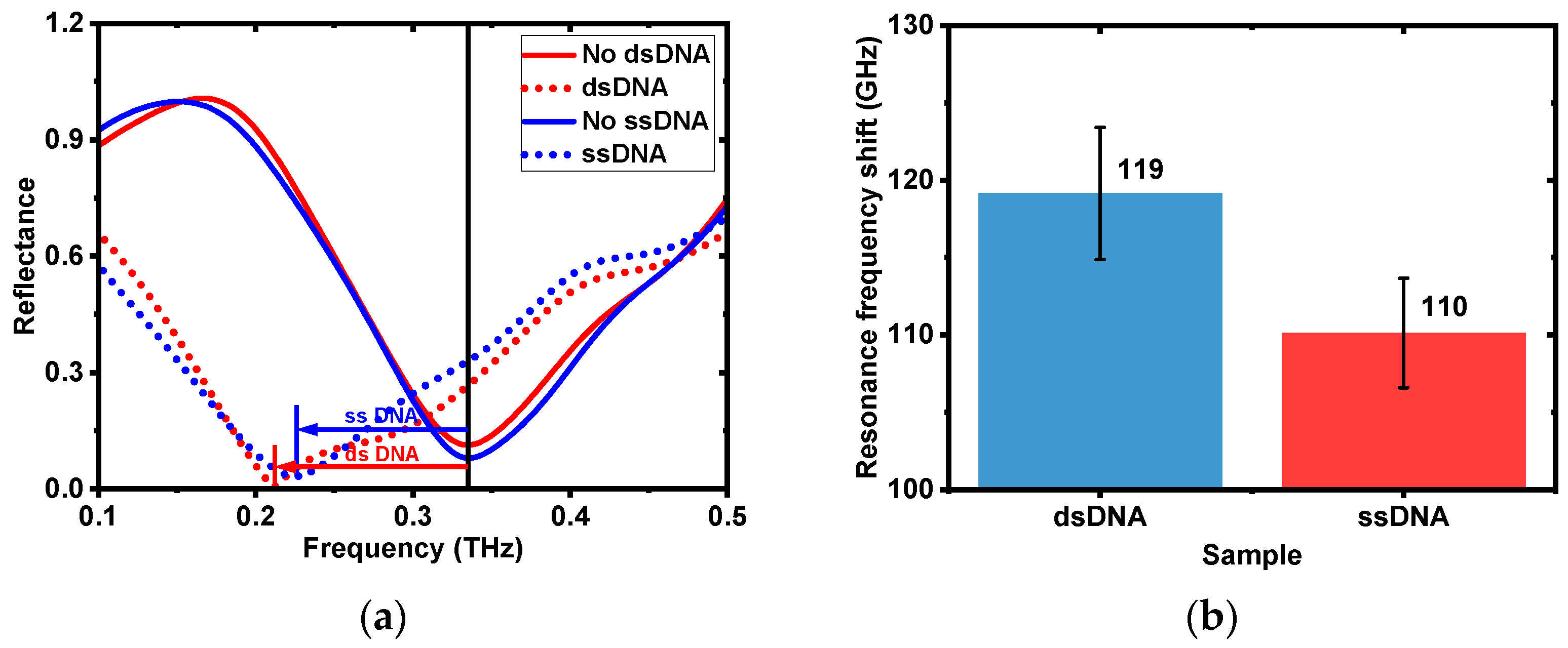
3.2.2. dsDNA and ssDNA Analysis
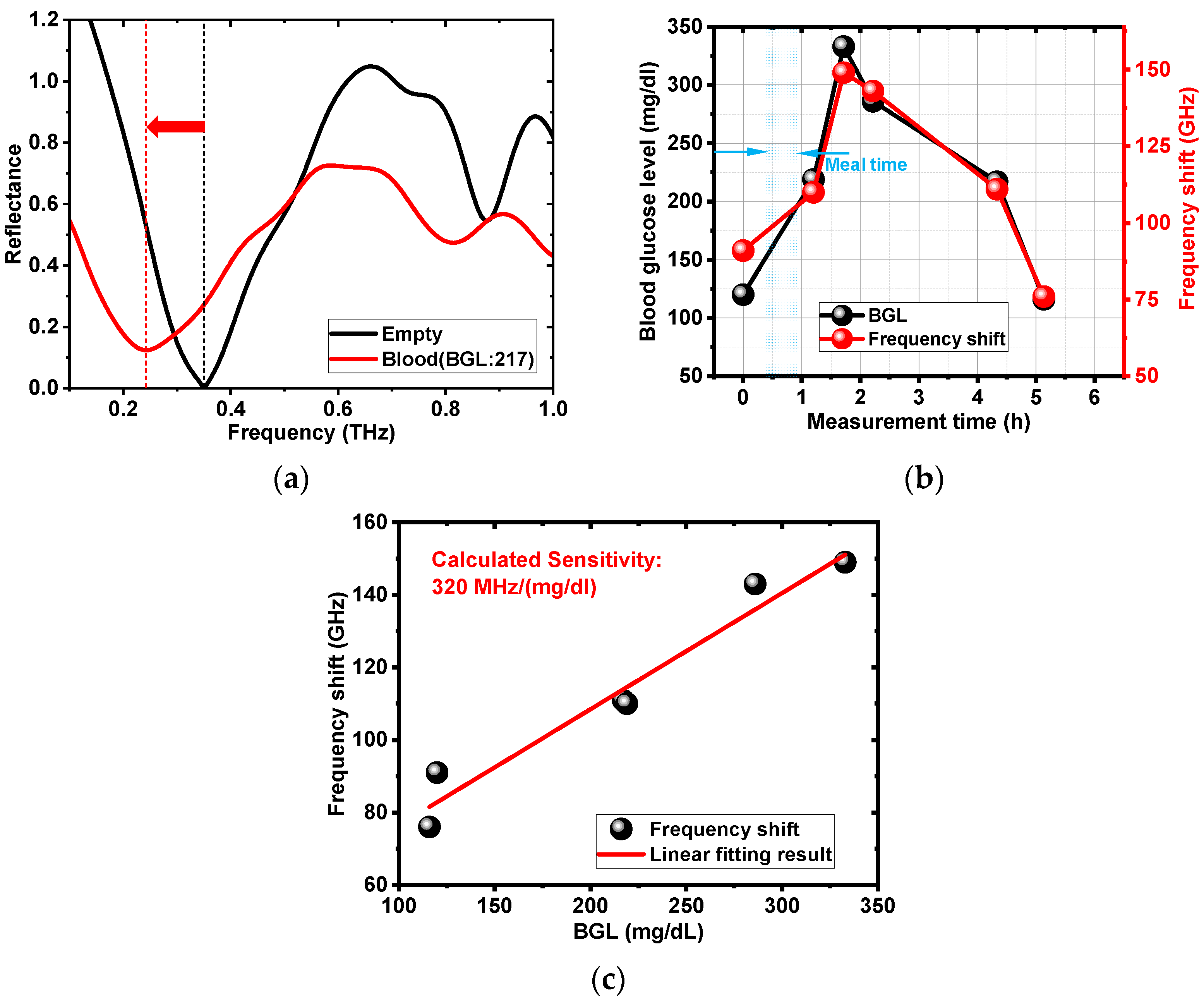
3.2.3. Blood Glucose Level Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leitenstorfer, A.; Moskalenko, A.S.; Kampfrath, T.; Kono, J.; Castro-Camus, E.; Peng, K.; Qureshi, N.; Turchinovich, D.; Tanaka, K.; Markelz, A.G.; et al. The 2023 Terahertz Science and Technology Roadmap. J. Phys. D Appl. Phys. 2023, 56, 223001. [Google Scholar] [CrossRef]
- Nagel, M.; Först, M.; Kurz, H. THz biosensing devices: Fundamentals and technology. J. Phys. Condens. Matter. 2006, 18, S601–S608. [Google Scholar] [CrossRef]
- Son, J.H. Terahertz Biomedical Science and Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 153–173. [Google Scholar]
- Pickwell, E.; Wallace, V.P. Biomedical applications of terahertz technology. J. Phys. D Appl. Phys. 2006, 39, R301–R310. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; He, Y.; Liu, K.; Fan, S.; Parrott, E.P.; Pickwell-MacPherson, E. Recent advances in terahertz technology for biomedical applications. Quant. Imaging Med. Surg. 2017, 7, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Oh, S.J.; Cheon, H. Potential clinical applications of terahertz radiation. J. Appl. Phys. 2019, 125, 190901. [Google Scholar] [CrossRef]
- Jepsen, P.U.; Cooke, D.G.; Koch, M. Terahertz spectroscopy and imaging–Modern techniques and applications. Laser Photon. Rev. 2011, 5, 124–166. [Google Scholar] [CrossRef]
- Heugen, U.; Schwaab, G.; Bründermann, E.; Heyden, M.; Yu, X.; Leitner, D.M.; Havenith, M. Solute-induced retardation of water dynamics probed directly by terahertz spectroscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, T.; Nagai, M.; Tanaka, K. Characterizing hydration state in solution using terahertz time-domain attenuated total reflection spectroscopy. Chem. Phys. Lett. 2008, 457, 12–17. [Google Scholar] [CrossRef]
- Thrane, L.; Jacobsen, R.H.; Jepsen, P.U.; Keiding, S.R. THz reflection spectroscopy of liquid water. Chem. Phys. Lett. 1995, 240, 330–333. [Google Scholar] [CrossRef]
- Fischer, B.M.; Walther, M.; Jepsen, P.U. Far-infrared vibrational modes of DNA components studied by terahertz time-domain spectroscopy. Phys. Med. Biol. 2002, 47, 3807. [Google Scholar] [CrossRef] [PubMed]
- Niessen, K.A.; Xu, M.; George, D.K.; Chen, M.C.; Ferré-D’Amaré, A.R.; Snell, E.H.; Cody, V.; Pace, J.; Schmidt, M.; Markelz, A.G. Protein and RNA dynamical fingerprinting. Nat. Commun. 2019, 10, 1026. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Serita, K.; Kitagishi, K.; Murakami, H.; Zhang, Z.H.; Tonouchi, M. Terahertz spectroscopy tracks proteolysis by a joint analysis of absorptance and Debye model. Biophys. J. 2020, 119, 2469–2482. [Google Scholar] [CrossRef] [PubMed]
- Landy, N.I.; Sajuyigbe, S.; Mock, J.J.; Smith, D.R.; Padilla, W.J. Perfect metamaterial absorber. Phys. Rev. Lett. 2008, 100, 207402. [Google Scholar] [CrossRef] [PubMed]
- Padilla, W.J.; Taylor, A.J.; Highstrete, C.; Lee, M.; Averitt, R.D. Dynamical electric and magnetic metamaterial response at terahertz frequencies. Phys. Rev. Lett. 2006, 96, 107401. [Google Scholar] [CrossRef] [PubMed]
- Withayachumnankul, W.; Abbott, D. Metamaterials in the terahertz regime. IEEE Photon. J. 2009, 1, 99–118. [Google Scholar] [CrossRef]
- Chen, H.T.; Padilla, W.J.; Zide, J.M.; Gossard, A.C.; Taylor, A.J.; Averitt, R.D. Active terahertz metamaterial devices. Nature 2006, 444, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, W.; Cheng, S.; Zhang, H.; Yi, Z.; Sun, T.; Wu, P.; Zeng, Q.; Raza, R. Tunable metamaterial absorption device based on Fabry–Perot resonance as temperature and refractive index sensing. Opt. Lasers Eng. 2024, 181, 108368. [Google Scholar] [CrossRef]
- Cao, T.; Lian, M.; Chen, X.; Mao, L.; Liu, K.; Jia, J.; Su, Y.; Ren, H.; Zhang, S.; Xu, Y.; et al. Multi-Cycle Reconfigurable THz Extraordinary Optical Transmission Using Chalcogenide Metamaterials. Opto-Electron. Sci. 2021, 1, 210010. [Google Scholar] [CrossRef]
- RoyChoudhury, S.; Rawat, V.; Jalal, A.H.; Kale, S.N.; Bhansali, S. Recent advances in metamaterial split-ring-resonator circuits as biosensors and therapeutic agents. Biosens. Bioelectron. 2016, 86, 595–608. [Google Scholar] [CrossRef]
- Xu, W.; Xie, L.; Ying, Y. Mechanisms and applications of terahertz metamaterial sensing: A review. Nanoscale 2017, 9, 13864–13878. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Gupta, N. Applications of metamaterial sensors: A review. Int. J. Microw. Wirel. Technol. 2022, 14, 19–33. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, J.; Yuan, Z.; Lin, Y.; Shang, W.; Chin, L.K.; Zhang, M. Terahertz Metamaterials for Biosensing Applications: A Review. Biosensors 2023, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Sium, F.S.; Islam, F.; Choudhury, S.M. A review on plasmonic and metamaterial based biosensing platforms for virus detection. Sens. Bio-Sens. Res. 2021, 33, 100429. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.; Vafapour, Z. Sensing avian influenza viruses using terahertz metamaterial reflector. IEEE Sens. J. 2019, 19, 5161–5166. [Google Scholar] [CrossRef]
- Lee, D.K.; Kang, J.H.; Kwon, J.; Lee, J.S.; Lee, S.; Woo, D.H.; Kim, J.H.; Song, C.; Park, Q.; Seo, M. Nano metamaterials for ultrasensitive Terahertz biosensing. Sci. Rep. 2017, 7, 8146. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z.; Kaushik, A.; Manickam, P.; Ghoreishi, S.A. Functionalized terahertz plasmonic metasensors: Femtomolar-level detection of SARS-CoV-2 spike proteins. Biosens. Bioelectron. 2021, 177, 112971. [Google Scholar] [CrossRef] [PubMed]
- Berrier, A.; Schaafsma, M.C.; Nonglaton, G.; Bergquist, J.; Rivas, J.G. Selective detection of bacterial layers with terahertz plasmonic antennas. Biomed. Opt. Express 2012, 3, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, K.; Luo, Y.; Fu, W. Terahertz spectroscopy for bacterial detection: Opportunities and challenges. Appl. Microbiol. Biotechnol. 2016, 100, 5289–5299. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Hong, J.T.; Choi, S.J.; Kim, H.S.; Park, W.K.; Han, S.T.; Park, J.Y.; Lee, S.; Kim, D.S.; Ahn, Y.H. Detection of microorganisms using terahertz metamaterials. Sci. Rep. 2014, 4, 4988. [Google Scholar] [CrossRef] [PubMed]
- Bhati, R.; Malik, A.K. Multiband terahertz metamaterial perfect absorber for microorganisms detection. Sci. Rep. 2023, 13, 19685. [Google Scholar] [CrossRef]
- Hasebe, T.; Kawabe, S.; Matsui, H.; Tabata, H. Metallic mesh-based terahertz biosensing of single-and double-stranded DNA. J. Appl. Phys. 2012, 112, 094702. [Google Scholar] [CrossRef]
- Weisenstein, C.; Richter, M.; Wigger, A.K.; Bosserhoff, A.K.; Haring Bolívar, P. Multifrequency investigation of single-and double-stranded DNA with scalable metamaterial-based THz biosensors. Biosensors 2022, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, C.; Huang, Y.; Huang, K.; Wang, Y.; Xu, W.; Xie, L.; Ying, Y. Label-free terahertz microfluidic biosensor for sensitive DNA detection using graphene-metasurface hybrid structures. Biosens. Bioelectron. 2021, 188, 113336. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Ogawa, Y.; Kawai, Y.; Hayashi, S.; Hayashi, A.; Otani, C.; Kato, E.; Miyamaru, F.; Kawase, K. Terahertz sensing method for protein detection using a thin metallic mesh. Appl. Phys. Lett. 2007, 91, 253901. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Hu, F.; Li, D.; Teng, H.; Rong, Q.; Zhang, W.; Han, J.; Liang, H. Four resonators based high sensitive terahertz metamaterial biosensor used for measuring concentration of protein. J. Phys. D 2019, 52, 095105. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, F.; Lang, T.; Liu, J.; Hong, Z.; Qin, J. All-metal terahertz metamaterial biosensor for protein detection. Nanoscale Res. Lett. 2021, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Elhelw, A.R.; Ibrahim, M.S.S.; Rashed, A.N.Z.; Mohamed, A.E.N.A.; Hameed, M.F.O.; Obayya, S.S.A. Highly Sensitive Triple-Band THz Metamaterial Biosensor for Cancer Cell Detection. IEEE Photonics J. 2023, 15, 3700113. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, N.; Liu, L.; Xie, J.; Feng, H.; Yao, J.; Chen, T.; Zhu, W. Highly sensitive detection of malignant glioma cells using metamaterial-inspired THz biosensor based on electromagnetically induced transparency. Biosens. Bioelectron. 2021, 185, 113241. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, M.; Zhang, Z.; Liang, L.; Wei, D.; Wang, M.; Zhang, M.; Wang, T.; Liu, L.; Xie, J.; et al. The terahertz electromagnetically induced transparency-like metamaterials for sensitive biosensors in the detection of cancer cells. Biosens. Bioelectron. 2019, 126, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, X.; Li, T.; Li, Y.; Tian, Z.; Wang, M. Highly sensitive terahertz metamaterial biosensor for bovine serum albumin (BSA) detection. Opt. Mater. Express 2021, 11, 2268–2277. [Google Scholar] [CrossRef]
- Li, D.; Hu, F.; Zhang, H.; Chen, Z.; Huang, G.; Tang, F.; Lin, S.; Zou, Y.; Zhou, Y. Identification of early-stage cervical cancer tissue using metamaterial terahertz biosensor with two resonant absorption frequencies. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 8600107. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, Z.; Fang, W. Exploring performance of THz metamaterial biosensor based on flexible thin-film. Opt. Express 2020, 28, 26370–26384. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choe, J.H.; Kim, C.; Bae, S.; Kim, J.S.; Park, Q.H.; Seo, M. Graphene assisted terahertz metamaterials for sensitive bio-sensing. Sens. Actuators B Chem. 2020, 310, 127841. [Google Scholar] [CrossRef]
- Guo, W.; Zhai, L.; El-Bahy, Z.M.; Lu, Z.; Li, L.; Elnaggar, A.Y.; Ibrahim, M.M.; Cao, H.; Lin, J.; Wang, B. Terahertz Metamaterial Biosensor Based on Open Square Ring. Adv. Compos. Hybrid Mater. 2023, 6, 92. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Q.; Liu, K.; Chen, Z.; Li, K.; Zhang, X.; Xu, J.; Pickwell-MacPherson, E. Terahertz microfluidic metamaterial biosensor for sensitive detection of small-volume liquid samples. IEEE Trans. Terahertz. Sci. Technol. 2019, 9, 209–214. [Google Scholar] [CrossRef]
- Serita, K.; Matsuda, E.; Okada, K.; Murakami, H.; Kawayama, I.; Tonouchi, M. Invited Article: Terahertz microfluidic chips sensitivity-enhanced with a few arrays of meta-atoms. APL Photonics 2018, 3, 051603. [Google Scholar] [CrossRef]
- Serita, K.; Murakami, H.; Kawayama, I.; Tonouchi, M. A terahertz-microfluidic chip with a few arrays of asymmetric meta-atoms for the ultra-trace sensing of solutions. Photonics 2019, 6, 12. [Google Scholar] [CrossRef]
- Serita, K.; Kobatake, S.; Tonouchi, M. I-design terahertz microfluidic chip for attomole-level sensing. J. Phys. Photonics 2022, 4, 034005. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Review of recent metamaterial microfluidic sensors. Sensors 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, K.; Tang, T.; Mao, Y.; Wang, R.; Li, C.; Shen, J. The terahertz metamaterials for sensitive biosensors in the detection of ethanol solutions. Opt. Commun. 2020, 475, 126287. [Google Scholar] [CrossRef]
- Xu, W.; Xie, L.; Zhu, J.; Tang, L.; Singh, R.; Wang, C.; Ma, Y.; Chen, H.; Ying, Y. Terahertz biosensing with a graphene-metamaterial heterostructure platform. Carbon 2019, 141, 247–252. [Google Scholar] [CrossRef]
- Shih, K.; Pitchappa, P.; Jin, L.; Chen, C.H.; Singh, R.; Lee, C. Nanofluidic terahertz metasensor for sensing in aqueous environment. Appl. Phys. Lett. 2018, 113, 071105. [Google Scholar]
- Zhao, X.; Zhang, M.; Wei, D.; Wang, Y.; Yan, S.; Liu, M.; Yang, X.; Yang, K.; Cui, H.; Fu, W. Label-free sensing of the binding state of MUC1 peptide and anti-MUC1 aptamer solution in fluidic chip by terahertz spectroscopy. Biomed. Opt. Express 2017, 8, 4427–4437. [Google Scholar] [CrossRef] [PubMed]
- Serita, K.; Mizuno, S.; Murakami, H.; Kawayama, I.; Takahashi, Y.; Yoshimura, M.; Mori, Y.; Darmo, J.; Tonouchi, M. Scanning laser terahertz near-field imaging system. Opt. Express 2012, 20, 12959–12965. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Serita, K.; Zang, Z.; Murakami, H.; Kawayama, I.; Cassar, Q.; Macgrogan, G.; Guillet, J.; Mounaix, P.; Tonouchi, M. Scanning laser terahertz near-field reflection imaging system. Appl. Phys. Express 2019, 12, 122005. [Google Scholar] [CrossRef]
- Azad, A.K.; Dai, J.; Zhang, W. Transmission properties of terahertz pulses through subwavelength double split-ring resonators. Opt. Lett. 2006, 31, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Viphavakit, C.; Komodromos, M.; Themistos, C.; Mohammed, W.S.; Kalli, K.; Rahman, B.A. Optimization of a horizontal slot waveguide biosensor to detect DNA hybridization. Appl. Opt. 2015, 54, 4881–4888. [Google Scholar] [CrossRef] [PubMed]
- Gusev, S.I.; Demchenko, P.S.; Litvinov, E.A.; Cherkasova, O.P.; Meglinski, I.V.; Khodzitsky, M.K. Study of glucose concentration influence on blood optical properties in THz frequency range. Nanosyst.-Phys. Chem. Math. 2018, 9, 389–400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Tonouchi, M.; Serita, K. A Terahertz Point Source Meta-Sensor in Reflection Mode for Trace-Amount Bio-Sensing Applications. Photonics 2024, 11, 766. https://doi.org/10.3390/photonics11080766
Zheng L, Tonouchi M, Serita K. A Terahertz Point Source Meta-Sensor in Reflection Mode for Trace-Amount Bio-Sensing Applications. Photonics. 2024; 11(8):766. https://doi.org/10.3390/photonics11080766
Chicago/Turabian StyleZheng, Luwei, Masayoshi Tonouchi, and Kazunori Serita. 2024. "A Terahertz Point Source Meta-Sensor in Reflection Mode for Trace-Amount Bio-Sensing Applications" Photonics 11, no. 8: 766. https://doi.org/10.3390/photonics11080766
APA StyleZheng, L., Tonouchi, M., & Serita, K. (2024). A Terahertz Point Source Meta-Sensor in Reflection Mode for Trace-Amount Bio-Sensing Applications. Photonics, 11(8), 766. https://doi.org/10.3390/photonics11080766




