Quantifying the Impact of Uneventful LASIK on the Cornea
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Analysis
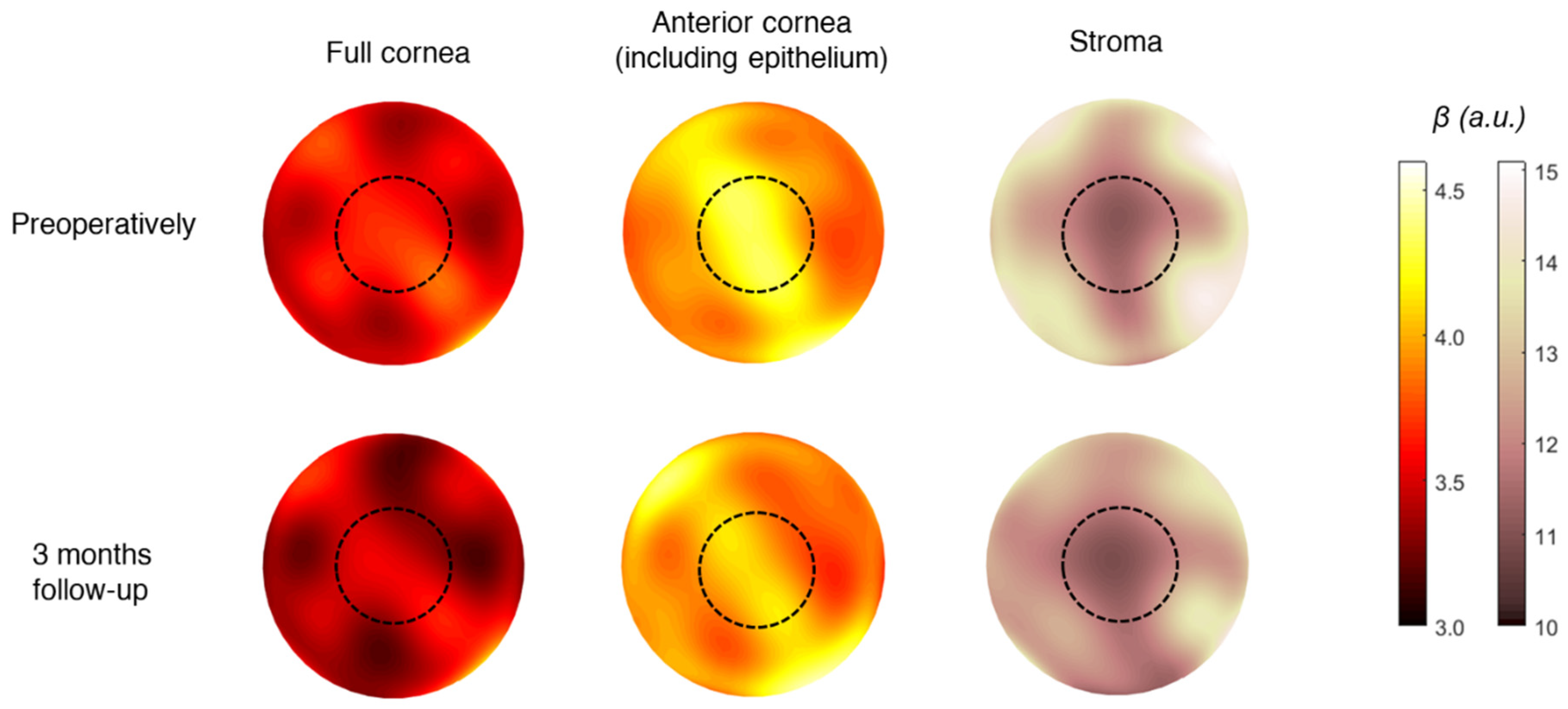
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, F.; Lopes, B.T.; Zheng, X.; Ji, Y.; Wang, J.; Elsheikh, A. Corneal Biomechanics Losses Caused by Refractive Surgery. Curr. Eye Res. 2023, 48, 137–143. [Google Scholar] [CrossRef]
- Netto, M.V.; Mohan, R.R.; Ambrósio, R.J.; Hutcheon, A.E.K.; Zieske, J.D.; Wilson, S.E. Wound Healing in the Cornea: A Review of Refractive Surgery Complications and New Prospects for Therapy. Cornea 2005, 24, 509–522. [Google Scholar] [CrossRef]
- Boulze-Pankert, M.; Dariel, R.; Hoffart, L. Corneal Scheimpflug Densitometry Following Photorefractive Keratectomy in Myopic Eyes. J. Refract. Surg. 2016, 32, 788–791. [Google Scholar] [CrossRef]
- Consejo, A.; Jiménez-García, M.; Issarti, I.; Rozema, J.J. Detection of Subclinical Keratoconus with a Validated Alternative Method to Corneal Densitometry. Transl. Vis. Sci. Technol. 2021, 10, 32. [Google Scholar] [CrossRef]
- Dhubhghaill, S.N.; Rozema, J.J.; Jongenelen, S.; Hidalgo, I.R.; Zakaria, N.; Tassignon, M.J. Normative Values for Corneal Densitometry Analysis by Scheimpflug Optical Assessment. Investig. Ophthalmol. Vis. Sci. 2013, 55, 162–168. [Google Scholar] [CrossRef]
- Savini, G.; Huang, J.; Lombardo, M.; Serrao, S.; Schiano-Lomoriello, D.; Venanzio, S.; Ducoli, P. Objective Monitoring of Corneal Backward Light Scattering After Femtosecond Laser-Assisted LASIK. J. Refract. Surg. 2016, 32, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fares, U.; Otri, A.M.; Al-Aqaba, M.A.; Faraj, L.; Dua, H.S. Wavefront-Optimized Excimer Laser in Situ Keratomileusis for Myopia and Myopic Astigmatism: Refractive Outcomes and Corneal Densitometry. J. Cataract Refract. Surg. 2012, 38, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Poyales, F.; Garzón, N.; Mendicute, J.; Illarramendi, I.; Caro, P.; Jáñez, O.; Argüeso, F.; López, A. Corneal Densitometry after Photorefractive Keratectomy, Laser-Assisted in Situ Keratomileusis, and Small-Incision Lenticule Extraction. Eye 2017, 31, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Consejo, A.; Gławdecka, K.; Karnowski, K.; Solarski, J.; Rozema, J.J.; Wojtkowski, M.; Iskander, D.R. Corneal Properties of Keratoconus Based on Scheimpflug Light Intensity Distribution. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3197–3203. [Google Scholar] [CrossRef] [PubMed]
- Consejo, A.; Solarski, J.; Karnowski, K.; Rozema, J.J.; Wojtkowski, M.; Iskander, D.R. Keratoconus Detection Based on a Single Scheimpflug Image. Transl. Vis. Sci. Technol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Consejo, A.; Jiménez-García, M.; Rozema, J.J. Age-Related Corneal Transparency Changes Evaluated with an Alternative Method to Corneal Densitometry. Cornea 2021, 40, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Consejo, A.; Trillo-Moreno, I.; Remon, L. Corneal Tissue Changes Following Short-Term Soft Contact Lens Wear of Different Materials. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2023, 43, 35–45. [Google Scholar] [CrossRef]
- Consejo, A.; Alonso-Caneiro, D.; Wojtkowski, M.; Vincent, S.J. Corneal Tissue Properties Following Scleral Lens Wear Using Scheimpflug Imaging. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2020, 40, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Tack, M.; Kreps, E.O.; De Zaeytijd, J.; Consejo, A. Scheimpflug-Based Analysis of the Reflectivity of the Cornea in Marfan Syndrome. Transl. Vis. Sci. Technol. 2021, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Miażdżyk, M.; Consejo, A.; Iskander, D.R. Assessing and Compensating for the Confounding Factors in Scheimpflug-Based Corneal Densitometry. Biomed. Opt. Express 2022, 13, 6258–6272. [Google Scholar] [CrossRef]
- Pisella, P.J.; Auzerie, O.; Bokobza, Y.; Debbasch, C.; Baudouin, C. Evaluation of Corneal Stromal Changes in Vivo after Laser in Situ Keratomileusis with Confocal Microscopy. Ophthalmology 2001, 108, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Latvala, T.; Linna, T.; Tervo, T. Corneal Nerve Recovery after Photorefractive Keratectomy and Laser in Situ Keratomileusis. Int. Ophthalmol. Clin. 1996, 36, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.G.; Holley, G.P.; Geroski, D.H.; Waring, G.O., 3rd; Grossniklaus, H.E.; Edelhauser, H.F. Ex Vivo Confocal Microscopy of Human LASIK Corneas with Histologic and Ultrastructural Correlation. Ophthalmology 2005, 112, 634–644. [Google Scholar] [CrossRef]
- Vesaluoma, M.; Pérez-Santonja, J.; Petroll, W.M.; Linna, T.; Alió, J.; Tervo, T. Corneal Stromal Changes Induced by Myopic LASIK. Investig. Ophthalmol. Vis. Sci. 2000, 41, 369–376. [Google Scholar]
- Erie, J.C.; Nau, C.B.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Long-Term Keratocyte Deficits in the Corneal Stroma after LASIK. Ophthalmology 2004, 111, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, P.; de Benito-Llopis, L.; Hernández-Verdejo, J.L.; Teus, M.A. Comparison of Keratocyte Density after Femtosecond Laser vs Mechanical Microkeratome from 3 Months up to 5 Years after LASIK. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Dupps, W.J., Jr.; Wilson, S.E. Biomechanics and wound healing in the cornea. Exp. Eye Res. 2006, 83, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Shajari, M.; Wanner, E.; Rusev, V.; Mir Mohi Sefat, S.; Mayer, W.J.; Kohnen, T.; Priglinger, S.; Kook, D. Corneal Densitometry after Femtosecond Laser-Assisted In Situ Keratomileusis (Fs-LASIK) and Small Incision Lenticule Extraction (SMILE). Curr. Eye Res. 2018, 43, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Li, M.; Yang, W.; Shen, Y.; Zhao, Y.; Fu, D.; Shang, J.; Zhang, J.; Choi, J.; Zhou, X. Corneal Densitometry After Small Incision Lenticule Extraction (SMILE) and Femtosecond Laser-Assisted LASIK (FS-LASIK): 5-Year Prospective Comparative Study. Front. Med. 2020, 7, 521078. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.W.; Bourne, W.M.; Maguire, L.J.; Patel, S.V. Changes in keratocyte density and visual function five years after laser in situ keratomileusis: Femtosecond laser versus mechanical microkeratome. Am. J. Ophthalmol. 2015, 160, 163–170. [Google Scholar] [CrossRef] [PubMed]

| Treatment Characteristic | Value |
|---|---|
| Refractive error Pre-Tx | |
| Sphere (D) | −3.6 ± 2.3 [−9.0, 5.2] |
| Cylinder (D) | 0.6 ± 1 [−5.0, 0.0] |
| Keratometry Pre-Tx | |
| K1F (D) | 43.1 ± 1.4 [40.4, 46.7] |
| K2F (D) | 44.1 ± 1.3 [41, 47.6] |
| Pachymetry Pre-Tx | |
| Pachy apex (μm) | 571 ± 35 [502, 687] |
| Pachy min (μm) | 569 ± 34 [497, 686] |
| Ablated tissue (μm) | 65 ± 24 [23, 118] |
| RSB (μm) | 394 ± 37 [312, 472] |
| α | β | ||||||
|---|---|---|---|---|---|---|---|
| Area | Pre-op | 3-Month FU | p-Value | Pre-op | 3-Month FU | p-Value | |
| Full cornea | 3.5 mm | 31 ± 4 [24, 43] | 32 ± 6 [23, 67] | 0.08 | 3.8 ± 0.5 [2.9, 5.2] | 3.8 ± 0.6 [2.5, 5.6] | 0.72 |
| 8 mm | 35 ± 6 [28, 44] | 35 ± 6 [25, 45] | 0.32 | 3.8 ± 0.3 [2.9, 4.9] | 3.7 ± 0.5 [2.4, 5.3] | 0.13 | |
| Anterior cornea (including epithelium) | 3.5 mm | 39 ± 7 [21, 51] | 39 ± 7 [22, 65] | 0.015 * | 4.9 ± 1.4 [3.4, 11.6] | 4.5 ± 0.9 [3.2, 8.1] | <0.001 * |
| 8 mm | 40 ± 6 [21, 53] | 41 ± 7 [23, 53] | 0.15 | 4.7 ± 1.0 [3.3, 8.3] | 4.4 ± 0.8 [3.0, 7.7] | 0.004 * | |
| Stroma | 3.5 mm | 26 ± 5 [20, 38] | 26 ± 5 [19, 54] | 0.57 | 13.3 ± 1.4 [9.0, 15.8] | 12.9 ± 1.9 [6.3, 16.2] | 0.08 |
| 8 mm | 29 ± 3 [23, 44] | 28 ± 4 [21, 37] | 0.21 | 11.9 ± 1.3 [7.8, 13.9] | 11.4 ± 1.5 [6.2, 14.0] | 0.03 * | |
| α | β | ||||||
|---|---|---|---|---|---|---|---|
| Pre-op (n = 90) | 3-Month FU (n = 90) | Difference Pre-op/3M FU (n = 90) | Pre-op (n = 90) | 3-Month FU (n = 90) | Difference Pre-op/3M FU (n = 90) | ||
| Full cornea | Age | 0.22 (0.03) * | 0.22 (0.03) * | 0.20 (0.03) * | 0.18 (0.04) * | 0.18 (0.04) * | 0.18 (0.04) * |
| CCT | 0.05 (0.30) | 0.05 (0.30) | 0.12 (0.12) | 0.08 (0.23) | 0.12 (0.12) | 0.04 (0.34) | |
| SE | 0.08 (0.23) | 0.08 (0.23) | 0.08 (0.23) | 0.06 (0.27) | 0.12 (0.12) | 0.06 (0.27) | |
| AT | 0.13 (0.10) | 0.05 (0.30) | 0.02 (0.40) | 0.02 (0.42) | 0.05 (0.33) | 0.02 (0.40) | |
| RSB | 0.08 (0.22) | <0.01 (0.50) | 0.08 (0.22) | 0.09 (0.19) | <0.01 (0.50) | 0.06 (0.29) | |
| Anterior cornea (including epithelium) | Age | 0.21 (0.02) * | 0.18 (0.04) * | 0.18 (0.04) * | 0.18 (0.04) * | 0.18 (0.04) * | 0.18 (0.04) * |
| CCT | 0.05 (0.30) | 0.04 (0.35) | 0.12 (0.12) | 0.16 (0.05) | 0.03 (0.36) | 0.12 (0.12) | |
| SE | 0.05 (0.31) | 0.02 (0.41) | 0.01 (0.45) | 0.13 (0.11) | 0.08 (0.23) | 0.03 (0.36) | |
| AT | 0.16 (0.06) | 0.07 (0.26) | 0.17 (0.06) | 0.02 (0.43) | −0.14 (0.09) | 0.17 (0.10) | |
| RSB | 0.15 (0.07) | 0.05 (0.29) | 0.06 (0.28) | <0.01 (0.50) | 0.07 (0.25) | 0.05 (0.32) | |
| Stroma | Age | 0.20 (0.03) * | 0.25 (0.01) * | 0.19 (0.03) * | 0.21 (0.02) * | 0.18 (0.04) * | 0.18 (0.04) * |
| CCT | 0.04 (0.35) | 0.08 (0.23) | 0.16 (0.05) | 0.08 (0.23) | 0.04 (0.35) | 0.12 (0.12) | |
| SE | 0.02 (0.41) | 0.01 (0.45) | 0.03 (0.36) | 0.08 (0.23) | 0.01 (0.45) | 0.13 (0.11) | |
| AT | 0.15 (0.08) | 0.03 (0.38) | 0.07 (0.24) | 0.14 (0.10) | 0.06 (0.28) | 0.14 (0.09) | |
| RSB | 0.06 (0.27) | 0.01 (0.18) | 0.14 (0.09) | 0.10 (0.17) | 0.03 (0.38) | 0.06 (0.28) | |
| Area | Pre-op | 3-Month FU | 1-Year FU | F-Stats | p-Value | |
|---|---|---|---|---|---|---|
| Parameter α | ||||||
| Full cornea | 3.5 mm | 31 ± 4 [24, 40] | 33 ± 7 [25, 56] | 31 ± 7 [22, 47] | F(2,42) = 0.73 | 0.49 |
| 8 mm | 34 ± 5 [28, 44] | 36 ± 7 [29, 61] | 32 ± 5 [25, 49] | F(2,42) = 2.61 | 0.08 | |
| Anterior cornea (including epithelium) | 3.5 mm | 36 ± 6 [25, 47] | 40 ± 9 [28, 63] | 36 ± 7 [26, 58] | F(2,42) = 1.89 | 0.056 |
| 8 mm | 40 ± 6 [29, 54] | 43 ± 9 [34, 65] | 38 ± 7 [27, 63] | F(2,42) = 2.13 | 0.13 | |
| Stroma | 3.5 mm | 26 ± 4 [20, 34] | 27 ± 6 [19, 47] | 27 ± 7 [19, 44] | F(2,42) = 0.41 | 0.66 |
| 8 mm | 28 ± 4 [24, 37] | 29 ± 6 [23, 49] | 26 ± 4 [22, 41] | F(2,42) = 1.63 | 0.20 | |
| Parameter β | ||||||
| Full cornea | 3.5 mm | 3.8 ± 0.4 [3.0, 4.7] | 3.7 ± 0.6 [2.5, 5.6] | 3.0 ± 0.6 [1.7, 4.6] | F(2,42) = 12.3 | <0.001 * |
| 8 mm | 3.8 ± 0.4 [3.3, 4.8] | 3.6 ± 0.6 [2.4, 5.3] | 3.2 ± 0.4 [2.5, 4.4] | F(2,42) = 10.84 | <0.001 * | |
| Anterior cornea (including epithelium) | 3.5 mm | 4.9 ± 1.0 [3.5, 8.0] | 4.4 ± 0.8 [3.2, 6.2] | 4.3 ± 0.9 [3.3, 6.9] | F(2,42) = 2.7 | 0.07 |
| 8 mm | 4.6 ± 0.7 [3.5, 6.1] | 4.3 ± 0.6 [3.2, 5.3] | 4.2 ± 0.5 [3.4, 5.4] | F(2,42) = 4.82 | 0.028 * | |
| Stroma | 3.5 mm | 13.4 ± 1.5 [9.6, 15.3] | 12.7 ± 2.2 [6.3, 15.2] | 10.2 ± 2.5 [4.3, 14.7] | F(2,42) = 13.9 | <0.001 * |
| 8 mm | 12 ± 1.5 [8.9, 13.9] | 11.4 ± 2.0 [6.3, 13.4] | 10.2 ± 1.6 [4.7, 12.4] | F(2,42) = 5.94 | 0.004 * | |
| Preop vs. 3-Month FU (n = 22) | Preop vs. 1-Year FU (n = 22) | 3-month FU vs. 1-Year FU (n = 22) | ||
|---|---|---|---|---|
| 3.5 mm | Full cornea | 0.94 | <0.001 * | 0.001 * |
| Anterior cornea (including epithelium) | 1.00 | 0.09 | 0.24 | |
| Stroma | 0.92 | <0.001 * | 0.001 * | |
| 8 mm | Full cornea | 0.218 | <0.001 * | 0.020 * |
| Anterior cornea (including epithelium) | 0.036 * | 0.024 * | 0.64 | |
| Stroma | 0.040 * | 0.003 * | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadnanansing, A.; Kreps, E.O.; Claerhout, I.; Kestelyn, P.G.; Consejo, A. Quantifying the Impact of Uneventful LASIK on the Cornea. Photonics 2024, 11, 315. https://doi.org/10.3390/photonics11040315
Jadnanansing A, Kreps EO, Claerhout I, Kestelyn PG, Consejo A. Quantifying the Impact of Uneventful LASIK on the Cornea. Photonics. 2024; 11(4):315. https://doi.org/10.3390/photonics11040315
Chicago/Turabian StyleJadnanansing, Arieke, Elke O. Kreps, Ilse Claerhout, Philippe G. Kestelyn, and Alejandra Consejo. 2024. "Quantifying the Impact of Uneventful LASIK on the Cornea" Photonics 11, no. 4: 315. https://doi.org/10.3390/photonics11040315
APA StyleJadnanansing, A., Kreps, E. O., Claerhout, I., Kestelyn, P. G., & Consejo, A. (2024). Quantifying the Impact of Uneventful LASIK on the Cornea. Photonics, 11(4), 315. https://doi.org/10.3390/photonics11040315





