1. Introduction
FLIM technology provides detailed information about the microenvironment in which a molecule resides by measuring the time it takes for a fluorescent molecule to return from the excited state to the ground state [
1]. Unlike conventional fluorescence intensity imaging, the fluorescence lifetime is independent of excitation intensity, fluorophore concentration, and photobleaching effects [
2]. Consequently, multiple biochemical parameters in the microenvironment, such as oxygen content [
3], viscosity [
4], ionic concentration [
5], and temperature [
6], can be quantitatively measured via the fluorescence lifetime. In recent years, researchers have made significant progress by utilizing FLIM technology to conduct comprehensive investigations into cell metabolism [
7], tumor microenvironment [
8], material properties [
9], and chemical reaction kinetics [
10]. However, in practical applications such as biomedical clinical diagnosis [
11,
12] and large-scale industrial detection [
13], there is a higher demand for the imaging speed of FLIM technology [
14].
FLIM techniques are mainly divided into two categories: time-domain (TD) and frequency-domain (FD) methods. The common implementations in time-domain FLIM are based on time-gating (TG), TCSPC, and a synchroscan streak camera (SC).
Table 1 presents a comparative analysis of the temporal resolution, spatial resolution, response sensitivity, and data acquisition speed of these FLIM techniques.
Although FD-FLIM and TG-FLIM systems can achieve Fast-FLIM by using CCD or CMOS to construct multi-pixel (channel) parallel acquisition, the spatial resolution and sensitivity are unsatisfactory. In addition, FD-FLIM indirectly calculates the fluorescence lifetime by measuring the phase delay and modulation depth of fluorescence signals, and the measurement accuracy is limited due to the modulation frequency (
Hz) and sampling frequency (
Hz) [
19]. Moreover, the process of measuring multi-exponential decay fluorescence is complex. TG-FLIM uses an electronic or optical shutter to turn the detector’s response on and off, and its temporal resolution depends on the width of the time gate (sub-nanoseconds) [
2]. In contrast, TCSPC-FLIM and SC-FLIM use high-gain detection modules (Vgain = 1V [
17]; gain =
[
18]) that can detect a single photon. A unique method of constructing fluorescence decay curves enables these two technologies to achieve a high temporal resolution [
12]. Consequently, this paper focuses on TCSPC and SC methods.
The TCSPC method effectively overcomes the signal broadening caused by the instrument response function (IRF) through single-photon counting and statistical analysis, resulting in high measurement accuracy [
20]. However, the excitation–detection process must be repeated tens of thousands of times to achieve the required accuracy and precision, leading to a slow acquisition speed and even photobleaching [
21]. In order to improve the acquisition speed of TCSPC, researchers have proposed two schemes to reduce repetitive measurements: (1) reducing the dead time of a single channel and (2) using a parallel multi-channel. When KOENIG M et al. [
22] used a combination of a hybrid photomultiplier tube (hybrid PMT) and a low dead-time (less than 1 ns) time-to-digital converter (TDC) to visualize fluorescent beads, the acquisition speed was 3 fps (128 × 128 pixels). BECKER W et al. [
23] employed a multi-channel parallel strategy to construct a FLIM system comprising eight parallel TCSPC channels, which required approximately 5 s to acquire a set of 256 × 256 pixels FLIM. POLAND S et al. [
24] developed a multi-channel parallel Fast-FLIM system using a single-photon avalanche diode (SPAD) line array. The system has a speed of up to 4 fps (516 × 516 pixels).
The synchroscan streak camera disperses photoelectrons that arrive at different moments to corresponding spatial positions, enabling high-resolution time measurements through spatio-temporal conversion [
25]. Currently, the temporal resolution of the synchroscan streak camera is less than 1ps [
26]. The streak camera method not only overcomes the slow speed problem caused by single-photon counting but also possesses a wide-field imaging capability [
20]. These advantages make it a new choice for Fast-FLIM. At present, Liu et al. [
27] have developed a high-speed SC-FLIM system by integrating a synchroscan streak camera with an ultra-high frame rate readout camera and a high-speed spatial scanning device.
The measurement principle of the synchroscan streak camera determines that it is an effective way to achieve Fast-FLIM. Therefore, this paper calculates the acquisition speed of the SC-FLIM system. We provide a detailed analysis of the factors that influence the acquisition speed in various processes of lifetime imaging, including fluorescence generation, fluorescence detection, spatial scanning, and data processing. Then, the acquisition time of TCSPC-FLIM and synchroscan SC-FLIM systems is calculated using a numerical simulation and a theoretical analysis. Finally, we quantitatively compare the data acquisition time of these FLIM systems by measuring the lifetime of a standard fluorescent dye, Erythrosin B, based on the developed acquisition time model.
This paper is organized as follows: In
Section 2, the method of constructing acquisition time models for TCSPC-FLIM and synchroscan SC-FLIM is described. In
Section 3, the simulated experimental conditions and the results are discussed, and a quantitative comparison of the speed of the FLIM system is presented. Conclusions are drawn in
Section 4.
2. Theory
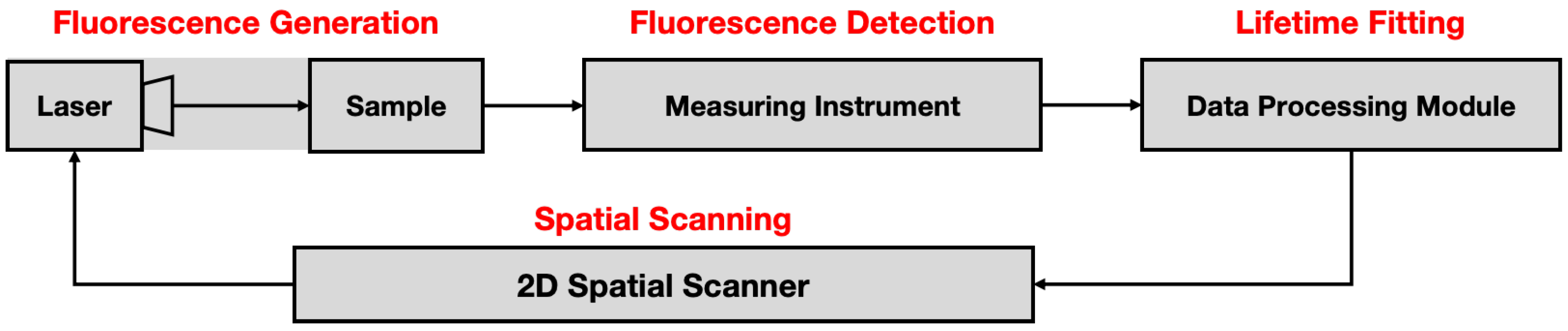
The FLIM technology, based on TCSPC or synchroscan streak camera, is regarded as a representative high-precision time-domain FLIM technology. As illustrated in
Figure 1, the 2D time-domain FLIM technology can be divided into four processes sequentially: fluorescence generation, fluorescence detection, lifetime fitting, and spatial scanning. After the sample is excited to produce fluorescence, the measuring instrument captures the fluorescence intensity decay signal and transmits the signal to the data processing module. The scanner completes 2D data acquisition by continuously changing the laser position and repeating the above process.
2.1. Factors Influencing the Acquisition Speed in the Fluorescence Generation Process
The photon emission rate of the sample exerts a direct influence on the acquisition time during the fluorescence generation process. When the fluorescence signal is weak, the system must repeat the excitation–detection process to obtain a high signal-to-noise ratio (SNR) signal, and the number of repetitions increases with the decrease in the photon emission rate. Therefore, the acquisition speed is influenced by the photon emission rate. Especially in multi-photon detection systems, such as the SC-FLIM system, the photon emission rate is closely related to the acquisition time.
2.2. Factors Influencing the Acquisition Speed in the Fluorescence Detection Process
The primary factor influencing the acquisition speed during the fluorescence detection process is the photon detection rate of the detection system. To assess the influence of photon detection rate on acquisition speed, it is essential to first comprehend the operational principles of diverse FLIM systems.
The TCSPC-FLIM system generates a histogram of photon arrival times by measuring the time interval between excitation and emission, as illustrated in
Figure 2. The laser beam is split into two signals, one of which is detected via the single-photon detection system to form a “start” signal, which is used to initiate the timing system. The other signal is employed to excite the sample, thereby producing a fluorescence signal. The fluorescence signal passes through the single-photon detection system to form a “stop” signal, which is used to terminate the timing system. The interval (
) between the “start” signal and the “stop” signal represents the delay time for fluorescent photon emission in the sample. The multi-channel analyzer records and accumulates the photon arrival time. This process is then repeated to form a histogram of the time distribution, as illustrated in
Figure 2c.
The synchroscan streak camera is capable of mapping temporal information onto the spatial axis, thereby facilitating the rapid and high-resolution measurement of temporal optical signals, as illustrated in
Figure 3. The fluorescence decay signals are projected onto the slit-shaped photocathode (PC) through a relay lens (L1), and they are converted into electrons sequentially via the photocathode. The electrons are accelerated via the accelerating electrode and deflected via high-voltage sweep electrodes. During the sweep, the electrons are deflected at slightly different angles, depending on the arrival time at the electrodes. The electrons are then multiplied via a microchannel plate (MCP) and converted back into photons via a phosphor screen (PS) to form a streak image. A high-speed CCD camera is used to digitize and transfer the streak image to a processing module for lifetime processing [
28].
In instances where the photon emission rate is high, the photon counting rate of the detection system assumes a primary role in influencing the acquisition speed, as it serves to determine the number of repetitions of the excitation–detection process. TCSPC-FLIM is a single-photon detection system that records at most one photon during a single excitation–detection process. Moreover, due to dead time in the detector, the photon detection rate is typically set to 20% of the laser frequency to minimize the waveform distortion of the recorded signal caused by the “pile-up” effect. The synchroscan streak camera realizes a multi-photon record in an excitation–detection process through a temporal-to-spatial mapping strategy with a high photon counting rate.
2.3. Factors Influencing the Acquisition Speed in the Lifetime Fitting Process
A fluorescence decay signal with a sufficient SNR is a prerequisite for accurately fitting fluorescence lifetimes. In the TCSPC-FLIM and SC-FLIM systems, the number of photons recorded in a time bin follows a Poisson distribution [
29], so the noise mainly comes from the Poisson noise of the photon counts, and the standard deviation of the noise can be approximated as the square root of the number of photons in the corresponding time bin. Assume that the photon histogram has a total of
M time bins, and the number of photons in the i-th time bin is
. The SNR can be expressed as follows:
The most straightforward method of enhancing the
is to increase the number of photons. However, this often leads to extended acquisition times and photobleaching, especially in dynamic scenes where sample motion can cause blurred imaging [
20]. Therefore, the most crucial step is determining the minimum
before calculating the acquisition time.
2.4. Factors Influencing the Acquisition Speed in the Spatial Scanning Process
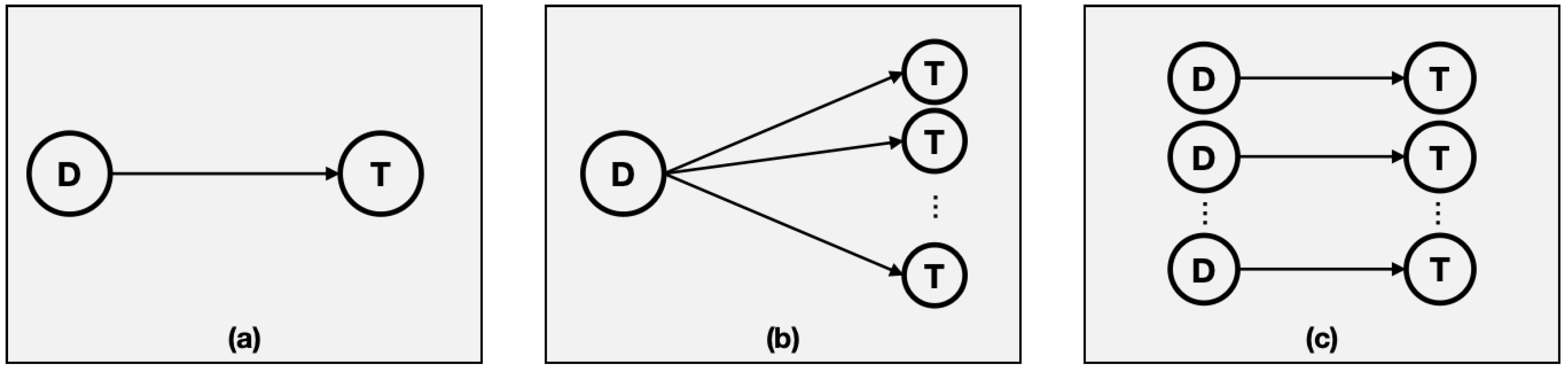
The most commonly utilized TCSPC-FLIM systems can be categorized into two principal types: laser scanning systems and wide-field imaging systems. The former can be further subdivided into single-channel and parallel multi-channel systems, as shown in
Figure 4. Depending on the arrangement of the detectors, wide-field imaging systems can be divided into line-scanning and surface-scanning systems. The FLIM system based on a synchroscan streak camera is a line-scanning system.
In 2D-FLIM, the total acquisition time is influenced by the dwell time (DT) of the scanner and the number of parallel channels of the measuring instrument: (1) the dwell time is defined as the time during which the laser beam is fixed at a specific position before moving to the next position; (2) the number of parallel channels is defined as the number of independent detection channels capable of simultaneously acquiring fluorescence lifetime data from multi-pixels. The Polygon mirror (BFI Optilas, Lincoln Laser; Minimum(DT) = 0.07
s) and galvanometer (Superscan SC-30-Y-Dig2, Raylase; Minimum(DT) = 1.05
s) are generally selected as high-speed scanners [
30].
Summary of the Factors Influencing the Acquisition Speed
Overall, in TCSPC-FLIM and synchroscan SC-FLIM systems, the factors influencing the acquisition speed include the photon emission rate, the photon counting rate, the required SNR, the dwell time, and the number of parallel channels.
Table 2 presents these factors and their definitions.
3. Method
Calculating the acquisition time of 2D-FLIM can be divided into two steps: (1) Calculate the single-channel acquisition time () of the FLIM system. (2) Calculate the total acquisition time () based on , the scanning speed, and the number of parallel channels. is determined using the photon counting rate and required photons. In SC-FLIM, the photon emission rate of the sample and the photon transfer efficiency of the system constitute the photon counting rate. Therefore, when calculating the of TCSPC-FLIM and synchroscan SC-FLIM, the photon emission rate, the photon transfer efficiency, the photon counting rate, the required photons, and the number of parallel channels are calculated sequentially.
3.1. Calculation of the Photon Emission Rate
The photon emission rate (PER) is the product of the number of fluorescence photons (
) produced in a single laser cycle and the laser frequency (
):
where
and
are represented as in Equations (
3) and (
4).
A is defined as the absorption efficiency of the sample at the excitation wavelength. It is worth noting that the value of
A is proportional to the number of chances for excitation;
is the fluorescence quantum yield,
and
are the average and peak power of the laser, respectively, and
is the pulse width.
The most straightforward method for enhancing the photon emission rate is to increase the laser power. However, this approach may lead to photobleaching or even photodamage [
31]. The use of multiphoton excitation effectively reduces the impact of laser light on sample stability. Single-photon excitation typically does not necessitate the use of high laser power, as the energy of a single photon is sufficient to prompt the molecule to transition directly to an excited state. In contrast, in multiphoton excitation, a fluorescent molecule enters the excited state by absorbing multiple low-energy photons simultaneously. This process necessitates the utilization of exceedingly high instantaneous laser power, with the objective of ensuring that effective nonlinear excitation occurs within the focal region of the laser. Single-photon excitation results in photon absorption across the entire laser path, affecting the sample in both the focal and non-focal regions. In contrast, the non-linear effect of multiphoton excitation occurs only in the focal region, significantly reducing photobleaching and photodamage in the non-focal region. In general, the laser requirements for multiphoton excitation are more demanding, necessitating the use of short-pulse, high-peak-power near-infrared lasers in conjunction with sophisticated optical focusing systems for the nonlinear imaging of deep biological samples [
24].
In addition, increasing the fluorophore concentration to improve the photon emission rate is also limited by application scenarios. For example, the fluorophore concentration must be kept non-invasive in live FLIM. In industrial and materials science applications, samples are typically more stable and less sensitive to light damage than biological samples.
3.2. Calculation of the Photon Counting Rate
In the case of a high photon emission rate, the photon counting rate of the FLIM system becomes a significant factor influencing the acquisition time. In a TCSPC-FLIM system, the photon counting rate is dependent on the dead time of the detector and the timing circuit. However, in a multi-photon detection system such as synchroscan SC-FLIM, the photon count rate of the system is dependent on the photon transfer efficiency.
3.2.1. TCSPC-FLIM
For TCSPC at a high count rate and a high signal repetition rate, two instrumental effects have to be considered: (1) the counting loss, the loss of a photon detected within the signal processing time (“Dead time”) of a previous one, and (2) the pile-up effect, the loss of a photon detected in the same signal period with a previous one [
17].
“Dead time” is the recovery time required for the photon detector after detecting a photon, during which the photon detector cannot detect new photons. In a time interval
, the detector can detect
photons without considering dead time. Due to the presence of the “Dead time”, the actual photon detection time of the detector in the time interval is
, where
is the dead time,
is the number of photons actually detected, and the
can be expressed in two ways:
where PCR is the photon counting rate, and PDR is the photon detection rate. Therefore, the photon count rate of the TCSPC system can be derived from Equations (
5) and (
6) as follows:
Unlike “Counting loss”, the “Pile-up” effect causes an error in the detected fluorescence lifetime:
where
is the fluorescence lifetime,
is the laser frequency, and
is the intensity-weighted lifetime of the measured decay profile. When the PDR is 20% of the excitation laser rate, the error is about 5%, which is small and acceptable in many applications [
20].
3.2.2. Synchroscan SC-FLIM
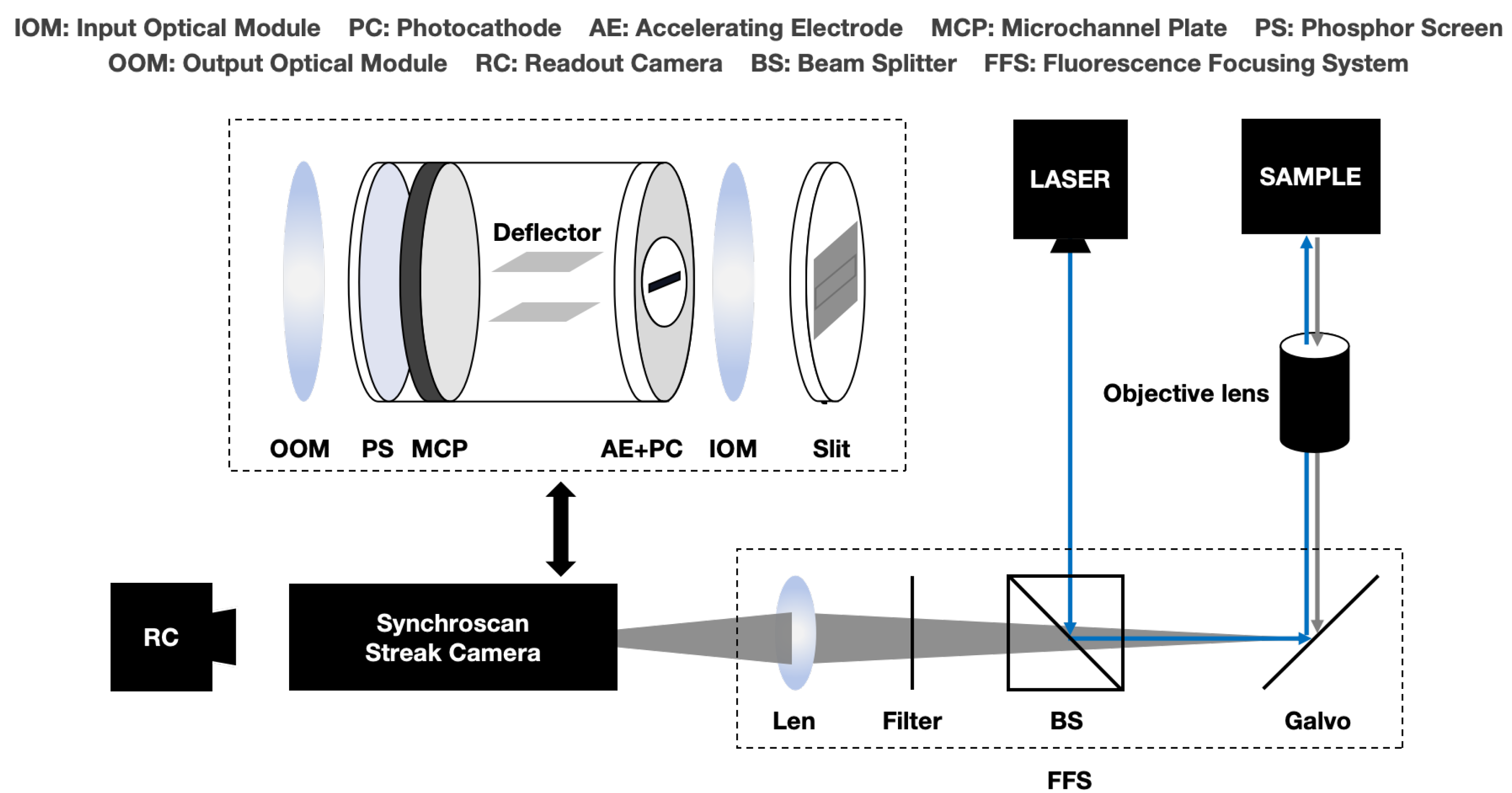
Due to the isotropy of fluorescence and the loss of photons during transmission, the photon counting rate of the SC-FLIM system is determined according to the photon emission rate and the photon transfer efficiency (PTE). Calculating the PTE is a prerequisite for calculating the photon counting rate. In the synchroscan SC-FLIM, as shown in
Figure 5, the PTE is composed of the fluorescence focusing system (FFS) and the synchroscan streak camera.
The numerical aperture (NA) determines the angular range over which the objective lens can collect photons. The photon transfer efficiency of an objective lens,
, can be expressed in Equations (
9) and (
10), where
is the photon collection range, and
n is the refractivity of the medium in which the objective lens is located.
Figure 5.
Schematic diagram of synchroscan SC-FLIM system.
Figure 5.
Schematic diagram of synchroscan SC-FLIM system.
The photon transfer efficiency of the fluorescence focusing system is denoted as , which is jointly determined by the reflectivity of the galvanometer, the splitting ratio of the beam splitter, and the transmittance of the beam filter and focusing lens, which can be obtained from the instrument manual.
The photon transfer efficiency of the streak camera is also influenced by the slit size, the photon-electric conversion efficiency of the photocathode, and the electron penetration efficiency of the accelerating electrode. It is assumed that the imaging area of the fluorescence focusing system in the plane where the slit is located is
S and that the width and height of the slit are
and
, respectively; the photon transfer efficiency of the slit (
) is as follows:
The photon-electric conversion efficiency of the photocathode is
, and the electron penetration efficiency of the accelerating electrode is
, which also can be obtained from the instrument manual. The photon transfer efficiency of the whole FLIM system (
), can be expressed as follows:
Synchroscan streak cameras employ a high-frequency sinusoidal scanning signal to convert a temporal signal into a spatial signal, rapidly accumulating it spatially to form a high-SNR spatial signal. Consequently, the photon counting rate of the streak camera is also dependent on the sinusoidal scanning signal frequency,
:
The selection of the frequency of the sinusoidal signal must take into account the necessity of ensuring the accuracy of the signal accumulation. In most cases, the is equal to the or equal to the , where .
3.3. Calculation of the Required Photons
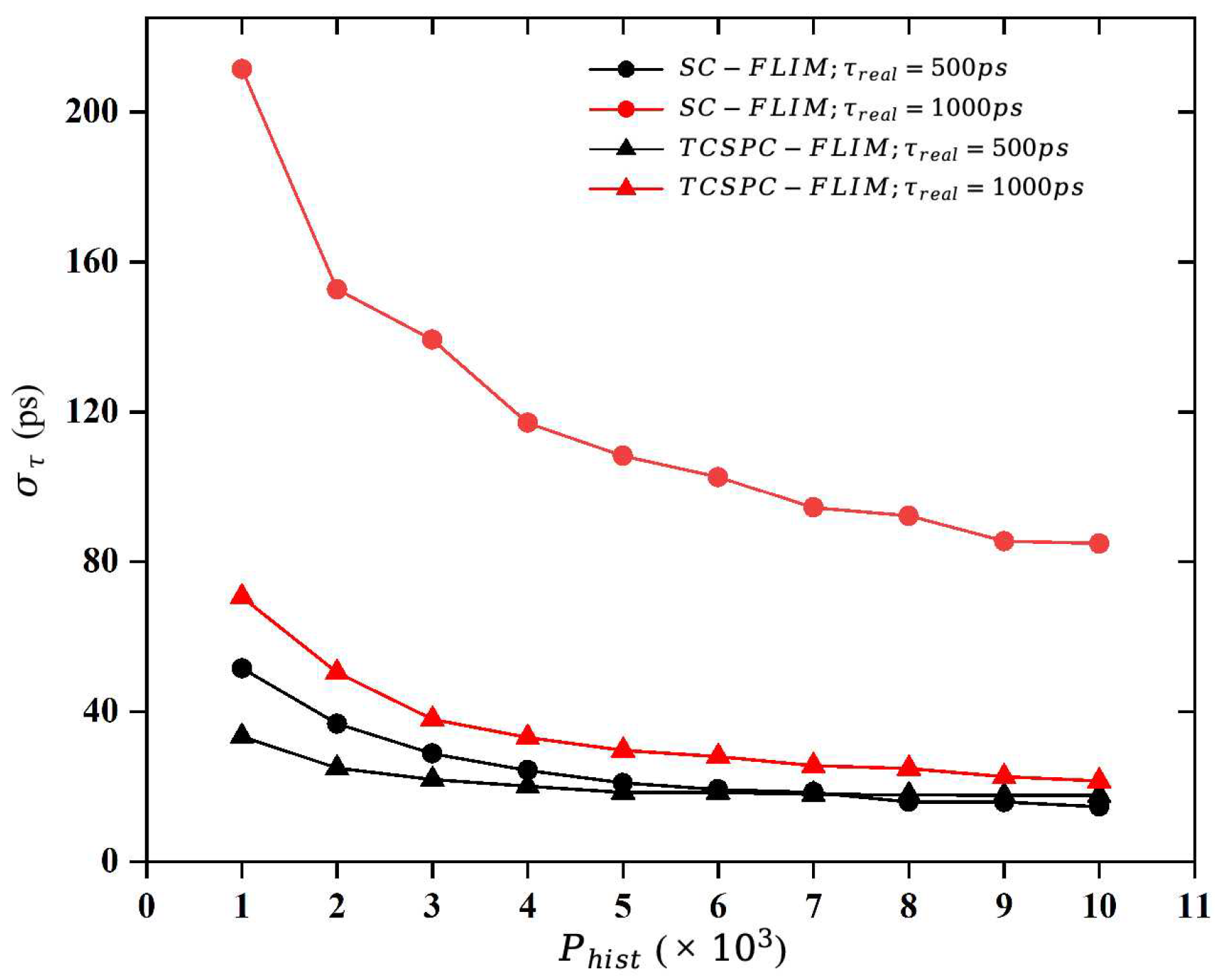
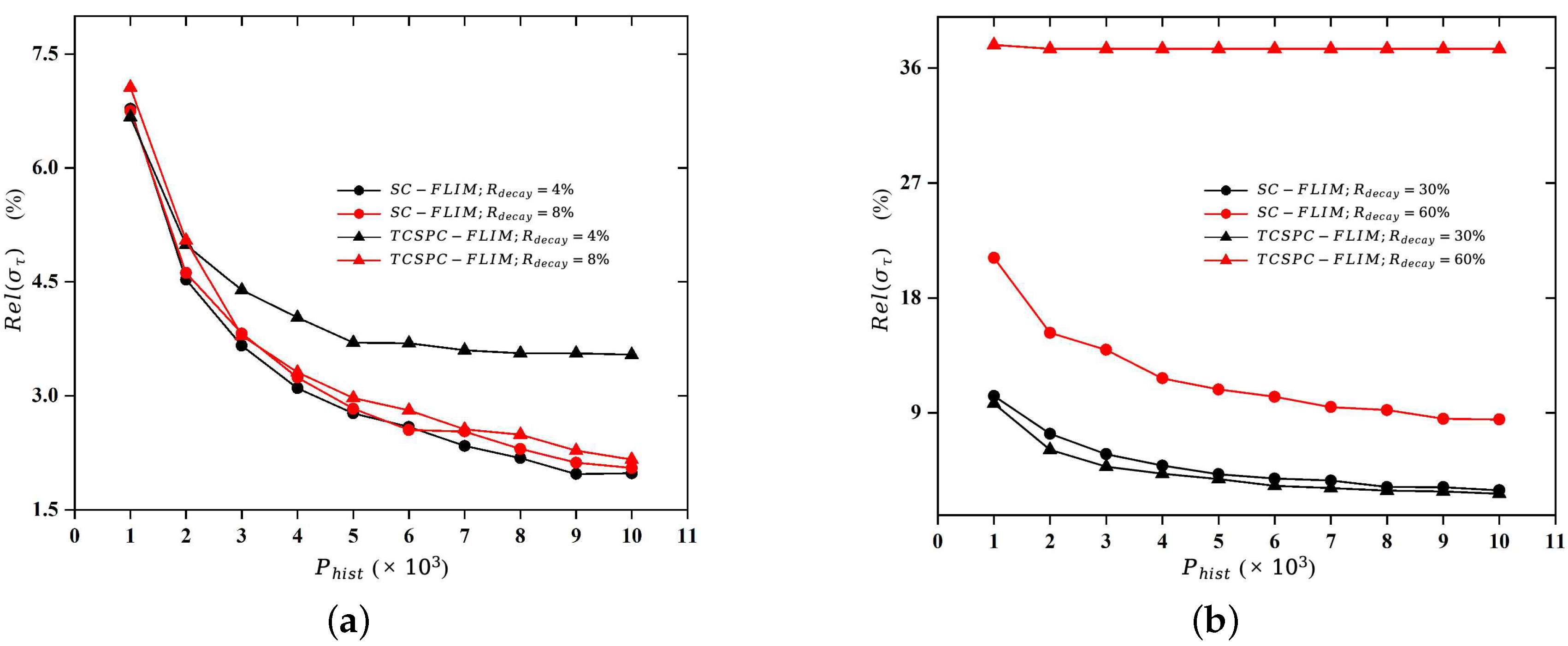
This paper uses numerical simulation methods to calculate the minimum number of photons required to form a fluorescence decay histogram when the fitting error is within a tolerable range, also known as the “Required Photons”. In the actual lifetime imaging process, the fitting error requirement, the systematic error, the fitting algorithm, and the decay model all affect the “Required Photons”.
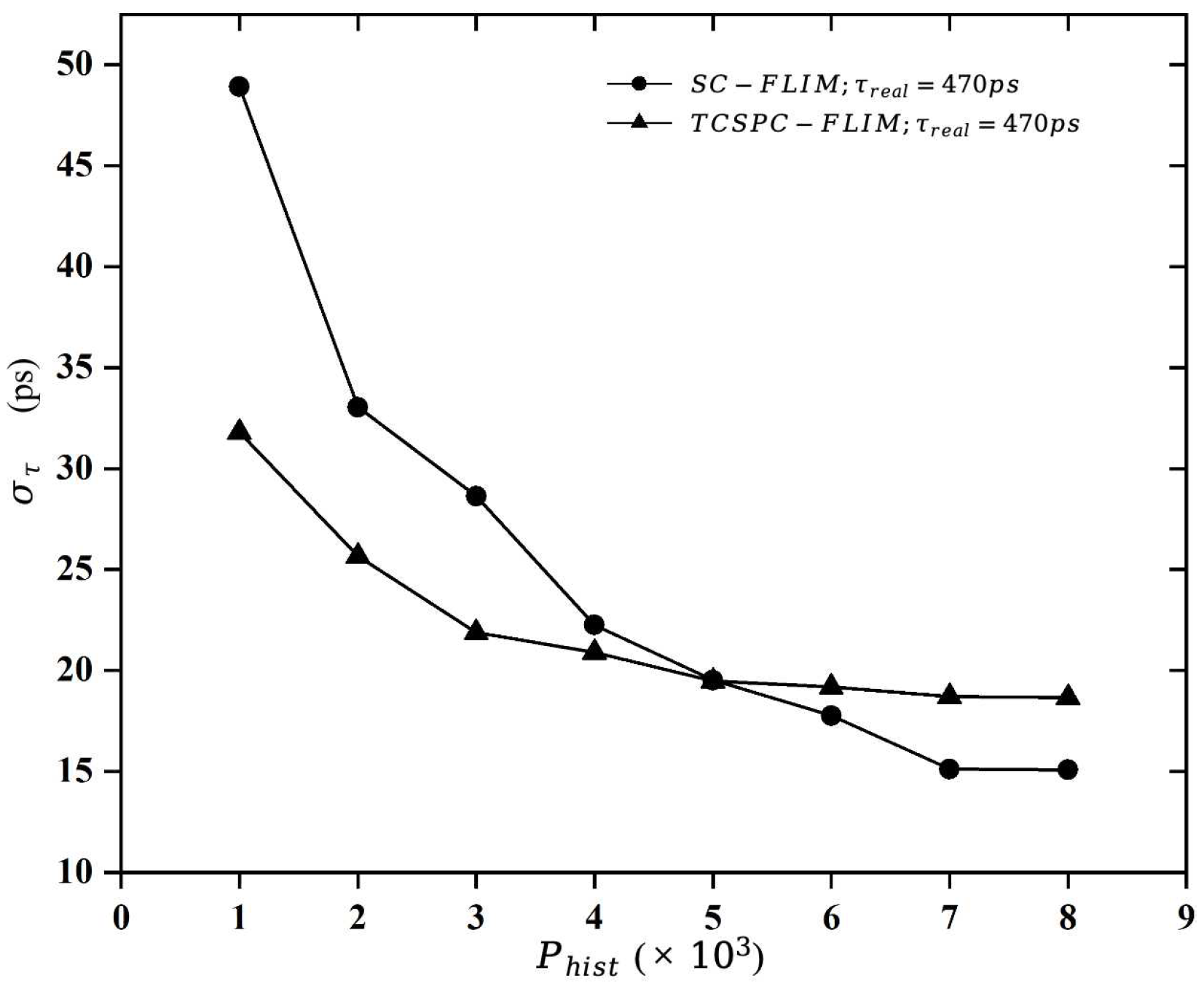
This study firstly generated a histogram of photon distribution based on the mono-exponential decay model and reconstructed the lifetime using the least-squares fitting algorithm to explore the relationship between the photon number and the fitting accuracy. Then, the study calculated the “Required Photons” according to the fitting error requirement of a standard fluorescent dye, Erythrosin B.
The mono-exponential decay model of fluorescence signal can be expressed as follows:
where
is the lifetime,
is the initial intensity, and
is the fluorescence intensity at time
t. In the numerical simulation, the uniformly distributed random numbers
are initially converted into mono-exponentially distributed random numbers,
t, based on the inverse transformation sampling method:
The number of mono-exponentially distributed random numbers,
t, represents the number of photons, and the value of
t represents the photon arrival time. Subsequently, a histogram of the photon arrival time distribution is generated through the mono-exponentially distributed random numbers,
t, based on the number of time bins and the width of the time bin. The simulation parameters of the FLIM system are set as shown in
Table 3. After the photons are dispersed into different time bins, the fluorescence decay curve is a discrete curve that can be expressed as
.
In a practical FLIM system, the laser pulse, the detector, and the electronics system all have their response times. In order to simulate the measurement signal more realistically, the instrument response function (IRF) is convolved with
to form the measurement signal
[
32]:
where
is a system noise obeying a Gaussian distribution, and
c is a low-frequency background noise. The IRF for two FLIM systems is defined in reference [
33]:
Among them, represents the full width at half the maximum of the IRF, follows a uniform distribution in the range of , and is the temporal resolution of the system. Histograms of fluorescence decay with different values are generated by varying . Then, the lifetime is calculated using the least-squares fitting method.
The ultimate goal of the numerical simulation experiment is to determine the “required photons”, which is the minimum number of photons required to form a fluorescence decay histogram (
). Suppose that the real value of the lifetime is set to
and the number of photons to
in each of the
M simulation experiments. Then, the standard deviation of the measured lifetime
is expressed as follows:
where
is the fitting lifetime of the k-th experiment. When
satisfies the requirements of the experimental scenario, the minimum value of
is the “required photons”.
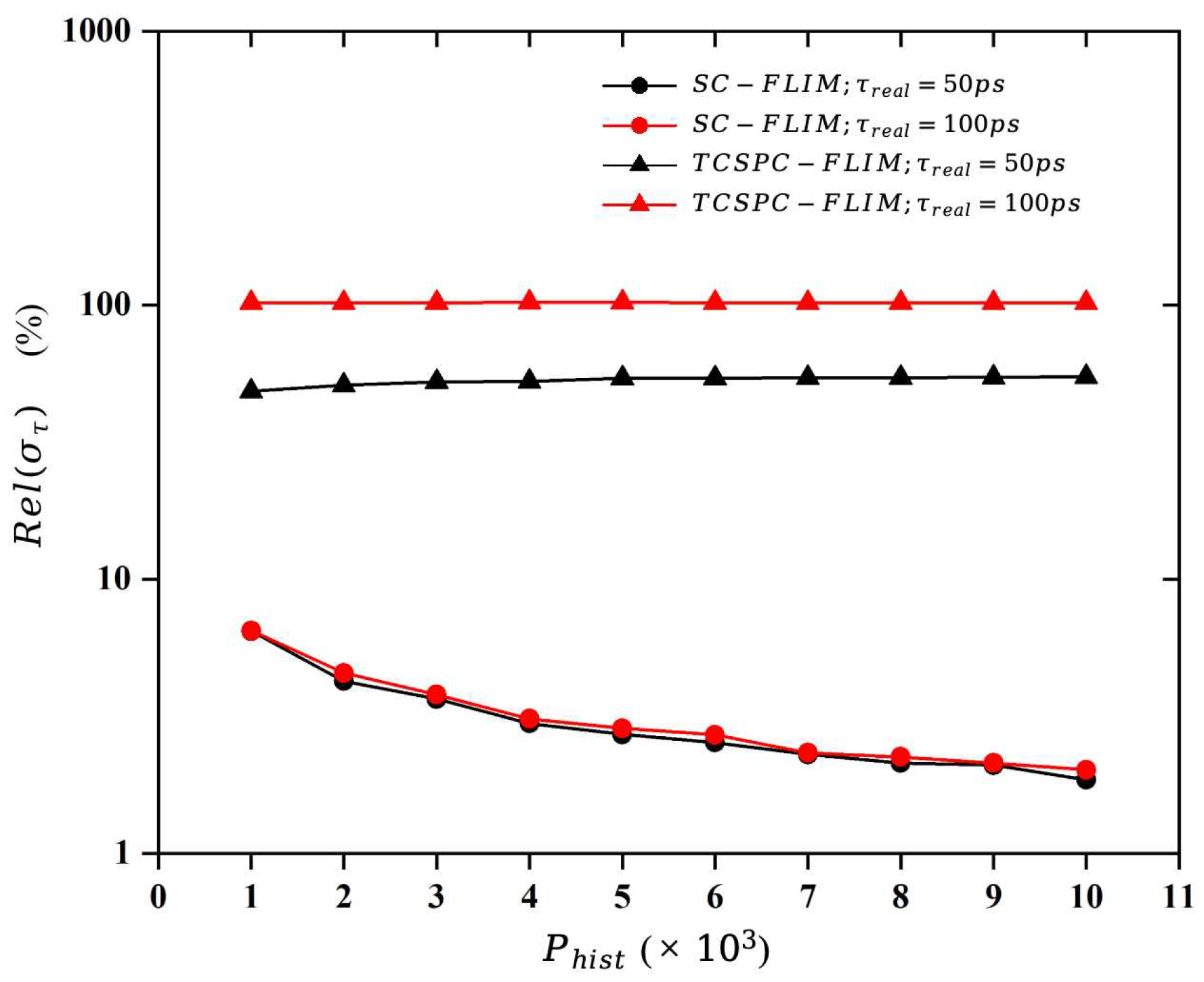
In the numerical simulation, the applicability of the two FLIM systems to the length of a lifetime is demonstrated by means of the relative standard deviation, which is defined as follows:
3.4. Calculation of the Number of Parallel Channels
The conventional TCSPC-FLIM system employs a pixel-by-pixel scanning approach to achieve 2D-FLIM, whereas the wide field TCSPC-FLIM utilizes linear or planar array detectors, which no longer permit pixel-by-pixel scanning for 2D-FLIM. It can be observed that the number of parallel channels in a TCSPC-FLIM system is determined by the number of single-photon detectors. However, the synchroscan streak camera has a 1D-FLIM capability, and the number of parallel channels is related to the resolution of the readout camera (RC).
In a synchroscan streak camera, the number of parallel channels is less than or equal to the number of pixels in the x-direction (
) of the readout frame, as illustrated in
Figure 6. This subsection needs to solve the problem of setting the spatial magnification of the fluorescence focusing system, the input optical module, the streak tube, and the output optical module so that the SC-FLIM system can maximize the number of parallel channels with a fixed sample spatial resolution (
) and a fixed readout frame size (
, and the pixel size is
). The number of parallel channels is greatest when the fluorescence emitted from an area of
on the sample can be captured via a pixel sensor with a size of
on the readout camera.
The function of the slit is to perform a 1D sampling of spatial light signals. Consequently, the light passing through the slit only retains width information. The slit width is defined as
, and when Equation (
20) is satisfied, all light passing through the slit can be recorded via the readout camera.
Among them, and are the magnification factors of the streak tube and the output optical system, respectively. Typically, is fixed before the streak camera leaves the factory. is affected by the area and distance of the effective detection area of the readout camera. Adjusting the enables the capture of all light signals passing through the slit with the readout camera. This maximizes the utilization of the effective imaging area of the readout camera.
The number of parallel channels is equal to
, the role of
is to ensure that the system has the required sample spatial resolution (C × C), and C can be expressed as follows:
Under the assumption that, in a FLIM experiment, the required spatial resolution is 10
m × 10
m (C = 10
m), the slit width is 5 cm (
cm), and the frame size is 1000 × 1000 (
), then
is as follows when NPC = 1000:
To ensure the one-dimensionality of the spatial signal, the slit height (
) is typically a few microns to a dozen microns. However, during weak signal measurements,
can be increased appropriately, provided that the following requirements are met:
3.5. Calculation of the Total Acquisition Time
In 2D-FLIM, the total acquisition time of a TCSPC-FLIM system is determined by the single-channel acquisition time (
), the scanning speed (represented by the dwell time (DT) of the scanner), and the number of parallel channels (NPC). When a resolution of 2D-FLIM is assumed to be
, the total acquisition time can be expressed as follows:
In the synchroscan SC-FLIM system, there is a mutual constraint between the speed of movement of the scanning device and the exposure time of the readout camera. Specifically, when the exposure time of the readout camera is long, the speed of the scanning device must be slowed down accordingly to ensure that, at each scanning position, the readout camera is able to fully complete the data acquisition and recording. Therefore, the total acquisition time for synchroscan SC-FLIM can be expressed as Equation (
25), where
is the exposure time of the readout camera.
5. Discussion
The acquisition time of FLIM systems is related to the photon emission rate, the photon counting rate, the number of “required photons”, the dwell time of the scanning device, and the number of parallel channels. Therefore, we provide a detailed analysis of these factors through numerical simulation and theoretical modeling, and we quantitatively compare the acquisition time of the TCSPC-FLIM and SC-FLIM systems when Erythromycin B is used as a sample. The results of this paper quantitatively illustrate that the synchroscan streak camera with a unique lifetime measurement approach has great potential for implementing Fast-FLIM, which has an acquisition speed about 14,000 times faster than the TCSPC-FLIM system.
Compared with TCSPC technology, the synchroscan streak camera is more suitable for the fluorescence lifetime imaging of samples with high fluorescence intensity and a short fluorescence lifetime. Although the acquisition speed of the TCSPC-FLIM system based on the SPAD array is similar to that of the SC-FLIM system (with a low photon detection rate), the TCSPC-FLIM system based on the SPAD array has a low spatial resolution and a low SNR for the acquired signal. As the readout camera frame rate increases, the acquisition speed of the SC-FLIM system will also be further improved. In addition, the synchroscan streak camera commonly has the single-photon detection mode of operation and a broader spectral response range, which can adapt to a more comprehensive range of application scenarios.
In the future, with the continuous development of streak camera technology, spatial scanning technology, and high frame rate camera technology, as well as the application of deep learning algorithms in lifetime fitting, synchroscan streak cameras will be able to be effectively applied to Fast-FLIM. This advancement will enable FLIM technology to play a crucial role in clinical diagnosis and industrial detection.