Non-Pharmacological Therapies for Management of Temporomandibular Myofascial Pain Syndrome: Laser Photobiomodulation or Dry Needling? Meta-Analyses of Human Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy for the Systematic Review
2.1.1. Research Question
- Is photobiomodulation treatment, which utilizes diode lasers with wavelengths ranging from 600 to 1200 nm, efficacious in treating myofascial pain associated with temporomandibular disorder (TMD) Axis 1?
- Does the DN method effectively control TPs in TMJD/MPS?
- Which therapy, PBMT or DNT, is more beneficial in treating myogenic pain of the temporomandibular joint (TMJ) in terms of efficacy, speed of recovery, and continuity of results?
2.1.2. Systematic Search Strategy
- PubMed/Medline electronic database;
- COCHRANE LIBRARY;
- ScienceDirect;
- Scopus;
- Google Scholar.
- Full-text clinical trials (CTs) or randomized clinical trials (RCTs) either blinded or not.
- Studies published in the English language in peer-reviewed journals between 1 January 2010 and 1 January 2024.
- Human studies involving both female and male genders > 18 years old.
- Studies that have patients with myofascial pain resulting from Axis 1 of TMD or MPS according to the RDC/TMD classification, regardless of the quality of life.
- Studies that compare extraoral photobiomodulation (PBM) therapy using diode lasers (600–1200 nm) with placebo or sham laser.
- Studies that compare SDN or DDN therapy with placebo.
- Studies that compare laser PBMT (600–1200 nm) with DNT in the management of TMJ myofascial pain.
- Systematic reviews and meta-analyses.
- In vitro studies.
- Studies that focus on specific age groups, such as adolescents or elders.
- Studies that contain subjects younger than 18 years old.
- Studies that have patients with a medical history of cancers or other syndromes in the head and neck region.
- Studies that have patients with pain not related to Axis 1 of TMD according to the RDC/TMD classification.
- Studies that use LEDs, or other laser equipment except diodes.
- Studies that use different laser wavelengths except 600–1200 nm.
- Studies that do not adopt the RDC/TMD classification in the diagnosis of TMJ diseases.
- Comparative studies that compare PBM with any aspect of therapy except the DN technique.
- Studies that adopt home PBM protocol.
- Patents, degrees, or doctoral theses.
- Republished articles or duplicated articles.
2.1.3. Study Selection and Data Extraction
2.2. Study Quality Assessment
2.2.1. PRISMA Guidelines
2.2.2. Risk of Bias
2.2.3. Meta Analysis of the Data
3. Results
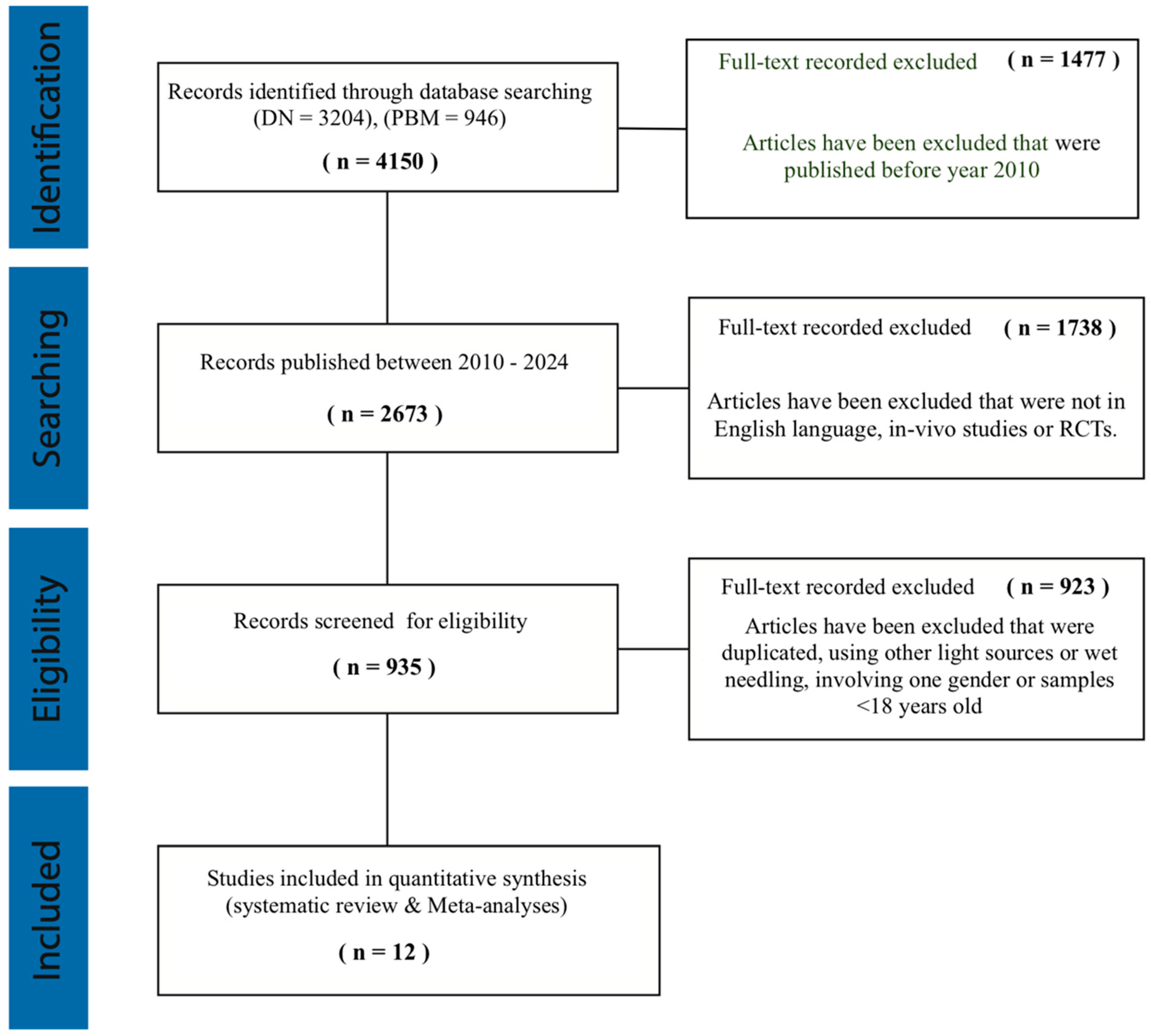
3.1. Literature Search Outcome
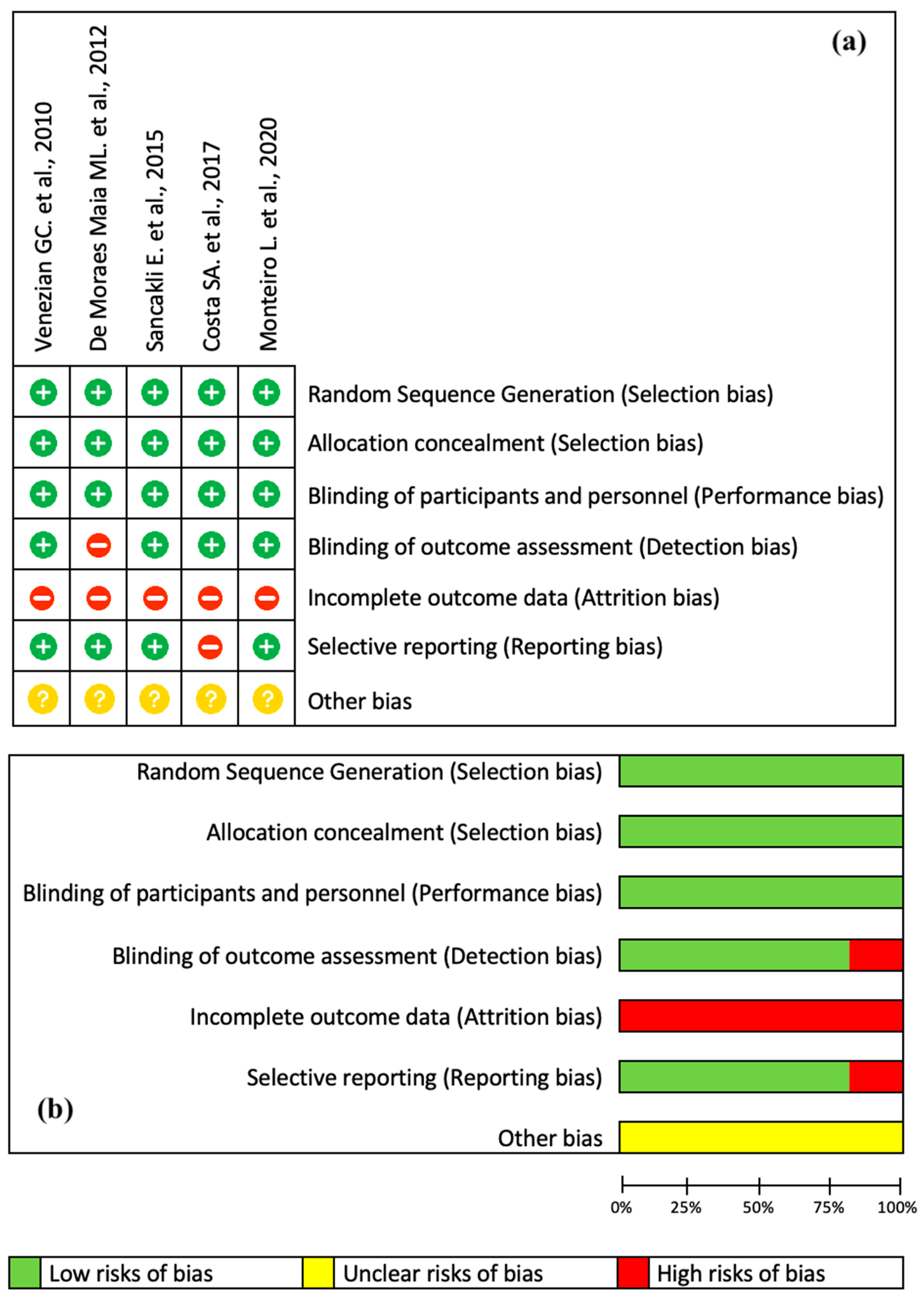
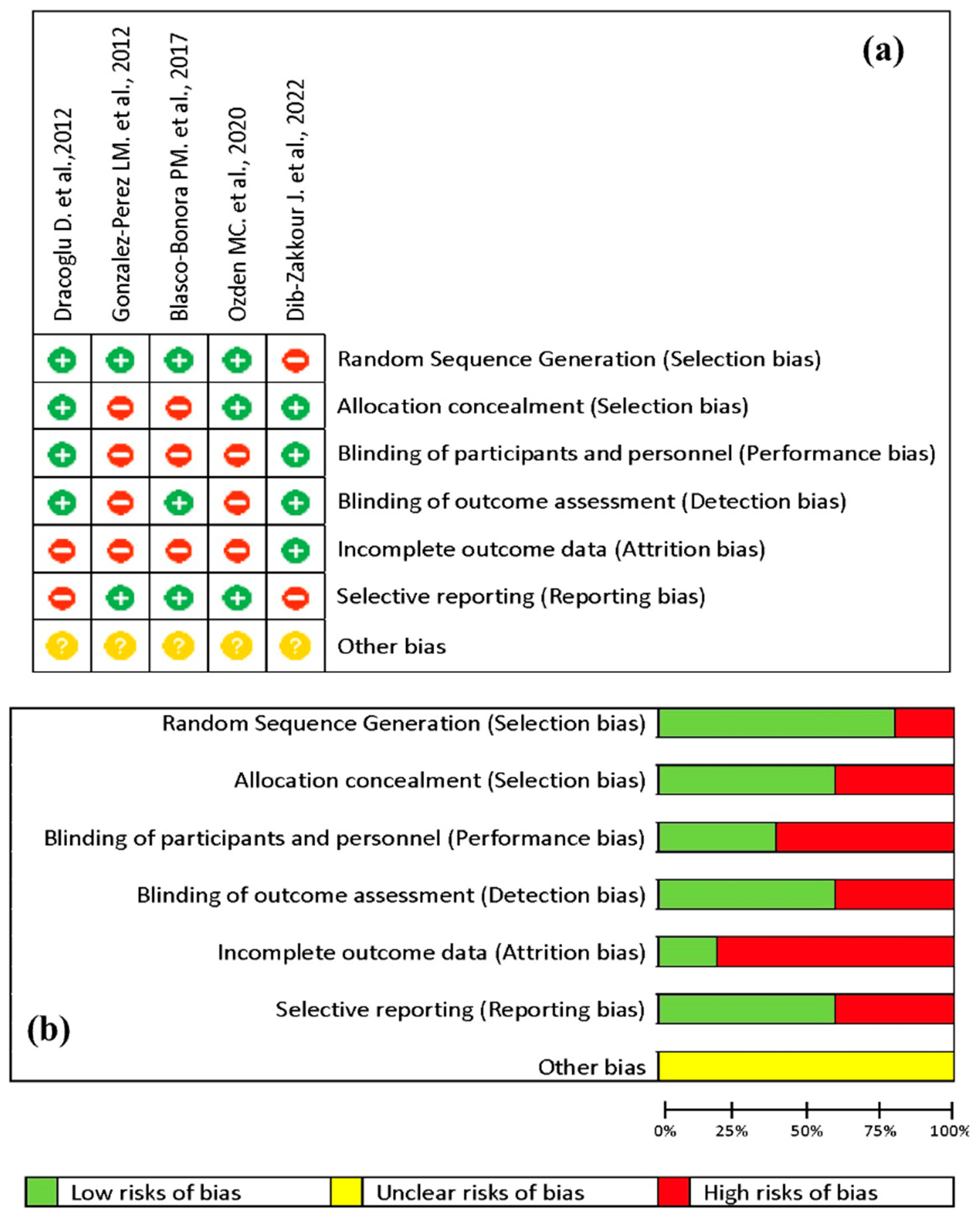
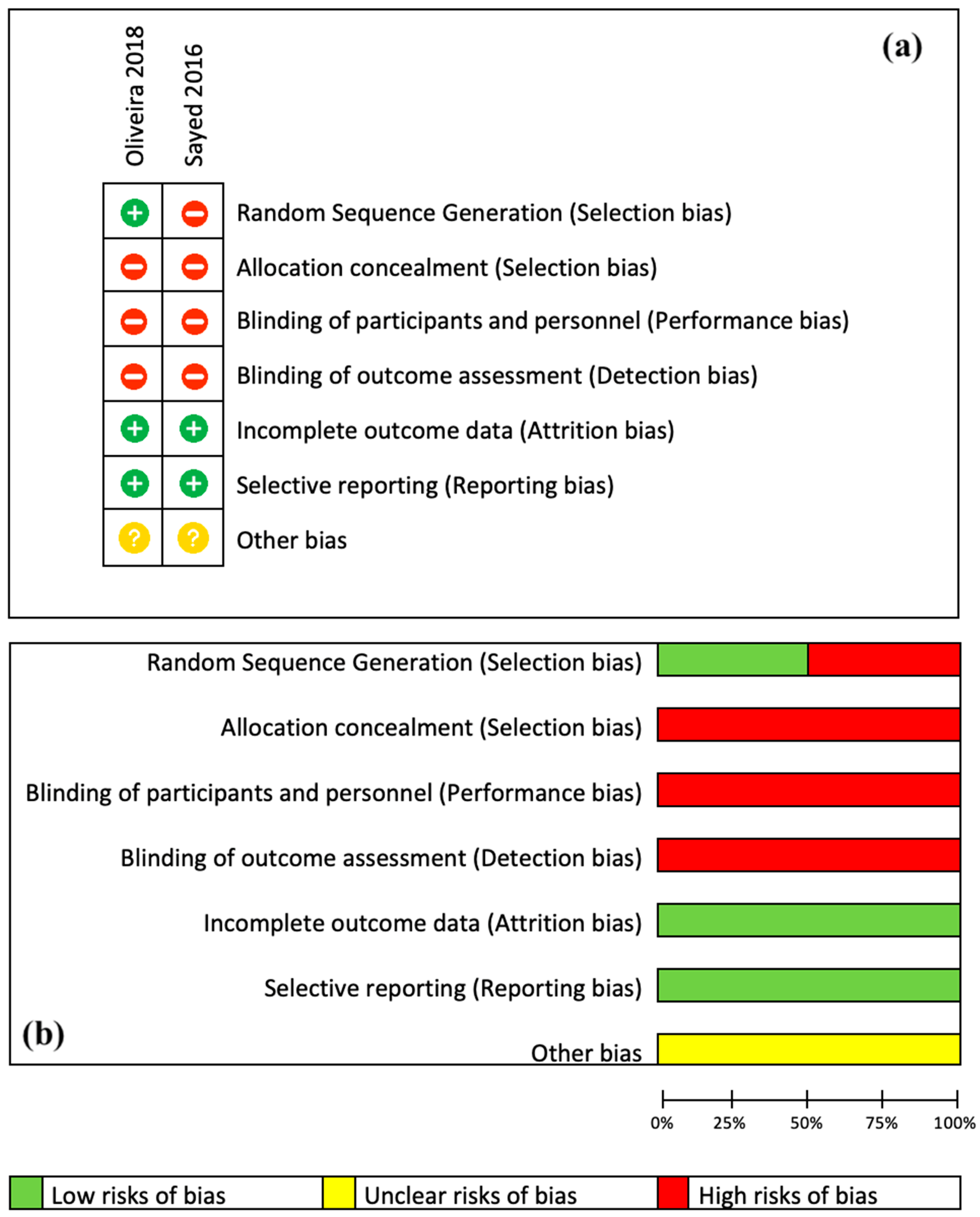
3.2. Risk of Bias
3.3. Study Characteristics
3.4. Meta-Analysis Results
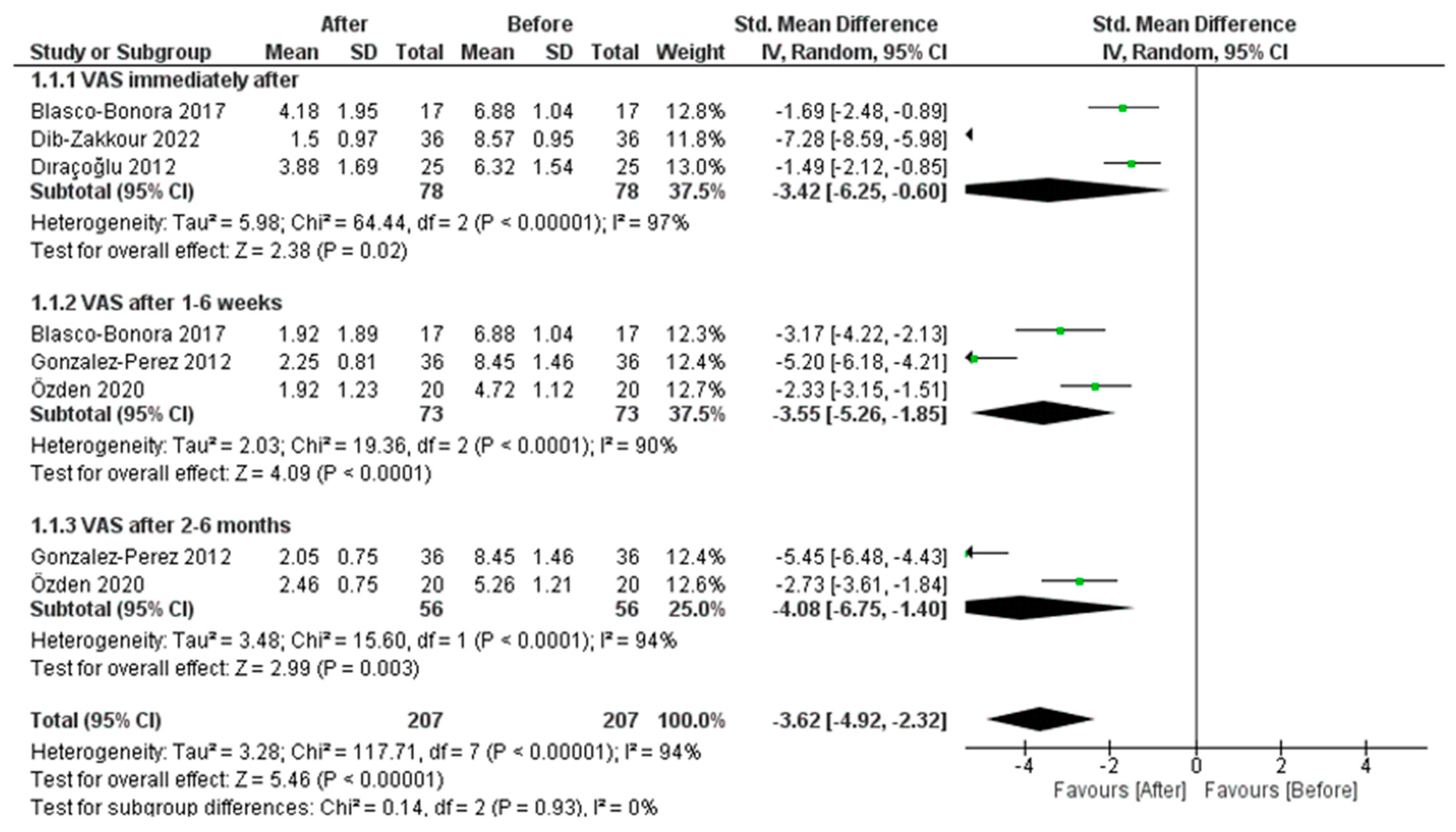
3.4.1. PBM Effect on Pain Measured by VAS
3.4.2. PBM Effect on Pressure Pain Threshold
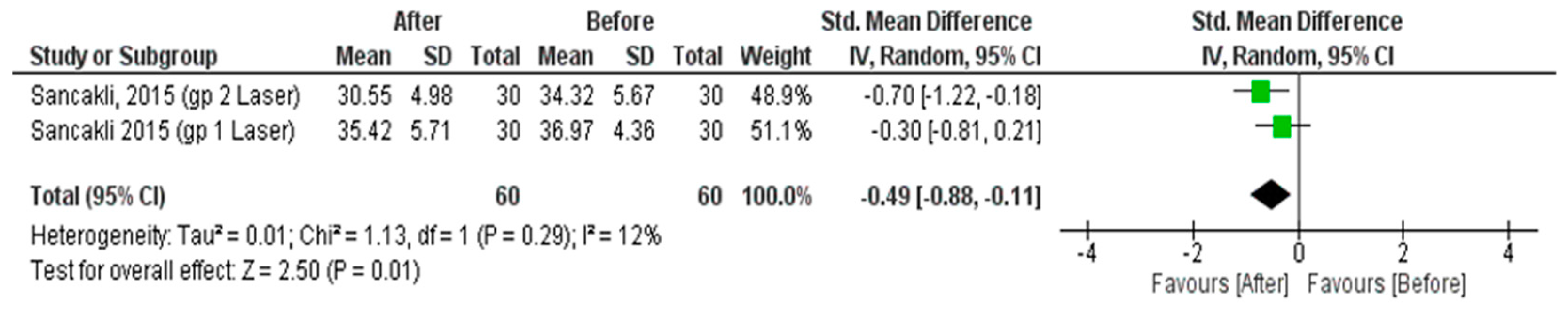
3.4.3. Dry Needle Effect on Pain Measured by VAS
3.4.4. Dry Needle Effect on Pressure Pain Threshold
3.4.5. Comparison between Dry Needle and PBM Therapies
3.5. Systematic Analysis of Results
4. Discussion
4.1. Exploring the Mechanisms and Practical Uses of the Anti-Inflammatory Properties of Photobiomodulation
4.2. Possible Management of Orofacial Pain with PBMT Compared to DNT
- Failing to assess the same set of masticatory muscles. Several studies examined the masseter muscle [51,52,53,54], while others investigated both the masseter and temporal muscles [43,44,45,46,47,48,50]. Additionally, one study specifically examined the lateral pterygoid muscle [49]. Monteiro et al. [47] evaluated the pain levels following laser PBM treatment in the masseter, temporal, lateral pterygoid muscles, and temporalis ligament. Furthermore, assessing the degrees of muscular pathology can be regarded as a complex task for evaluation.
- In the DN studies [48,49,50,51,52], the authors treated the trigger points identified by patients without specifying the number of ones treated per patient, leading to an indeterminate number of needles used in each session. The investigations [48,49,52,53,54] did not describe the depth of needle entry, either. Likewise, the comparison of trigger-point locations and pain intensity levels among participants, as well as across various clinical trials, presents substantial obstacles that may compromise the integrity and reliability of the quality of the articles.
- The studies [46,50,52] had a single treatment session, which may significantly impact the long-term viability of their findings. Other authors recommended using palliative methods such as thermotherapy and exercising following the treatment sessions. These procedures can potentially enhance the joint’s self-healing ability and may impact the accuracy of their findings [53,54].
- The included studies dealt with both genders, except Oliveira et al. [54], who treated 10 women. Studies have shown that gender characteristics may have an important impact on the course and outcomes of the therapies. The prevalence of TMD decreases after menopause, indicating a significant relationship with the hormonal oscillation, and it is higher in women of reproductive age. Estrogen and prolactin, which are present in higher concentrations in women, can heighten the symptoms of TMJ dysfunction. These hormones can accelerate the breakdown of articular cartilage and bone, triggering a cascade of immunological reactions within these joints. One contributing factor is the higher prevalence of psychosomatic disorders in women, which is a direct result of their higher stress indices compared to men [8,11,12].
- The presence of diverse operating laser characteristics, as seen in Table 3 and Table 7, highlights the lack of consistency in delivering a valid, reliable, and accurate PBM protocol and doses. Furthermore, the variations in laser operational parameters, the manner of laser application, and other factors will undoubtedly impact the reliability and consistency of the results. For instance, a key element to take into account is the velocity of manual motion, which was not addressed in any of the research considered. The speed of the application is crucial, as it allows for enhanced regulation of the energy release onto the tissue. A gradual and slow motion of the hand enables a higher energy discharge per unit of surface area over a period of time. Conversely, a rapid movement may result in an inefficient transmission of photonic energy. Furthermore, the clinical experiments presented do not demonstrate consistency in the fluence or the amount of energy density supplied per trigger site. The energy density is a vital parameter that governs all interactions between lasers and tissues. It denotes the quantity of energy transferred to a given region within a specific timeframe. Typically, clinically evaluating this metric is usually quite challenging. The variables that have a direct correlation with this include the velocity of manual motion, the length of the procedure, the size of the fiber tip, and the concept of focusing or defocusing the laser beam in a Gaussian-profile delivery system [80]. Consequently, the general acceptance of PBM therapy as a viable treatment for managing severe disorders like TMD/MPS pain would be restricted. It is important to be aware that TMD/MPS disorders vary in intensity, and certain acute symptoms may improve on their own without treatment. Occasionally, they have a tendency to restrict themselves, and as a result, they may spontaneously improve without any external intervention in certain instances [6,8].
- Another important factor to consider is the utilization of an optical power meter, which is a device used to measure the optical power (the amount of energy delivered per unit of time) in a light beam, such as a laser beam. It is widely recognized that light gradually dissipates its energy over time, and this principle also applies to laser light. Several factors can contribute to a decrease in power, such as unclean optics, electrical issues, and a limited lifespan [81]. The majority of the PBM research studies included (five out of seven) did not specify the utilization of a power meter. In these studies, the average power levels examined may be less precise.
- Furthermore, none of the laser studies included provided any information regarding the specific properties of the beam profile, which undermines the ability to replicate the therapy in the selected research. The energy density delivered into the treated area is directly correlated with the beam profile. Using a traditional laser handpiece, the spatial beam profile naturally follows a Gaussian distribution. Typically, as the distance between the tip and the tissue increases, the energy density decreases [82]. This variable poses a significant difficulty for researchers and is a crucial consideration to take into account in PBM studies [83]. Utilizing a flat-top handpiece may effectively address and resolve these problems [80].
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Desai, A.P.; Roy, S.K.; Semi, R.S.; Balasundaram, T. Efficacy of Low-Level Laser Therapy in Management of Temporomandibular Joint Pain: A Double-Blind and Placebo-Controlled Trial. J. Maxillofac. Oral Surg. 2022, 21, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Alsarhan, J.; El Feghali, R.; Alkhudari, T.; Benedicenti, S.; Pasquale, C. Can Photobiomodulation Support the Management of Temporomandibular Joint Pain? Molecular Mechanisms and a Systematic Review of Human Clinical Trials. Photonics 2022, 9, 420. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Vier, C.; Almeida, M.B.; Neves, M.L.; Santos, A.R.S.D.; Bracht, M.A. The effectiveness of dry needling for patients with orofacial pain associated with temporomandibular dysfunction: A systematic review and meta-analysis. Braz. J. Phys. Ther. 2019, 23, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Chaurand, J.; Pacheco-Ruíz, L.; Orozco-Saldívar, H.; López-Valdés, J. Efficacy of botulinum toxin therapy in treatment of myofascial pain. J. Oral Sci. 2017, 59, 351–356. [Google Scholar] [CrossRef]
- Kalladka, M.; Young, A.; Khan, J. Myofascial pain in temporomandibular disorders: Updates on etiopathogenesis and management. J. Bodyw. Mov. Ther. 2021, 28, 104–113. [Google Scholar] [CrossRef]
- Peck, C.C.; Goulet, J.P.; Lobbezoo, F.; Schiffman, E.L.; Alstergren, P.; Anderson, G.C.; de Leeuw, R.; Jensen, R.; Michelotti, A.; Ohrbach, R.; et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J. Oral Rehabil. 2014, 41, 2–23. [Google Scholar] [CrossRef]
- Golanska, P.; Saczuk, K.; Domarecka, M.; Kuć, J.; Lukomska-Szymanska, M. Temporomandibular Myofascial Pain Syndrome-Aetiology and Biopsychosocial Modulation. A Narrative Review. Int. J. Environ Res. Public Health 2021, 18, 7807. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Nijs, J. Trigger point dry needling for the treatment of myofascial pain syndrome: Current perspectives within a pain neuroscience paradigm. J. Pain Res. 2019, 12, 1899–1911. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Dommerholt, J. Myofascial trigger points: Peripheral or central phenomenon? Curr. Rheumatol. Rep. 2014, 16, 395. [Google Scholar] [CrossRef]
- Alrizqi, A.H.; Aleissa, B.M. Prevalence of Temporomandibular Disorders Between 2015–2021: A Literature Review. Cureus 2023, 15, e37028. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.; Zaps, D.; Rüger, L.J.; Lehmeyer, L.; Freiberg, F.; Lang, P.M.; Irnich, D. Discrepancy between prevalence and perceived effectiveness of treatment methods in myofascial pain syndrome: Results of a cross-sectional, nationwide survey. BMC Musculoskelet. Disord. 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Svensson, P. Myofascial Temporomandibular Disorder. Curr. Rheumatol. Rev. 2015, 12, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Kuć, J.; Szarejko, K.D.; Gołębiewska, M. Evaluation of soft tissue mobilization in patients with temporomandibular disorder-myofascial pain with referral. Int. J. Environ. Res. Public Health 2020, 17, 9576. [Google Scholar] [CrossRef]
- List, T.; Jensen, R.H. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia 2017, 37, 692–704. [Google Scholar] [CrossRef]
- Fricton, J. Myofascial Pain: Mechanisms to Management. Oral Maxillofac. Surg. Clin. N. Am. 2016, 28, 289–311. [Google Scholar] [CrossRef]
- Brighenti, N.; Battaglino, A.; Sinatti, P.; Abuín-Porras, V.; Sánchez Romero, E.A.; Pedersini, P.; Villafañe, J.H. Effects of an Interdisciplinary Approach in the Management of Temporomandibular Disorders: A Scoping Review. Int. J. Environ. Res. Public Health. 2023, 20, 2777. [Google Scholar] [CrossRef]
- Herranz-Aparicio, J.; Vázquez-Delgado, E.; Arnabat-Domínguez, J.; España-Tost, A.; Gay-Escoda, C. The use of low-level laser therapy in the treatment of temporomandibular joint disorders. Review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e603–e612. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.; Wiland, P.; Shiau, Y.Y.; Paradowska-Stolarz, A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J. Headache Pain 2015, 16, 106. [Google Scholar] [CrossRef]
- Budakoti, A.; Puri, N.; Dhillon, M.; Ahuja, U.; Rathore, A.; Choudhary, A.; Kour, M. A comparative evaluation of the effectiveness of low-level laser therapy, ultrasound therapy, and transcutaneous electric nerve stimulation in the treatment of patients with TMDs: A prospective study. Laser Dent. Sci. 2019, 3, 257–267. [Google Scholar] [CrossRef]
- Yehoshua, I.; Rimon, O.; Mizrahi Reuveni, M.; Peleg, R.; Adler, L. Dry needling for the treatment of acute myofascial pain syndrome in general practitioners’ clinics: A cohort study. BMC Prim. Care 2022, 23, 339. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.D.; Bavaresco, C.S.; Grossmann, E. The use of acupuncture versus dry needling in the treatment of myofascial temporomandibular dysfunction. Rev. Dor. 2017, 18, 342–349. [Google Scholar] [CrossRef]
- Halle, J.S.; Halle, R.J. Pertinent dry needling considerations for minimizing adverse effects—Part One. Int. J. Sports Phys. Ther. 2016, 11, 651–662. [Google Scholar] [PubMed]
- Ansari, M.; Baradaran Mahdavi, S.; Vahdatpour, B.; Lahijanian, A.; Khosrawi, S. Effects of Dry Needling and Low-Power Laser for the Treatment of Trigger Points in the Upper Trapezius Muscle: A Randomized Clinical Trial. J. Chiropr. Med. 2022, 21, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Gattie, E.; Cleland, J.A.; Snodgrass, S. The Effectiveness of Trigger Point Dry Needling for Musculoskeletal Conditions by Physical Therapists: A Systematic Review and Meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 133–149. [Google Scholar] [CrossRef]
- Brady, S.; McEvoy, J.; Dommerholt, J.; Doody, C. Adverse events following trigger point dry needling: A prospective survey of chartered physiotherapists. J. Man. Manip. Ther. 2014, 22, 134–140. [Google Scholar] [CrossRef]
- Boyce, D.; Wempe, H.; Campbell, C.; Fuehne, S.; Zylstra, E.; Smith, G.; Wingard, C.; Jones, R. Adverse events associated with therapeutic dry needling. Int. J. Sports Phys. Ther. 2020, 15, 103–113. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation or low-level laser therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation affects key cellular pathways of all life-forms: Considerations on old and new laser light targets and the calcium issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying mechanism and clinical applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [PubMed]
- El Feghali, R.; Tatarian, K.; Zogheib, C.; Benedicenti, S.; Pasquale, C.; Amaroli, A. The 1064-nm Nd: YAG photobiomodulation vs. 20% benzocaine topical gel in inducing mucosal anaesthetic effect: A double-blind randomized clinical trial. Photonics 2022, 9, 519. [Google Scholar] [CrossRef]
- Ferrando, S.; Agas, D.; Mirata, S.; Signore, A.; De Angelis, N.; Ravera, S.; Utyuzh, A.S.; Parker, S.; Sabbieti, M.G.; Benedicenti, S.; et al. The 808 nm and 980 nm infrared laser irradiation affects spore germination and stored calcium homeostasis: A comparative study using delivery hand-pieces with standard (Gaussian) or flat-top profile. J. Photochem. Photobiol. B. 2019, 199, 111627. [Google Scholar] [CrossRef]
- Amaroli, A.; Agas, D.; Laus, F.; Cuetri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The effects of photobiomodulation of 808 nm diode laser therapy at higher fluence on the in vitro osteogenic differentiation of bone marrow stromal cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Assis, L.; Moretti, A.I.; Abrahao, T.B.; Cury, V.; Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg. Med. 2012, 44, 726–735. [Google Scholar] [CrossRef]
- Gonzalez-Perez, L.M.; Infante-Cossio, P.; Granados-Nunez, M.; Urresti-Lopez, F.J.; Lopez-Martos, R.; Ruiz-Canela-Mendez, P. Deep dry needling of trigger points located in the lateral pterygoid muscle: Efficacy and safety of treatment for management of myofascial pain and temporomandibular dysfunction. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e326–e333. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carnero, J.; La Touche, R.; Ortega-Santiago, R.; Galan-del-Rio, F.; Pesquera, J.; Ge, H.Y.; Fernández-de-Las-Peñas, C. Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J. Orofac. Pain 2010, 24, 106–112. [Google Scholar]
- Carrasco, T.G.; Guerisoli, L.D.; Guerisoli, D.M.; Mazzetto, M.O. Evaluation of low intensity laser therapy in myofascial pain syndrome. Cranio 2009, 27, 243–247. [Google Scholar] [CrossRef]
- Uemoto, L.; Garcia, M.A.; Gouvêa, C.V.; Vilella, O.V.; Alfaya, T.A. Laser therapy and needling in myofascial trigger point deactivation. J. Oral Sci. 2013, 55, 175–181. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, 1000097. [Google Scholar] [CrossRef]
- Berger, V.W.; Alperson, S.Y. A general framework for the evaluation of clinical trial quality. Rev. Recent. Clin. Trials 2009, 4, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Savovic, J.; Page, M.; Elbers, R.; Sterne, J. Assessing Risk of Bias in Randomized Trials. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019; pp. 205–228. [Google Scholar]
- Venezian, G.C.; da Silva, M.A.M.R.; Mazzetto, R.G.; Oliveira Mazzetto, M. Low level laser effects on pain to palpation and electromyographic activity in TMD patients: A double-blind, randomized, placebo-controlled study. Cranio 2010, 28, 84–91. [Google Scholar] [CrossRef]
- de Moraes Maia, M.L.; Ribeiro, M.A.; Maia, L.G.; Stuginski-Barbosa, J.; Costa, Y.M.; Porporatti, A.L.; Conti, P.C.; Bonjardim, L.R. Evaluation of low-level laser therapy effectiveness on the pain and masticatory performance of patients with myofascial pain. Lasers Med. Sci. 2012, 29, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sancakli, E.; Gökçen-Röhlıg, B.; Balık, A.; Öngül, D.; Kıpırdı, S.; Keskın, H. Early results of low-level laser application for masticatory muscle pain: A double-blind randomized clinical study. BMC Oral Health 2015, 15, 131. [Google Scholar] [CrossRef]
- Costa, S.A.P.; Florezi, G.P.; Artes, G.E.; Costa, J.R.D.; Gallo, R.T.; Freitas, P.M.; Witzel, A.L. The analgesic effect of photobiomodulation therapy (830 nm) on the masticatory muscles: A randomized, double-blind study. Braz. Oral Res. 2017, 31, e107. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Ferreira, R.; Resende, T.; Pacheco, J.J.; Salazar, F. Effectiveness of photobiomodulation in temporomandibular disorder-related pain using a 635 nm diode laser: A randomized, blinded, and placebo-controlled clinical trial. Photobiomodul. Photomed. Laser Surg. 2020, 38, 280–288. [Google Scholar] [CrossRef]
- Dıraçoğlu, D.; Vural, M.; Karan, A.; Aksoy, C. Effectiveness of Dry Needling for the Treatment of Temporomandibular Myofascial Pain: A Double-blind, Randomized, Placebo Controlled Study. J. Back Musculoskelet. Rehabil. 2012, 25, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, L.M.; Infante-Cossio, P.; Granados-Nuñez, M.; Urresti-Lopez, F.J. Treatment of temporomandibular myofascial pain with deep dry needling. Med. Oral Patol. Oral Cir. Buca. 2012, 17, e781–e785. [Google Scholar] [CrossRef]
- Blasco-Bonora, P.M.; Martín-Pintado-Zugasti, A. Effects of myofascial trigger point dry needling in patients with sleep bruxism and temporomandibular disorders: A prospective case series. Acupunct. Med. 2017, 35, 69–74. [Google Scholar] [CrossRef]
- Özden, M.C.; Atalay, B.; Özden, A.V.; Çankaya, A.; Kolay, E.; Yıldırım, S. Efficacy of dry needling in patients with myofascial temporomandibular disorders related to the masseter muscle. Cranio 2020, 38, 305–311. [Google Scholar] [CrossRef]
- Dib-Zakkour, J.; Flores-Fraile, J.; Montero-Martin, J.; Dib-Zakkour, S.; Dib-Zaitun, I. Evaluation of the Effectiveness of Dry Needling in the Treatment of Myogenous Temporomandibular Joint Disorders. Medicina 2022, 58, 256. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.; Refai, H.; Hassanin, N.; Zaky, A. Low-Level Laser Therapy versus Dry Needling for Inactivation of Myofascial Trigger Points. Egypt. Dent. J. 2016, 62, 539–545. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Pinto, R.; Reis, L.; Dias, I.; Leite, I.; Leite, F. Clinical effectiveness evaluation of laser therapy and dry needling in the treatment of patients with myofascial pain in masseter muscle. Int. J. Orofac. Myol. 2018, 44, 22–41. [Google Scholar] [CrossRef]
- Serrage, H.; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M.R. Under the spotlight: Mechanisms of photobiomodulation concentrating on blue and green light. Photochem. Photobiol. Sci. 2019, 18, 1877–1909. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Cardoso, F.d.S.; Mansur, F.C.B.; Araújo, B.H.S.; Gonzalez-Lima, F.; da Silva, S.G. Photobiomodulation Improves the Inflammatory Response and Intracellular Signaling Proteins Linked to Vascular Function and Cell Survival in the Brain of Aged Rats. Mol. Neurobiol. 2022, 59, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Guermonprez, C.; Peno-Mazzarino, L.; Lati, E.; Rousseaud, A.; Declercq, L.; Kerdine-Römer, S. Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation. Antioxidants 2023, 12, 766. [Google Scholar] [CrossRef]
- Ketz, A.K.; Byrnes, K.R.; Grunberg, N.E.; Kasper, C.E.; Osborne, L.; Pryor, B.; Tosini, N.L.; Wu, X.; Anders, J.J. Characterization of Macrophage/Microglial Activation and Effect of Photobiomodulation in the Spared Nerve Injury Model of Neuropathic Pain. Pain Med. 2017, 18, 932–946. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Benedicenti, S.; Bianco, B.; Bosco, A.; Vargas, M.R.C.; Hanna, R.; Ramakrishnan, P.K.; Raffetto, M.; Ravera, S. Electromagnetic Dosimetry for Isolated Mitochondria Exposed to Near-Infrared Continuous-Wave Illumination in Photobiomodulation Experiments. Bioelectromagnetics 2021, 42, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Floravanti, M.; Boccassini, A.; Gaimari, G.; Vestri, A.; Di Paolo, C.; Romeo, U. Evaluation of the efficacy of a new low-level laser therapy home protocol in the treatment of temporomandibular joint disorder-related pain: A randomized, double-blind, placebo-controlled clinical trial. Cranio 2021, 39, 141–150. [Google Scholar] [CrossRef]
- da Cunha, L.A.; Firoozmand, L.M.; da Silva, A.P.; Camargo, S.E.; Oliveira, W. Efficacy of low-level laser therapy in the treatment of temporomandibular disorder. Int. Dent. J. 2008, 58, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.C.; Scheffer, D.d.L.; Glaser, V.; Remor, A.P.; Oinho, R.A.; Aguiar Junior, A.S.; Latini, A. Low-level laser therapy attenuates the acute inflammatory response induced by muscle traumatic injury. Free Radic. Res. 2016, 50, 503–513. [Google Scholar] [CrossRef]
- Douplik, A.; Saiko, G.; Schelkanova, I.; Tuchin, V.V. The response of tissue to laser light. In Lasers for Medical Applications Diagnostics, Therapy and Surgery; Jelinkova, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 47–109. [Google Scholar] [CrossRef]
- Luo, L.Y.; Lee, J.; Li, K.Y.; Leung, Y.Y.; Li, D.T. Psychological outcomes on anxiety and depression after interventions for temporomandibular disorders: A systematic review and meta-analysis. Diagnostics 2023, 13, 653. [Google Scholar] [CrossRef]
- Wilkie, G.; Al-Ani, Z. Temporomandibular joint anatomy, function and clinical relevance. Br. Dent. J. 2022, 233, 539–546. [Google Scholar] [CrossRef]
- da Silva, M.A.; Botelho, A.L.; Turim, C.V.; da Silva, A.M. Low level laser therapy as an adjunctive technique in the management of temporomandibular disorders. Cranio 2012, 30, 264–271. [Google Scholar] [CrossRef]
- Barbero, M.; Schneebeli, A.; Koetsier, E.; Maino, P. Myofascial pain syndrome and trigger points: Evaluation and treatment in patients with musculoskeletal pain. Curr. Opin. Support Palliat. Care 2019, 13, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Brochado, F.T.; Jesus, L.H.; Martins, M.D.; Chaves, K.D. Non-invasive therapies for management of temporomandibular disorders: A systematic review. Clinic. Biomed. Res. 2019, 39, 230–243. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Ge, M.; Gao, M. Efficacy of low-level laser therapy in the treatment of TMDs: A meta-analysis of 14 randomised controlled trials. J. Oral Rehabil. 2015, 42, 291–299. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Y.; Huo, K.; Liu, J.; Huang, X.; Bao, J. Efficacy of laser therapy for temporomandibular disorders: A systematic review and meta-analysis. Complement. Ther. Med. 2023, 74, 102945. [Google Scholar] [CrossRef]
- Tesch, R.S.; Macedo, L.C.D.S.P.; Fernandes, F.S.; Goffredo Filho, G.S.; Goes, C.P.Q.F. Effectiveness of dry needling on the local pressure pain threshold in patients with masticatory myofascial pain. Cranio 2021, 39, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Salmos-Brito, J.A.; de Menezes, R.F.; Teixeira, C.E.; Gonzaga, R.K.; Rodrigues, B.H.; Braz, R.; Bessa-Nogueira, R.V.; Gerbi, M.E. Evaluation of low-level laser therapy in patients with acute and chronic temporomandibular disorders. Lasers Med. Sci. 2013, 28, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Pollo, A.; Colloca, L. Opioid-mediated placebo responses boost pain endurance and physical performance: Is it doping in sport competitions? J. Neurosci. 2007, 27, 11934–11939. [Google Scholar] [CrossRef]
- Amaroli, A.; Arany, P.; Pasquale, C.; Benedicenti, S.; Bosco, A.; Ravera, S. Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. Int. J. Mol. Sci. 2021, 22, 7788. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Bordin-Aykroyd, S.R.; Lynch, E. Systematic Review of Delivery Parameters Used in Dental Photobiomodulation Therapy. Photobiomodul. Photomed. Laser Surg. 2019, 37, 784–797. [Google Scholar] [CrossRef]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A Comparative Study Between the Effectiveness of 980 nm Photobiomodulation Delivered by Hand-Piece with Gaussian vs. Flat-Top Profiles on Osteoblasts Maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef]
- Sommer, A.P.; Pinheiro, A.L.; Mester, A.R.; Franke, R.P.; Whelan, H.T. Biostimulatory windows in low-intensity laser activation: Lasers, scanners, and NASA’s light-emitting diode array system. J. Clin. Laser Med. Surg. 2001, 19, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ilbuldu, E.; Cakmak, A.; Disci, R.; Aydin, R. Comparison of laser, dry needling, and placebo laser treatments in myofascial pain syndrome. Photomed. Laser Surg. 2004, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
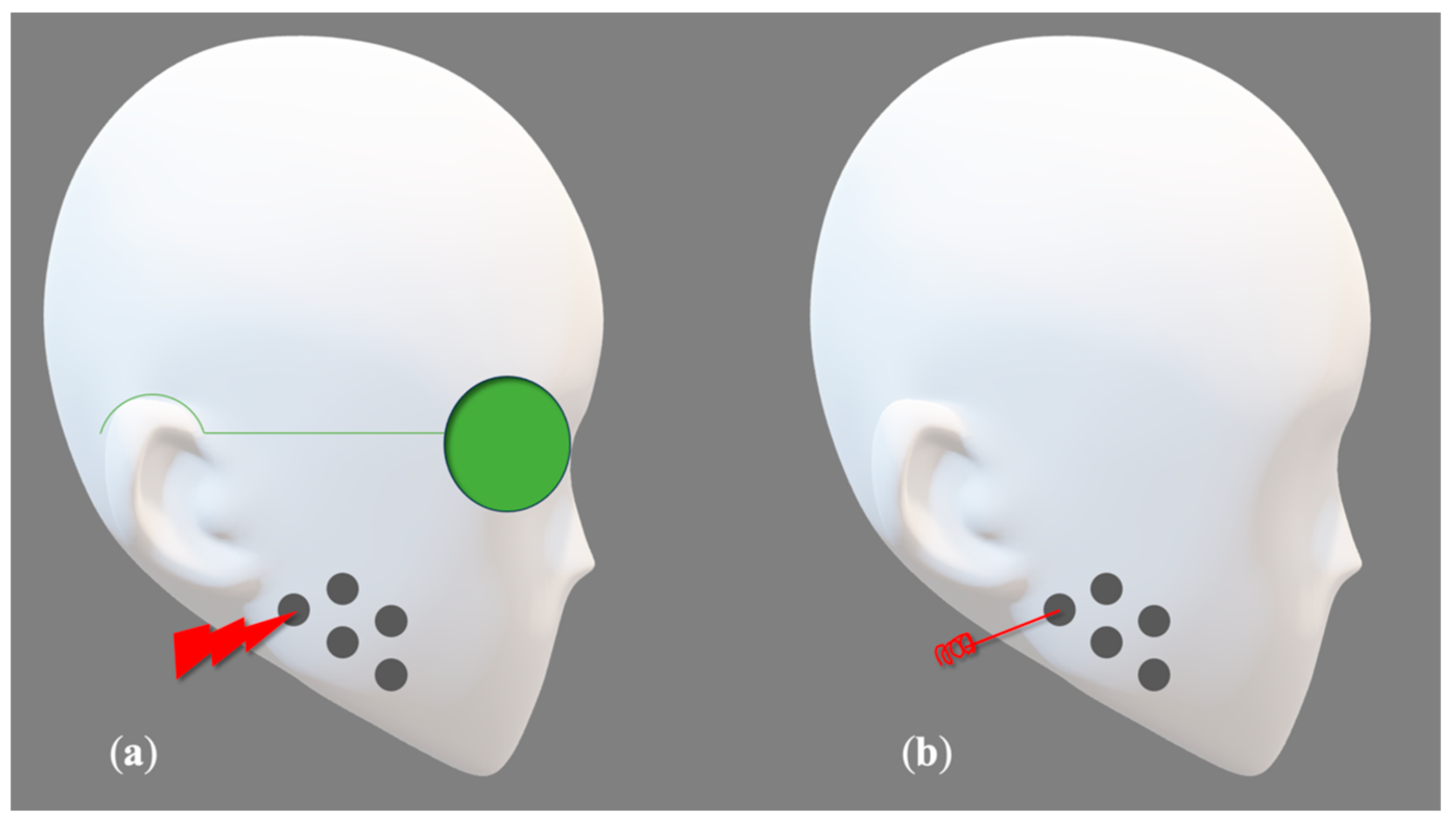



| RDC/TMD Classification |
|---|
| Axis 1: Muscular disorders, a—(with pain), b—(with pain and limited opening) |
| Axis 2: Disc Displacement disorders, a—(disc displacement with reduction) b—(disc displacement without reduction) |
| Axis 3: Any joint pain (arthralgia, osteoarthritis) |
| Author/Year | Groups | Number of Patients Gender Age | Number of Application | Points of Application | Variables | Scale | Follow Up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Venezian et al. (2010) [43] | 1. Diode (PBM) 2. Placebo. 3. Diode (PBM) 4. Placebo | 43 women 5 men 18–60 years | 2 times/week 4 weeks | Extra-orally 1 pt/Anterior Temporalis 3 pts/ Masseter (UM, MM, LM) | Pain surface electromyograph charging | VAS Electromyographic (EMG) device | Pain: Before After the treatment After 30 days of last treatment EMG: Before After the treatment | The results showed no significant statistical differences between groups for both variables. |
| De Moraes Maia et al. (2012) [44] | 1. Diode (PBM) 2. Placebo. | 19 women 2 men Mean of ages 27.76 + 10.44 | 2 times/week 4 weeks | Extra-Orally 5 pts/Masseter 5 pts/ Anterior Temporal | Pain intensity PPT MP | VAS Analog Compression Dynamometer Optocal tablets | Baseline Weekly End of the therapy 30 days of final session | The results showed no significant difference between the groups according to pain intensity. |
| Sancakli et al. (2015) [45] | 1. Diode (PBM) 2. Diode (PBM) 3. Placebo | 21 women 9 men 18–60 years | 3 times/week 4 weeks | Extra-orally 3pts/masseter 3pts/temporal | Pain intensity PPT MP | VAS Muscle palpation | Baseline End of the therapy | Laser groups showed significant reduction of pain and improvement in other Variables compared to placebo group. |
| Costa et al. (2017) [46] | 1. Diode (PBM) 2. Placebo | 54 women 6 men 18–76 years | Single session | Extra-orally: 3 pts/temporal 2 pts/ masseter | Pain Pain during muscular palpation MMO | VAS Muscular AlgometerDigital Caliper | Before After the treatment | The laser group showed a significant differences and improvement in pain reported with palpation compared to placebo group, while there was no significant improvement in range of mandibular movements. |
| Monteiro et al. (2020) [47] | 1. Diode (PBM) 2. Placebo | 32 women 10 men age > 18 | 1 time /week 4 weeks | Extra-orally: Trigger points determined by patients | Pain MMO Pain tenderness Mandibular movement (P, LL, RL) | VAS Patchmeter Muscular Palpation | Baseline 1 month after the last session | The laser group showed significant improvement for all variables compared to placebo group. |
| Study | Wavelength | Power | Tip Diameter | Irradiation Time | Speed of Movement | Tip-tissue Distance | Delivery Mode | Contact/ Non-Contact | Energy Density | Power Meter |
|---|---|---|---|---|---|---|---|---|---|---|
| Venezian et al. (2010) [43] | 780 nm (Ga Al As) | 50 mW 60 mW | NM | 20 s 40 s | NM | 0 mm | CW | Contact | 25 J/cm2 60 J/cm2 | No |
| De Moraes Maia et al. (2012) [44] | 808 nm (Ga Al As) | 100 mW | NM | 19 s/point | NM | 0 mm | CW | Contact | 70 J/cm2 | Yes |
| Sancakli et al. (2015) [45] | 820 nm (Ga Al As) | 300 mW | 6 mm | 10 s | NM | 2 mm | CW | Non-Contact | 3 J/cm2 | Yes |
| Costa et al. (2017) [46] | 830 nm | 100 mW | NM | 28 s/point | NM | 0 mm | CW | Contact | 100 J/cm2 | Yes |
| Monteiro et al. (2020) [47] | 635 nm | 200 mW | 8 mm | 20 s | NM | 0 mm | CW | Contact | 8 J/cm2 | Yes |
| Author/Year | Groups | Number of Patients Gender Age | Number of Application | Points of Application | Variables | Scale | Follow Up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Dracoglu et al. (2012) [48] | 1. DN 2. Placebo | 45 women 7 men 18–57 years | 1 time/week 3 weeks | Trigger points determined by patients in the masseter and temporalis muscles | Pain intensity PPT MO | VAS Pressure algometry Millimeter ruler | Baseline After one week of last session | The results showed there were no differences between the groups in terms of pain and mouth opening, while the needling group showed significant improvement in PPTcompared to placebo group. |
| Gonzales-Perez et al. (2012) [49] | 1. DN No control group | 30 women 6 men | 1 time/week 3 weeks | Trigger points determined by patients in the external pterygoid muscle | Pain Range of mandibular movement (MO, L, P) | VAS Therabite System | Before After 2 weeks After 1 month After 2 months After 6 months of last session | The results showed significant improvements for the variables After the therapeutic intervention |
| Blasco-Bonora et al. (2017) [50] | 1. DN No control group | 11 women 6 men 23–66 years | Single session | Trigger points determined by patients in the masseter and temporalis muscles | Pain PPT MMO Jaw disability | VAS MPA Millimeter ruler JDC list of RDC/TMD | Baseline After treatment for all Variables except jaw disability which was assessed after 1 week of the treatment. | The results showed significant improvements in the study group for all variables after the treatment. |
| Ozden et al. (2018) [51] | 1. SDN 2. DDN 3.Control group | 31 women 29 men 18–65 year | 1 time/week 3 weeks | Points of Application Trigger points determined by patients in the masseter muscle | Pain PPT MJO | VAS MA | Before At the third week At the sixth week of the last intervention | The results showed significant improvements in both groups. While SDN group showed significantly better Pain reduction compared to DDN group. |
| Dib-Zakkour et al. (2022) [52] | 1. DDN 2. Control group | 36 patients 18–40 years | Single session | Trigger points determined by patients in the masseter muscle | Pain Muscular palpation MO Articular sounds Tone of masseter muscle | VAS Algometer Digital caliper Auscultate Electromyography | Before After 10 min of the session After 15 days of the intervention | The results showed significant reduction in the fascial pain and muscle activity in the study group compared to controlling group. |
| Study | Needle Size | Needling Method (Superficial/Deep) | Penetration Depth | Number of Needles in One Session |
|---|---|---|---|---|
| Dracoglu et al. (2012) [48] | 0.22 × 30 mm | (Deep/Superficial) NM | NM | NM |
| Gonzales-Perez et al. (2012) [49] | 0.25 × 40 mm | Deep | NM | NM |
| Blasco-Bonora et al. (2017) [50] | 0.16 × 25 mm | NM | 15–25 mm | NM |
| Ozden et al. (2018) [51] | 0.25 × 25 mm | SDN DDN | SDN 5–10 mm DDN > 10 mm | NM |
| Dib-Zakkour et al. (2022) [52] | 0.30 × 30 mm | NM | NM | NM |
| Author/Year | Groups | Number of Patients Gender Age | Laser Parameters | Needling Method | Number of Application | Variable/Scale | Follow Up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Sayed S. et al. (2016) [53] | 1. PBM 2. DN | 17 women 1 man 18–42 years | Wavelength (980 nm) P (0,2 W) E (12 J) T (50 SEC) | 23 ×1.5 inch Depth 1–2 cm | 1 time/week 4 weeks | Pain intensity /NRS MMO/in mm using Vernier graduated caliper | Before After 2 weeks After 4 weeks | The results showed insignificant difference between the groups. |
| Oliveira D.A. et al. (2018) [54] | 1. PBM 2. DN | 10 women 18–70 years | Wavelength (660 nm) ED (40 J/cm2) AP (40 mW) E (1.6 J) T (40 sec) CW | 0.25× 30 mm Length 5 cm Time 1 min | 1 time/week 12 weeks | Pain/VAS MO/in millimeter ruler | Before After 1 week | The results showed insignificant difference between the groups. |
| Study | Wavelength | Power | Tip Diameter | Irradiation Time | Speed of Movement | Tip-Tissue Distance | Delivery Mode | Contact/ Non-Contact | Energy Density | Power Meter |
|---|---|---|---|---|---|---|---|---|---|---|
| Sayed et al. (2016) [53] | 980 nm | 200 mW | NM | 50 s/session | NM | NM | NM | NM | NM | NM |
| Oliveira et al. (2018) [54] | 660 nm | 40 mW | NM | 40 s/point | NM | 0 mm | CW | Contact | 40 J/cm2 | NM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsarhan, J.; El Feghali, R.; Alkhudari, T.; Benedicenti, S. Non-Pharmacological Therapies for Management of Temporomandibular Myofascial Pain Syndrome: Laser Photobiomodulation or Dry Needling? Meta-Analyses of Human Clinical Trials. Photonics 2024, 11, 965. https://doi.org/10.3390/photonics11100965
Alsarhan J, El Feghali R, Alkhudari T, Benedicenti S. Non-Pharmacological Therapies for Management of Temporomandibular Myofascial Pain Syndrome: Laser Photobiomodulation or Dry Needling? Meta-Analyses of Human Clinical Trials. Photonics. 2024; 11(10):965. https://doi.org/10.3390/photonics11100965
Chicago/Turabian StyleAlsarhan, Jumana, Rita El Feghali, Thaer Alkhudari, and Stefano Benedicenti. 2024. "Non-Pharmacological Therapies for Management of Temporomandibular Myofascial Pain Syndrome: Laser Photobiomodulation or Dry Needling? Meta-Analyses of Human Clinical Trials" Photonics 11, no. 10: 965. https://doi.org/10.3390/photonics11100965
APA StyleAlsarhan, J., El Feghali, R., Alkhudari, T., & Benedicenti, S. (2024). Non-Pharmacological Therapies for Management of Temporomandibular Myofascial Pain Syndrome: Laser Photobiomodulation or Dry Needling? Meta-Analyses of Human Clinical Trials. Photonics, 11(10), 965. https://doi.org/10.3390/photonics11100965







