Abstract
This study explores the effects of Si and Si-P heteroatoms doping and co-doping on a monolayer graphene surface through density functional analysis. The results suggest that doping with Si and co-doping with Si-P significantly alters the bonding arrangement of the atoms surrounding the graphene sheet. Additionally, the surface of the graphene material had a high concentration of electrons in both Si doping and Si-P co-doping, based on electron population analysis. The HOMO–LUMO gap of graphene sheets was found to decrease in the following order: pristine graphene sheet > Si-doped graphene sheet > Si-P co-doped graphene sheet. Furthermore, a TD-DFT study revealed that the absorption wavelength of Si and Si-P co-doped graphene systems had a greater shift to a lower range compared to pristine graphene. The order of decreasing absorption wavelength is Si-P co-doped graphene, Si doped graphene, and pristine graphene. These materials are suggested to have a high potential for photodetector applications due to their broad absorption range.
1. Introduction
Material scientists have been fascinated with carbon nanostructures, such as fullerenes and graphene, over the past thirty years [1,2]. Graphene is one of these promising materials that has been extensively applied in nanotechnology [3]. A single layer of graphite was discovered in 1995, which exhibited several new and promising chemical, physical, and mechanical characteristics. This material has a great fracture strength (~125 GPa), elevated Youngs modulus (~1 TPa), excellent light transmittance (~97.4%), superior electron mobility exceeding 2 × 105 cm2 V−1 s−1, high ampacity (1012–1013 A m−2), and a large surface area (theoretically 2630 m2 g−1) [4,5]. These diverse, outstanding properties make graphene an eminent material in several fields. Such characteristics, combined with the atomic thickness of the graphene layer, open the door to a conceptually novel class of materials. This gives a fresh insight into the field of low-dimensional carbon materials that have never stopped surprising and remains so far, the source of different applications [6].
Since 2004, 2D graphene has been successfully prepared through different approaches, such as electrophoretic, Chemical vapor deposition (CVD), and exfoliation techniques [7,8]. This encourages the usage of 2D materials in next-generation lithium-ion batteries, optoelectronics, electronics, optical sensors, energy storage, gas sensing, biosensing, and Nano adsorbent applications [9,10,11,12,13,14]. For instance, high-speed graphene transistors are already being used in consumer gadgets [15,16]. When an electric field is provided, a smart window that employs Liquid Crystal Display (LCD) technology transforms from being opaque to transparent. The technology includes sandwiching two flexible electrodes constructed of graphene and flexible polymers between a layer of liquid crystals [17,18]. There is a growing interest in using flexible graphene electrodes in Organic Light Emitting Diodes (OLEDs) due to their high practicality. Unlike the brittle and scarce indium tin oxide counter electrodes used in current OLED technology, graphene is virtually limitless and flexible [19,20,21]. The possibility of utilizing graphene-based lithium-ion batteries or supercapacitors in electronic vehicles like cars, trains, and potentially even airplanes in the future is exciting [22,23,24,25,26,27]. The efficiency of a graphene electrode in solar cells was 7.8%, which is only 0.2% less efficient than a counter electrode made of platinum [28,29,30]. The science of electroanalysis is one of graphene’s potential fields of applications. Following the graphene revolution, several articles reported its high ability for catalysis and electrochemical sensing reactions [31,32]. Graphene’s unique properties are often altered when blended with other substances, including metals, ceramics, liquids, biomaterials, polymers, and semiconductors, to fit specific applications. The optical properties of graphene are enhanced when combined with these materials.
Pure graphene has a zero-energy band gap, which makes it inert despite having all these enticing features. Adding impurities of any form, whether by doping foreign atoms or functional groups, automatically affects its properties. These kinds of disruptions can alter the pure structure of graphene and cause overlapping of the energy bands, which opens doors for its application in new fields. An N-doped graphene-based flexible supercapacitor was the subject of study by Jeong et al. through electrochemical measurements in both aqueous and organic electrolytes. This study shows an elevated supercapacitor’s capacitance, i.e., four times greater than that prepared by pristine graphene [33,34,35]. According to Kim et al., dye solar cells with graphene electrodes have high performance compared to those with Pt counter electrodes (Jsc = 13.83 mA cm2) [36]. Thus, p-n junction solar cells can be explored with doped-graphene materials. On the other hand, a notable enhancement has been seen in the work function (≈0.4 eV) of the solar cell while using N-doped graphene [37]. Furthermore, such doping favors active site appearances like those prepared in WSe2 flakes grown on N-doped graphene (WSe2/NG), which makes it a dual-functional host able to supply the S cathode and Li anode simultaneously [38]. Moreover, doped graphene can enhance and modify optical characteristics. A red shift in the optical edge also results from N-doped graphene [39]. Liu et al. show an important change in the absorption of visible light for graphene doped with Nitrogen, where these latest reaches semiconductor species with photons [40]. Based on the examples above, the capacity to tune the optical characteristics of graphene and then the electronic applications has been generated via a variety of defect-producing techniques.
Doping with heteroatoms has proven to be the most effective method of controlling the band gap engineering, optical phenomena management, and structure-property tuning in graphene, opening up new possibilities in nano-optoelectronic applications, particularly in energy-related devices [41]. While impurities within graphene are frequent and random, the placement of dopants on the periodic structure of graphene has shown promising results in theoretical research [42].
In addition, graphene oxide has been found to have excellent optical properties that can improve the usage of polyaniline/PbS nanocomposites as a photoelectrode for hydrogen generation [43]. Furthermore, the incorporation of graphene oxide with carbon nanotubes has been utilized for water-splitting production through photocatalytic roles under car exhaust. This reaction uses exhaust as an electrolyte for hydrogen gas production, taking advantage of the outstanding optical properties of graphene oxide [44]. These promising developments in graphene-based materials demonstrate the potential for innovative and sustainable energy solutions in the future.
The most successful method for controlling band gap engineering, optical phenomena management, and structure-property tuning through doping with heteroatoms. This leads to new possibilities in nano-optoelectronic applications, particularly as energy-related devices [41]. Although the presence of impurities within graphene is frequent and random, regarding the placement of dopants on the periodic structure of graphene, theoretical research has shown encouraging results [42]. Additionally, graphene oxide has great optical properties oriented towards improving the usage of polyaniline/PbS nanocomposites as photoelectrodes for hydrogen generation [43]. Likewise, the incorporation of graphene oxide with carbon nanotubes is utilized for water-splitting production via photocatalytic roles under car exhaust. This reaction uses the exhaust as an electrolyte for hydrogen gas production based on the great optical properties of graphene oxide [44].
Theoretical calculations, including first-principles calculations, have been employed to study various aspects, such as energy bands, optical properties, energy storage, and gas sensing of graphene [45,46,47,48,49,50,51,52]. However, an assessment of how optical excitations are affected by the type and quantity of dopants is currently needed. Optical excitations in materials are influenced by the presence and quantity of dopants, which are atoms or molecules intentionally introduced into a material to modify its properties. The type and quantity of dopants can have significant effects on the electronic structure and optical properties of a material, making it important to understand their influence on the design and optimize materials for specific applications.
The optical properties of a material are related to its electronic structure, which is affected by the dopant atoms or molecules. For example, dopants can introduce new energy levels in the material’s electronic band structure, which can impact the absorption and emission of light by the material. The type of dopant can also change the energy level and position of these new states, which, in turn, can influence the material’s optical properties. The quantity of dopants can also have an important effect on the optical properties of a material. At low doping levels, the dopants can act as localized perturbations in the material’s electronic structure, leading to small changes in its optical properties. However, at higher doping levels, the dopants can begin to interact with each other, leading to more significant changes in the material’s electronic and optical properties.
In summary, the type and quantity of dopants can have a significant impact on the optical excitations of a material. Understanding these effects is important for designing and optimizing materials for specific applications, such as optoelectronic devices or solar cells. Further research is needed to fully explore and understand the influence of dopants on the optical properties of materials.
In this study, we explore how the optical behavior of pristine graphene varies under doping with Si and Si-P dopants, including changes in chemical and electrical properties. For example, our calculations illustrate the impact of the addition of Si and Si-P on turning graphene into an electron-rich system. To simulate the UV-Vis absorption spectra, we used density functional theory (DFT). UV-Vis absorption spectroscopy is a commonly used experimental technique for characterizing the electronic transitions in material. To simulate the UV-Vis absorption spectra of a material using DFT, one would need to calculate the energies of the electronic transitions that give rise to the absorption peaks. This can be done by first optimizing the geometry of the system using DFT, which involves finding the configuration of the atoms that minimizes the total energy of the system. Once the optimized geometry is obtained, the electronic structure can be calculated using DFT, which provides information about the energy levels and transitions in the material. From this electronic structure, one can calculate the absorption spectra using theoretical models, such as the Kubo–Greenwood equation.
It is important to note that the accuracy of Density Functional Theory (DFT) calculations for electronic transitions is highly dependent on the choice of exchange-correlation functional used in the calculation. The choice of functional can significantly affect the calculated energies and intensities of electronic transitions, making it crucial to carefully select an appropriate functional for the material being studied. Moreover, other factors, such as the inclusion of solvent effects and the use of time-dependent DFT, may be necessary for accurate simulations of UV-Vis’s absorption spectra.
In this study, the light absorption spectra of Si-doped graphene and Si-P co-doped graphene are compared to those of pristine graphene. The results illustrate a migration towards lower wavelengths, highlighting the effect of doping on the electronic properties of graphene. This research adds to our understanding of heteroatom-doped graphene systems, potentially paving the way for experimental scientists to design better graphene-based materials for optoelectronics, electronics, and energy storage applications.
2. Theoretical Study
2.1. Structure Optimization
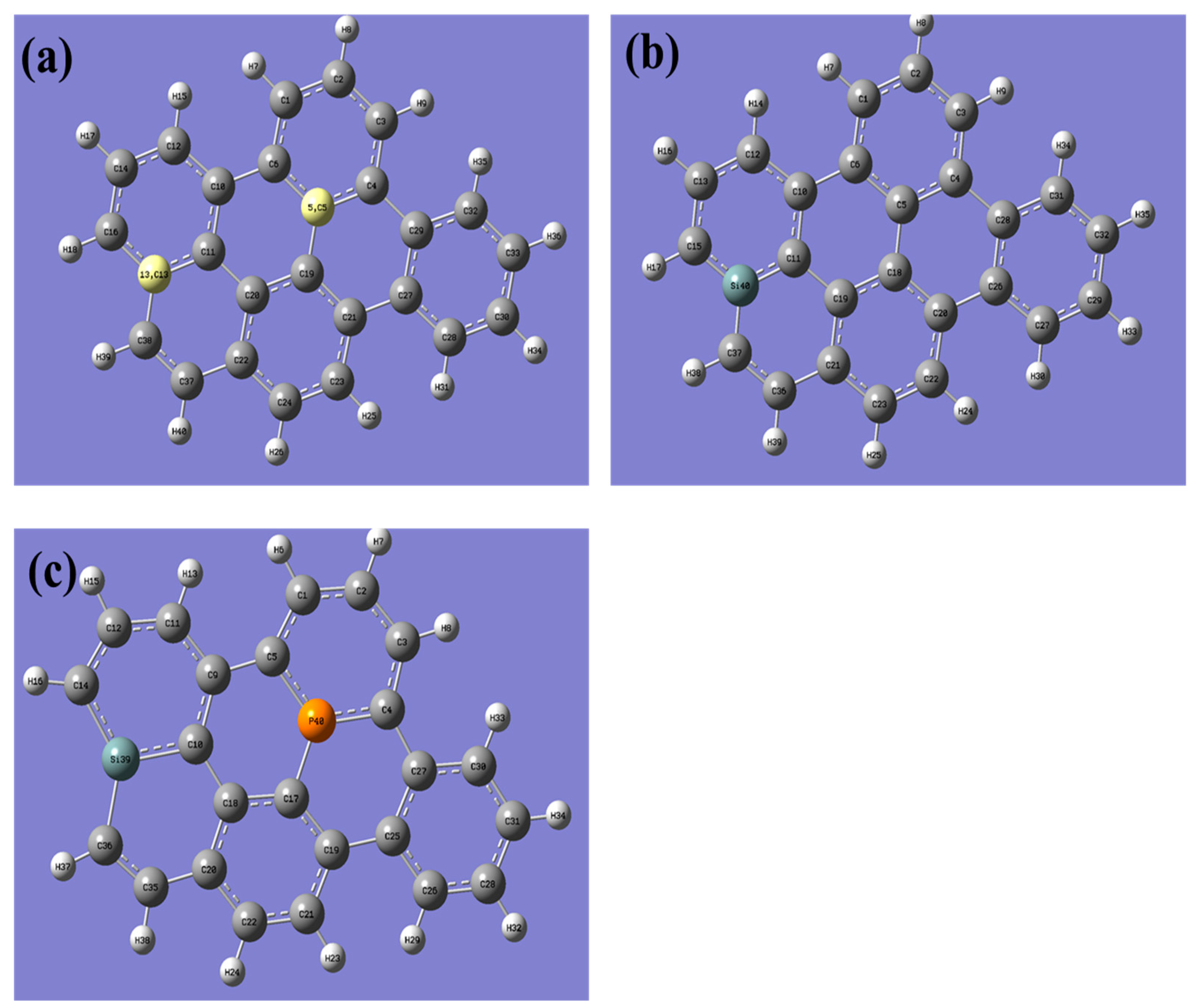
In this study, a graphene sheet was constructed with a total of 40 atoms, comprising 26 carbon atoms and 14 hydrogen atoms (Figure 1). The hydrogen atoms were added to eliminate dangling bonds on the edges of the graphene sheet, thus avoiding any edge effects. A geometry optimization was performed on the pristine graphene, during which the optimized graphene was not subjected to any symmetry constraints, and no imaginary frequencies were observed. This confirms that graphene has reached its lowest energy state. The absence of symmetrical constraints during the optimization implies that the optimized structure can exhibit any symmetry and is not limited to any particular symmetry group. This allows the optimization process to explore a wider range of potential configurations and identify the most energetically stable one.
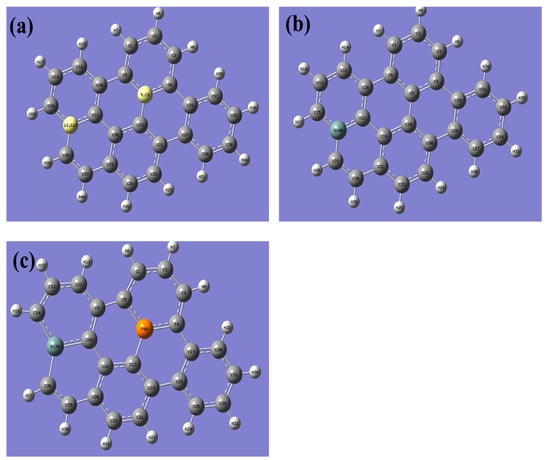
Figure 1.
The figure presented showcases the optimized structures of three different graphene samples: (a) pristine graphene, (b) silicon-doped graphene, and (c) graphene co-doped with silicon and phosphorus. The (a) panel shows the structure of pristine graphene, which has no doping. The (b) panel displays the doping site for silicon atoms in graphene, which are located on top of carbon atoms. The (c) panel illustrates the co-doping site of both silicon and phosphorus atoms in graphene, where the silicon atom is located on top of a carbon atom, and the phosphorus atom is placed on top of an adjacent carbon atom. The optimized structures are presented in a 3D representation, highlighting the different atomic positions and the overall shape of each graphene sample. This figure serves as a useful reference for understanding the differences between the three graphene samples and the impact of doping on their structural properties. (The computational resources provided by the High Performance Computing (HPC) Cluster at UNC Charlotte).
Indeed, the absence of imaginary frequencies found here indicates that the optimized structure is at a minimum energy state. In molecular or materials simulations, imaginary frequencies are indicative of a transition state or a higher-energy structure than the optimized one. Therefore, the absence of imaginary frequencies confirms that the optimized graphene structure represents the most stable configuration of the system.
To investigate the impact of doping on the electronic and optical properties of graphene, we doped graphene with Si and co-doped it with Si-P, despite the structural changes. Nonetheless, the geometry optimization of pristine graphene remains a crucial step in comprehending the structural and energetic characteristics of graphene and can serve as a fundamental basis for future calculations or simulations of graphene-based materials.
2.2. Charge Transfer
The B3LYP functional and 6-31G (d, p) basis set are both utilized in the analysis of natural population (NPA), which is performed using the Gaussian 16 program. Additionally, the study involves assessing the charge transfer between the pristine and dopant atom through NBO (Natural Bonding Orbital Analysis) [53,54,55,56,57]. This approach is based on constructing localized orbitals, or natural bond orbitals, for each bond and lone pair in a molecule or material. These orbitals depict the electron density associated with each bond or lone pair and can be employed to quantify the extent of electron sharing or transfer between atoms.
In the case of a doped material, NBO analysis can be applied to examine the degree of charge transfer between the pristine and dopant atoms. This can offer insight into the electronic structure of the material and assist in explaining the impact of the dopant on its properties.
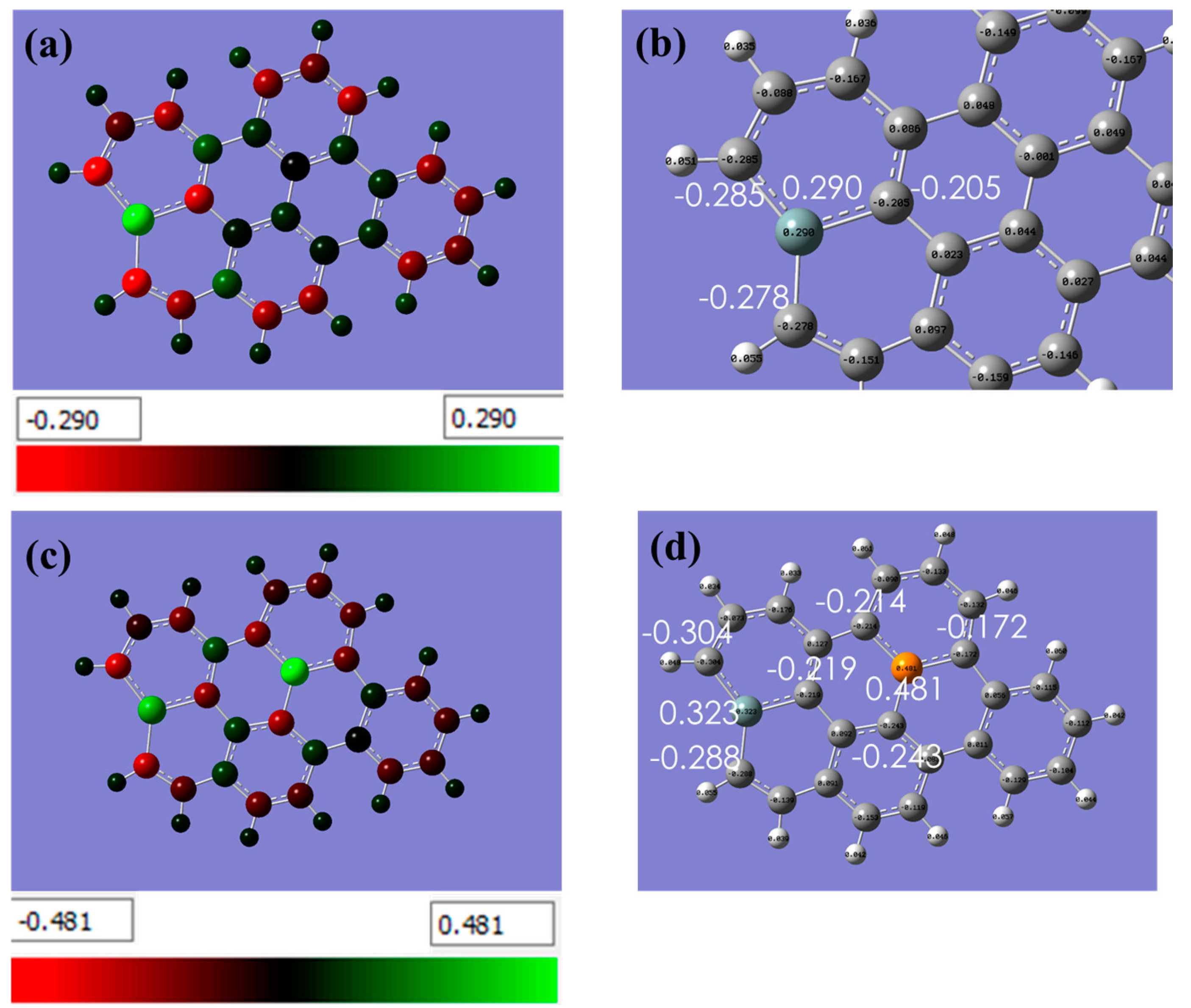
Figure 2 displays the partial charge distribution with a color scheme, where the color bar represents the range of partial charges. Green indicates positive charges and red signifies negative charges. The analysis of Si-doped graphene demonstrates a transfer of approximately −0.285 e, −0.278 e, and −0.205 e charges from Si to its neighboring C atoms. This illustrates Si role in acting as an electron donor in the system, transferring electrons to the neighboring carbon atoms.
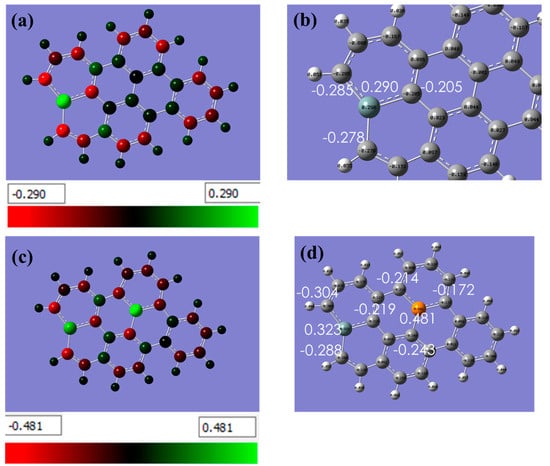
Figure 2.
Shows the partial charge distribution with a color scheme, where the color bar represents the range of partial charges. Green indicates positive charges and red signifies negative charges. (a,b) In the case of Si-doped graphene, the by Natural Bond Orbital (NBO) analysis demonstrated a transfer of approximately −0.285 e, −0.278 e, and −0.205 e charges from Si to its neighboring C atoms. This indicates that Si is acting as an electron donor in the system, transferring electrons to the neighboring carbon atoms, (c,d) The same analysis but for graphene co-doped with silicon and phosphorus. This information provides insight into the electronic structure of the material and helps to explain the effects of the dopant on its properties. (The computational resources provided by the High Performance Computing (HPC) Cluster at UNC Charlotte).
In the case of Si-P co-doped graphene, the analysis found a transfer of −0.243 e, −0.172 e, and −0.214 e charges from P. This suggests that P acts as an electron acceptor in the system, attracting electrons from the neighboring atoms.
When comparing the charge transfer of Si in both doped and co-doped graphene structures, the study found that a larger charge transfer is supported through Si-P co-doped graphene (−0.304 e, −0.288 e, and −0.219 e) compared to Si-doped graphene (−0.285 e, −0.278 e, and −0.205 e). This suggests that the presence of P in the system enhances the charge transfer from Si to the neighboring C atoms.
Overall, these results provide important insight into the electronic properties of doped graphene sheets and can help to guide the design and optimize graphene-based materials for various applications.
2.3. Electron Density Distribution
The molecular electrostatic potential (MEP) of both pristine graphene and doped/co-doped graphene sheets has been simulated to gain insights into their electronic properties. This simulation allows for the visualization of the electron density distribution around the dopant atoms using a color-coded scale, thereby providing information about the electronic interactions between the dopant and pristine atoms.
To calculate the MEP, the Schrödinger equation for the electronic wave function of the system is solved based on the principle that electrons are attracted to positively charged nuclei and repelled by other electrons. As a result, regions of high and low electron density can be observed and analyzed.
Comparing the MEP of pristine graphene with that of doped and co-doped graphene enables the observation of variations in electron density near the dopant atoms. These variations can help explain the effects of the dopant on the electronic properties of the material.
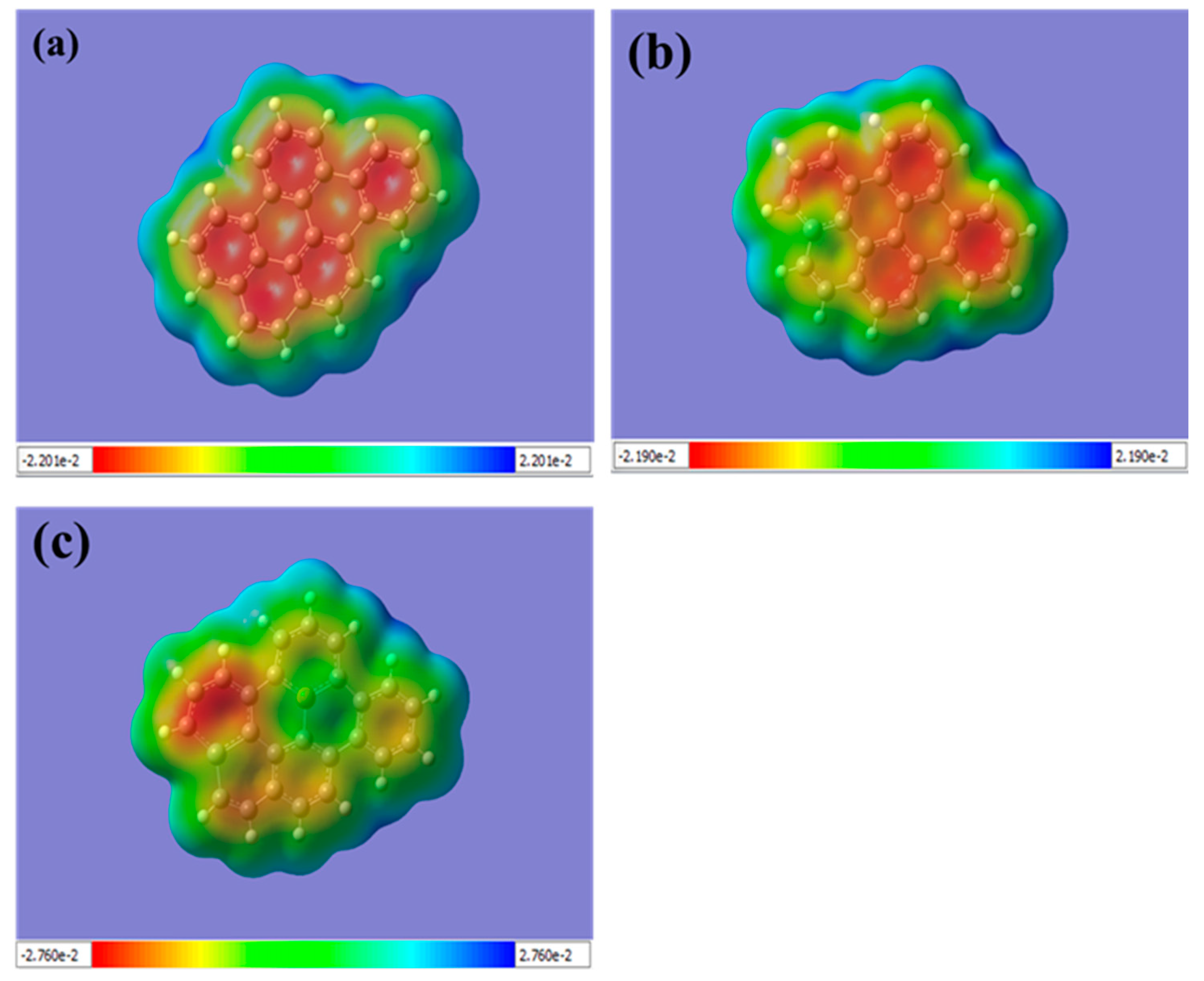
The MEP simulation of graphene sheets is a valuable tool for understanding the electronic properties of doped and co-doped graphene and can guide the design and optimization of graphene-based materials for various applications. The color-coded scale used in the MEP simulation displays the electrostatic potential distribution value, with blue representing a more positive portion of the potential surface and the range from yellow to red representing the more negative part [52,53].
For pristine graphene, the top surface has a negative charge, represented by the red color (see, Figure 3a). The edges of the graphene sheet are indicated by a positive charge, represented by the blue color. This is due to the delocalization of the electrons over the whole graphene sheet, which results in a uniform distribution of electron density, while the positively charged hydrogen atoms at the edges are attracted to the negatively charged carbon atoms.
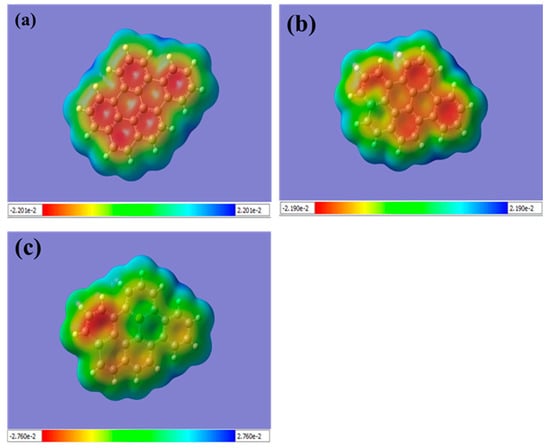
Figure 3.
Displays the molecular electrostatic potential (MEP) for (a) pristine graphene, (b) Si-doped graphene, and (c) Si-P co-doped graphene. The color bar represents the range of the electrostatic potential distribution values, with blue indicating positive charges and red representing negative charges. For pristine graphene (a), the color map shows a uniform distribution of electron density with a negative charge on the top surface (represented by the red color), while the edges of the graphene sheet have a positive charge (represented by the blue color). This can be attributed to the delocalization of electrons over the entire graphene sheet and the positively charged hydrogen atoms at the edges being attracted to the negatively charged carbon atoms. In Si-doped graphene (b), the potential surface near the Si atom is more positive (represented by the green color), indicating that the Si atom donates electrons to the graphene sheet, resulting in a positively charged region around the Si atom. For Si-P co-doped graphene (c), the top surface near the Si atom is weakly positive, while the potential surface near the P atom is more positively charged (represented by the blue color), suggesting that the P atom attracts electrons from the graphene sheet, creating a region of positive charge around the P atom. (The computational resources provided by the High Performance Computing (HPC) Cluster at UNC Charlotte).
Additionally, the MEP analysis can provide insight into the interaction between dopant atoms and the graphene lattice. In the case of Si-doped graphene, the MEP shows that the Si atom is strongly attracted to the adjacent carbon atoms, as indicated by the blue and green colors in Figure 3b. This suggests a strong interaction between the Si atom and the graphene lattice, which can lead to changes in the electronic and structural properties of the material. Similarly, in Si-P co-doped graphene, the MEP shows a strong interaction between the P atom and the adjacent carbon atoms, as well as a weaker interaction with the Si atom, as indicated by the red color near the P atom in Figure 3c. This indicates that the co-doping of Si and P can lead to complex electronic interactions in the graphene lattice. Overall, the MEP analysis provides important insights into the electronic properties and interactions in doped and co-doped graphene sheets, which can help to guide the design and optimization of graphene-based materials for various applications.
2.4. HOMO and LUMO Orbitals
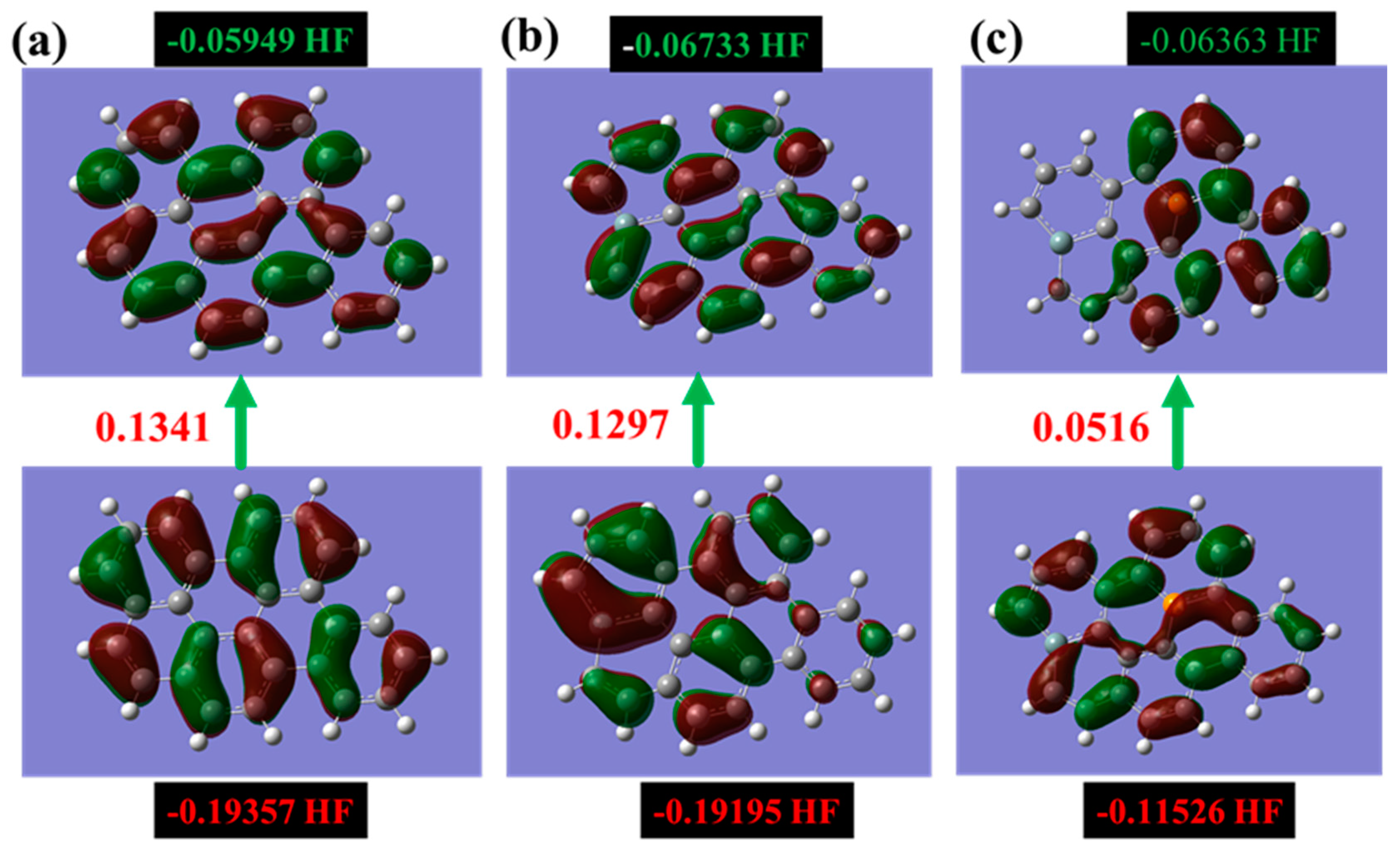
Figure 4 illustrates the correlation between the Highest Occupied Molecular Orbital (HOMO), the Lowest Unoccupied Molecular Orbital (LUMO), and the band gap energy required for an electron to transition between these orbitals. The band gap energy is a crucial factor in determining the electrical conductivity and optical properties of materials, with a larger band gap energy indicating a wider separation between the HOMO and LUMO and typically resulting in a material with lower electrical conductivity and higher optical transparency.
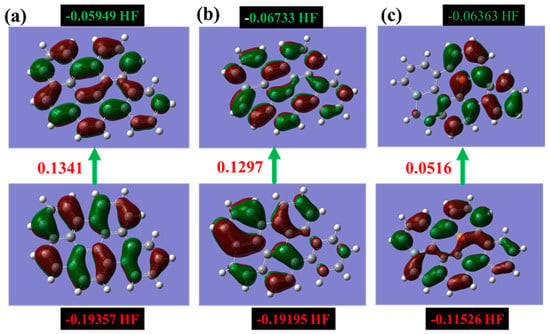
Figure 4.
Showcases the intricate molecular orbitals of the alpha HOMO and alpha LUMO for three different types of graphene: (a) pure graphene, (b) silicon-doped graphene, and (c) graphene co-doped with silicon and phosphorus. The figure emphasizes the highest occupied and lowest unoccupied states, providing a detailed illustration of the unique characteristics of each graphene type. (The computational resources provided by the High Performance Computing (HPC) Cluster at UNC Charlotte).
The mapping of HOMO and LUMO orbitals with the band gap energy is shown for various types of graphene sheets in Figure 4. By comparing the band gap energy for different graphene sheets, it is possible to assess their electronic properties and potential applications.
The HOMO/LUMO orbitals of pristine graphene, Si-doped graphene, and the alpha HOMO of Si-P co-doped graphene exhibit highly symmetrical configurations. This indicates that the electrons are uniformly distributed in both the ground and excited states through these orbitals, which is a characteristic feature of sp2 hybridized carbon atoms in graphene.
In contrast, the alpha LUMO orbitals of Si-P co-doped graphene show an asymmetrical distribution, with the orbitals being predominantly concentrated around the added P atom. This suggests that the P atom introduces a local perturbation in the electronic structure of the graphene sheet, creating a region of higher electron density around the P atom.
The symmetry of HOMO and LUMO orbitals is a critical aspect of the electronic properties of materials, as it can impact their chemical reactivity, optical properties, and electronic transport properties. The observation of an asymmetrical distribution in the alpha LUMO orbitals of Si-P co-doped graphene highlights the potential of this material for applications in areas, such as catalysis and sensing, where localized electronic perturbations can be used to control chemical reactions or detect specific molecules.
The process of diffusion, whereby atoms or molecules move from a region of higher concentration to a region of lower concentration, is an important phenomenon in the study of doped graphene. In particular, the potential for Si or P dopant atoms to diffuse to a shorter position on the graphene sheet has been the subject of analysis. This diffusion behavior is of great interest as it can significantly impact the electronic and optical properties of the material.
Understanding the diffusion behavior of dopant atoms in graphene is crucial for the development of practical applications, as it can affect the stability and performance of the material. For instance, if dopant atoms are prone to diffusion or clustering, this can result in the loss of electronic and optical properties over time, which can limit the effectiveness of the material for applications, such as electronic devices or energy storage.
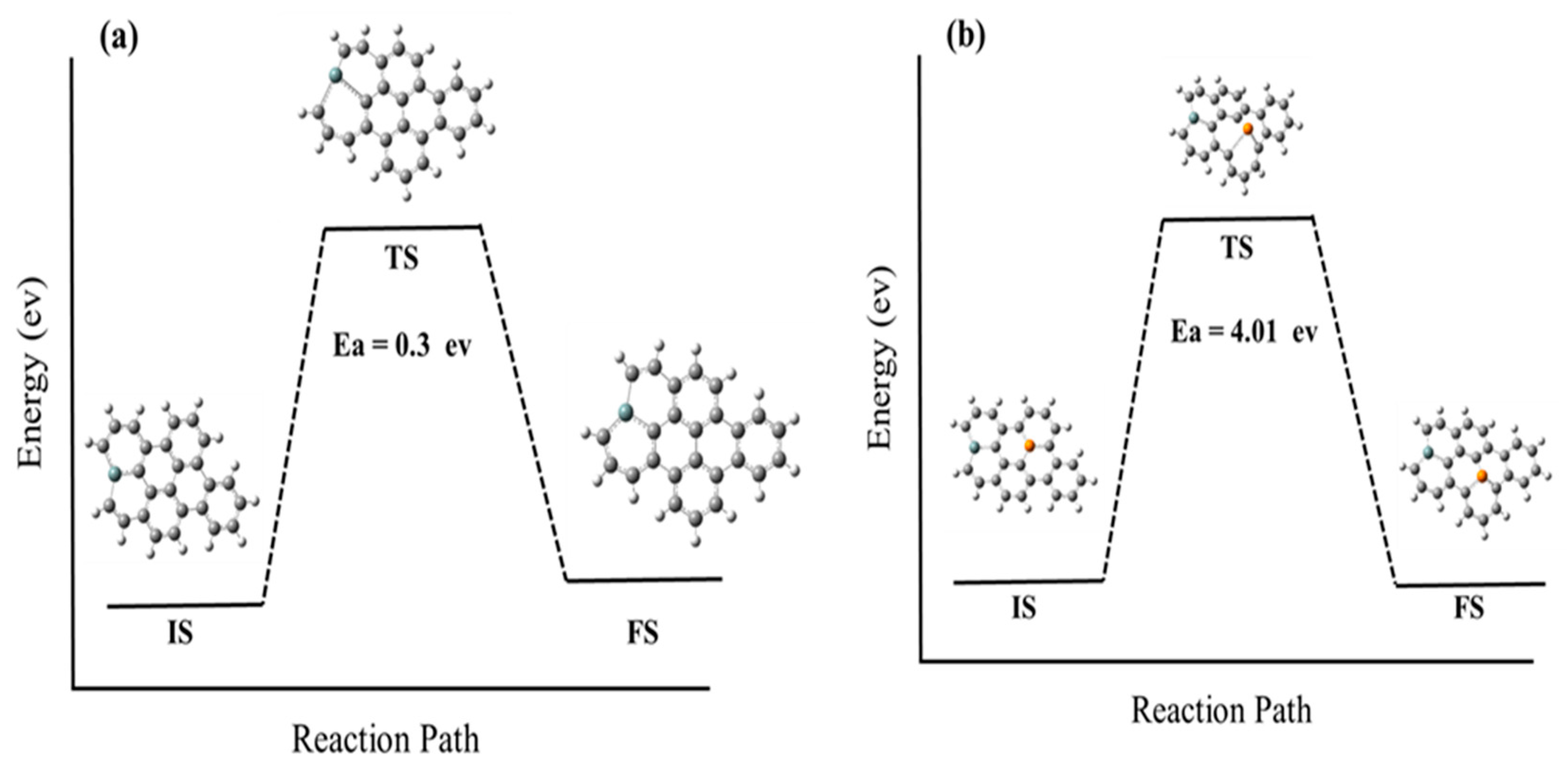
To better understand this diffusion behavior, we must study diffusion barriers for Si and P atoms on a graphene sheet, which refers to the energy required for an atom or molecule to overcome the energy barrier and move from one position to another. The adsorption energies of these dopants on graphene vacancy sites are also analyzed. The diffusion barriers and adsorption energies are important factors that can affect the mobility and stability of dopant atoms on the graphene sheet. By examining these factors, we can gain a better understanding of the diffusion behavior of dopant atoms in graphene, which can ultimately inform the development of more stable and efficient doped graphene materials for a variety of practical applications. Figure 5 illustrates the diffusion barriers for Si and P atoms, which are found to be 0.3 eV and 4.01 eV, respectively. This suggests that Si atoms are more mobile and less stable on the graphene sheet compared to P atoms, which require more energy to move and are, therefore, more stable in their positions.
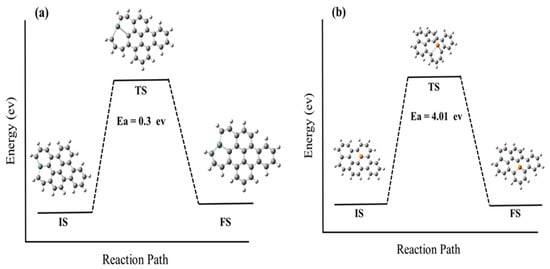
Figure 5.
Provides a schematic representation of the energetical structure that is associated with the diffusion process of dopant atoms on graphene. Specifically, the figure displays the energy diagram for the diffusion process of both silicon (a) and phosphorus (b) atoms on graphene. The figure includes representations of the initial structure (IS), transition structure (TS), and final structure (FS) for each diffusion process. These structures represent the starting point, intermediate step, and final position of the dopant atoms during the diffusion process. (The computational resources provided by the High Performance Computing (HPC) Cluster at UNC Charlotte).
Additionally, the study has also evaluated the adsorption energies of these dopants on graphene vacancy sites. Adsorption energy refers to the energy released or absorbed when a molecule or atom is absorbed onto a surface. In the case of doped graphene, high adsorption energies can help stabilize the dopant atoms on the graphene sheet and prevent clustering.
We believe that the relatively high adsorption energies of Si and P dopants on graphene vacancy sites ensure that Si dopants can be dispersed on graphene in a stable manner without clustering. This implies that Si dopants are less prone to clustering and can be dispersed more uniformly on the graphene sheet, making them potentially more suitable for certain applications.
The energy diagram in Figure 5 illustrates the energy changes that occur during the diffusion process, indicating the energy barriers that need to be overcome for the dopant atoms to move from one position to another on the graphene sheet. The energy barrier, which is represented by the transition structure, indicates the minimum amount of energy required for the dopant atoms to move from the initial position to the final position.
2.5. UV-Vis Adsorption Calculation
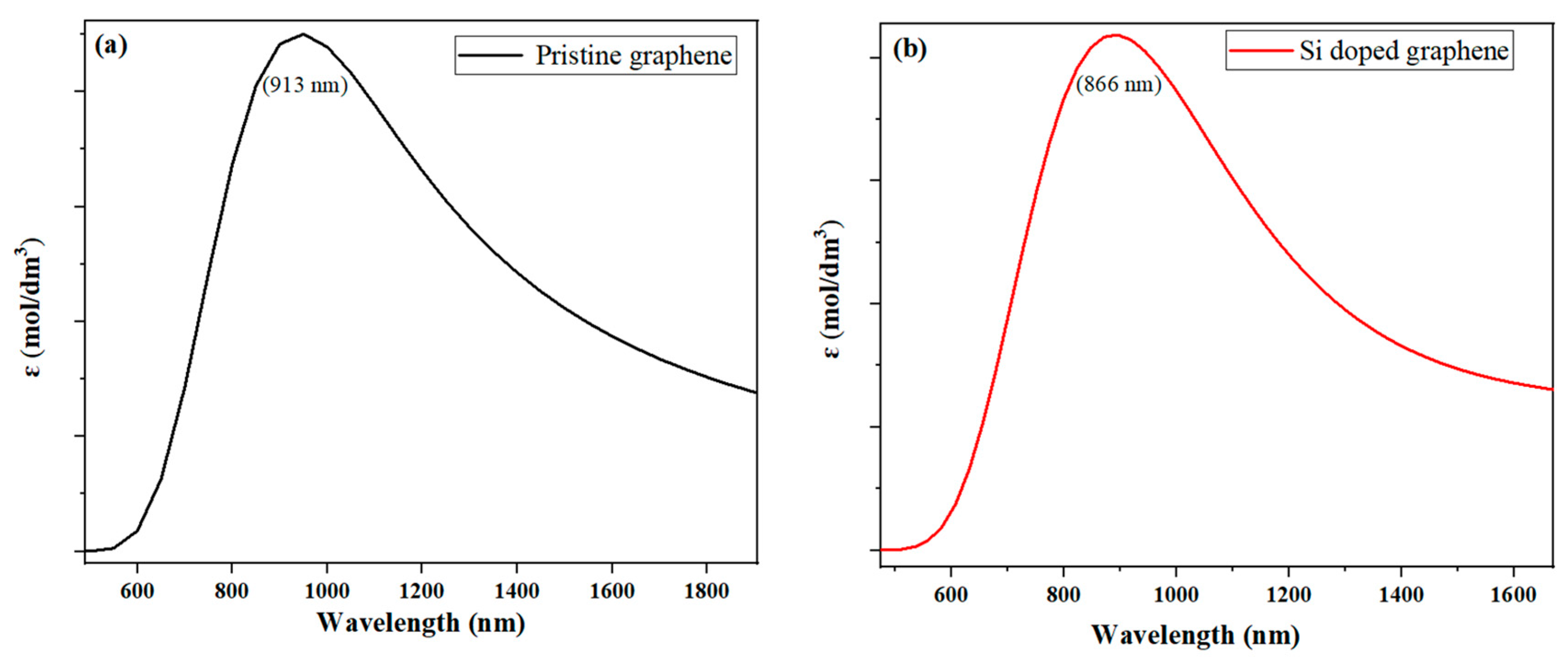
Figure 6 shows the UV-Vis absorption spectra obtained from TD-DFT simulations at the B3LYP/6-31G (d, p) level. The results reveal a blue shift in the absorption spectra, with a shift towards shorter wavelengths, indicating a decrease in the maximum absorption wavelength. The order of this shift is as follows: Si-P co-doped graphene < Si-doped graphene (at approximately 866 nm) < pristine graphene (at approximately 913 nm). These results suggest that both the type and amount of dopants in graphene significantly affect its optical properties, with potential implications for applications, such as optoelectronics and photocatalysis.
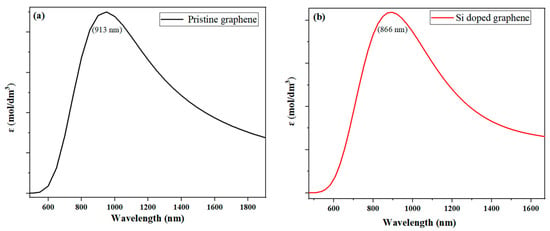
Figure 6.
Depicts the UV-Vis absorption spectra obtained from TD-DFT simulations at the B3LYP/6-31G (d, p) level. Here, we give the spectra for (a) pristine graphene and (b) silicon-doped graphene, as measured by the absorbance coefficient (ε), which is the imaginary part of the dielectric function. The spectra show a blue shift, indicating a decrease in the maximum absorption wavelength, with a shift towards shorter wavelengths. (The computational resources provided by the High Performance Computing (HPC) Cluster at UNC Charlotte).
The blue shift observed in the absorption spectra can be attributed to the shorter bond lengths in the doped and co-doped graphene systems. These bond lengths alter the electronic structure of the material, resulting in a shift in the energy levels required for electronic excitations. Additionally, the introduction of dopants can cause changes in the band structure of graphene, which may further affect its electronic and optical properties. The shift towards shorter wavelengths in the absorption spectra indicates that the doped and co-doped graphene systems have a higher probability of absorbing photons with higher energies, making them useful for applications requiring high optical absorption, such as solar energy harvesting.
The results suggest that both pristine and doped graphene materials have the capability to absorb photons and detect light in a wide optical range, making them promising materials for photodetector applications in optical communication systems, image sensing, and other optoelectronic devices. The ability of doped graphene materials to tune their optical properties could be particularly useful for designing photodetectors with specific wavelength selectivity and sensitivity.
3. Conclusions
Here we utilized density functional theory (DFT) to investigate the electronic and optical properties of monolayer graphene sheets doped with silicon (Si) and silicon-phosphorus (Si-P). The results revealed significant alterations in the bond lengths of neighboring atoms within the graphene sheet due to Si and Si-P co-doping, indicating that the presence of dopants can affect the structural integrity of graphene.
The analysis of molecular orbitals demonstrated changes in the energy gap (Eg) between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) in the doped and co-doped graphene sheets. The obtained data showed that the Eg value decreased in the order of pristine graphene > Si-doped graphene > Si-P co-doped graphene, suggesting that doping graphene with Si and Si-P can lead to a decrease in the required energy levels for electronic excitations.
In addition to analyzing the electronic properties of the doped and co-doped graphene sheets, we evaluated the charge transfer between the dopant atoms and the neighboring carbon atoms in the graphene sheet. The findings revealed a larger charge transfer in Si-P co-doped graphene compared to Si-doped graphene, suggesting that the presence of phosphorus in the dopant mixture can enhance the charge transfer effect.
Furthermore, we investigated the potential for diffusion of Si and P atoms on the graphene sheet. The analysis showed that the high adsorption energies of these dopants prevent clustering and ensure stable dispersion on the graphene sheet, which is essential for their potential applications in electronic devices.
Finally, the UV-Vis absorption spectrum was simulated using time-dependent DFT (TD-DFT), revealing a blue shift in the maximum absorption wavelength value in the doped and co-doped systems compared to pristine graphene. These findings suggest that Si and Si-P doped graphene sheets have potential applications as photodetectors in a wide optical range. While analyzing obtained data, we also found that the Si-P co-doped graphene had the highest photon sensitivity, making it a promising material for light detection.
Overall, we believe that our results provide valuable insights into the effect of Si and Si-P co-doping on the electronic and optical properties of graphene sheets, which could have significant implications for various applications in optoelectronics, photodetectors, and other electronic devices.
Author Contributions
Methodology, A.A.A.A.; formal analysis, A.A.A.A., A.B.G.T., F.H.A. and M.R.; investigation, S.A., M.S., T.A.A., F.H.A., K.S.A. and F.V.K.; supervision, A.B.G.T., F.H.A., S.A., M.S., T.A.A., K.S.A. and F.V.K.; writing—original draft preparation, A.A.A.A., A.B.G.T., F.H.A., F.V.K. and M.R.; writing—review and editing, A.A.A.A., A.B.G.T., F.H.A., F.V.K. and M.R.; funding acquisition, A.B.G.T. and F.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1442-0034).
Acknowledgments
The authors express their gratitude to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1442-0034).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geim, A.; Novoselov, K. Nanoscience and Technology: A Collection of Reviews from Nature Journals; Rodgers, P., Ed.; World Scientific: Singapore, 2010. [Google Scholar]
- Hornyak, G.L.; Joydeep, D.; Tibbals, H.F.; Anil, R. Introduction to Nanoscience and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Pati, S.K. Novel properties of graphene nanoribbons: A review. J. Mater. Chem. 2010, 20, 8207–8223. [Google Scholar] [CrossRef]
- Liao, L.; Peng, H.; Liu, Z. Chemistry makes graphene beyond graphene. J. Am. Chem. Soc. 2014, 136, 12194–12200. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, F.; Han, S.; Deng, W.; Du, X.; Yu, H.; Gou, J.; Wang, Q.J.; Wang, J. Recent Progress in 2D Inorganic/Organic Charge Transfer Heterojunction Photodetectors. Adv. Funct. Mater. 2022, 32, 2205150. [Google Scholar] [CrossRef]
- Abdelazeez, A.A.A.; Trabelsi, A.B.G.; Alkallas, F.H.; Rabia, M. Successful 2D MoS2 nanosheets synthesis with SnSe grid-like nanoparticles: Photoelectrochemical hydrogen generation and solar cell applications. Sol. Energy 2022, 248, 251–259. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Hasan, F.; Kim, J.; Song, H.; Lee, S.H.; Sung, J.H.; Kim, J.; Yoo, H. D, Effect of Particle Size and Doping on the Electrochemical Characteristics of Ca-doped LiCoO2 Cathodes. J. Electrochem. Sci. Technol. 2020, 11, 352–360. [Google Scholar]
- Abdelazeez, A.A.A.; Hadia, N.M.A.; Alzaid, M.; Shaban, M.; Mourad, A.H.I.; Fernández, S.; Rabia, M. Development of CuO nanoporous material as a highly efficient optoelectronic device. Appl. Phys. A 2022, 128, 321. [Google Scholar] [CrossRef]
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.P.; Zhang, H.; Kim, J.S. Omnipotent phosphorene: A next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef] [PubMed]
- Glavin, N.R.; Rao, R.; Varshney, V.; Bianco, E.; Apte, A.; Roy, A.; Ringe, E.; Ajayan, P.M. Emerging applications of elemental 2D materials. Adv. Mater. 2020, 32, 1904302. [Google Scholar] [CrossRef] [PubMed]
- Rabia, M.; Hadia, N.M.A.; Farid, O.M.; Abdelazeez, A.A.A.; Mohamed, S.H.; Shaban, M. Poly(m-toluidine)/rolled graphene oxide nanocomposite photocathode for hydrogen generation from wastewater. Int. J. Energy Res. 2022, 46, 11943–11956. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Huang, Q.; Xie, C.; Li, N. Graphene Transistors. Nanocarbon Electron. 2020, 213–272. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.; Choe, M.; Lee, S.; Park, W.; Kahng, Y.H.; Lee, T. The application of graphene as electrodes in electrical and optical devices. Nanotechnology 2012, 23, 112001. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef]
- Wu, J.; Agrawal, M.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic Light-Emitting Diodes on Solution-Processed Graphene Transparent Electrodes. ACS Nano 2010, 4, 43–48. [Google Scholar] [CrossRef]
- Wu, J.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic solar cells with solution-processed graphene transparent electrodes. Appl. Phys. Lett. 2008, 92, 237. [Google Scholar] [CrossRef]
- Han, T.-H.; Lee, Y.; Choi, M.R.; Woo, S.-H.; Bae, S.-H.; Hong, B.H.; Ahn, J.-H.; Lee, T.-W. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar] [CrossRef]
- Zhang, B.; Grassano, D.; Pulci, O.; Liu, Y.; Luo, Y.; Conte, A.M.; Kusmartsev, F.V.; Kusmartseva, A. Covalent bonded bilayers from germanene and stanene with topological giant capacitance effects. NPJ 2D Mater. Appl. 2023, 7, 27. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Ren, W.; Li, F.; Cheng, H.-M. Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates. Proc. Natl. Acad. Sci. USA 2012, 109, 17360–17365. [Google Scholar] [CrossRef]
- Leng, K.; Zhang, F.; Zhang, L.; Zhang, T.; Wu, Y.; Lu, Y.; Huang, Y.; Chen, Y. Graphene-based Li-ion hybrid supercapacitors with ultrahigh performance. Nano Res. 2013, 6, 581–592. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Luo, B.; Zhi, L. Design and construction of three-dimensional graphene-based composites for lithium-ion battery applications. Energy Environ. Sci. 2015, 8, 456–477. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D Honeycomb-Like Structured Graphene and Its High Efficiency as a Counter-Electrode Catalyst for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2013, 52, 9210–9214. [Google Scholar] [CrossRef]
- Zhuo, Q.-Q.; Wang, Q.; Zhang, Y.-P.; Zhang, D.; Li, Q.-L.; Gao, C.-H.; Sun, Y.-Q.; Ding, L.; Sun, Q.-J.; Wang, S.-D.; et al. Transfer-Free Synthesis of Doped and Patterned Graphene Films. ACS Nano 2015, 9, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fang, W.; Brenes, R.; Kong, J. Challenges and opportunities for graphene as transparent conductors in optoelectronics. Nano Today 2015, 10, 681–700. [Google Scholar] [CrossRef]
- Wu, S.; He, Q.; Tan, C.; Wang, Y.; Zhang, H. Graphene-based electrochemical sensors. Small 2013, 9, 1160–1172. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Wu, L.; Sun, S. Co/CoO nanoparticles assembled on graphene for electrochemical reduction of oxygen. Angew. Chem. 2012, 124, 11940–11943. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Graphene for High-Performance Ultracapacitors and the Importance of Nitrogen-Doped Sites at Basal Planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Luo, M.; Ye, M.; Ahmed, T.; Yu, X.; Lien, D.-H.; He, Q.; Lei, D.; Ho, J.C.; Bullock, J.; et al. Infrared photodetectors based on 2D materials and nanophotonics. Adv. Funct. Mater. 2022, 32, 2111970. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Ju, M.J.; Kim, J.C.; Choi, H.-J.; Choi, I.T.; Kim, S.G.; Lim, K.; Ko, J.; Lee, J.-J.; Jeon, I.-Y.; Baek, J.-B.; et al. N-Doped Graphene Nanoplatelets as Superior Metal-Free Counter Electrodes for Organic Dye-Sensitized Solar Cells. ACS Nano 2013, 7, 5243–5250. [Google Scholar] [CrossRef]
- Jun, G.H.; Jin, S.H.; Lee, B.; Kim, B.H.; Chae, W.-S.; Hong, S.H.; Jeon, S. Enhanced conduction and charge-selectivity by N-doped graphene flakes in the active layer of bulk-heterojunction organic solar cells. Energy Environ. Sci. 2013, 6, 3000–3006. [Google Scholar] [CrossRef]
- Hidalgo, N.; Moreno, J.J.; García-Rubio, I.; Campos, J. WSe2 Flakelets on N-Doped Graphene for Accelerating Polysulfide Redox and Regulating Li Plating. Angew. Chem. Int. Ed. 2022, 61, e202116048. [Google Scholar]
- Pei, F.; Xu, S.; Zuo, W.; Zhang, Z.; Liu, Y.; Cao, S. Effective improvement of photocatalytic hydrogen evolution via a facile in-situ solvothermal N-doping strategy in N-TiO2/N-graphene nanocomposite. Int. J. Hydrogen Energy 2014, 39, 6845–6852. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Xu, S.; Cao, S. Evident improvement of nitrogen-doped graphene on visible light photocatalytic activity of N-TiO2/N-graphene nanocomposites. Mater. Res. Bull. 2015, 65, 27–35. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Gao, H.-L.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J. Mater. Chem. 2012, 22, 390–395. [Google Scholar] [CrossRef]
- Denis, P.A. Band gap opening of monolayer and bilayer graphene doped with aluminium, silicon, phosphorus, and sulfur. Chem. Phys. Lett. 2010, 492, 251–257. [Google Scholar] [CrossRef]
- Helmy, A.; Rabia, M.; Shaban, M.; Ashraf, A.M.; Ahmed, S.; Ahmed, A.M. Graphite/rolled graphene oxide/carbon nanotube photoelectrode for water splitting of exhaust car solution. Int. J. Energy Res. 2020, 44, 7687–7697. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic properties of PbS/graphene oxide/polyaniline electrode for hydrogen generation. Sci. Rep. 2017, 7, 14100. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, L.; Liu, L.-L.; Zhao, L.-S.; Chen, C.-P.; Zhang, Y.; Wang, X.-C. Adsorption of formaldehyde molecule on the pristine and transition metal doped graphene: First-principles study. Appl. Surf. Sci. 2017, 396, 1020–1025. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Lai, P.-J.; Jiang, J.-C. Efficient hydrogen storage in boron doped graphene decorated by transition metals–A first-principles study. Carbon 2014, 73, 132–140. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Xu, X. Single atom catalysts supported on N-doped graphene toward fast kinetics in Li–S batteries: A theoretical study. J. Mater. Chem. A 2021, 9, 12225–12235. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Chen, D.; Miao, J.; Zhi, X.; Deng, S.; Lin, S.; Jin, H.; Cui, D. Nitrogen Dioxide Gas Sensor Based on Ag-Doped Graphene: A First-Principle Study. Chemosensors 2021, 9, 227. [Google Scholar] [CrossRef]
- Ghanbari, M.; Afshari, S.; Amri, S.A.N. New capability of graphene as hydrogen storage by Si and/or Ge doping: Density functional theory. Int. J. Hydrogen Energy 2020, 45, 23048–23055. [Google Scholar] [CrossRef]
- Mei, H.Y.; Pang, Y.; Liu, D.Y.; Cheng, N.; Zheng, S.; Song, Q.; Wang, M. Electronic and mechanic properties of trigonal boron nitride by first-principles calculations. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 101, 16–21. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.-J.; Wang, H.-X.; Zhao, J.-X.; Cai, Q.-H.; Wang, X.-Z.; Ding, Y.-H. Silicon-doped graphene: An effective and metal-free catalyst for NO reduction to N2O? ACS Appl. Mater. Interfaces 2013, 5, 5994–6000. [Google Scholar] [CrossRef]
- Lv, R.; Santos, M.C.D.; Antonelli, C.; Feng, S.; Fujisawa, K.; Berkdemir, A.; Cruz-Silva, R.; Elías, A.L.; Perea-Lopez, N.; López-Urías, F.; et al. Large-area Si-doped graphene: Controllable synthesis and enhanced molecular sensing. Adv. Mater. 2014, 26, 7593–7599. [Google Scholar] [CrossRef]
- Wang, J.; Cao, S.; Ding, Y.; Ma, F.; Lu, W.; Sun, M. Theoretical investigations of optical origins of fluorescent graphene quantum dots. Sci. Rep. 2016, 6, 24850. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, A.; Khosroshahi, E.S.; Nejati, K.; Edjlali, E.; Vessally, E. A DFT study on graphene, SiC, BN, and AlN nanosheets as anodes in Na-ion batteries. J. Mol. Model. 2017, 23, 354. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Li, Y.; Shu, H.; Niu, X.; Wang, J. Electronic and optical properties of edge-functionalized graphene quantum dots and the underlying mechanism. J. Phys. Chem. C 2015, 119, 24950–24957. [Google Scholar] [CrossRef]
- Enriquez, J.I.G.; Villagracia, A.R.C. Hydrogen adsorption on pristine, defected, and 3d-block transition metal-doped penta-graphene. Int. J. Hydrogen Energy 2016, 41, 12157–12166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).