A Blue-Light-Emitting 3 nm-Sized CsPbBr3 Perovskite Quantum Dot with ZnBr2 Synthesized by Room-Temperature Supersaturated Recrystallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
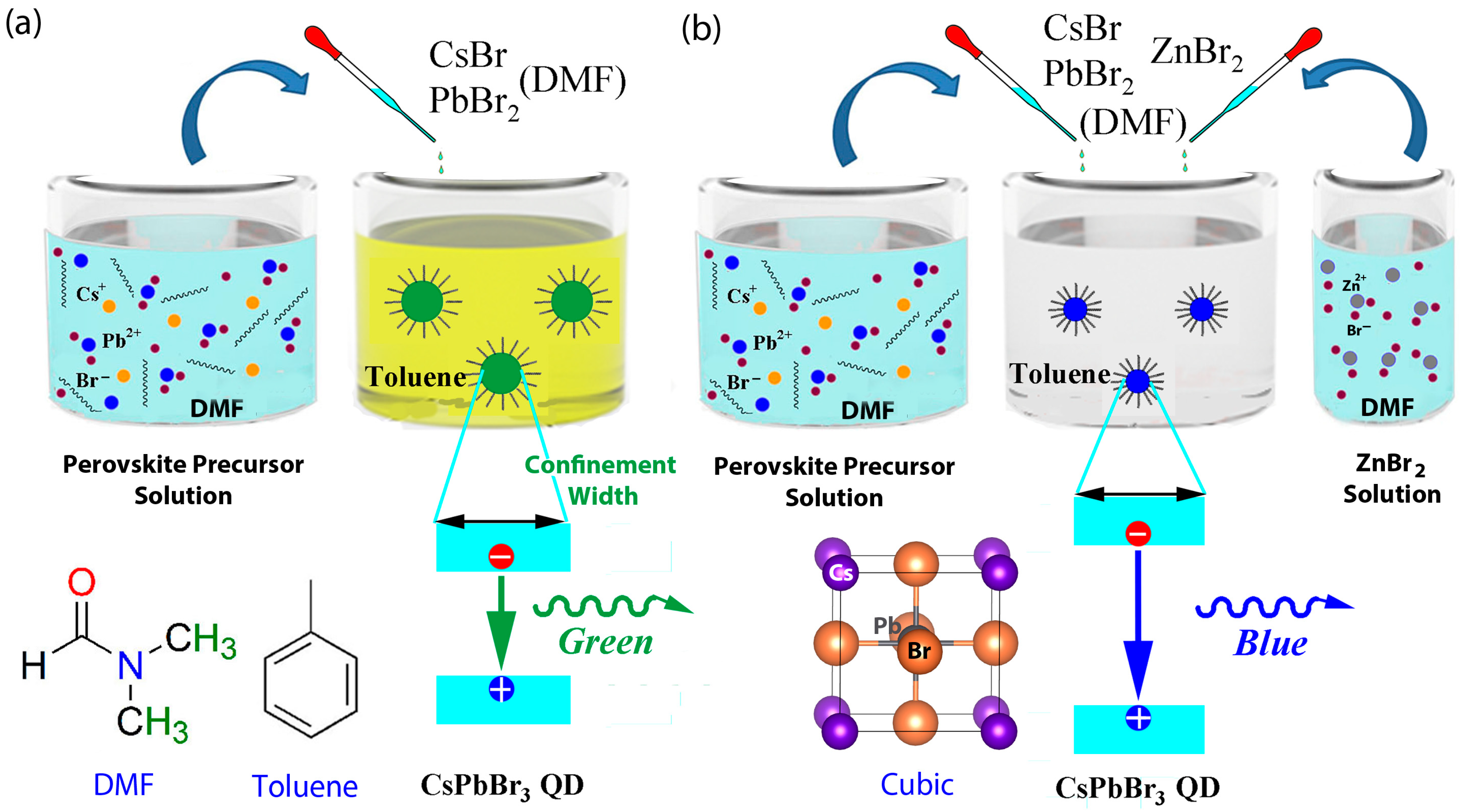
2.2. Synthesis of ZnBr2-CsPbBr3 QDs
2.3. Characterization
2.4. Computational Methods
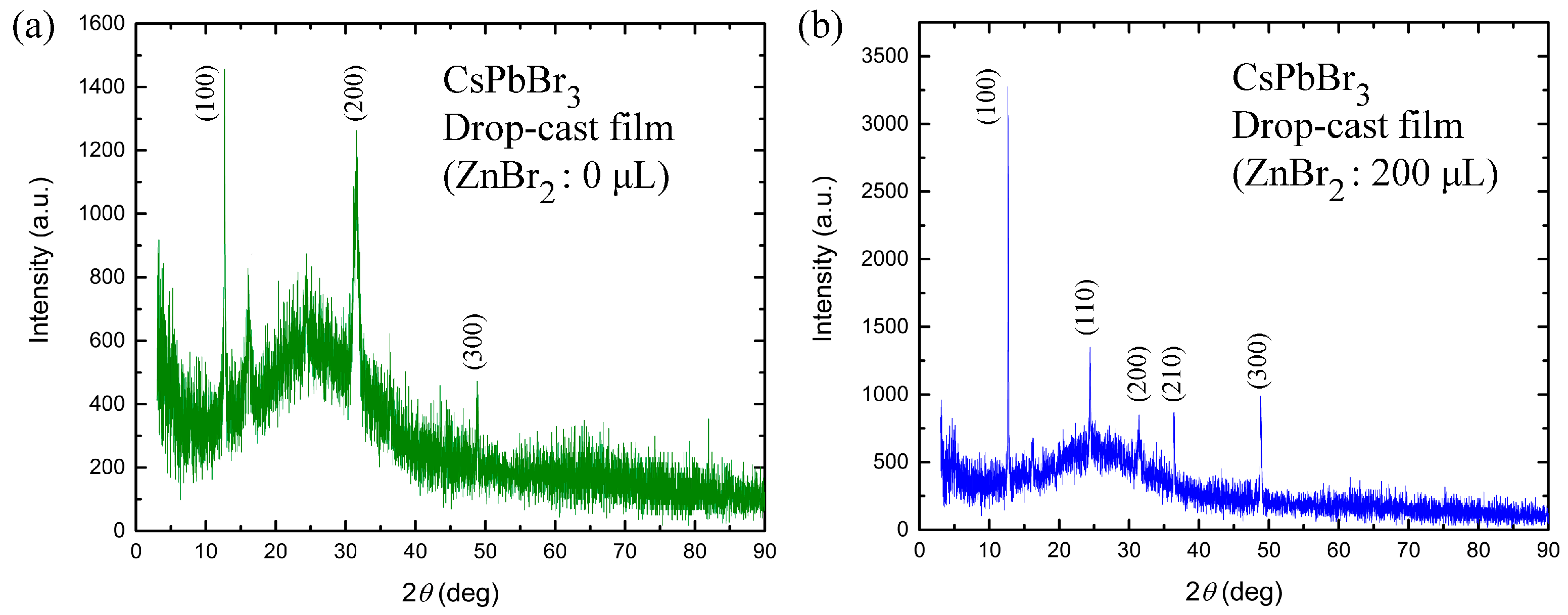
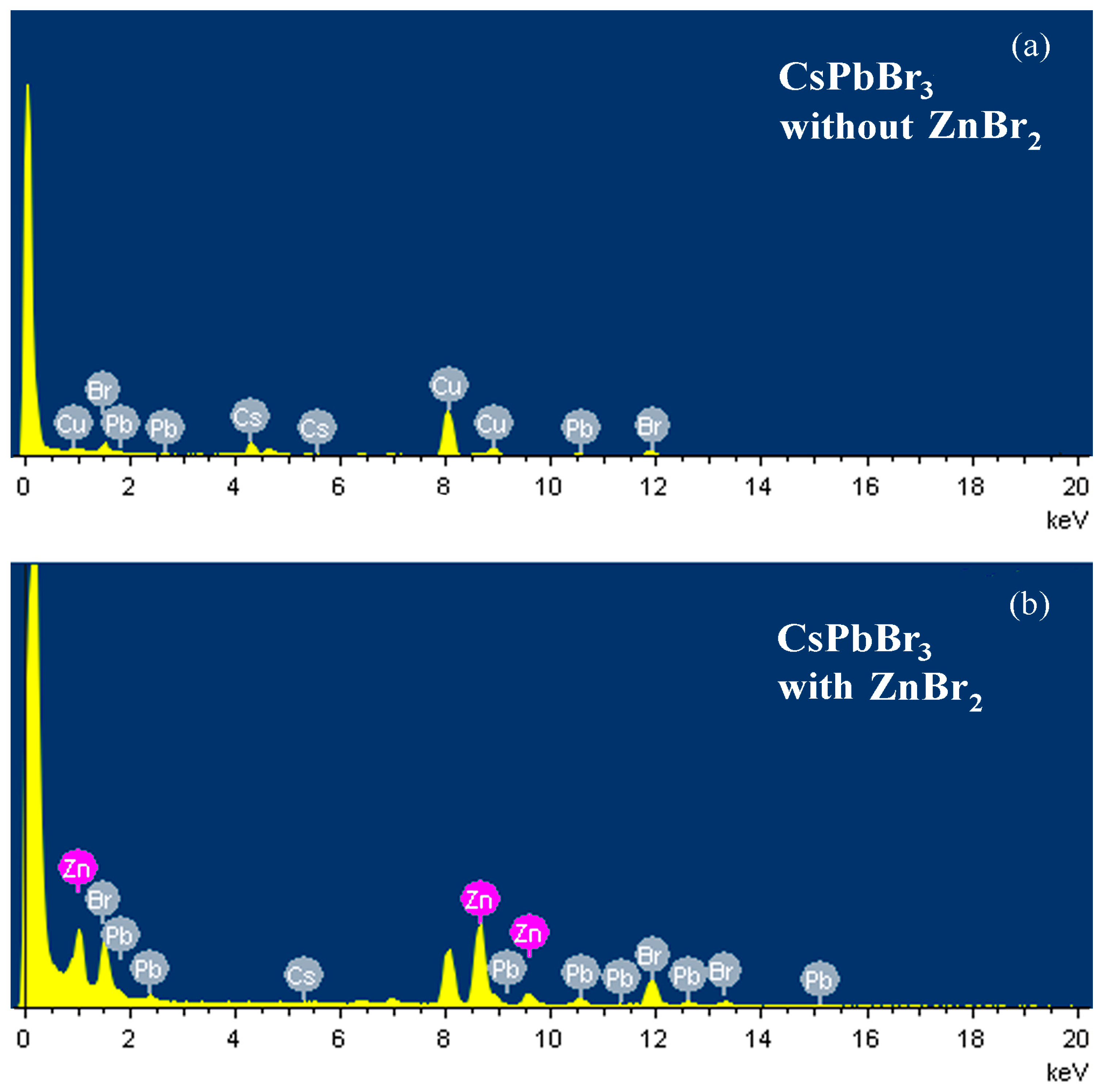
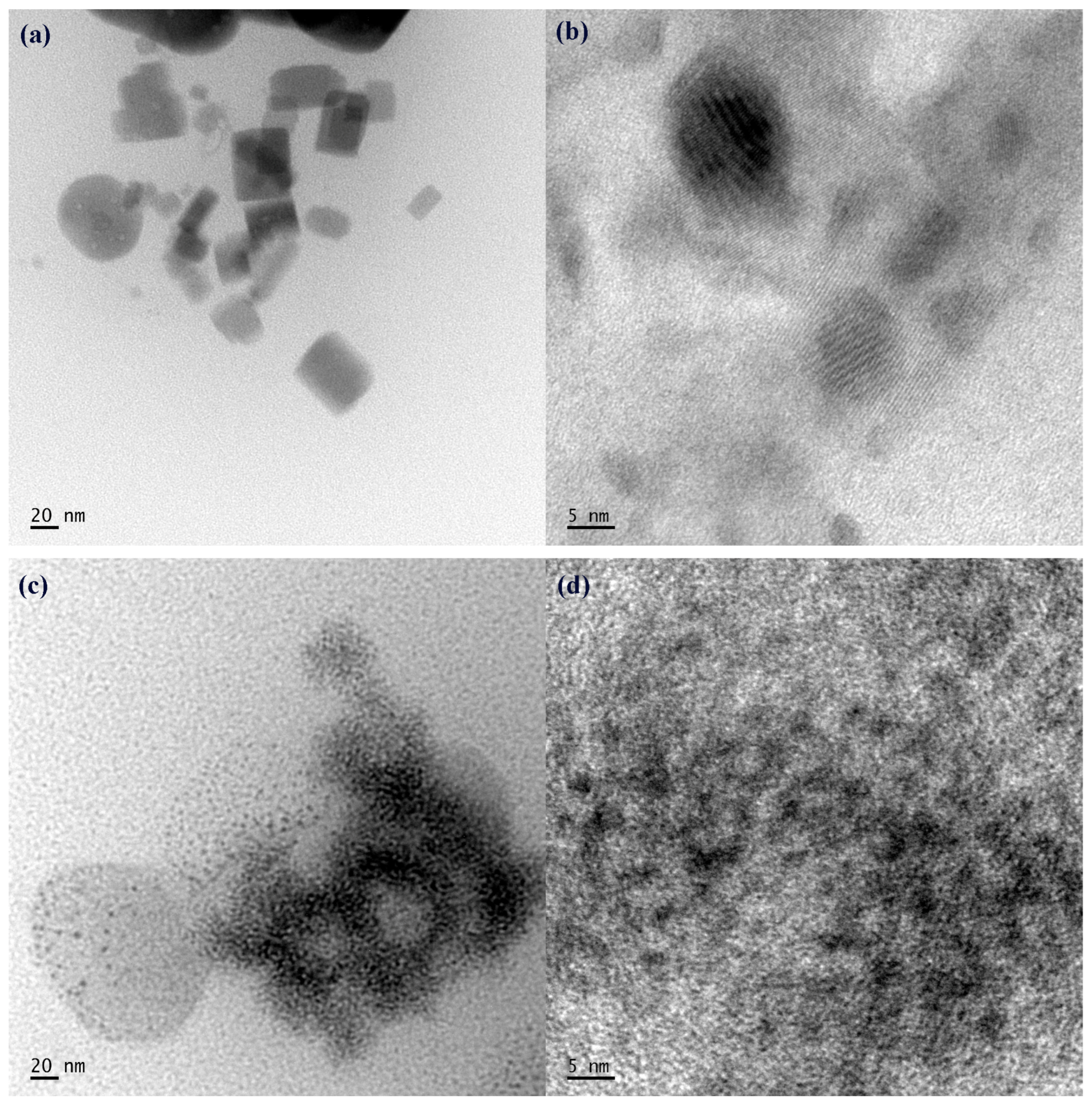
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Li, J.; Cai, B.; Song, J.; Zhang, F.; Fang, T.; Zeng, H. A Bilateral Interfacial Passivation Strategy Promoting Efficiency and Stability of Perovskite Quantum Dot Light-Emitting Diodes. Nat. Commun. 2020, 11, 3900. [Google Scholar] [CrossRef]
- Karlsson, M.; Yi, Z.; Reichert, S.; Luo, X.; Lin, W.; Zhang, Z.; Bao, C.; Zhang, R.; Bai, S.; Zheng, G.; et al. Mixed Halide Perovskites for Spectrally Stable and High-Efficiency Blue Light-Emitting Diodes. Nat. Commun. 2021, 12, 361. [Google Scholar] [CrossRef]
- Tsai, H.; Huang, H.H.; Watt, J.; Hou, C.H.; Strzalka, J.; Shyue, J.J.; Wang, L.; Nie, W. Cesium Lead Halide Perovskite Nanocrystals Assembled in Metal-Organic Frameworks for Stable Blue Light Emitting Diodes. Adv. Sci. 2022, 14, 210580. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Jiang, Q.; Wang, P.; Yin, Z.; Zhang, X.; Tan, H.; Yang, Y.M.; Wei, M.; Sutherland, B.R.; et al. Ultra-Bright and Highly Efficient Inorganic Based Perovskite Light-Emitting Diodes. Nat. Commun. 2017, 8, 15640. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, K.; Sun, X.W.; Choy, W.C.H. Strategies Toward Efficient Blue Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2100516. [Google Scholar] [CrossRef]
- Shi, W.; Zhao, Y.; Xie, C.; Yang, P. Na-Doping CsPbBr3 Quantum Dots Prepared via Ion Exchange for Bright and Stable Blue-to-Red Photoluminescence. J. Lumin. 2021, 233, 117886. [Google Scholar] [CrossRef]
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites for Light-Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, J.; Zhu, H.; Li, Y.; Tang, J.X.; Jiang, Y. Surface Ligand Engineering for Near-Unity Quantum Yield Inorganic Halide Perovskite QDs and High-Performance QLEDs. Chem. Mater. 2018, 30, 6099–6107. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-Shell Resurfacing for Blue LEDs Based on Strongly Confined Perovskite Quantum Dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef]
- Chen, C.; Xuan, T.; Bai, W.; Zhou, T.; Huang, F.; Xie, A.; Wang, L.; Xie, R.J. Highly Stable CsPbI3:Sr2+ Nanocrystals with near-Unity Quantum Yield Enabling Perovskite Light-Emitting Diodes with an External Quantum Efficiency of 17.1%. Nano Energy 2021, 85, 106033. [Google Scholar] [CrossRef]
- Bi, C.; Wang, S.; Li, Q.; Kershaw, S.V.; Tian, J.; Rogach, A.L. Thermally Stable Copper(II)-Doped Cesium Lead Halide Perovskite Quantum Dots with Strong Blue Emission. J. Phys. Chem. Lett. 2019, 10, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, K. Blue Perovskite Light-Emitting Diodes (LEDs): A Minireview. Instrum. Sci. Technol. 2020, 48, 616–636. [Google Scholar] [CrossRef]
- Shao, H.; Zhai, Y.; Wu, X.; Xu, W.; Xu, L.; Dong, B.; Bai, X.; Cui, H.; Song, H. High Brightness Blue Light-Emitting Diodes Based on CsPb(Cl/Br)3 perovskite QDs with Phenethylammonium Chloride Passivation. Nanoscale 2020, 12, 11728–11734. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yuan, S.; Liu, J.; Yin, J.; Yuan, F.; Shen, W.S.; Yao, K.; Wei, M.; Zhou, C.; Song, K.; et al. Chlorine Vacancy Passivation in Mixed Halide Perovskite Quantum Dots by Organic Pseudohalides Enables Efficient Rec. 2020 Blue Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 793–798. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Li, X.; Wang, S. Enhancing Quantum Yield of CsPb(BrxCl1−x)3 Nanocrystals through Lanthanum Doping for Efficient Blue Light-Emitting Diodes. Nano Energy 2020, 77, 105302. [Google Scholar] [CrossRef]
- Ochsenbein, S.T.; Krieg, F.; Shynkarenko, Y.; Rainò, G.; Kovalenko, M.V. Engineering Color-Stable Blue Light-Emitting Diodes with Lead Halide Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 21655–21660. [Google Scholar] [CrossRef]
- He, H.; Mei, S.; Wen, Z.; Yang, D.; Yang, B.; Zhang, W.; Xie, F.; Xing, G.; Guo, R. Recent Advances in Blue Perovskite Quantum Dots for Light-Emitting Diodes. Small 2022, 18, 2103527. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, M.; Yang, Z.; Wang, H.; Padhiar, M.A.; Qiu, H.; Dang, J.; Miao, Y.; Zhou, Y.; Bhatti, A.S. Strong Violet Emission from Ultra-Stable Strontium-Doped CsPbCl3 superlattices. Nanoscale 2022, 14, 2359–2366. [Google Scholar] [CrossRef]
- Liu, Y.; Ono, L.K.; Tong, G.; Bu, T.; Zhang, H.; Ding, C.; Zhang, W.; Qi, Y. Spectral Stable Blue-Light-Emitting Diodes via Asymmetric Organic Diamine Based Dion-Jacobson Perovskites. J. Am. Chem. Soc. 2021, 143, 19711–19718. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Zhang, R.; Liu, X.; Zhang, W.; Zhang, J.B.; Gao, F.; Wang, L. Efficient and Spectrally Stable Blue Perovskite Light-Emitting Diodes Based on Potassium Passivated Nanocrystals. Adv. Funct. Mater. 2020, 30, 1908760. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Van der Stam, W.; Geuchies, J.J.; Altantzis, T.; Van Den Bos, K.H.W.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; De Mello Donega, C. Highly Emissive Divalent-Ion-Doped Colloidal CsPb1−XMxBr3 Perovskite Nanocrystals through Cation Exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhong, G.; Yin, Y.; Miao, J.; Li, K.; Wang, C.; Xu, X.; Shen, C.; Meng, H. Aluminum-Doped Cesium Lead Bromide Perovskite Nanocrystals with Stable Blue Photoluminescence Used for Display Backlight. Adv. Sci. 2017, 4, 1700345. [Google Scholar] [CrossRef]
- Zeng, F.; Tan, Y.; Hu, W.; Tang, X.; Zhang, X.; Yin, H. A Facile Strategy to Synthesize High Colour Purity Blue Luminescence Aluminium-Doped CsPbBr3 Perovskite Quantum Dots. J. Lumin. 2022, 245, 118788. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Hu, Y.; Pei, Y.; Wang, S.; Shi, Z.; Colvin, V.L.; Wang, S.; Zhang, Y.; Yu, W.W. Strong Blue Emission from Sb3+-Doped Super Small CsPbBr3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 1750–1756. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Liu, H.; Li, F.; Zhao, J.; Wang, Y. Strongly Quantum-Confined Mn2+-Doped CsPbBr3 Nanocrystals in MCM-41 with Pure Blue Emission. New J. Chem. 2020, 44, 2980–2985. [Google Scholar] [CrossRef]
- Qiao, T.; Son, D.H. Synthesis and Properties of Strongly Quantum-Confined Cesium Lead Halide Perovskite Nanocrystals. Acc. Chem. Res. 2021, 54, 1399–1408. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, Z.; Fang, F.; Wang, L.; Wang, G.; Liu, C.; Huang, Q.; Sun, J.; Huang, Y.; Mao, L.; et al. A Synergetic Codoping Strategy Enabling Performance Improvement of Pure-Blue Perovskite Quantum Dots Light-Emitting Diodes. Adv. Opt. Mater. 2022, 10, 2102569. [Google Scholar] [CrossRef]
- Almeida, G.; Goldoni, L.; Akkerman, Q.; Dang, Z.; Khan, A.H.; Marras, S.; Moreels, I.; Manna, L. Role of Acid-Base Equilibria in the Size, Shape, and Phase Control of Cesium Lead Bromide Nanocrystals. ACS Nano 2018, 12, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Yao, Z.; Sun, X.; Wei, X.; Wang, J.; Tian, J. Perovskite Quantum Dots with Ultralow Trap Density by Acid Etching-Driven Ligand Exchange for High Luminance and Stable Pure-Blue Light-Emitting Diodes. Adv. Mater. 2021, 33, 2006722. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Qiao, T.; Kim, D.; Parobek, D.; Rossi, D.; Son, D.H. Precise Control of Quantum Confinement in Cesium Lead Halide Perovskite Quantum Dots via Thermodynamic Equilibrium. Nano Lett. 2018, 18, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Chang, Y.; Xu, E.; Yang, S.; Zhang, J.; Jiang, Y.; Cheng, X.; Yu, D. Highly Efficient and Blue-Emitting CsPbBr3 quantum Dots Synthesized by Two-Step Supersaturated Recrystallization. Nanotechnology 2021, 32, 145721. [Google Scholar] [CrossRef] [PubMed]
- Sitapure, N.; Qiao, T.; Hee, D.; Kwon, J.S. Kinetic Monte Carlo Modeling of the Equilibrium-Based Size Control of CsPbBr3 Perovskite Quantum Dots in Strongly Confined Regime. Comput. Chem. Eng. 2020, 139, 106872. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Roomerature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Ghorai, A.; Mahato, S.; Srivastava, S.K.; Ray, S.K. Atomic Insights of Stable, Monodispersed CsPbI3−xBrx (x = 0, 1, 2, 3) Nanocrystals Synthesized by Modified Ligand Cell. Adv. Funct. Mater. 2022, 32, 2202087. [Google Scholar] [CrossRef]
- Huangfu, C.; Feng, L. High-performance fluorescent sensor based on CsPbBr3 quantum dots for rapid analysis of total polar materials in edible oils. Sens. Actuators B Chem. 2021, 344, 130193. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Wang, J.; Liu, W. Thermal Stability of CsPbBr3 Perovskite Quantum Dots Assembled with SBA-15. Coatings 2021, 11, 953. [Google Scholar] [CrossRef]
- Brinck, S.T.; Infante, I. Surface Termination, Morphology, and Bright Photoluminescence of Cesium Lead Halide Perovskite Nanocrystals. ACS Energy Lett. 2016, 1, 1266–1272. [Google Scholar] [CrossRef]
- Cui, K.; Wen, Y.; Han, X.; Hao, Z.; Zhang, J.; Xie, J. Intense Blue Emission from One-Pot Synthesized Quaternary CsZnxPb1−XBr3 Perovskite Quantum Dots. Opt. Mater. 2023, 136, 113441. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Liu, Y.F.; Bi, Y.G.; Li, C.H.; Wang, Y.F.; Li, H.W.; Zhang, Q.W.; Lv, C.; Wu, Y.Q. Improved Performance of CsPbBr3 Light-Emitting Diodes Based on Zinc Bromide Passivated Quantum Dots. Org. Electron. 2023, 116, 106775. [Google Scholar] [CrossRef]
- Smock, S.R.; Chen, Y.; Rossini, A.J.; Brutchey, R.L. The Surface Chemistry and Structure of Colloidal Lead Halide Perovskite Nanocrystals. Acc. Chem. Res. 2021, 54, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, P.; Brutchey, R.L. Depressed Phase Transitions and Thermally Persistent Local Distortions in CsPbBr3 Quantum Dots. Chem. Mater. 2018, 30, 6711–6716. [Google Scholar] [CrossRef]
- Kong, X.; Wu, Y.; Xu, F.; Yang, S.; Cao, B. Ultrasmall CsPbBr3 Quantum Dots with Bright and Wide Blue Emissions. Phys. Status Solidi—Rapid Res. Lett. 2021, 15, 2100134. [Google Scholar] [CrossRef]
- Rainò, G.; Yazdani, N.; Krieg, F.; Rossell, M.D.; Kovalenko, M.V.; Boehme, S.C.; Kober-czerny, M.; Zhu, C.; Erni, R.; Wood, V.; et al. Ultra-Narrow Room-Temperature Emission from Single CsPbBr3 Perovskite Quantum Dots. Nat. Commun. 2022, 13, 2587. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, B.; Bravić, I.; Yu, Y.; Dong, Y.; Liang, R.; Ou, Q.; Monserrat, B.; Zhang, S. Highly Efficient Blue-Emitting CsPbBr3 Perovskite Nanocrystals through Neodymium Doping. Adv. Sci. 2020, 7, 2001698. [Google Scholar] [CrossRef]
- Cao, J.; Yan, C.; Luo, C.; Li, W.; Zeng, X.; Xu, Z.; Fu, X.; Wang, Q.; Chu, X.; Huang, H.; et al. Cryogenic-Temperature Thermodynamically Suppressed and Strongly Confined CsPbBr3 Quantum Dots for Deeply Blue Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2100300. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, M.; Yang, Z.; Qiu, H.; Kou, S.; Padhiar, M.A.; Bhatti, A.S.; Gaponenko, N.V. Pressure-Driven Transformation of CsPbBrI2 Nanoparticles into Stable Nanosheets in Solution through Self-Assembly. J. Phys. Chem. Lett. 2020, 11, 9862–9868. [Google Scholar] [CrossRef]
- Zhu, Y.; Cui, Q.; Chen, J.; Chen, F.; Shi, Z.; Zhao, X.; Xu, C. Inhomogeneous Trap-State-Mediated Ultrafast Photocarrier Dynamics in CsPbBr3Microplates. ACS Appl. Mater. Interfaces 2021, 13, 6820–6829. [Google Scholar] [CrossRef] [PubMed]
- Ghaithan, H.M.; Alahmed, Z.A.; Qaid, S.M.; Hezam, M.; Aldwayyan, A.S. Density Functional Study of Cubic, Tetragonal, and Orthorhombic. ACS Omega 2020, 5, 7468–7480. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Su, J.; Wang, L.; Lin, Z.; Hao, Y.; Chang, J. Improved Doping and Optoelectronic Properties of Zn-Doped CsPbBr3 Perovskite through Mn Codoping Approach. J. Phys. Chem. Lett. 2021, 12, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
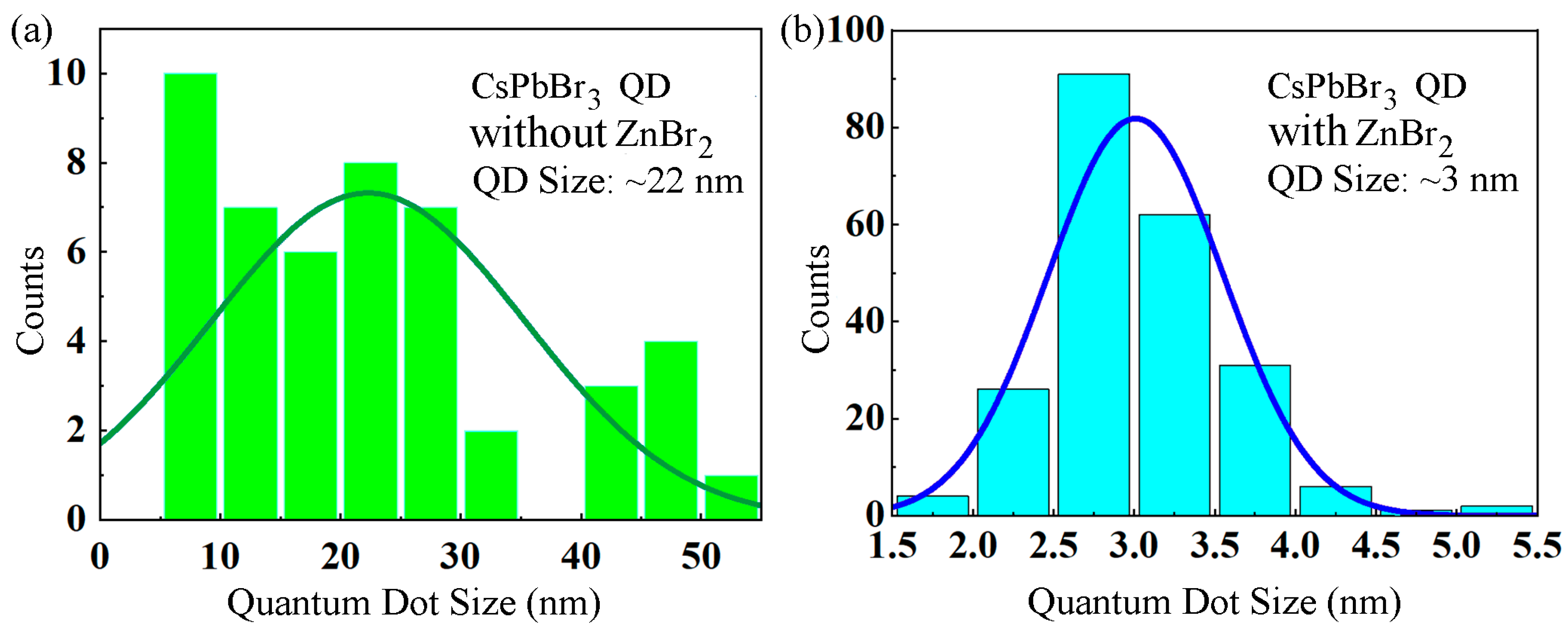
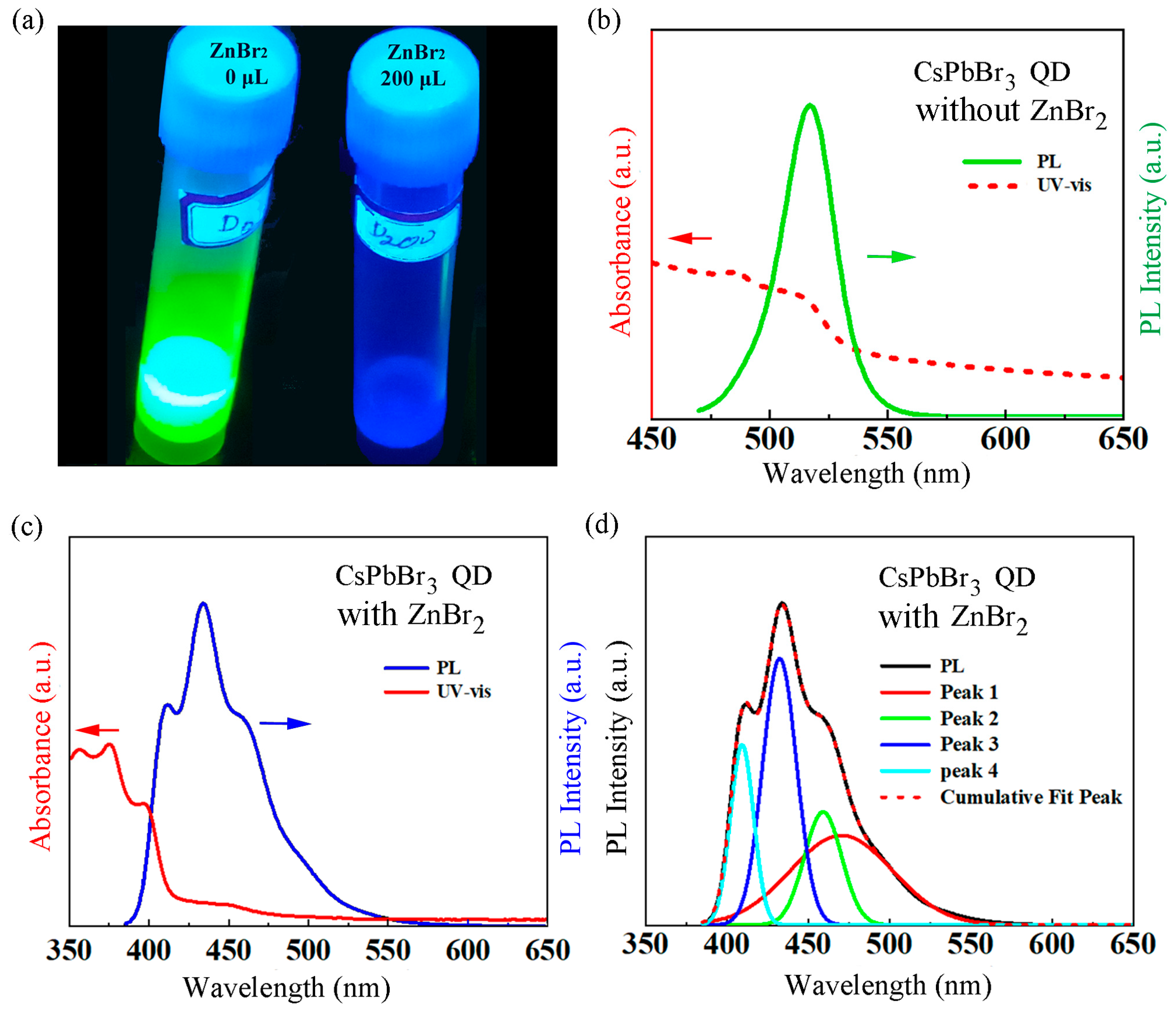
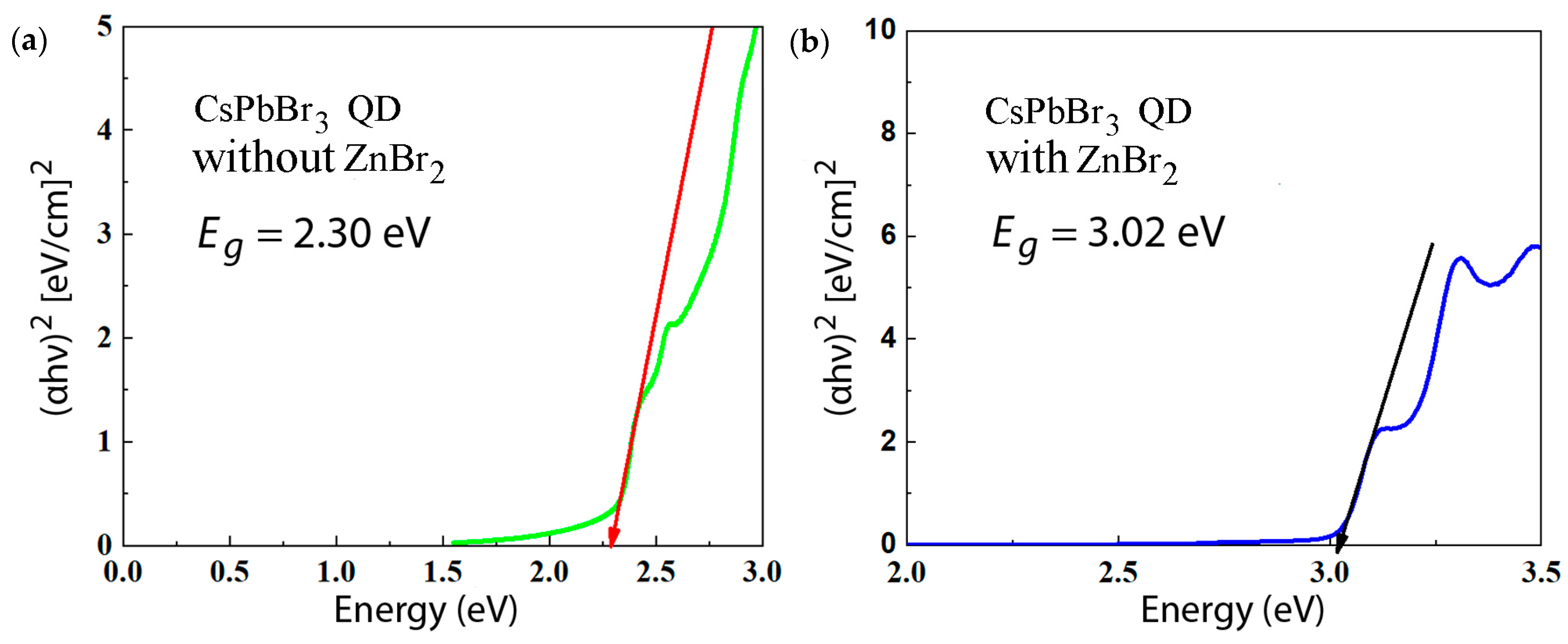

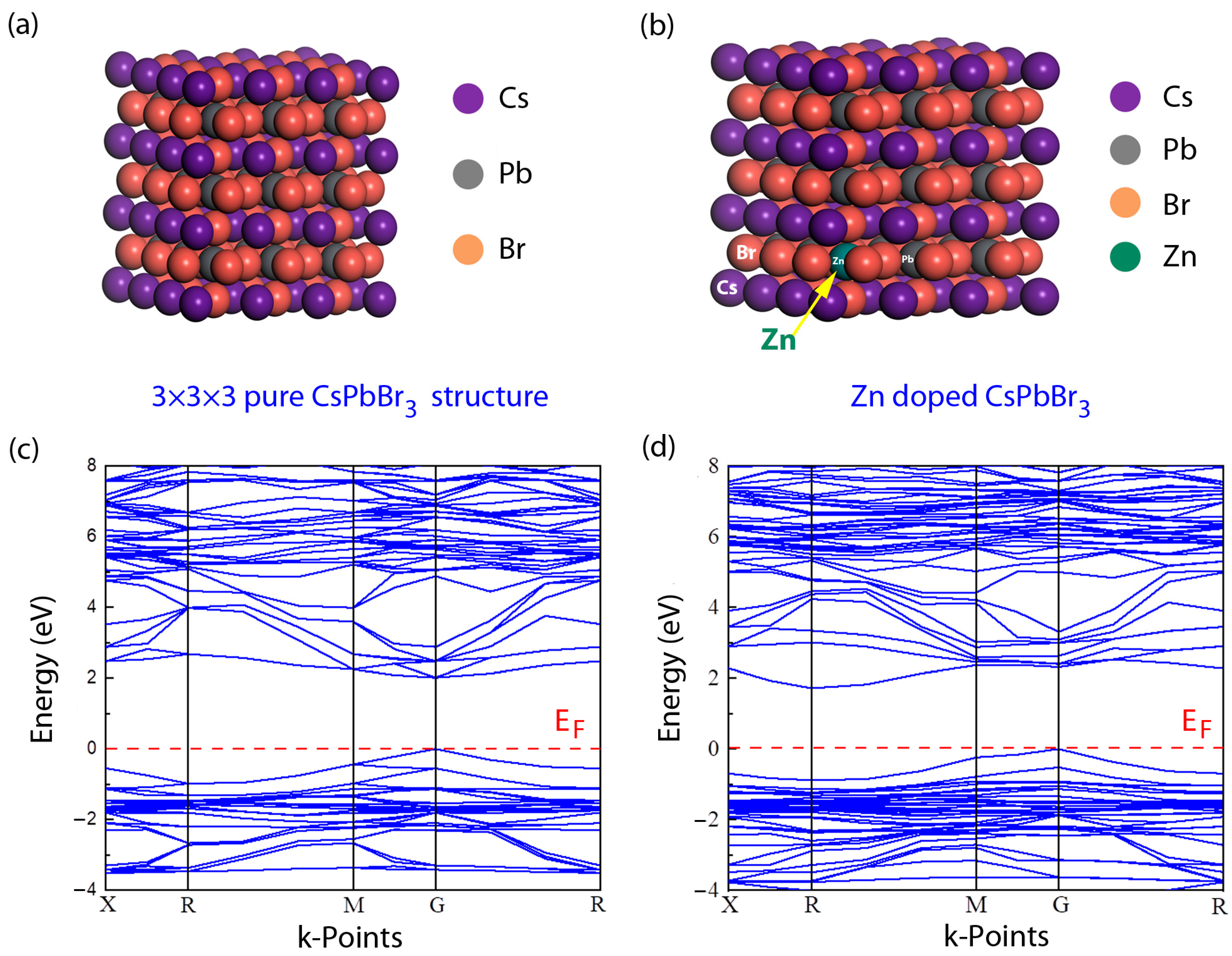

| Samples | A1 (%) | τ1 (ns) | A2 (%) | τ2 (ns) | τave (ns) |
|---|---|---|---|---|---|
| CsPbBr3 QDs without ZnBr2 | 98.10 | 3.67 | 1.90 | 14.23 | 4.41 |
| CsPbBr3 QDs with ZnBr2 | 41.29 | 1.45 | 58.71 | 1.45 | 1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idosa, D.A.; Abebe, M.; Mani, D.; Thankappan, A.; Thomas, S.; Aga, F.G.; Kim, J.Y. A Blue-Light-Emitting 3 nm-Sized CsPbBr3 Perovskite Quantum Dot with ZnBr2 Synthesized by Room-Temperature Supersaturated Recrystallization. Photonics 2023, 10, 802. https://doi.org/10.3390/photonics10070802
Idosa DA, Abebe M, Mani D, Thankappan A, Thomas S, Aga FG, Kim JY. A Blue-Light-Emitting 3 nm-Sized CsPbBr3 Perovskite Quantum Dot with ZnBr2 Synthesized by Room-Temperature Supersaturated Recrystallization. Photonics. 2023; 10(7):802. https://doi.org/10.3390/photonics10070802
Chicago/Turabian StyleIdosa, Dula Adugna, Mulualem Abebe, Dhakshnamoorthy Mani, Aparna Thankappan, Sabu Thomas, Fekadu Gochole Aga, and Jung Yong Kim. 2023. "A Blue-Light-Emitting 3 nm-Sized CsPbBr3 Perovskite Quantum Dot with ZnBr2 Synthesized by Room-Temperature Supersaturated Recrystallization" Photonics 10, no. 7: 802. https://doi.org/10.3390/photonics10070802
APA StyleIdosa, D. A., Abebe, M., Mani, D., Thankappan, A., Thomas, S., Aga, F. G., & Kim, J. Y. (2023). A Blue-Light-Emitting 3 nm-Sized CsPbBr3 Perovskite Quantum Dot with ZnBr2 Synthesized by Room-Temperature Supersaturated Recrystallization. Photonics, 10(7), 802. https://doi.org/10.3390/photonics10070802







