Abstract
The use of lasers in endourology has grown exponentially, leading to technological advancement and to miniaturization of the procedures. We aim to provide an overview of the lasers used in endourology and the associated future perspectives. Using MEDLINE, a non-systematic review was performed including articles between 2006 and 2023. English language original articles, reviews and editorials were selected based on their clinical relevance. Guidelines recommend ureteroscopy in case of stones <2 cm and a percutaneous approach for renal stones ≥2 cm. High-power holmium (Ho:YAG) lasers and the new thulium fibre laser (TFL) may change the future, offering shorter procedures for complex stones, with good outcomes. Increased intrarenal temperature associated with these new technologies may be overcome with adaptive strategies and optimal settings. For upper-tract urothelial carcinoma (UTUC), the combination of laser techniques and these new lasers may reduce the risk of stenosis and allow for a more accurate tumour ablation, potentially reducing the recurrence rates. Laser enucleation procedures are gaining a major role in benign prostate enlargement (BPE), especially in patients with larger prostates or under anticoagulant therapy. However, the superiority of one laser over the other has not been established yet, and the choice of technique is mainly deferred to the surgeon’s expertise. In conclusion, lasers will further expand their horizon in endourology, allowing for instrument adaptation to challenging anatomy. Prospective, randomized clinical trials are however needed to confirm available results and to provide the optimal settings for each pathology.
Keywords:
endourology; lasers; kidney calculi; intrarenal temperature; UTUC; BPH; TFL; holmium; thulium fibre laser; ureteroscopy; PCNL 1. Introduction
The use of lasers in endourology seems has grown exponentially over the last three decades [1]. Due to equipment advancements and the improvement of surgeon skills, minimally invasive laser treatments are an alternative to traditional open surgery in the context of kidney and ureteric stones, bladder stones, upper-tract urothelial tumours and benign prostatic hyperplasia (BPH) [2,3,4,5,6,7,8,9,10].
While holmium lasers are still the mainstay lasers, the landscape has changed with the recent introduction of thulium fibre lasers (TFLs). Their use has also allowed minimization of percutaneous nephrolithotomy (PCNL) and enucleation of prostate procedures. Because of the elongation of life expectancy, older patients with cardiac pathologies requiring anticoagulant prevention might benefit from a safer treatment using laser technology than classic procedures [1]. Laser procedures are less invasive and safer in more fragile patients; they are sometimes more precise and accurate than traditional surgeries and allow for shorter hospitalization time [1].
In this review article we look at the role of different lasers for endourology, the usefulness of energy, frequency and pulse modulation, and the current concepts around intrarenal temperature and the future of laser technology.
2. Lasers and Urolithiasis
The prevalence of kidney stone disease has increased worldwide, especially during the last two decades, with a lifetime risk in Europe of up to 14% [2]. This has led to a broadening of numerous advancements in laser technologies, equipment, and settings. According to the European Association of Urologists (EAU) Guidelines, ureteroscopy (URS), either ante- or retrograde, might be the first treatment option for proximal or distal ureteral stones > or <10 mm, and for kidney stones <10 mm, or lower pole stones between 10 and 20 mm in patients with unfavourable factors for Shock Wave Lithotripsy (SWL) [3]. Percutaneous nephrolithotomy (PCNL) is the first-choice treatment for renal stones >20 mm and for lower pole stones between 10 and 20 mm in patients with unfavourable factors for SWL [3]. However, with technological advancements and improved surgical skills, flexible URS has been increasingly used for renal stones >2 cm, with similar stone-free rates (SFR) and a lower complication rate than PCNL [4].
Available lasers for stone treatment include the holmium:yttrium-aluminium-garnet (Ho:YAG) and the thulium (Tm:YAG and fibre (TFL)) lasers (Table 1 and Figure 1).

Table 1.
Summary of laser properties for urolithiasis (use, advancements, benefits and disadvantages, suggested laser settings).
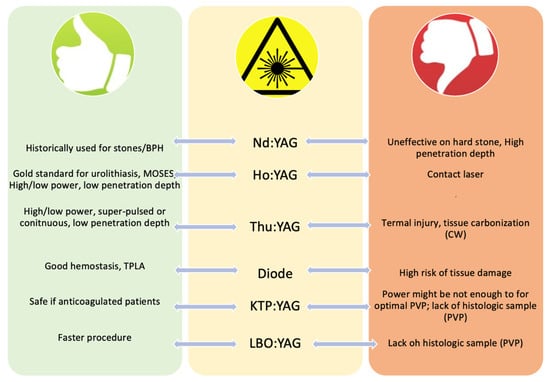
Figure 1.
Advantages and disadvantages of each laser (BPH—benign prostatic hyperplasia, TPLA—transperineal laser ablation).
2.1. Neodymium-Doped Yttrium Aluminium Garnet Laser: Nd:YAG Laser
The Nd:YAG laser was one of the first lasers to be used for urolithiasis due to its ability to release energy in pulses [11]. In its FREDDY (frequency-doubled double pulse) mode, the Nd:YAG laser demonstrates good fragmentation potential when operated within 3 mm of the target stone [12]. Due to its higher ability to fragment harder stones and to its ability to release “packets” of energy, improving fragmentation speed and efficacy, the Ho:YAG laser is now preferred over the Nd:YAG laser, and it is now considered the optimum standard for both URS and flexible nephroscopy [3].
2.2. Holmium Laser: Ho:YAG Laser
The first report on the Ho:YAG laser in the treatment of urolithiasis was published about three decades ago [5]. Ho:YAG is a 2140 nm wavelength laser with a high water absorption, that reflects in a fluid medium at a distance of about 0.3–0.5 mm. This makes the Ho:YAG laser ideal for urological use in limited spaces such as the ureter or the renal pelvis [6].
The Ho:YAG laser can be used in a high- or low-power modality. The lower power Ho:YAG devices have a maximum power up to 30–35 Watts (W) with a maximum laser pulse frequency of 25–30 Hz [7], while high-power Ho:YAG laser machines might reach higher energy output, up to 100–120 W [8]. These characteristics and variation in the settings such as pulse, energy, frequency, and pulse width can allow for other treatment aspects apart from fragmentation and the retrieval of stones.
High-energy and low-frequency settings are used for fragmentation, while low-energy and high-frequency settings are used for dusting [6]. Dusting has been demonstrated to have an advantage over fragmentation, in terms of decreased use of the ureteral access sheath (UAS), basket-associated complications, and reduced operative time [9,10,11].
If fragmentation is performed in the first stage of the lithotripsy procedure, a second-stage (“completion”) non-contact lithotripsy may be needed, intended to produce smaller particles that might spontaneously pass [11]. Two different techniques may be used for this, the “popcorn” and the “pop-dusting”. The “popcorn” technique uses high pulse energy (1.5 J), usually associated with high frequency (20–40 Hz), and long-pulse mode to produce clinically insignificant fragments [11,13]. The second one, the “pop-dusting” technique, uses instead low-power, long-pulse energy (0.5–0.8 J), resulting in finer fragments [11]. The “pop-dusting” technique has been demonstrated to enhance the speed of clearance for harder stones both in the adult and paediatric populations [11,14,15].
Additionally, the modern high-powered Ho:YAG lasers can be equipped with the MOSES “pulse modulation” technology. MOSES technology (Lumenis) divides the laser pulse into two peaks: the first pulse separates the fluid in front of the stone (Moses effect), while the second pulse is delivered directly to the stone surface by the intervening fluid, leading to better fragmentation, lower retropulsion, and less time taken for the procedure [2,6,16,17,18]. A recent systematic review showed that the advantage of high-power lasers was lost for larger stones [19]; however Pietropaolo et al. conducted a retrospective study demonstrating that MOSES technology with high-power lasers reduces the fragmentation time and infective complications, even in patients with larger stones and those who had a stent placed, and minimized the number of patients who needed a second procedure to achieve a stone-free status [2]. They conclude that MOSES technology has a definitive advantage, especially when used with a mid-power laser, in treating complex stones, usually without the need for secondary procedures.
2.3. Thulium Fibre Laser (TFL)
The super-pulsed thulium fibre lasers (TFL) use electronically modulated laser diodes, allowing the delivery of a higher and more constant peak power with a wider range of laser parameters through a fibre [6,20]. This makes the TFL a highly versatile laser, with pulse frequencies up to 2200 Hz, very low to very high pulse energies (0.005–6J), short- to very long-pulse durations (200 μm to 12 ms), and a total power level up to 55 W [20], with optimal laser settings proposed as 0.5 J × 30 Hz for fragmentation and 0.15 J × 100 Hz for dusting [21]. Its wavelength is 1940 nm and has an optical penetration depth in water four-times shorter than the Ho:YAG laser [20]. This means that water absorbs TFL energy approximately four times higher than Ho:YAG, resulting in lower stone and tissue ablation thresholds. The higher water tropism of the TFL is at the base of the “micro-explosions” mechanism, that is to say, an increase in the breakability of the stone due to increased water absorption and larger stone pore size induced by laser stimulation [22]. Additionally, the TFL can use smaller laser fibres (up to 50 μm core), improving irrigation, scope deflection, retropulsion reduction, accessibility, and visibility [20].
Since the approval of the TFL by the FDA and the EMA, numerous studies have been published showing that the TFL outperforms the Ho:YAG laser in terms of dusting quantity and quality, lower retropulsion, and shorter operation time for any kind of stone [21,23,24,25,26,27]. In a comparative study by Ulvik et al., the TFL showed a higher SFR (92% vs. 67%, p = 0.002), lower operative time (49 vs. 57 min, p = 0.008), and lower intraoperative adverse event (5% vs. 22%, p = 0.014), compared to the Ho:YAG laser [25]. In a large multicenter study involving 4208 patients who underwent RIRS, Keat et al. showed that overall, the TFL was associated with lesser residual fragments (>2 mm), a finding that was confirmed by a multivariate analysis for stones both more and less than 1000 HU [26]. Since residual fragments require second treatment, this is best avoided. Ryan et al. confirmed a decrease in operative time for the TFL vs. the Ho:YAG without pulse modulation for any stone size (<15 mm, p = 0.007; <10 mm, p = 0.002), and showed that the mean 13 min reduction in operative time resulted in a saving of USD 440/case in direct operating room costs, resulting in a range of USD 294,000–381,900 savings per year [27].
Even if the results are promising, the TFL still needs to be tested in further randomized prospective trials on larger sample sizes with the associated cost analysis.
3. Clinical Influence of Laser in Urolithiasis
3.1. PCNL and Laser
With the advent of laser technology and the increased ability to produce smaller residual fragments or dust, PCNL underwent a process of miniaturization, that was coupled to a decrease in bleeding, leading to higher chance of tubeless procedures and reduced hospital stay [28,29,30]. According to their size, miniaturized techniques are defined as mini-PCNL (16–20 Fr), ultramini-PCNL (UMP, 11–14 Fr), and micro-PCNL (microPNL, <10 Fr) [31].
Compared to RIRS, PCNL is associated with higher blood loss, complication rates, and readmissions [32]. On the contrary, the main advantages of PCNL are that it generally ensures SFR in usually a single and faster procedure, usually with fragmentation [33,34]. This might have clinical advantages, in terms of anaesthetic length and infective complications. One of the main problems related to the increased time of miniaturized PCNL was due to stone retropulsion. However, both in vitro and in vivo studies have demonstrated that TFL has low or zero retropulsion [35]. With Ho:YAG lasers, retropulsion becomes evident at 0.2 J, while with the TFL, retropulsion begins at around 1 J, being therefore clinically insignificant in many cases [35,36]. The debate is now open on whether TFL has less retropulsion with respect to Ho:YAG when combined with MOSES technology, but there is no a definitive answer as yet [35].
Interestingly, in a recent retrospective study, Patil et al. compared the high-power Ho:YAG with MOSES Technology vs. the TFL for miniPCNL [37]. They found that the TFL produced a greater proportion of fragments ≥3 mm (36% vs. 22.68%, p = 0.002), but a subset analysis based on stone density showed that the TFL had a shorter total operative time (p < 0.005) [37].
The introduction of the TFL raises a question: could the new kid on the block change the game? Taratkin et al. compared results for patients who underwent RIRS or mini-PCNL (16.5–17.5 Fr) for kidney stones > 20 mm, and found no differences in procedure duration, complications, or hospital stay [38]. In another prospective randomized comparison between RIRS and mini-PCNL for stones between 10 and 20 mm, Perri et al. demonstrated that both procedures were effective in obtaining a postoperative SFR with the TFL, but that according to stone position, either RIRS (upper calyceal stones) or mini-PCNL (lower calyceal stones) were superior [28].
It is therefore difficult to give a definitive answer, due to the lack of standardization of the studies and the heterogeneity in the reported results.
3.2. Role of Temperature with Laser Use
Thermal control when dealing with the upper tract and during lasering is of pivotal importance. During lithotripsy, the stone absorbs the laser energy itself, causing melting, fragmentation, chemical disintegration, and vaporization of the interstitial water [39]. Heat energy passes beyond the direct laser absorption zone, affecting the surrounding tissues. It has been demonstrated that the impact on biologic tissue ranges between 41 °C and 47 °C, but that an increase in temperature above the safe threshold of 43 °C has a detrimental cytotoxic effect [39]. This effect might cause ureteral scarring and in turn cause stricture.
Temperature rises according to power, so that high-power lasers cause higher thermal injury. According to a research by Tokas et al., when Ho:YAG and Tm:YAG are used with laser powers below 40 W, their temperature profiles are safe [40]. However, in vitro, with powers above 40 W, the temperature rises exponentially, thus making them dangerous for in vivo use [40]. For this reason, several factors might be used by the operators to keep the temperature in a safe profile [39]. With irrigation, cooler water circulates through the system, dissipating heat [40,41]. The use of a larger calibre ureteral access sheath (UAS) (12/14 F or 14/16 Fr) results in temperatures around 43 °C, and in improved irrigation and lower intrarenal pressures [39,40]. Shorter on/off laser activation intervals (5 s/5 s) are also recommended to keep temperatures low [39,42].
The TFL on the other hand has a heat generation that is four times larger than that of the Ho:YAG laser; for this reason, Tokas et al. suggest to maintain pulse rate below 500 Hz with higher saline irrigation rates [40]. Recently, several in vitro or animal studies have been conducted to investigate the thermal damage induced by the TFL [43,44]. Belle et al. have demonstrated in an in vitro silicone kidney-ureter model, that the TFL generated more heat at all settings tested (3.6, 10, 20 and 30 W) compared to the holmium laser, with higher risk of complications from thermal damage [43]. On the contrary, in their animal study, Sierra et al. compared the temperature increase and histologic changes in porcine urinary tracts treated with either TFL (left side) or Ho:YAG (right side) lasers [44]. In their experimental setting, they worked with 20 W in the kidney, 12 W in the ureter with a continuous irrigation at 40 cm H2O that provided a temperature of 36.8 °C and 34.6 °C for TFL and Ho:YAG, respectively [44]. They found that both technologies were equally safe. However, all authors agree on advocating low power settings for the urothelium, especially in the ureter, associated with continuous irrigation.
It is important to bear in mind that due to its anatomical features and lower surrounding vasculature, the ureter has a higher risk with respect to the renal pelvis to incur mucosal injury and to develop strictures. This has a special clinical value when treating UTUC or stones impacted in the mucosa, where the depth of penetration of the laser acquires fundamental importance.
4. Lasers and Urothelial Tumours
Non-muscle-invasive bladder cancer (NMIBC) is the most prevalent of bladder cancers, accounting for 70–75% of cases at first presentation [45,46]. According to EAU Guidelines, NMIBC can be stratified as low, intermediate, or high risk. Low-risk tumours have a low risk of progression, but a high risk of recurrence [46]. For this reason, treatment options are aimed at decreasing the risk of recurrence, and even if the gold standard for its treatment remains the transurethral resection of the bladder tumour (TURBT), office-based laser ablation or diathermy and surveillance could be offered.
Upper-tract urothelial carcinoma (UTUC) is a relatively rare cancer, representing <10% of all urothelial tumours [45]. According to EAU Guidelines, URS is considered as the first line for low-risk disease with oncological equipoise to radical nephroureterectomy (RNU) [46]. Usually, URS is coupled with adjuvant intracavitary therapy (with BCG or MMC), but nowadays there is limited evidence [46]. Since a higher association with local recurrence rate and risk of progression to RNU exists, for minimally invasive therapy in the context of UTUC, a careful patient selection and a strict follow-up and surveillance (both for upper tract and bladder recurrences) according to the risk-stratified strategy of the EAU guidelines, are necessary [46].
4.1. Ho:YAG, Nd:YAG and Thulium Lasers for Urothelial Cancer
The available lasers that might be used for urothelial ablation are the Ho:YAG, Nd:YAG, Thu:YAG and TFL, and the diode lasers (Table 2 and Figure 1). These lasers differ in terms of laser–tissue interaction, ablation depth, coagulation efficacy, and cutting speed [39,40,47].

Table 2.
Summary of laser properties for urothelial cancer (use, advancements, benefits and disadvantages, techniques used).
The Ho:YAG and the Nd:YAG lasers have been widely described and used for the management of upper-tract urothelial cancer. The Ho:YAG laser works in the pulse mode with a wavelength of 2.09 μm. Its energy is readily absorbed by water and the laser tip must be always in contact with the tissue to achieve tumour ablation. Due to its great availability, Ho:YAG is considered the gold standard for the treatment of UTUC [48]. The Ho:YAG laser provides a deeper incision and a higher tissue penetration compared to thulium lasers, whereas the coagulation and total laser areas are more extensive with the Tm:YAG laser [49]. Compared to Ho:YAG, the Nd:YAG laser has a greater depth of penetration (4–6 mm) and provides a deeper coagulation and ablative effect on the tumour that might be useful when treating larger tumours [50]. Direct contact with the tumour is not required and should be avoided to prevent a decrease in the effectiveness of the fibre [51]. Additionally, the Nd:YAG laser can be used with albumin as a solder [51]. In laser tissue soldering (LTS), laser energy is applied to a solder in conjunction with laser wavelength-specific chromophores, to facilitate light absorption [52]. The increase in temperature denatures the solder which forms a coagulum, at a speed of 1 min per centimetre of incision length. This property is particularly useful in the context of the repair of bladder, urethra, or uretero-pelvic junction (UPJ) [52].
Three machines are available nowadays using thulium lasers operating within a wavelength range of 1.94–2.0 μm: the TFL QCW mode (pulse max 120 W, 1.94 μm), the Tm:YAG CW mode (pulse max 200 W, 2.0 μm), and the TFL SuperPulsed mode (pulse max 500 W, 1.94 μm) [53]. The Tm:YAG CW mode ensures continuous ablation and low tissue penetration, resulting in good vaporization and coagulation properties for soft tissue. However, this laser causes a higher tissue necrosis and carbonization due to the low peak power and the absence of thermal relaxation [53]. This might represent a problem in terms of both defining ablation completion and pathologic sampling. The TFL QWC mode has very controllable behaviour, as both the incision depth and the damage dose increase with increasing laser power [49]. The TFL offers maximum water absorption. It can be used in a quasi-continuous and in a SuperPulsed (SP) mode that allow for increased pulse power, improved cutting efficiency, and reduced carbonization [49,54]. SP TFL offers a Ho:YAG incision and its coagulation depth remains nearly constant within the laser fibre–tissue distance range of 0.3–0.4 mm, without major complications [53,55].
4.2. Diode Laser
Semiconductor or diode lasers are made of two semiconductor material layers that can be combined to create larger more powerful versions [6]. Historically, diode lasers were used as an alternative to the 120 W lithium triborate (LBO) lasers, due to their higher haemostatic ability [56]. Diode lasers have been used for direct tumour ablation or as a “photosensitizer” in the contest of photodynamic therapy (PDT), due to their ability to match the desired wavelengths of many photosensitizer drugs (630–760 nm) and increase their penetration in the tumoral tissue [51,57]. Recently, Wu et al. conducted a non-inferiority study to assess the efficacy and safety of the newer 450 nm wavelength blue diode laser compared to the conventional electrocautery performed with the plasmakinetic loop for non-muscular invasive bladder cancer [58]. Patients in the blue-laser arm showed longer operative times (p = 0.001), but lower blood loss (p = 0.003) and faster wound healing three months after operation [58]. However, survival outcomes were not reported, and authors state that longer follow-up is needed to confirm recurrence-free survival benefit [58].
4.3. Combination of Treatments and Oncologic Outcomes
In terms of what is the best laser for the conservative treatment of UTUC, Cornu et al. and Villa et al. reported recurrence rates of 60% and 76.1% of UTUC after Ho:YAG laser ablation, respectively [48,59]. Other studies have demonstrated that the use of Ho:YAG or Nd:YAG showed a progression to RNU in 16.6–35.9%, vs. 8.9–13.5% using Tm:YAG. UTUC recurrence was 24.6–90.5% with the use of Ho:YAG or Nd:YAG, vs. 19.2–49% for Tm:YAG [50]. Even if it seems that Ho:YAG lasers had a higher recurrence rate and progression than RNU, Ho-YAG laser studies have generally a longer follow-up period (30–50 months), although the surgical view can be compromised due to their lower haemostatic ability compared to other lasers, thus worsening the oncological outcomes [60].
In a retrospective comparative study on 59 patients, Defidio et al. demonstrated the non-inferiority of Tm:YAG vs. Ho:YAG laser ablation with better median parameter performance scores in fibre-tip stability, precision in ablating tumours <1.5 cm, and reduced bleeding or mucosal perforation [61]. Other reports later also corroborated these initial findings [62,63,64].
Additionally, there is a general tendency to use a combination of dual-wavelength Tm-Ho:YAG and/or other energies for optimal tumour ablation and coagulation. Defidio et al. evaluated the ablative safety and efficacy of the Tm-Ho:YAG Duo laser for UTUC, and found, at 144 months of follow-up, a recurrence-free survival rate of 69.3%, with a kidney preserving rate of 91% in the intention-to-treat population and 87.5% in imperative indications [62]. Sanguedolce et al. used Tm:YAG for larger lesions for its higher ability in ablation and coagulation, switching to Ho:YAG to cut and dislodge the carbonized tissue to uncover residual neoplastic tissue to be targeted with Tm:YAG for complete ablation [65].
However, these results must be carefully interpreted, since they are usually retrospective, heterogeneous in their populations, with small numbers of patients. Some lack information of tumour grading, and they generally differ in their follow-up times. As a general rule, all authors suggest that for ablative therapy in the context of UTUC, the real game-changers are with patient selection, with potentially smaller papillary lesions (<2 cm) and those able to pursue a strict endoscopic follow-up [48,66].
A further ally to decrease tumour recurrence might be confocal laser endomicroscopy. This technology, previously applied to GI tumours [67], aims to perform real-time tumour characterization. In this regard, Vanacore et al. analysed results from 186 patients who underwent URS with CLE for UTUC and ablation with Ho:YAG, Tm:YAG, or a combination of both [68]. Authors found 84.6%, 66%, and 100% concordance for low-grade, intermediate-grade, and in situ carcinoma, respectively [68]. Other authors suggest that CLE provides prompt intraoperative data with high sensitivity for high-grade tumours, but that it still has a low specificity [65]. For these reasons, more robust data and larger studies are warranted.
4.4. Complications: Stricture/Recurrence
Major complications (≥Clavien-Dindo IIIb) associated with flexible ureteroscopy, including major perforation, perirenal hematoma, subcapsular hematoma, ureteral avulsion, arteriovenous fistula, and acute sepsis, are generally extremely rare (<1%) [60]. The most common complication seems to be the ureteric stricture and its incidence varies between 0 and 27%, according to the follow-up period and to the energy load used for tumour ablation [50,60].
5. Lasers and Benign Prostatic Hyperplasia (BPH)
Lower urinary tract symptoms (LUTS) secondary to BPH are frequent in aging men and can lead to a significant loss of quality of life. While transurethral resection of the prostate (TURP) is still the standard therapy for patients with small to medium prostates in whom medical therapies were unsuccessful, the use of lasers has emerged as an alternative treatment modality in these patients. To date, a variety of lasers can be used either for the enucleation, vaporization, or resection of prostate tissue including holmium, thulium, and Greenlight lasers [69] (Table 3 and Figure 1).

Table 3.
Summary of laser properties for BPH (Use, advancements, benefits and disadvantages, techniques used).
5.1. Nd:YAG Laser
The Nd:YAG laser was used mainly in the past for non-contact “visual laser ablation of the prostate” (VLAP), contact ablation, or interstitial laser coagulation (ILC) [1]. It is characterized by more than 1 cm tissue penetration and by a 1064 wavelength. For this reason, it causes deep coagulative necrosis and thermal tissue injury, with an oedema occurring after the procedure leading to LUTS and urinary retention [1].
5.2. Holmium Laser
TURP remains the gold standard in the surgical management of benign prostatic enlargement (BPE), especially in small and mid-sized prostates; however, challenges in the surgical treatment of larger prostates and technological advances led to the development of new treatment strategies. Holmium laser enucleation of the prostate (HoLEP) has been found to be an efficient and safe treatment option for all prostate sizes in several randomized controlled trials [70,71,72,73,74]. This technique allows for the coagulation of tissue simultaneous to the enucleation of the prostate tissue from the capsule [70,71,75]. HoLEP has shown favourable functional outcomes, better haemostasis, shorter hospitalization and indwelling catheter times compared to TURP, and lower complication and morbidity rates compared to simple open prostatectomy and is therefore considered a valid alternative treatment to both procedures [70,71,75,76].
HoLEP is usually conducted using high power settings of 80–100 W with 40–50 Hz frequency and 2 J energy and a reduction of power for coagulation and apical enucleation [77,78]. However, high-power devices require costly equipment and high-power outlets, leading to higher costs and preventing their widespread use. In comparison, low-power lasers with power settings of 20–50 W do not require special outlets and are less expensive than high-power lasers. Rassweiler et al. found the use of low power settings (24 W, 2 J, and 12 Hz; or 39.6 W, 2.2 J, and 18 Hz) to be an efficient and safe alternative to high power settings [79]. After conducting an analysis of the existing literature, Gkolezakis et al. concluded that low-power HoLEP is safe, feasible, and efficient. Furthermore, low-power HoLEP was found to reduce postoperative storage and irritative symptoms. Complications including intra- and postoperative complications were found to be independent of the laser power [77].
A recent meta-analysis included seven studies comparing HoLEP to holmium enucleation of the prostate using MOSES technology (MoLEP). The enucleation time was significantly shorter for MoLEP (mean difference [MD] −7.27 min, 95% confidence interval [CI] −11.26 to −3.28; p = 0.0004). Furthermore, postoperative length of hospital stay was significantly longer in the HoLEP group (MD 0.3 d, 95% CI −0.24–0.85, p < 0.0001). The mean maximum peak flow was higher for patients treated with HoLEP (MD 0.95 mL/s, 95% CI −1.66 to 3.57; p = 0.47) and the mean postvoid residual volume was lower in the MoLEP group (MD −10.08 mL, 95% CI −53.54 to 33.37; p = 0.65). The authors concluded that the use of MOSES technology for prostate enucleation showed advantages in enucleation, haemostasis, and procedural time and length of hospital stay, and therefore even could make the enucleation of the prostate feasible as a day surgery procedure [80].
5.3. Thulium Laser
Thulium laser has been reported to achieve higher ablation rates with only minimal thermal tissue injury and minimal blood loss compared to other lasers, resulting from its 2 μm wavelength which is close to the 1.92 μm water absorption peak and the possibility of using continuous wave mode [72]. Thulium laser resection was first used as a treatment alternative to TURP in 2008 and has since been established in the treatment for LUTS secondary to BPH as thulium laser enucleation of the prostate (ThuLEP) and thulium laser vapo-enucleation (ThuVEP) [72,81,82]. Endoscopic enucleation of the prostate can be conducted with several sources of energy. Enucleation of the prostate using the thulium laser is considered an efficient and safe alternative to TURP and HoLEP [69]. Like HoLEP, ThuLEP has been found to be an efficient and safe treatment modality for BPH independent of prostate size [72]. A meta-analysis comparing the enucleation of the prostate using holmium and thulium laser technology found both methods to have low morbidity and result in satisfactory micturition improvement. However, enucleation with a thulium laser (ThuLEP or ThuVEP) proved to be advantageous in efficacy, enucleation time, perioperative blood loss, flow rate and postvoid residual at one month, and international prostate symptom score at 12 months postoperatively compared to HoLEP [72]. Similarly, another recent meta-analysis found no significant differences in operating time, enucleation weight, catheterization time, functional measures and symptom scores, or hospital stay with ThuLEP and HoLEP. Furthermore, the decrease in haemoglobin was significantly lower in patients receiving ThuLEP (mean difference −0.54 g/dL, 95% CI −0.93 to −0.15; p < 0.001), while transient urinary incontinence was more common in patients treated with HoLEP (odds ratio 0.56, 95% CI 0.32–0.99; p = 0.045), only with, however, a low certainty of evidence. Therefore, the authors concluded that the choice of treatment should be made according to surgeon’s expertise and local conditions [83]. A recent randomized controlled trial compared endoscopic enucleation of the prostate using a thulium: yttrium–aluminum–garnet (Tm:YAG) laser and a super-pulsed thulium fibre laser set in continuous-wave (CW) mode. In total, 110 patients were randomized and treated either with ThuLEP or CW thulium fibre laser enucleation of the prostate (CW-ThuFLEP). There was no significant difference between ThuLEP and CW-ThuFLEP in operative time (70.69 vs. 72.41 min), enucleation time (50.23 vs. 53.33 min), enucleated tissue weight (40.2 vs. 41.9 g), enucleation efficiency (0.80 vs. 0.79 g/min), catheterization time (2.45 vs. 2.57 days), hospital stay (2.82 vs. 2.95 days), and haemoglobin drop (1.05 vs. 1.27 g/dL). Furthermore, no significant differences were found in IPSS (5.09 vs. 5.81), peak urinary flow rates (26.51 vs. 27.13 mL/s), post-void residual volume (25.22 vs. 23.81 mL), and IIEF-5 (14.01 vs. 14.54) at the 3-month follow-up. The authors concluded that the theoretical advantages of the super-pulsed thulium fibre laser, such as the shallower penetration depth and improved vaporization capacity, did not lead to significant differences in patient outcomes [84].
As with ThuLEP, the goal of ThuVEP is the enucleation of prostate tissue along the capsule. Both techniques differ in energy settings and applied mechanical force [84,85]. ThuVEP is a safe treatment option, especially in patients with large prostates or patients on anticoagulant or antiplatelet therapy [69]. Potential benefits of ThuVEP are smoother vaporization and enhanced haemostasis due to the possibility of using continuous wave and pulsed modes. Significant improvements in short-term outcomes, both subjective and objective, were observed. Netsch et al. conducted a prospective, randomized trial comparing ThuVEP with HoLEP with 48 and 46 patients, respectively. In both groups, peak urinary flow rates (10.7 vs. 22 mL/s), post-void residual volumes (100 vs. 20 mL), international prostate symptom score (20 vs. 10) and quality of life (4 vs. 3) improved significantly at 1-month follow-up. There was no significant difference between both groups [86]. Compared to the baseline parameters, the same cohort showed peak urinary flow rates (10.7 vs. 25.9 mL/s), post-void residual urine (100 vs. 6.5 mL), IPSS (20 vs. 5), quality of life (4 vs. 1), PSA (4.14 vs. 0.71 µg/L), and prostate volume (80 vs. 16 mL) had improved significantly (p < 0.001) at 6-month follow-up, without significant differences between both groups [87]. According to the existing evidence, ThuVEP is a safe and efficient treatment option for patients with symptomatic BPE. However, large scale randomized trials are still scarce [88].
5.4. Potassium-Titanyl-Phosphate (KTP) and Lithium Triborate (LBO) Lasers: Greenlight-Based PVP
Photoselective vaporization of the prostate (PVP) is another possible surgical treatment alternative for patients suffering from LUTS secondary to BPE [89]. Currently, three different Greenlight lasers are in use, the 80 W KTP, the 120 W HPS LBO and the 180 W XPS LBO [90]. The main technical difference between the PVP and the enucleation techniques, is that PVP ablates tissue by vaporization of the tissue from the prostatic urethra toward the capsule (inside-out), while enucleating techniques are used to enter the plane of the prostatic capsule and dissect the prostatic lobes (outside-in) [90]. PVP is considered to be a side-firing laser ablation of the prostate using a 532 nm wavelength laser which is absorbed by haemoglobin, resulting in better coagulation during the procedure [91]. The main advantage of the Greenlight technology is its intraoperative haemostasis and therefore a clear surgical view [72]. Therefore, PVP seems a safe treatment option for anticoagulated patients, even though the RCTs were conducted on just small amounts of patients and, therefore, it is recommended with a weak rating as per the Guidelines [3,91]. Disadvantages of PVP are the limitation of resectable volume and no histopathological analysis due to the vaporization of the prostate tissue [72].
A meta-analysis comparing PVP using 80 W and 120 W lasers to TURP analysed 9 trials with a total of 448 patients undergoing PVP (80 W in 5 trials and 120 W in 4 trials) and 441 undergoing TURP. Overall data showed shorter catheterisation time and shorter length of stay in patients treated with PVP compared to TURP, with 1.91 day (95% CI, 1.47–2.35; p < 0.00001) and 2.13 days (95% CI, 1.78–2.48; p < 0.00001), respectively. Operation time was shorter in patients treated with TURP by 19.64 min (95% CI, 9.05–30.23; p = 0.0003). The transfusion rate was significantly lower in the PVP group (risk ratio: 0.16; 95% CI, 0.05–0.53; p = 0.003). No significant difference was found for other complications [91]. Similarly, a large PVP of the prostate was noninferior to TURP in terms of international prostate symptom score, peak flow rate, and freedom from complications [92]. Noninferiority was sustained at the two-year follow-up [93]. A recent randomized controlled trial with 154 consecutive enrolled patients investigated a new surgical procedure, an enucleation technique involving photoselective sharp enucleation of the prostate (PSEP), with a front-firing 532-nm laser for LUTS caused by BPE in order to address the limitations of PVP, such as a lack of tissue for histologic analysis and long operating times for large prostates. The authors compared this novel technique to traditional PVP. Both groups showed an improvement of lower urinary tract symptoms at one, six and twelve months postoperatively. Furthermore, both groups had equivalent international prostate symptom scores, quality-of-life scores, postvoid residual urine volume, maximum urine flow rates, as well as prostate-specific antigen at each follow-up (p > 0.05). The median operative time for patients treated with PSEP was significantly shorter than for patients treated with PVP, at 35 min vs. 47 min (p < 0.001), respectively. The median prostate volume was significantly smaller in patients treated with PSEP compared to those treated with PVP (p < 0.05) at six and twelve months. There was no significant difference in complication rates. While the authors found both treatments to safe and efficient, PSEP could resolve the limitations of PVP [94].
5.5. Diode Laser
In the context of BPH, diode lasers with a wavelength of 940, 980, 1318, and 1470 nm are marketed for vaporization and enucleation [69]. Historically, diode lasers have been associated with higher complications such as postoperative irritative symptoms, transient urinary incontinence, and epididymitis when compared to other lasers, such as the Greenlight [95]. However, more recent RCTs did not show a difference in complication rates, blood loss, IPSS, or QoL when compared to bipolar resection or enucleation of the prostate [96,97,98]. However, since these studies are limited and of mainly low quality with controversial data on retreatment rates, diode laser enucleation of the prostate is recommended only with a weak rating for men with moderate-to-severe LUTS, while diode laser vaporization is not recommended yet [69]. In 2022, the FDA approved the SoracteLite transperineal interstitial laser ablation (TPLA) to induce coagulative necrosis of tissue through a continuous 1064 nm wavelength for men who desire to maintain ejaculation [99]. TPLA may be performed in an office-based setting under local anaesthesia with a highly custom and precise ablation through the transperineal route [99]. Because of the novelty of this technique, the Canadian, American, and European urological guidelines have not yet endorsed it [69,100,101]. Additionally, an open-label randomized trial comparing the functional outcomes between TPLA and TURP is in the recruitment phase [99].
6. Limitations
The present review has some limitations. First, this is a literature review that has a narrative character. Second, we lack granular information on laser fibres, irrigation settings, activation/deactivation intervals, and laser tip/tissue distance; indeed, the included studies show heterogeneity in their endpoints. Third, most included studies are mainly retrospective with small sample sizes because of a low availability, due to the relatively recent acceptance of TFLs by the FDA and the EMA. Fourth, there is a lack of comparative clinical studies between the different technologies, especially dealing with TFLs. However, we believe that with this review we were able to give a general overview of the current state of lasers in endourology, to provide some practical information for beginners, and to give some perspectives for future studies.
7. Future Perspectives
Laser technology is continuously evolving. Being relatively new, a lot of attention is given to TFLs in the context of stone treatment. One advantage is that smaller laser fibre sizes that might be used with TFLs, coupled with the new miniaturized technologies and the smaller single-use flexible scopes, might extend the use of RIRS for larger and lower pole stones. Additionally, high-power lasers might widen the horizon of RIRS, since some studies have already demonstrated advantages in terms of shorter operative time, reduced use of ureteral access sheath (UAS), and postoperative stent, which in turn mean a reduction in sepsis-related complication rates [102]. Large comparative studies comparing TFL and Ho:YAG lasers with MOSES technology might shed new light on what the best treatment is in terms of surgical outcomes, emphasizing the outcome measures such as stone-free rate and the imaging used to achieve it, quality of life, costs, and environmental impact [103,104,105].
In the field of UTUC, while no study has shown the superiority of one laser type over the other, over time, clinicians have reported an increasing success with thulium lasers, especially TFLs. Proietti et al. suggested an ideal setting of 1 J and 10 Hz to provide optimal haemostasis without generating excessive carbonization that might hinder the radicality of tumour extirpation [53]. However, TFL laser technology is still in its infancy and larger prospective and randomized studies are needed to find the optimal settings, and longer follow-up is needed to corroborate the survival outcomes [106].
In view of the great success of lasers in the field of BPH for medium-sized prostates, in the near future this technology is likely to have a role even for larger prostates. One of the main biases related to these procedures is that despite the widespread use of the technique, the learning curve might be steep for laser enucleation procedures. For this reason, medical industries should improve training programs similar to what happens for robotic surgery.
8. Conclusions
Laser and laser-related techniques are very versatile and have been demonstrated to be particularly useful in the context of urolithiasis, urothelial cancer, and BPH. For urolithiasis, lasers may offer the opportunity to use ureteroscopy and lasertripsy even for larger stones. When newer high-power lasers are used, the related increase in intrarenal or ureteral temperature should be managed by titrating the irrigation and using a ureteral access sheath. However, at powers <40 W, all lasers exhibit a safe temperature profile.
In the context of UTUC, Ho:YAG has a deeper incision in comparison to thulium, while CW Tm:YAG offers fast, deep and precise cutting but has a broader coagulation area with increased carbonization. The TFL offers a Ho:YAG-like incision and at an ideal setting of 1 J and 10 Hz seems to provide optimal haemostasis without generating excessive carbonization. TURP is still the standard therapy for patients with small to medium prostates who require surgical treatment. However, intervention using laser technologies can be considered as a safe and efficient alternative treatment modality, especially in patients with larger prostates or anticoagulated patients. For patients who want to maintain ejaculation, TPLA might become an alternate option. Ultimately, the choice of treatment should be made considering the individual surgeon’s expertise, local availability of various treatment options, patient counselling, and shared decision making.
Author Contributions
Conceptualization, C.C. and B.S.; methodology, C.C. and B.S.; investigation, C.C. and B.S.; data curation, C.C., V.J. and C.N.; writing— original draft preparation, C.C. and V.J.; writing—review and editing, C.C., V.J. and B.S.; supervision, A.P. and B.S.; project administration, B.S.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the studies included in the review and were discussed in the present manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dołowy, Ł.; Krajewski, W.; Dembowski, J.; Zdrojowy, R.; Kołodzej, A. The role of lasers in modern urology. Cent. Eur. J. Urol. 2015, 68, 175–182. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Hughes, T.; Mani, M.; Somani, B. Outcomes of ureteroscopy and laser stone fragmentation (URSL) for kidney stone disease (KSD): Comparative cohort study using moses technology 60 w laser system versus regular holmium 20 w laser. J. Clin. Med. 2021, 10, 2742. [Google Scholar] [CrossRef]
- Skolarikos, A.; Neisius, A.; Petřík, A.; Somani, B.; Thomas, K.; Gambaro, G. EAU Guidelines on Urolithiasis. 2023. Available online: https://uroweb.org/guidelines/urolithiasis (accessed on 9 April 2023).
- Barone, B.; Crocetto, F.; Vitale, R.; Di Domenico, D.; Caputo, V.; Romano, F.; De Luca, L.; Bada, M.; Imbimbo, C.; Prezioso, D. Retrograde intra renal surgery versus percutaneous nephrolithotomy for renal stones >2 cm. A systematic review and meta-analysis. Minerva Urol. Nephrol. 2020, 72, 441–450. [Google Scholar] [CrossRef]
- Erhard, M.J.; Bagley, D.H. Urologic Applications of the Holmium Laser: Preliminary Experience. J. Endourol. 1995, 9, 383–386. [Google Scholar] [CrossRef]
- Brewin, A.; Somani, B. What is new in lasers for endourology: Looking into the future. Urol. News 2021, 25, 2. [Google Scholar]
- Schembri, M.; Sahu, J.; Aboumarzouk, O.; Pietropaolo, A.; Somani, B.K. Thulium fiber laser: The new kid on the block. Turk. J. Urol. 2020, 46 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Basulto-Martínez, M.; Proietti, S.; Yeow, Y.; Rapallo, I.; Saitta, G.; Cimino, S.; Luciani, L.; Bellinzoni, P.; Gaboardi, F.; Giusti, G. Holmium laser for RIRS. Watts are we doing? Arch. Esp. Urol. 2020, 73, 735–744. [Google Scholar] [PubMed]
- Matlaga, B.R.; Chew, B.; Eisner, B.; Humphreys, M.; Knudsen, B.; Krambeck, A.; Lange, D.; Lipkin, M.; Miller, N.L.; Monga, M.; et al. Ureteroscopic Laser Lithotripsy: A Review of Dusting vs. Fragmentation with Extraction. J. Endourol. 2018, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.E.; Hollander, A.B.; Soni, S.D.; Link, R.E.; Mayer, W.A. To Dust or Not To Dust: A Systematic Review of Ureteroscopic Laser Lithotripsy Techniques. Curr. Urol. Rep. 2017, 18, 32. [Google Scholar] [CrossRef]
- Kronenberg, P.; Somani, B. Advances in Lasers for the Treatment of Stones—A Systematic Review. Curr. Urol. Rep. 2018, 19, 45. [Google Scholar] [CrossRef]
- Fuh, E.; Haleblian, G.E.; Norris, R.D.; Albala, W.D.M.; Simmons, N.; Zhong, P.; Preminger, G.M. The Effect of Frequency Doubled Double Pulse Nd: YAG Laser Fiber Proximity to the Target Stone on Transient Cavitation and Acoustic Emission. J. Urol. 2007, 177, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Emiliani, E.; Talso, M.; Cho, S.-Y.; Baghdadi, M.; Mahmoud, S.; Pinheiro, H.; Traxer, O. Optimal Settings for the Noncontact Holmium: YAG Stone Fragmentation Popcorn Technique. J. Urol. 2017, 198, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, A.; Jones, P.; Whitehurst, L.; Somani, B.K. Role of ‘dusting and pop-dusting’ using a high-powered (100 W) laser machine in the treatment of large stones (≥15 mm): Prospective outcomes over 16 months. Urolithiasis 2019, 47, 391–394. [Google Scholar] [CrossRef]
- Reeves, T.; Griffin, S.; Pietropaolo, A.; Somani, B.K. Feasibility of dusting and pop-dusting using high-power (100W) holmium YAG (HO: YAG) laser in treatment of paediatric stones: Results of first worldwide clincial study. Cent. Eur. J. Urol. 2019, 72, 398–401. [Google Scholar]
- Ibrahim, A.; Badaan, S.; Elhilali, M.M.; Andonian, S. Moses technology in a stone simulator. Can. Urol. Assoc. J. 2018, 12, 127–130. [Google Scholar] [CrossRef]
- Keller, E.X.; De Coninck, V.; Audouin, M.; Doizi, S.; Bazin, D.; Daudon, M.; Traxer, O. Fragments and dust after Holmium laser lithotripsy with or without “Moses technology”: How are they different? J. Biophotonics 2019, 12, e201800227. [Google Scholar] [CrossRef] [PubMed]
- Winship, B.; Wollin, D.A.; Carlos, E.C.; Li, M.J.; Peters, M.C.; Simmons, W.N.; Preminger, G.M.; Lipkin, M.E. Dusting Efficiency of the Moses Holmium Laser: An Automated In Vitro Assessment. J. Endourol. 2018, 32, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, E.; Pauchard, F.; Quadrini, F.; Sindhubodee, S.; Kamkoum, H.; Godínez, A.J.; Doizi, S.; Traxer, O. High- and Low-Power Laser Lithotripsy Achieves Similar Results: A Systematic Review and Meta-Analysis of Available Clinical Series. J. Endourol. 2021, 35, 1146–1152. [Google Scholar] [CrossRef]
- Kronenberg, P.; Traxer, O. The laser of the future: Reality and expectations about the new thulium fiber laser—A systematic review. Transl. Androl. Urol. 2019, 8, S398–S417. [Google Scholar] [CrossRef]
- Enikeev, D.; Grigoryan, V.; Fokin, I.; Morozov, A.; Taratkin, M.; Klimov, R.; Kozlov, V.; Gabdullina, S.; Glybochko, P. Endoscopic lithotripsy with a SuperPulsed thulium-fiber laser for ureteral stones: A single-center experience. Int. J. Urol. 2021, 28, 261–265. [Google Scholar] [CrossRef]
- Hardy, L.A.; Vinnichenko, V.; Fried, N.M. High power holmium: YAG versus thulium fiber laser treatment of kidney stones in dusting mode: Ablation rate and fragment size studies. Lasers Surg. Med. 2019, 51, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Vaddi, C.M.; Ramakrishna, P.; Ganeshan, S.; Swamy, S.; Anandan, H.; Babu, M.; Panda, R. The clinical efficiency and safety of 60W superpulse thulium fiber laser in retrograde intrarenal surgery. Indian J. Urol. 2022, 38, 191–196. [Google Scholar] [PubMed]
- Taratkin, M.; Azilgareeva, C.; Korolev, D.; Barghouthy, Y.; Tsarichenko, D.; Akopyan, G.; Chinenov, D.; Ali, S.; Kozlov, V.; Mikhailov, V.; et al. Prospective Single-Center Study of SuperPulsed Thulium Fiber Laser in Retrograde Intrarenal Surgery: Initial Clinical Data. Urol. Int. 2022, 106, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ulvik, Ø.; Æsøy, M.S.; Juliebø-Jones, P.; Gjengstø, P.; Beisland, C. Thulium Fibre Laser versus Holmium: YAG for Ureteroscopic Lithotripsy: Outcomes from a Prospective Randomised Clinical Trial. Eur. Urol. 2022, 82, 73–79. [Google Scholar] [CrossRef]
- Keat, W.O.L.; Somani, B.K.; Pietropaolo, A.; Chew, B.H.; Chai, C.A.; Inoue, T.; Ragoori, D.; Biligere, S.; Galosi, A.B.; Pavia, M.P.; et al. Do Hounsfield Units have any significance in predicting intra- and postoperative outcomes in retrograde intrarenal surgery using Holmium and Thulium fiber laser? Results from the flexible ureteroscopy Outcomes Registry (FLEXOR). World J. Urol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Ryan, J.R.; Nguyen, M.H.; Linscott, J.A.; Nowicki, S.W.; James, E.; Jumper, B.M.; Ordoñez, M.; Ingimarsson, J.P. Ureteroscopy with thulium fiber laser lithotripsy results in shorter operating times and large cost savings. World J. Urol. 2022, 40, 2077–2082. [Google Scholar] [CrossRef]
- Perri, D.; Berti, L.; Pacchetti, A.; Morini, E.; Maltagliati, M.; Besana, U.; Pastore, A.L.; Romero-Otero, J.; Saredi, G.; Centrella, D.; et al. A comparison among RIRS and MiniPerc for renal stones between 10 and 20 mm using thulium fiber laser (Fiber Dust): A randomized controlled trial. World J. Urol. 2022, 40, 2555–2560. [Google Scholar] [CrossRef]
- Rice, P.; Somani, B.K. Percutaneous laser nephrolithotripsy: Is it here to stay? Results of a systematic review. Curr. Opin. Urol. 2022, 32, 185–191. [Google Scholar] [CrossRef]
- Ghani, K.R.; Andonian, S.; Bultitude, M.; Desai, M.; Giusti, G.; Okhunov, Z.; Preminger, G.M.; de la Rosette, J. Percutaneous Nephrolithotomy: Update, Trends, and Future Directions. Eur. Urol. 2016, 70, 382–396. [Google Scholar] [CrossRef]
- Moore, S.L.; Bres-Niewada, E.; Cook, P.; Wells, H.; Somani, B.K. Optimal management of lower pole stones: The direction of future travel. Cent. Eur. J. Urol. 2016, 69, 274–279. [Google Scholar]
- De, S.; Autorino, R.; Kim, F.J.; Zargar, H.; Laydner, H.; Balsamo, R.; Torricelli, F.C.; Di Palma, C.; Molina, W.R.; Monga, M.; et al. Percutaneous Nephrolithotomy versus Retrograde Intrarenal Surgery: A Systematic Review and Meta-analysis. Eur. Urol. 2015, 67, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, H.; Yu, X.; Chen, Z.; Ye, Z.; Yuan, H. The “all-seeing needle” micro-PCNL versus flexible ureterorenoscopy for lower calyceal stones of ≤2 cm. Urolithiasis 2019, 47, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hong, T.Y.; Li, G.; Jiang, N.; Hu, C.; Cui, X.; Chu, C.; Zhao, J. Comparison of the Efficacy of Ultra-Mini PCNL, Flexible Ureteroscopy, and Shock Wave Lithotripsy on the Treatment of 1–2 cm Lower Pole Renal Calculi. Urol. Int. 2019, 102, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, P.; Hameed, B.M.Z.; Somani, B. Outcomes of thulium fibre laser for treatment of urinary tract stones: Results of a systematic review. Curr. Opin. Urol. 2021, 31, 80–86. [Google Scholar] [CrossRef]
- Andreeva, V.; Vinarov, A.; Yaroslavsky, I.; Kovalenko, A.; Vybornov, A.; Rapoport, L.; Enikeev, D.; Sorokin, N.; Dymov, A.; Tsarichenko, D.; et al. Preclinical comparison of superpulse thulium fiber laser and a holmium: YAG laser for lithotripsy. World J. Urol. 2020, 38, 497–503. [Google Scholar] [CrossRef]
- Patil, A.; Reddy, N.; Shah, D.; Singh, A.; Ganpule, A.; Sabnis, R.; Desai, M. High-Power Holmium with MOSES Technology or Thulium Fiber Laser in MiniPerc with Suction: A New Curiosity. J. Endourol. 2022, 36, 1348–1354. [Google Scholar] [CrossRef]
- Taratkin, M.; Azilgareeva, C.; Chinenov, D.; Mikhailov, V.; Inoyatov, J.; Ali, S.; Korolev, D.; Tsarichenko, D.; Corrales, M.; Enikeev, D. Retrograde intrarenal surgery versus percutaneous nephro-lithotomy in larger kidney stones. Could superpulsed thulium-fiber laser change the game? Cent Eur. J. Urol. 2021, 74, 229–234. [Google Scholar]
- Rice, P.; Somani, B.K.; Nagele, U.; Herrmann, T.R.W.; Tokas, T. Generated temperatures and thermal laser damage during upper tract endourological procedures using the holmium: Yttrium–aluminum-garnet (Ho: YAG) laser: A systematic review of experimental studies. World J. Urol. 2022, 40, 1981–1992. [Google Scholar] [CrossRef]
- Tokas, T.; Rice, P.; Seitz, C.; Gauhar, V.; Somani, B. Temperature change during laser upper-tract endourological procedures: Current evidence and future perspective. Curr. Opin. Urol. 2023, 33, 108–115. [Google Scholar] [CrossRef]
- Yamashita, S.; Inoue, T.; Imai, S.; Kohjimoto, Y.; Fujisawa, M.; Hara, I. Thermography-based comparison of irrigation temperatures between Moses Mode and Virtual Basket Mode: An in-vitro phantom study. J. Endourol. 2023, 37, 179–184. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Y.; Jia, Z.; Guan, Y.; Fei, W.; Ai, X. Temperature profiles of calyceal irrigation fluids during flexible ureteroscopic Ho: YAG laser lithotripsy. Int. Urol. Nephrol. 2021, 53, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Belle, J.D.; Chen, R.; Srikureja, N.; Amasyali, A.S.; Keheila, M.; Duane Baldwin, D. Does the Novel Thulium Fiber Laser Have a Higher Risk of Urothelial Thermal Injury than the Conventional Holmium Laser in an in Vitro Study? J. Endourol. 2022, 36, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Corrales, M.; Somani, B.; Traxer, O. Laser Efficiency and Laser Safety: Holmium YAG vs. Thulium Fiber Laser. J. Clin. Med. 2023, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Compérat, E.; Cowan, N.; Gontero, P. EAU Guidelines on Upper Urinary Tract Urothelial Carcinoma. 2023. Available online: https://uroweb.org/guidelines/upper-urinary-tract-urothelial-cell-carcinoma (accessed on 9 April 2023).
- Gkolezakis, V.; Rice, P.; Somani, B.K.; Tokas, T. Thulium Fiber Laser Behavior on Tissue During Upper- and Lower-Tract Endourology. Curr. Urol. Rep. 2022, 23, 271–278. [Google Scholar] [CrossRef]
- Villa, L.; Haddad, M.; Capitanio, U.; Somani, B.; Cloutier, J.; Doizi, S.; Salonia, A.; Briganti, A.; Montorsi, F.; Traxer, O. Which Patients with Upper Tract Urothelial Carcinoma Can be Safely Treated with Flexible Ureteroscopy with Holmium: YAG Laser Photoablation? Long-Term Results from a High Volume Institution. J. Urol. 2018, 199, 66–73. [Google Scholar] [CrossRef]
- Huusmann, S.; Lafos, M.; Meyenburg, I.; Muschter, R.; Teichmann, H.O.; Herrmann, T. Tissue effects of a newly developed diode pumped pulsed Thulium: YAG laser compared to continuous wave Thulium: YAG and pulsed Holmium: YAG laser. World J. Urol. 2021, 39, 3503–3508. [Google Scholar] [CrossRef]
- Ng Chieng Hin, J.; Hettiarachchilage, D.; Gravestock, P.; Rai, B.; Somani, B.K.; Veeratterapillay, R. Role of Ureteroscopy in Treatment of Upper Tract Urothelial Carcinoma. Curr. Urol. Rep. 2021, 22, 49. [Google Scholar] [CrossRef]
- Zarrabi, A.; Gross, A.J. The evolution of lasers in urology. Ther. Adv. Urol. 2011, 3, 81–89. [Google Scholar] [CrossRef]
- Lee, J.; Gianduzzo, T.R.J. Advances in Laser Technology in Urology. Urol. Clin. N. Am. 2009, 36, 189–198. [Google Scholar] [CrossRef]
- Proietti, S.; Johnston, T.; Pupulin, M.; Di Pietro, S.; Spagna, S.; Rico, L.; Lucianò, R.; Ventimiglia, E.; Villa, L.; Gaboardi, F.; et al. Effectiveness and Safety of Thulium Fiber Laser in the Conservative Management of Patients with Upper Tract Urothelial Carcinoma. Eur. Urol. Open Sci. 2022, 46, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Huusmann, S.; Wolters, M.; Kramer, M.W.; Bach, T.; Teichmann, H.-O.; Eing, A.; Bardosi, S.; Herrmann, T.R.W. Tissue damage by laser radiation: An in vitro comparison between Tm: YAG and Ho: YAG laser on a porcine kidney model. Springerplus 2016, 5, 266. [Google Scholar] [CrossRef] [PubMed]
- Ortner, G.; Rice, P.; Nagele, U.; Herrmann, T.R.W.; Somani, B.K.; Tokas, T. Tissue thermal effect during lithotripsy and tissue ablation in endourology: A systematic review of experimental studies comparing Holmium and Thulium lasers. World J. Urol. 2023, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, E.; Wendt-Nordahl, G.; Honeck, P.; Alken, P.; Knoll, T.; Michel, S.M.; Häcker, A. 120 W lithium triborate laser for photoselective vaporization of the prostate: Comparison with 80 W potassium-titanyl-phosphate laser in an ex-vivo model. J. Endourol. 2010, 24, 75–79. [Google Scholar] [CrossRef]
- Rahman, K.M.M.; Giram, P.; Foster, B.A.; You, Y. Photodynamic Therapy for Bladder Cancers, A Focused Review. Photochem. Photobiol. 2023, 99, 420–436. [Google Scholar] [CrossRef]
- Wu, K.; Jiang, D.; Zhang, L.; Jiang, S.; Lin, T.; Luo, Y.; Fan, J.; Yang, T.; Chen, H.; Zhang, P.; et al. Efficacy and safety of a novel 450 nm blue diode laser versus plasmakinetic electrocautery for the transurethral resection of non-muscle invasive bladder cancer: The protocol and result of a multicenter randomized con-trolled trial. Front. Oncol. 2023, 12, 1065735. [Google Scholar] [CrossRef]
- Cornu, J.-N.; Rouprêt, M.; Carpentier, X.; Geavlete, B.; Medina, S.G.D.D.; Cussenot, O.; Traxer, O. Oncologic control obtained after exclusive flexible ureteroscopic management of upper urinary tract urothelial cell carcinoma. World J. Urol. 2010, 28, 151–156. [Google Scholar] [CrossRef]
- Bozzini, G.; Gastaldi, C.; Besana, U.; Calori, A.; Casellato, S.; Parma, P.; Pastore, A.L.; Macchi, A.; Breda, A.; Gozen, A.; et al. Thulium-laser Retrograde Intra Renal Ablation (T-RIRA) of upper urinary tract transitional cell carcinoma: An ESUT study. Minerva Urol. Nephrol. 2021, 73, 114–121. [Google Scholar] [CrossRef]
- Defidio, L.; De Dominicis, M.; Di Gianfrancesco, L.; Fuchs, G.; Patel, A. First collaborative experience with thulium laser ablation of localized upper urinary tract urothelial tumors using retrograde intra-renal surgery. Arch. Ital. Urol. Androl. 2011, 83, 147–153. [Google Scholar]
- Defidio, L.; Antonucci, M.; De Dominicis, M.; Fuchs, G.; Patel, A. Thulium-Holmium: YAG Duo Laser in Conservative Upper Tract Urothelial Cancer Treatment: 13 Years Experience from a Tertiary National Referral Center. J. Endourol. 2019, 33, 902–908. [Google Scholar] [CrossRef]
- Wen, J.; Ji, Z.G.; Li, H.Z. Treatment of upper tract urothelial carcinoma with ureteroscopy and thulium laser: A retrospective single center study. BMC Cancer 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Musi, G.; Mistretta, F.A.; Marenghi, C.; Russo, A.; Catellani, M.; Nazzani, S.; Conti, A.; Luzzago, S.; Ferro, M.; Matei, D.V.; et al. Thulium Laser Treatment of Upper Urinary Tract Carcinoma: A Multi-Institutional Analysis of Surgical and Oncological Outcomes. J. Endourol. 2018, 32, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Fontana, M.; Turco, M.; Territo, A.; Lucena, J.B.; Cortez, J.C.; Vanacore, D.; Meneghetti, I.; Gallioli, A.; Gaya, J.M.; et al. Endoscopic Management of Upper Urinary Tract Urothelial Carcinoma: Oncologic Outcomes and Prognostic Factors in a Contemporary Cohort. J. Endourol. 2021, 35, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, C.M.; Cracco, C.M.; Poggio, M.; Cossu, M.; Scarpa, R.M. Treatment of the pyelocalyceal tumors with laser. Arch. Esp. Urol. 2008, 61, 1080–1087. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Abu Dayyeh, B.K.; Bhat, Y.M.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; Manfredi, M.A.; Maple, J.T.; et al. Confocal laser endomicroscopy. Gastrointest. Endosc. 2014, 80, 928–938. [Google Scholar] [CrossRef]
- Vanacore, D.; Sanguedolce, F.; Territo, A.; Roca, B.M.; Saitta, G.; Tallè, M.; Martinez, C.; Mosquera, L.; Meneghetti, J.; Bevilacqua, G.; et al. Evolving techniques of endoscopic UTUC management: Optimising outcomes with the appropriate use of latest technologies. Eur. Urol. Open Sci. 2020, 19, e2374–e2375. [Google Scholar] [CrossRef]
- Cornu, J.N.; Gacci, M.; Hashim, H.; Herrmann, T.R.W.; Malde, S.; Netsch, C.; Rieken, M.; Sakalis, V.; Tutolo, M.; Baboudjian, M.; et al. EAU Guidelines on Non-Neurogenic Male LUTS. 2023. Available online: https://uroweb.org/guidelines/management-of-non-neurogenic-male-luts (accessed on 9 April 2023).
- Herrmann, T.R.W.; Liatsikos, E.N.; Nagele, U.; Traxer, O.; Merseburger, A.S. EAU guidelines on laser technologies. Eur. Urol. 2012, 61, 783–795. [Google Scholar] [CrossRef]
- Vincent, M.W.; Gilling, P.J. HoLEP has come of age. World J. Urol. 2015, 33, 487–493. [Google Scholar] [CrossRef]
- Xiao, K.-W.; Zhou, L.; He, Q.; Gao, X.-S.; Chen, G.; Ma, Y.-C.; Li, H.; Wang, K.-J. Enucleation of the prostate for benign prostatic hyperplasia thulium laser versus holmium laser: A systematic review and meta-analysis. Lasers Med. Sci. 2019, 34, 815–826. [Google Scholar] [CrossRef]
- Naspro, R.; Suardi, N.; Salonia, A.; Scattoni, V.; Guazzoni, G.; Colombo, R.; Cestari, A.; Briganti, A.; Mazzoccoli, B.; Rigatti, P.; et al. Holmium Laser Enucleation of the Prostate versus Open Pros-tatectomy for Prostates >70 g: 24-Month Follow-up. Eur. Urol. 2006, 50, 563–568. [Google Scholar] [CrossRef]
- Kuntz, R.M.; Lehrich, K.; Ahyai, S.A. Holmium Laser Enucleation of the Prostate versus Open Prostatectomy for Prostates Greater than 100 Grams: 5-Year Follow-Up Results of a Randomised Clinical Trial. Eur. Urol. 2008, 53, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Cornu, J.-N.; Ahyai, S.; Bachmann, A.; de la Rosette, J.; Gilling, P.; Gratzke, C.; McVary, K.; Novara, G.; Woo, H.; Madersbacher, S. A Systematic Review and Meta-analysis of Functional Outcomes and Complications Following Transurethral Procedures for Lower Urinary Tract Symptoms Resulting from Benign Prostatic Obstruction: An Update. Eur. Urol. 2015, 67, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Alzweri, L.; Rai, B.P.; Somani, B.K.; Bates, C.; Aboumarzouk, O.M. Holmium laser enucleation versus simple prostatectomy for treating large prostates: Results of a systematic review and meta-analysis. Arab J. Urol. 2016, 14, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Gkolezakis, V.; Somani, B.K.; Tokas, T. Low- vs. High-Power Laser for Holmium Laser Enucleation of Prostate. J. Clin. Med. 2023, 12, 2084. [Google Scholar] [CrossRef]
- Gong, Y.-G.; He, D.-L.; Wang, M.-Z.; Li, X.-D.; Zhu, G.-D.; Zheng, Z.-H.; Du, Y.-F.; Chang, L.S.; Nan, X.-Y. Holmium Laser Enucleation of the Prostate: A Modified Enucleation Technique and Initial Results. J. Urol. 2012, 187, 1336–1340. [Google Scholar] [CrossRef]
- Rassweiler, J.; Roder, M.; Schulze, M.; Muschter, R. Transurethral enucleation of the prostate with the holmium: YAG laser system: How much power is necessary? Urol. A 2008, 47, 441–448. [Google Scholar] [CrossRef]
- Gauhar, V.; Gilling, P.; Pirola, G.M.; Chan, V.W.-S.; Lim, E.J.; Maggi, M.; Teoh, J.Y.-C.; Krambeck, A.; Castellani, D. Does MOSES Technology Enhance the Efficiency and Outcomes of Standard Holmium Laser Enucleation of the Prostate? Results of a Systematic Review and Meta-analysis of Comparative Studies. Eur. Urol. Focus 2022, 8, 1362–1369. [Google Scholar] [CrossRef]
- Bach, T.; Netsch, C.; Haecker, A.; Michel, M.S.; Herrmann, T.R.W.; Gross, A.J. Thulium: YAG laser enucleation (VapoEnucleation) of the prostate: Safety and durability during intermediate-term follow-up. World J. Urol. 2010, 28, 39–43. [Google Scholar] [CrossRef]
- Herrmann, T.R.; Bach, T.; Imkamp, F.; Georgiou, A.; Burchardt, M.; Oelke, M.; Gross, A.J. Thulium laser enucleation of the prostate (ThuLEP): Tran-surethral anatomical prostatectomy with laser support. Introduction of a novel technique for the treatment of benign prostatic obstruction. World J. Urol. 2010, 28, 45–51. [Google Scholar] [CrossRef]
- Hartung, F.O.; Kowalewski, K.-F.; von Hardenberg, J.; Worst, T.S.; Kriegmair, M.C.; Nuhn, P.; Herrmann, T.R.; Michel, M.S.; Herrmann, J. Holmium versus Thulium Laser Enucleation of the Prostate: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Eur. Urol. Focus 2022, 8, 545–554. [Google Scholar] [CrossRef]
- Bozzini, G.; Berti, L.; Maltagliati, M.; Besana, U.; Micali, S.; Roche, J.B.; Romero-Otero, J.; Pacchetti, A.; Perri, D.; Morini, E.; et al. Thulium: YAG vs. continuous-wave thulium fiber laser enucleation of the prostate: Do potential advantages of thulium fiber lasers translate into relevant clinical differences? World J. Urol. 2023, 41, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.R.W.; Wolters, M. Transurethral anatomical enucleation of the prostate with Tm: YAG support (ThuLEP): Evolution and variations of the technique. The inventors’ perspective. Andrologia 2020, 52, e13587. [Google Scholar] [CrossRef] [PubMed]
- Netsch, C.; Becker, B.; Tiburtius, C.; Moritz, C.; Becci, A.V.; Herrmann, T.R.W.; Gross, A.J. A prospective, randomized trial comparing thulium vapoenu-cleation with holmium laser enucleation of the prostate for the treatment of symptomatic benign prostatic obstruction: Perioperative safety and efficacy. World J. Urol. 2017, 35, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Herrmann, T.R.W.; Gross, A.J.; Netsch, C. Thulium vapoenucleation of the prostate versus holmium laser enucleation of the prostate for the treatment of large volume prostates: Preliminary 6-month safety and efficacy results of a prospective randomized trial. World J. Urol. 2018, 36, 1663–1671. [Google Scholar] [CrossRef]
- Jones, P.; Rai, B.P.; Somani, B.K.; Aboumarzouk, O.M. A review of thulium laser vapo-enucleation of the prostate: A novel laser-based strategy for benign prostate enlargement. Arab J. Urol. 2015, 13, 209–211. [Google Scholar] [CrossRef]
- Broggi, E.; May, A.; Giretti, G.; Tabchouri, N.; Lorphelin, H.; Brichart, N.; Bruyère, F. Long-Term Outcomes of 80-Watt KTP and 120-Watt HPS GreenLight Photoselective Vaporization of the Prostate. Urol. Int. 2014, 93, 229–236. [Google Scholar] [CrossRef]
- Gomez Sancha, F.; Rivera, V.C.; Georgiev, G.; Botsevski, A.; Kotsev, J.; Herrmann, T. Common trend: Move to enucleation—Is there a case for GreenLight enucleation? Development and description of the technique. World J. Urol. 2015, 33, 539–547. [Google Scholar] [CrossRef]
- Thangasamy, I.A.; Chalasani, V.; Bachmann, A.; Woo, H.H. Photoselective vaporisation of the prostate using 80-W and 120-W laser versus tran-surethral resection of the prostate for benign prostatic hyperplasia: A systematic review with meta-analysis from 2002 to 2012. Eur. Urol. 2012, 62, 315–323. [Google Scholar] [CrossRef]
- Bachmann, A.; Tubaro, A.; Barber, N.; D’ancona, F.; Muir, G.; Witzsch, U.; Grimm, M.-O.; Benejam, J.; Stolzenburg, J.-U.; Riddick, A.; et al. 180-W XPS greenlight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European multicentre ran-domised trial—The GOLIATH study. Eur. Urol. 2014, 65, 931–942. [Google Scholar] [CrossRef]
- Thomas, J.A.; Tubaro, A.; Barber, N.; D’Ancona, F.; Muir, G.; Witzsch, U.; Grimm, M.-O.; Benejam, J.; Stolzenburg, J.-U.; Riddick, A.; et al. A Multicenter Randomized Noninferiority Trial Comparing GreenLight-XPS Laser Vaporization of the Prostate and Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: Two-yr Outcomes of the GOLIATH Study. Eur. Urol. 2016, 69, 94–102. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yan, D.; Jiang, T.; Fu, J.; Zheng, J.; Zhou, Y.; Zhou, Z.; Shen, W. Photoselective sharp enucleation of the prostate with a front-firing 532-nm laser versus photoselective vaporization of the prostate in the treatment of benign prostatic hyperplasia: A randomised controlled trial with 1-year followup results. BMC Urol. 2022, 22, 173. [Google Scholar]
- Chiang, P.H.; Chen, C.H.; Kang, C.H.; Chuang, Y.C. GreenLight HPS laser 120-W versus diode laser 200-W vaporization of the prostate: Comparative clinical experience. Lasers Surg. Med. 2010, 42, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Lusuardi, L.; Myatt, A.; Sieberer, M.; Jeschke, S.; Zimmermann, R.; Janetschek, G. Safety and efficacy of eraser laser enucleation of the prostate: Preliminary report. J. Urol. 2011, 186, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Zhang, Y.; Shi, C.; Tu, M.; Shi, G. 1470 nm Diode Laser Enucleation vs. Plasmakinetic Resection of the Prostate for Benign Prostatic Hyperplasia: A Randomized Study. J. Endourol. 2019, 33, 211–217. [Google Scholar] [CrossRef]
- Zou, Z.; Xu, A.; Zheng, S.; Chen, B.; Xu, Y.; Li, H.; Duan, C.; Zheng, J.; Chen, J.; Li, C.; et al. Dual-centre randomized-controlled trial comparing transurethral endoscopic enucleation of the prostate using diode laser vs. bipolar plasmakinetic for the treatment of LUTS secondary of benign prostate obstruction: 1-year follow-up results. World J. Urol. 2018, 36, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Li, T.; Ferreira, R.; Berjaoui, M.B.; Nguyen, A.L.V.; Chughtai, B.; Zorn, K.Z.; Bhojani, N.; Elterman, D. Ablative minimally invasive surgical therapies for benign prostatic hyperplasia: A review of Aquablation, Rezum, and transperineal laser prostate ablation. Prostate Cancer Prostatic Dis. 2023. ahead of print. [Google Scholar] [CrossRef]
- Lerner, L.B.; McVary, K.T.; Barry, M.J.; Bixler, B.R.; Dahm, P.; Das, A.K.; Gandhi, M.C.; Kaplan, S.A.; Kohler, T.S.; Martin, L.; et al. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE PART II-Surgical Evaluation and Treatment. J. Urol. 2021, 206, 818–826. [Google Scholar] [CrossRef]
- Elterman, D.; Aubé-Peterkin, M.; Evans, H.; Elmansy, H.; Meskawi, M.; Zorn, K.C.; Naeem, B. UPDATE—2022 Canadian Urological Association guideline on male lower urinary tract symptoms/benign prostatic hyperplasia (MLUTS/BPH). Can. Urol. Assoc. J. 2022, 16, 245–256. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Mani, M.; Hughes, T.; Somani, B.K. Role of low- versus high-power laser in the treatment of lower pole stones: Prospective non-randomized outcomes from a university teaching hospital. Ther. Adv. Urol. 2022, 14, 17562872221097345. [Google Scholar] [CrossRef]
- Somani, B.K.; Robertson, A.; Kata, S.G. Decreasing the Cost of Flexible Ureterorenoscopic Procedures. Urology 2011, 78, 528–530. [Google Scholar] [CrossRef]
- Chapman, R.A.; Somani, B.K.; Robertson, A.; Healy, S.; Kata, S.G. Decreasing the cost of flexible ureterorenoscopy: Single-use laser fiber cost analysis. Urology 2014, 83, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.M.; Jones, P.; Herrmann, T.R.; Aboumarzouk, O.; Somani, B.K. Ureteroscopy is more cost effective than shockwave lithotripsy for stone treatment: Systematic review and meta-analysis. World J. Urol. 2018, 36, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.M.; Davis, N.F.; Tzelves, L.; Lombardo, R.; Yuan, C.; Thomas, K.; Petrik, A.; Neisius, A.; Türk, C.; Gambaro, G.; et al. Best Practice in Interventional Management of Urolithiasis: An Update from the European Association of Urology Guidelines Panel for Urolithiasis 2022. Eur. Urol. Focus 2023, 9, 199–208. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
