Upturn Strategies for Arachidonic Acid-Induced MC3T3-E1—625 nm Irradiation in Combination with NSAIDs: Dissipating Inflammation and Promoting Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Instruments
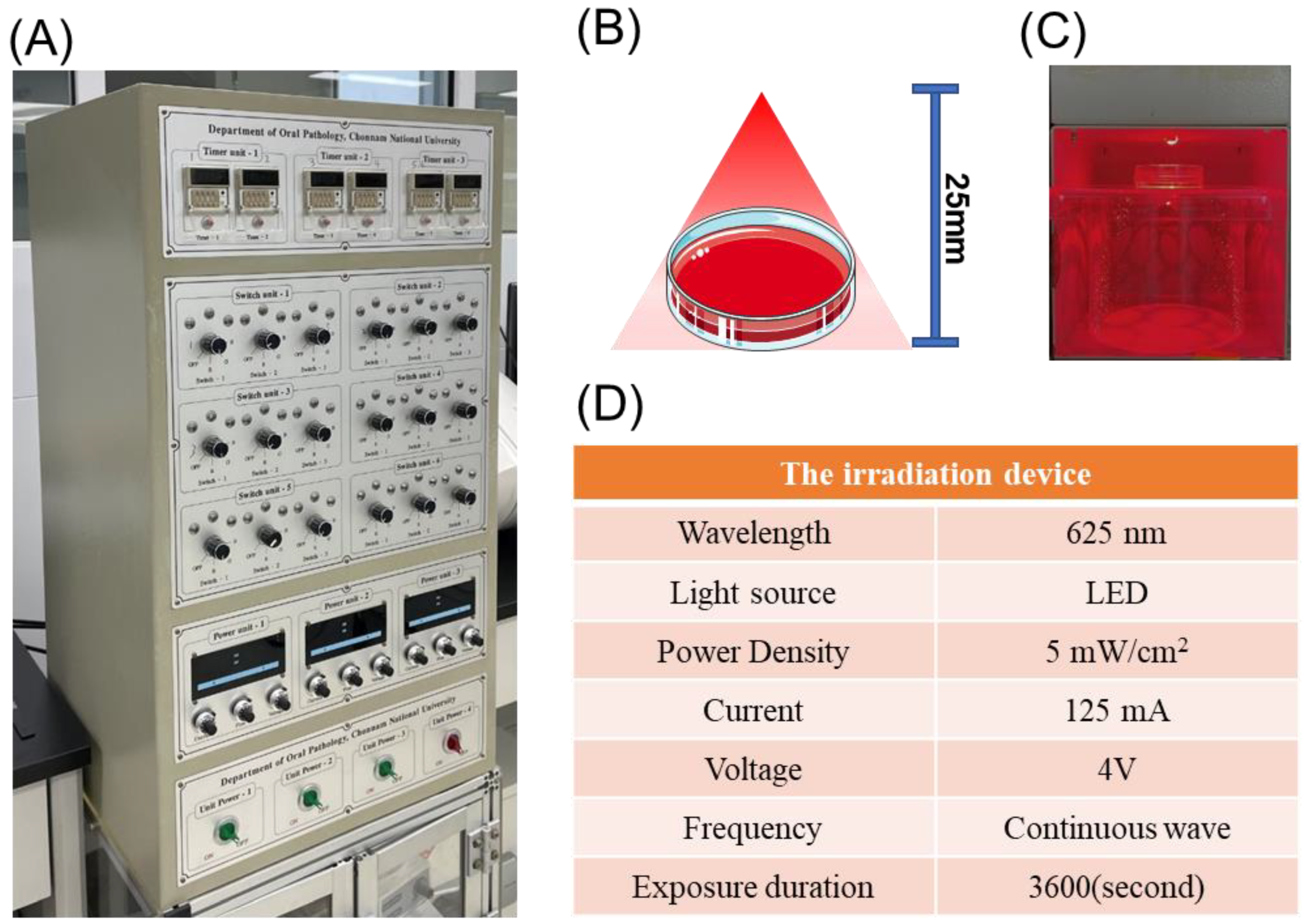
2.2. LEDI Treatment
2.3. Cell Culture and Design
2.4. Cell Viability Assay
2.5. PGE2 Release Assay
2.6. Western Blotting
2.7. BrdU Cell Proliferation Assay
2.8. Migration Assay
2.9. ALP Activity and ALP Staining
2.10. Statistical Analysis
3. Results
3.1. Effects of AA on MC3T3-E1
3.2. Effects of 625 nm LEDI on AA-Induced Inflammation in MC3T3-E1
3.3. Effects of NSAIDs on Cell Viability and PGE2 in MC3T3-E1
3.4. Effects of 625 nm LEDI and NSAIDs on Cell Migration in MC3T3-E1
3.5. Effects of Combined 625 nm LEDI and NSAIDs on Cell Migration and Proliferation in AA-Induced MC3T3-E1
3.6. Effects of the Combined Use of 625 nm Irradiation and NSAIDs on the Bone Formationin AA-Induced MC3T3-E1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, N.; Bakathir, A.; Pasha, M.; Al-Sudairy, S. Complications of Third Molar Extraction: A retrospective study from a tertiary healthcare centre in Oman. Sultan Qaboos Univ. Med. J. 2019, 19, e230. [Google Scholar] [CrossRef] [PubMed]
- Bouloux, G.F.; Steed, M.B.; Perciaccante, V.J. Complications of third molar surgery. Oral Maxillofac. Surg. Clin. 2007, 19, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ogle, O.E.; Mahjoubi, G. Local anesthesia: Agents, techniques, and complications. Dent. Clin. 2012, 56, 133–148. [Google Scholar] [CrossRef]
- Decloux, D.; Ouanounou, A. Local anaesthesia in dentistry: A review. Int. Dent. J. 2021, 71, 87–95. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Adeyemo, W.L. Oral health-related quality of life following third molar surgery with or without application of ice pack therapy. Oral Maxillofac. Surg. 2016, 20, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V.; Magnusson, P.; LeQuang, J.A.; Gharibo, C.; Varrassi, G. The pharmacological management of dental pain. Expert Opin. Pharmacother. 2020, 21, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontology 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Swift, A. Understanding pain and the human body’s response to it. Nurs. Times 2018, 114, 22–26. [Google Scholar]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Khouly, I.; Braun, R.S.; Ordway, M.; Alrajhi, M.; Fatima, S.; Kiran, B.; Veitz-Keenan, A. Post-operative pain management in dental implant surgery: A systematic review and meta-analysis of randomized clinical trials. Clin. Oral Investig. 2021, 25, 2511–2536. [Google Scholar] [CrossRef] [PubMed]
- Kokki, H. Nonsteroidal anti-inflammatory drugs for postoperative pain: A focus on children. Pediatr. Drugs 2003, 5, 103–123. [Google Scholar] [CrossRef]
- Miller, C.S.; Oyler, D.R. Post-operative pain management in dental implant surgery should consider nonsteroidal anti-inflammatory drugs as best practice. J. Oral Maxillofac. Anesth. 2021, 1, 1–3. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Blokhuis, T.J.; Pape, H.C.; Giannoudis, P.V. Pharmacological agents and impairment of fracture healing: What is the evidence? Injury 2008, 39, 384–394. [Google Scholar] [CrossRef]
- Bergenstock, M.; Min, W.; Simon, A.M.; Sabatino, C.; O’Connor, J.P. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J. Orthop. Trauma 2005, 19, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Kumchai, H.; Taub, D.; Tomlinson, R. Role of NSAIDs in Osseointegration of Dental Implants. J. Oral Maxillofac. Surg. 2021, 79, e76–e77. [Google Scholar] [CrossRef]
- Simon, A.M.; O’Connor, J.P. Dose and Time-Dependent Effects of Cyclooxygenase-2 Inhibition on Fracture-Healing. JBJS 2007, 89, 500–511. [Google Scholar] [CrossRef]
- Wheatley, B.M.; Nappo, K.E.; Christensen, D.L.; Holman, A.M.; Brooks, D.I.; Potter, B.K. Effect of NSAIDs on Bone Healing Rates: A Meta-analysis. J. Am. Acad. Orthop. Surg. 2019, 27, e330–e336. [Google Scholar] [CrossRef]
- Su, B.; O’Connor, J.P. NSAID therapy effects on healing of bone, tendon, and the enthesis. J. Appl. Physiol. 2013, 115, 892–899. [Google Scholar] [CrossRef]
- Rather, A.M. Effectiveness of Cold Therapy in Reducing Post-Surgery Oedema, Pain and Trismus after Impacted Mandibular Third Molar Surgery: A Randomized Observer-Blind Split-Mouth Clinical Trial. Saudi J. Oral Dent. Res. 2021, 6, 323–328. [Google Scholar]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Lee, J.-H.; Chiang, M.-H.; Chen, P.-H.; Ho, M.-L.; Lee, H.-E.; Wang, Y.-H. Anti-inflammatory effects of low-level laser therapy on human periodontal ligament cells: In vitro study. Lasers Med. Sci. 2018, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.S.; Ahrari, F.; Nasiri, F.; Abtahi, M.; Tunér, J. Low-level laser therapy for management of TMJ osteoarthritis. Cranio 2014, 32, 38–44. [Google Scholar] [CrossRef]
- de Paula Eduardo, C.; Aranha, A.C.C.; Simões, A.; Bello-Silva, M.S.; Ramalho, K.M.; Esteves-Oliveira, M.; de Freitas, P.M.; Marotti, J.; Tunér, J. Laser treatment of recurrent herpes labialis: A literature review. Lasers Med. Sci. 2014, 29, 1517–1529. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Shen, N.; Wu, N.; Sun, J. A systematic review and meta-analysis of the effect of low-level laser therapy (LLLT) on chemotherapy-induced oral mucositis in pediatric and young patients. Eur. J. Pediatr. 2018, 177, 7–17. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Zhao, L. The effect of low-level laser therapy as an adjunct to periodontal surgery in the management of postoperative pain and wound healing: A systematic review and meta-analysis. Lasers Med. Sci. 2021, 36, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. Effect of diode low-level lasers on fibroblasts derived from human periodontal tissue: A systematic review of in vitro studies. Lasers Med. Sci. 2016, 31, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kim, H.-E.; Cho, H.; Shi, S.; Kim, B.; Kim, O. Red light-emitting diode irradiation regulates oxidative stress and inflammation through SPHK1/NF-κB activation in human keratinocytes. J. Photochem. Photobiol. B Biol. 2018, 186, 31–40. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Effect of red light and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 188, 60–68. [Google Scholar] [CrossRef] [PubMed]
- de Carli, M.L.; Guerra, M.B.; Nunes, T.B.; di Matteo, R.C.; de Luca, C.E.; Aranha, A.C.; Bolzan, M.C.; Witzel, A.L. Piroxicam and laser phototherapy in the treatment of TMJ arthralgia: A double-blind randomised controlled trial. J. Oral Rehabil. 2013, 40, 171–178. [Google Scholar] [CrossRef]
- Nabi, S.; Amin, K.; Masoodi, A.; Farooq, R.; Purra, A.R.; Ahangar, F.A. Effect of preoperative ibuprofen in controlling postendodontic pain with and without low-level laser therapy in single visit endodontics: A randomized clinical study. Indian J. Dent. Res. 2018, 29, 46. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Carvalho, R.L.; Leal-Junior, E.C.; Petrellis, M.C.; Marcos, R.L.; de Carvalho, M.H.; De Nucci, G.; Lopes-Martins, R.A. Effects of low-level laser therapy (LLLT) and diclofenac (topical and intramuscular) as single and combined therapy in experimental model of controlled muscle strain in rats. Photochem. Photobiol. 2013, 89, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Thanikaivelan, P. Bionic, porous, functionalized hybrid scaffolds with vascular endothelial growth factor promote rapid wound healing in Wistar albino rats. RSC Adv. 2016, 6, 19252–19264. [Google Scholar] [CrossRef]
- Shabestani Monfared, G.; Ertl, P.; Rothbauer, M. An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure. Sci. Rep. 2020, 10, 16192. [Google Scholar] [CrossRef] [PubMed]
- Miroshnychenko, A.; Ibrahim, S.; Azab, M.; Roldan, Y.; Martinez, J.P.D.; Tamilselvan, D.; He, L.; Little, J.W.; Urquhart, O.; Tampi, M.; et al. Acute Postoperative Pain Due to Dental Extraction in the Adult Population: A Systematic Review and Network Meta-analysis. J. Dent. Res. 2023, 102, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A. Do NSAIDs Really Interfere with Healing after Surgery? J. Clin. Med. 2021, 10, 2359. [Google Scholar] [CrossRef]
- Etikala, A.; Tattan, M.; Askar, H.; Wang, H.L. Effects of NSAIDs on Periodontal and Dental Implant Therapy. Compend. Contin. Educ. Dent. 2019, 40, e1–e9. [Google Scholar]
- Silveira, P.C.; da Silva, L.A.; Fraga, D.B.; Freitas, T.P.; Streck, E.L.; Pinho, R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J. Photochem. Photobiol. B Biol. 2009, 95, 89–92. [Google Scholar] [CrossRef]
- Dima, R.; Tieppo Francio, V.; Towery, C.; Davani, S. Review of Literature on Low-level Laser Therapy Benefits for Nonpharmacological Pain Control in Chronic Pain and Osteoarthritis. Altern Ther. Health Med. 2018, 24, 8–10. [Google Scholar]
- Dawood, M.S.; Al-Habib, M.F.; Salman, S.D. A histological study of the effect of low level diode laser therapy on wound healing. Al-Nahrain J. Eng. Sci. 2009, 12, 80–88. [Google Scholar]
- Enwemeka, C.S.; Parker, J.C.; Dowdy, D.S.; Harkness, E.E.; Sanford, L.E.; Woodruff, L.D. The efficacy of low-power lasers in tissue repair and pain control: A meta-analysis study. Photomed. Laser Surg. 2004, 22, 323–329. [Google Scholar] [CrossRef]
- Yang, T.-S.; Nguyen, L.-T.-H.; Hsiao, Y.-C.; Pan, L.-C.; Chang, C.-J. Biophotonic Effects of Low-Level Laser Therapy at Different Wavelengths for Potential Wound Healing. Photonics 2022, 9, 591. [Google Scholar] [CrossRef]
- Woodruff, L.D.; Bounkeo, J.M.; Brannon, W.M.; Dawes, K.S.; Barham, C.D.; Waddell, D.L.; Enwemeka, C.S. The efficacy of laser therapy in wound repair: A meta-analysis of the literature. Photomed. Laser Surg. 2004, 22, 241–247. [Google Scholar] [CrossRef]
- Fulop, A.M.; Dhimmer, S.; Deluca, J.R.; Johanson, D.D.; Lenz, R.V.; Patel, K.B.; Douris, P.C.; Enwemeka, C.S. A meta-analysis of the efficacy of phototherapy in tissue repair. Photomed Laser Surg 2009, 27, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. HNE as an inducer of COX-2. Free Radic. Biol. Med. 2017, 111, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Mbonye, U.R.; DeLong, C.J.; Wada, M.; Smith, W.L. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 2007, 46, 108–125. [Google Scholar] [CrossRef]
- Cho, H.; Kim, O.-S.; Kim, B.; Yang, Y.; Song, J.; Liu, D.; Kim, Y.; Jeon, S.; Kim, O. 635 nm LED irradiation may prevent endoplasmic reticulum stress in MC3T3-E1 cells. J. Mol. Histol. 2022, 53, 75–83. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-inflammatory Drugs (NSAIDs). In StatPearls; StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Matzelle, M.M.; Gallant, M.A.; Condon, K.W.; Walsh, N.C.; Manning, C.A.; Stein, G.S.; Lian, J.B.; Burr, D.B.; Gravallese, E.M. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum 2012, 64, 1540–1550. [Google Scholar] [CrossRef]
- Thomas, M.; Puleo, D. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef]
- Conway, J.R.W.; Jacquemet, G. Cell matrix adhesion in cell migration. Essays Biochem. 2019, 63, 535–551. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369. [Google Scholar] [PubMed]
- Lewis, J.E.; Jensen, P.J.; Johnson, K.R.; Wheelock, M.J. E-cadherin mediates adherens junction organization through protein kinase C. J. Cell Sci. 1994, 107, 3615–3621. [Google Scholar] [CrossRef]
- Ostrowska-Podhorodecka, Z.; Ding, I.; Lee, W.; Tanic, J.; Abbasi, S.; Arora, P.D.; Liu, R.S.; Patteson, A.E.; Janmey, P.A.; McCulloch, C.A. Vimentin tunes cell migration on collagen by controlling β1 integrin activation and clustering. J. Cell Sci. 2021, 134, jcs254359. [Google Scholar] [CrossRef]
- Hazan, R.B.; Phillips, G.R.; Qiao, R.F.; Norton, L.; Aaronson, S.A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000, 148, 779–790. [Google Scholar] [CrossRef]
- Haraguchi, M.; Fukushige, T.; Kanekura, T.; Ozawa, M. E-cadherin loss in RMG-1 cells inhibits cell migration and its regulation by Rho GTPases. Biochem. Biophys. Rep. 2019, 18, 100650. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.; Yamada, S. N-cadherin-mediated cell–cell adhesion promotes cell migration in a three-dimensional matrix. J. Cell Sci. 2012, 125, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, W.; Jiang, Z.; Su, Z.; Ma, X.; Li, Y.; Jiang, R.; Zhang, J.; Yang, X. Hypothermia inhibits the proliferation of bone marrow-derived mesenchymal stem cells and increases tolerance to hypoxia by enhancing SUMOylation. Int. J. Mol. Med. 2017, 40, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Low-Level Laser Irradiation Promotes Proliferation and Differentiation of Human Osteoblasts in Vitro. Photomed. Laser Surg. 2005, 23, 161–166. [CrossRef]
- Thiel, A.; Reumann, M.K.; Boskey, A.; Wischmann, J.; von Eisenhart-Rothe, R.; Mayer-Kuckuk, P. Osteoblast migration in vertebrate bone. Biol. Rev. 2018, 93, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, L.; Kou, N.; Bai, Y.; Zhang, Y.; Lu, Y.; Gao, L.; Wang, F. Low level laser therapy promotes bone regeneration by coupling angiogenesis and osteogenesis. Stem Cell Res. Ther. 2021, 12, 432. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Kim, B.; Fu, W.; Zhu, S.; Kang, J.; Kim, O.; Kim, O. Upturn Strategies for Arachidonic Acid-Induced MC3T3-E1—625 nm Irradiation in Combination with NSAIDs: Dissipating Inflammation and Promoting Healing. Photonics 2023, 10, 535. https://doi.org/10.3390/photonics10050535
Liu D, Kim B, Fu W, Zhu S, Kang J, Kim O, Kim O. Upturn Strategies for Arachidonic Acid-Induced MC3T3-E1—625 nm Irradiation in Combination with NSAIDs: Dissipating Inflammation and Promoting Healing. Photonics. 2023; 10(5):535. https://doi.org/10.3390/photonics10050535
Chicago/Turabian StyleLiu, Danyang, Byunggook Kim, Wenqi Fu, Siyu Zhu, Jaeseok Kang, Oksu Kim, and Okjoon Kim. 2023. "Upturn Strategies for Arachidonic Acid-Induced MC3T3-E1—625 nm Irradiation in Combination with NSAIDs: Dissipating Inflammation and Promoting Healing" Photonics 10, no. 5: 535. https://doi.org/10.3390/photonics10050535
APA StyleLiu, D., Kim, B., Fu, W., Zhu, S., Kang, J., Kim, O., & Kim, O. (2023). Upturn Strategies for Arachidonic Acid-Induced MC3T3-E1—625 nm Irradiation in Combination with NSAIDs: Dissipating Inflammation and Promoting Healing. Photonics, 10(5), 535. https://doi.org/10.3390/photonics10050535




