In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. UVB Exposure
2.4. Cell Viability Assessment
2.5. Cellular Morphology
2.6. Identification of the Nuclear Structure and Actin Filaments
2.7. Expression of Bcl-2 and Bax Genes
2.8. Caspase Activity
2.9. Statistical Analysis
3. Results
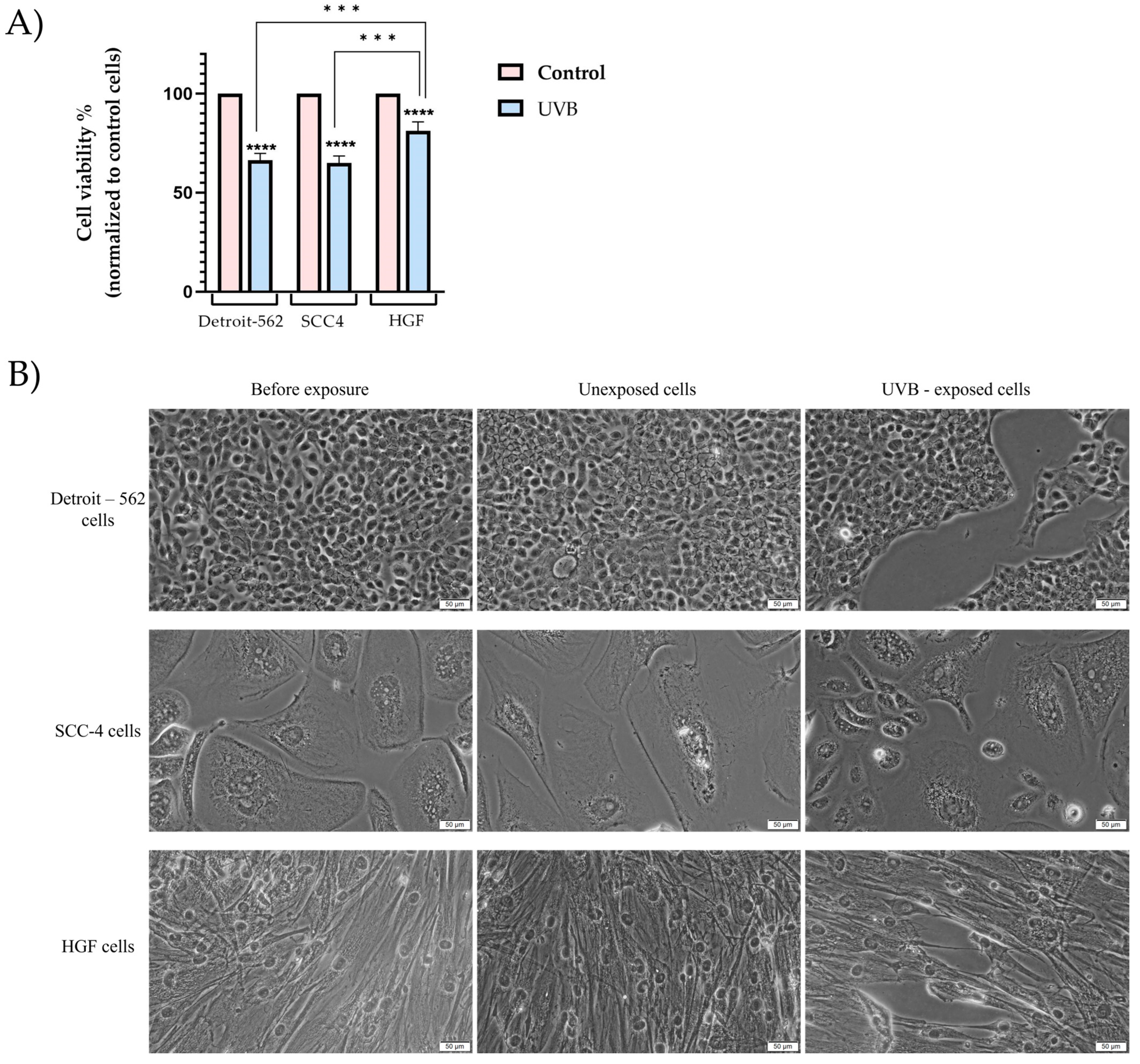
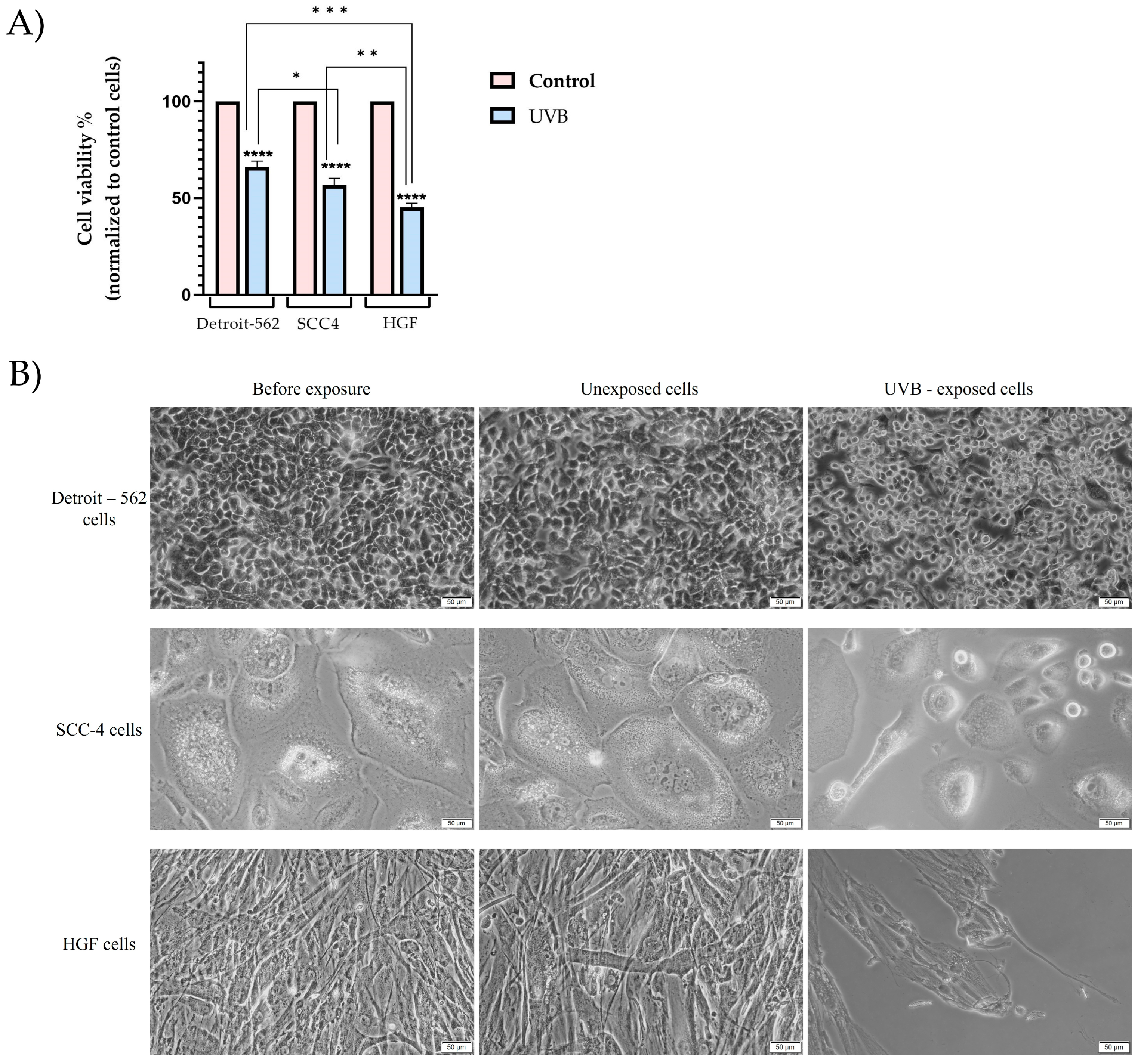
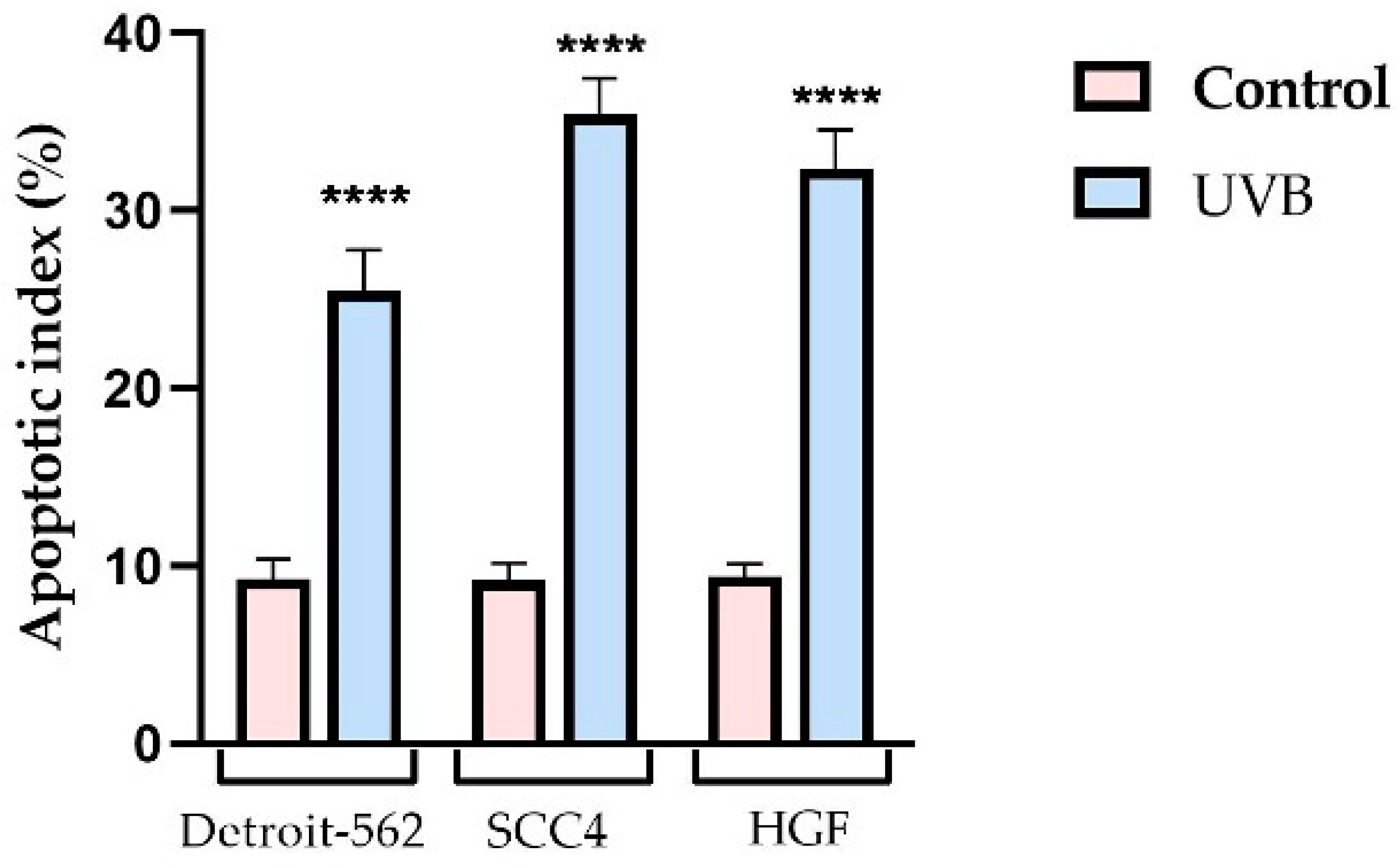
3.1. Cell Viability and Morphology Assessment
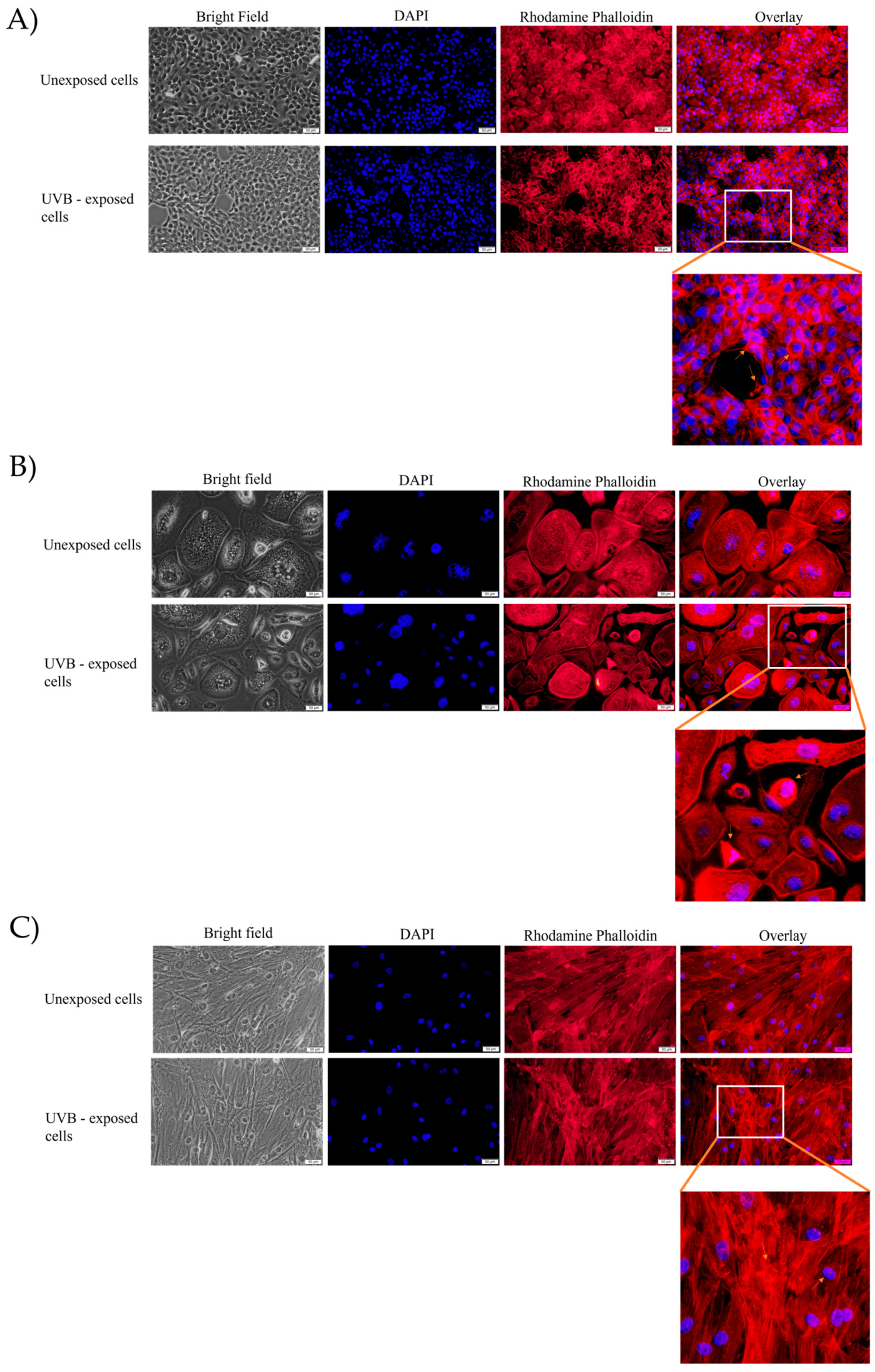
3.2. Fluorescence Immunocytochemistry
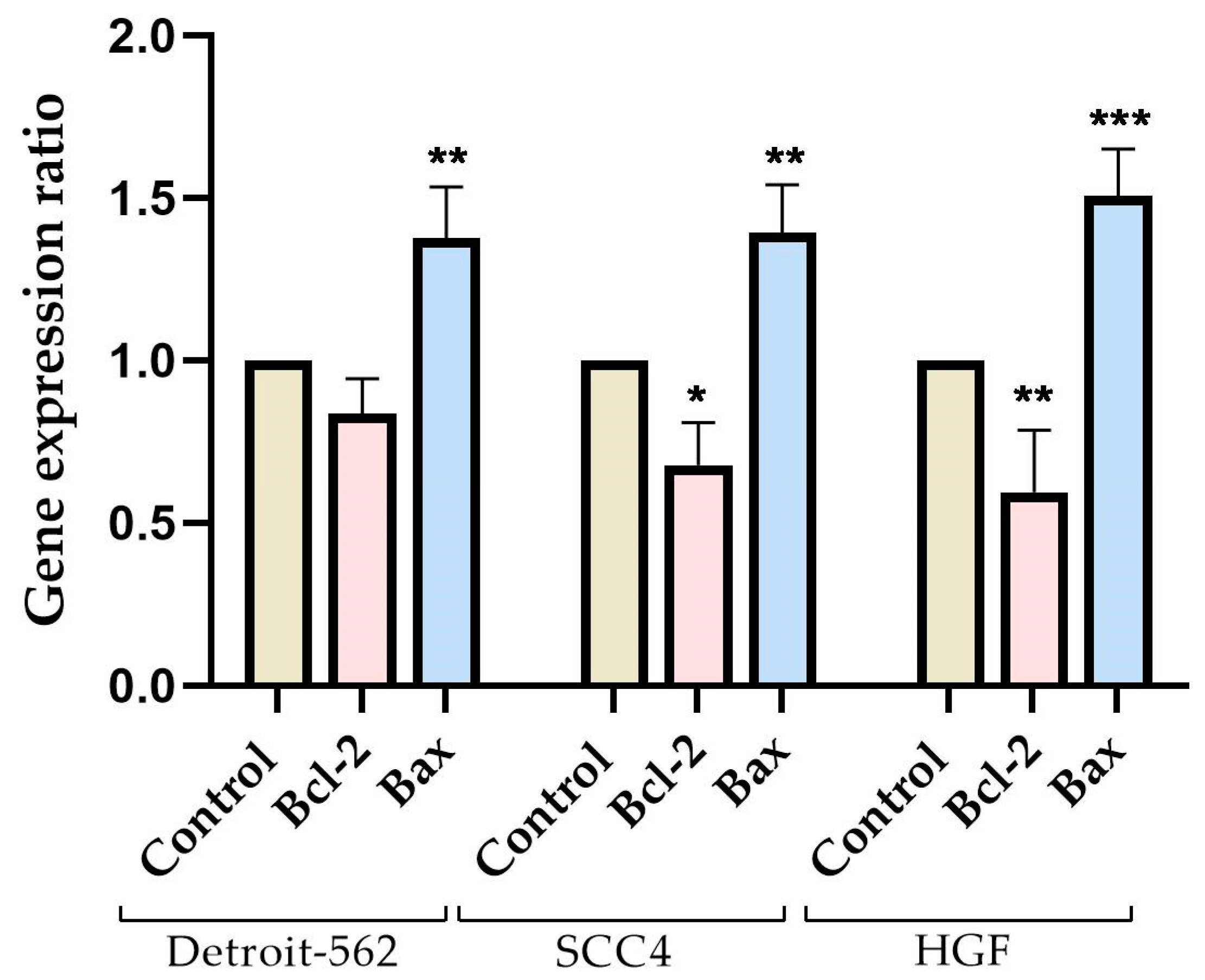
3.3. Gene Expression Ratio
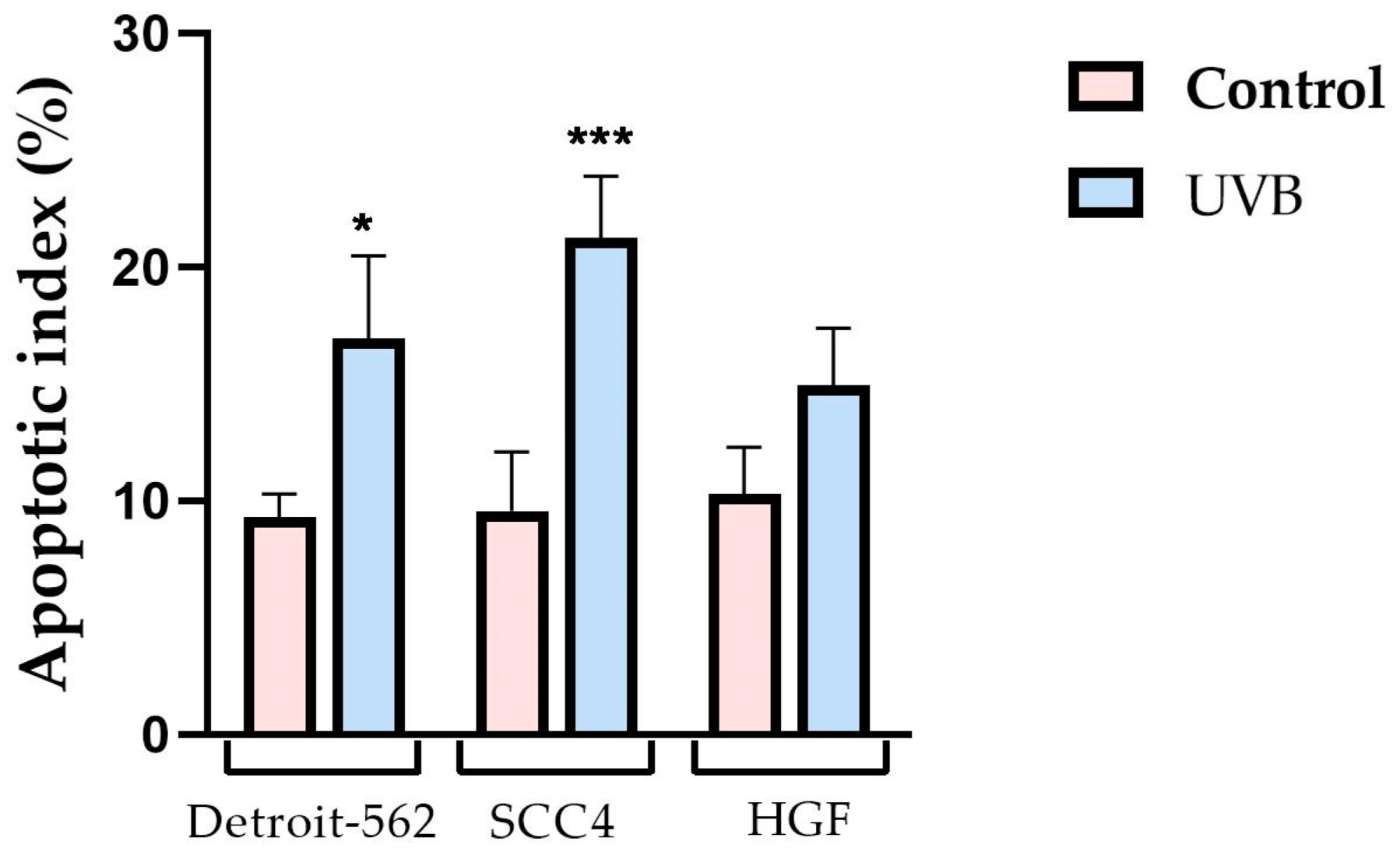
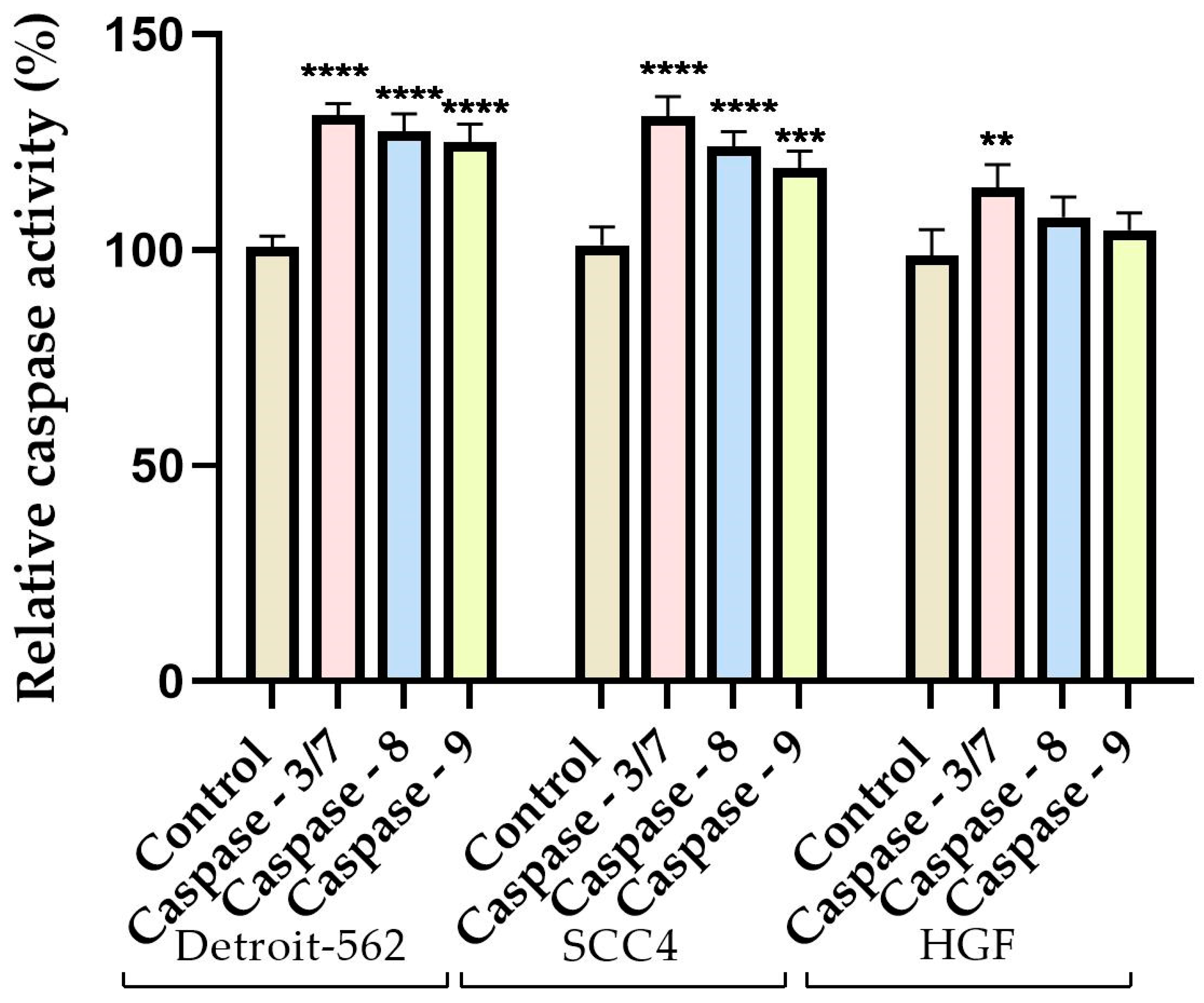
3.4. Caspase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moan, J.; Grigalavicius, M.; Baturaite, Z.; Dahlback, A.; Juzeniene, A. The Relationship between UV Exposure and Incidence of Skin Cancer. Photodermatol. Photoimmunol. Photomed. 2015, 31, 26–35. [Google Scholar] [CrossRef]
- Adams, S.; Lin, J.I.E.; Brown, D.; Shriver, C.D.; Zhu, K. Ultraviolet Radiation Exposure and the Incidence of Oral, Pharyngeal and Cervical Cancer and Melanoma: An Analysis of the SEER Data. Anticancer. Res. 2016, 36, 233–237. [Google Scholar]
- Dale Wilson, B.; Moon, S.; Armstrong, F. Comprehensive Review of Ultraviolet Radiation and the Current Status on Sunscreens. J. Clin. Aesthetic Dermatol. 2012, 5, 18. [Google Scholar]
- Kumar, R.; Deep, G.; Agarwal, R. An Overview of Ultraviolet B Radiation-Induced Skin Cancer Chemoprevention by Silibinin. Curr. Pharmacol. Rep. 2015, 1, 206–215. [Google Scholar] [CrossRef]
- El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A Review of Human Carcinogens—Part D: Radiation. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Singh, T.; Prasad, R.; Sun, Q.; Vaid, M. Epigenetic Alterations in Ultraviolet Radiation-Induced Skin Carcinogenesis: Interaction of Bioactive Dietary Components on Epigenetic Targets. Photochem. Photobiol. 2012, 88, 1066–1074. [Google Scholar] [CrossRef]
- de Oliveira, N.F.; de Souza, B.F.; de Castro Coêlho, M. UV Radiation and Its Relation to DNA Methylation in Epidermal Cells: A Review. Epigenomes 2020, 4, 23. [Google Scholar] [CrossRef]
- Gentile, M.; Latonen, L.; Laiho, M. Cell Cycle Arrest and Apoptosis Provoked by UV Radiation-induced DNA Damage Are Transcriptionally Highly Divergent Responses. Nucleic Acids Res. 2003, 31, 4779–4790. [Google Scholar] [CrossRef]
- Cha Jun, H.; Kim, O.; Lee Tai, G.; Lee Sik, K.; Lee Ho, J.; Park, I.; Lee, S.; Kim Ri, Y.; Ahn Joong, K.; An, I.; et al. Identification of Ultraviolet B Radiation-induced MicroRNAs in Normal Human Dermal Papilla Cells. Mol. Med. Rep. 2014, 10, 1663–1670. [Google Scholar] [CrossRef]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef]
- Bush, M.A.; Hermanson, A.S.; Yetto, R.J.; Wieczkowski, G.J. The Use of Ultraviolet LED Illumination for Composite Resin Removal: An In Vitro Study. Gen. Dent. 2010, 58, e214–e218. [Google Scholar]
- McGee, S.; Mirkovic, J.; Mardirossian, V.; Elackattu, A.; Yu, C.-C.; Kabani, S.; Gallagher, G.; Pistey, R.; Galindo, L.; Badizadegan, K.; et al. Model-Based Spectroscopic Analysis of the Oral Cavity: Impact of Anatomy. J. Biomed. Opt. 2008, 13, 64034. [Google Scholar] [CrossRef]
- McGee, S.; Mardirossian, V.; Elackattu, A.; Mirkovic, J.; Pistey, R.; Gallagher, G.; Kabani, S.; Yu, C.-C.; Wang, Z.; Badizadegan, K.; et al. Anatomy-Based Algorithms for Detecting Oral Cancer Using Reflectance and Fluorescence Spectroscopy. Ann. Otol. Rhinol. Laryngol. 2009, 118, 817–826. [Google Scholar] [CrossRef]
- Duarte, I.A.G.; Hafner, M.D.F.S.; Malvestiti, A.A. Ultraviolet Radiation Emitted by Lamps, TVs, Tablets and Computers: Are There Risks for the Population? An. Bras. Dermatol. 2015, 90, 595–597. [Google Scholar] [CrossRef]
- Morais, P. Artificial Tanning Devices (Sunbeds): Where Do We Stand? Cutan. Ocul. Toxicol. 2022, 41, 123–128. [Google Scholar] [CrossRef]
- Bruzell, E.M.; Johnsen, B.; Aalerud, T.N.; Dahl, J.E.; Christensen, T. In Vitro Efficacy and Risk for Adverse Effects of Light-Assisted Tooth Bleaching. Photochem. Photobiol. Sci. 2009, 8, 377–385. [Google Scholar] [CrossRef]
- Rudhart, S.A.; Günther, F.; Dapper, L.; Thangavelu, K.; Gehrt, F.; Stankovic, P.; Wilhelm, T.; Guenzel, T.; Stuck, B.A.; Hoch, S. UV Light-Based Decontamination: An Effective and Fast Way for Disinfection of Endoscopes in Otorhinolaryngology? Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2363–2369. [Google Scholar] [CrossRef]
- Liu, K.; Shang, C.; Wang, Z.; Qi, Y.; Miao, R.; Liu, K.; Liu, T.; Fang, Y. Non-Contact Identification and Differentiation of Illicit Drugs Using Fluorescent Films. Nat. Commun. 2018, 9, 1695. [Google Scholar] [CrossRef]
- Herrington, W.F.; Singh, G.P.; Wu, D.; Barone, P.W.; Hancock, W.; Ram, R.J. Optical Detection of Degraded Therapeutic Proteins. Sci. Rep. 2018, 8, 5089. [Google Scholar] [CrossRef]
- Mojeski, J.A.; Almashali, M.; Jowdy, P.; Fitzgerald, M.E.; Brady, K.L.; Zeitouni, N.C.; Colegio, O.R.; Paragh, G. Ultraviolet Imaging in Dermatology. Photodiagnosis Photodyn. Ther. 2020, 30, 101743. [Google Scholar] [CrossRef]
- Xu, Q.; McMichael, P.; Creagh-Flynn, J.; Zhou, D.; Gao, Y.; Li, X.; Wang, X.; Wang, W. Double-Cross-Linked Hydrogel Strengthened by UV Irradiation from a Hyperbranched PEG-Based Trifunctional Polymer. ACS Macro Lett. 2018, 7, 509–513. [Google Scholar] [CrossRef]
- Branisteanu Elena, D.; Dirzu Stefania, D.; Toader Paula, M.; Branisteanu Constantin, D.; Nicolescu Codrut, A.; Brihan, I.; Bogdanici Margareta, C.; Branisteanu, G.; Dimitriu, A.; Anton, N.; et al. Phototherapy in Dermatological Maladies (Review). Exp. Ther. Med. 2022, 23, 259. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; Aguiar, C.M.; Câmara, A.C.; de Albuquerque, M.M.; Correia, A.C.R.D.B.; Soares, M.F.D.L.R. The Efficacy of Photodynamic Therapy and Sodium Hypochlorite in Root Canal Disinfection by a Single-File Instrumentation Technique. Photodiagnosis Photodyn. Ther. 2015, 12, 436–443. [Google Scholar] [CrossRef]
- Haraguchi, A.; Yoshida, S.; Takeshita, M.; Sumi, Y.; Mitarai, H.; Yuda, A.; Wada, H.; Nishimura, F.; Maeda, H.; Wada, N. Effects of Ultraviolet Irradiation Equipment on Endodontic Disease–Related Bacteria. Lasers Dent. Sci. 2022, 6, 31–40. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of Oral Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884. [Google Scholar]
- Dzebo, S.; Mahmutovic, J.; Erkocevic, H. Quality of Life of Patients with Oral Cavity Cancer. Mater. Sociomed. 2017, 29, 30–34. [Google Scholar] [CrossRef]
- Thavarool, S.B.; Muttath, G.; Nayanar, S.; Duraisamy, K.; Bhat, P.; Shringarpure, K.; Nayak, P.; Tripathy, J.P.; Thaddeus, A.; Philip, S.; et al. Improved Survival among Oral Cancer Patients: Findings from a Retrospective Study at a Tertiary Care Cancer Centre in Rural Kerala, India. World J. Surg. Oncol. 2019, 17, 15. [Google Scholar] [CrossRef]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and Incidence of Oral Cancer in Low- and Middle-Income Countries: A Scoping Review. Eur. J. Cancer Care 2020, 29, e13207. [Google Scholar] [CrossRef]
- Leverrier, S.; Bergamaschi, D.; Ghali, L.; Ola, A.; Warnes, G.; Akgül, B.; Blight, K.; García-Escudero, R.; Penna, A.; Eddaoudi, A.; et al. Role of HPV E6 Proteins in Preventing UVB-Induced Release of pro-Apoptotic Factors from the Mitochondria. Apoptosis 2007, 12, 549–560. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective Assessment of Changes in Nuclear Morphology and Cell Distribution Following Induction of Apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef]
- Parikh, R.; Sorek, E.; Parikh, S.; Michael, K.; Bikovski, L.; Tshori, S.; Shefer, G.; Mingelgreen, S.; Zornitzki, T.; Knobler, H.; et al. Skin Exposure to UVB Light Induces a Skin-Brain-Gonad Axis and Sexual Behavior. Cell Rep. 2021, 36, 109579. [Google Scholar] [CrossRef]
- Pfeifer, G.P. Mechanisms of UV-Induced Mutations and Skin Cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef]
- Cuomo, R.E.; Mohr, S.B.; Gorham, E.D.; Garland, C.F. What Is the Relationship between Ultraviolet B and Global Incidence Rates of Colorectal Cancer? Dermato-Endocrinol. 2013, 5, 181–185. [Google Scholar] [CrossRef]
- Ibbotson, S.H. A Perspective on the Use of NB-UVB Phototherapy vs. PUVA Photochemotherapy. Front. Med. 2018, 5, 184. [Google Scholar] [CrossRef]
- Young, A.R.; Morgan, K.A.; Harrison, G.I.; Lawrence, K.P.; Petersen, B.; Wulf, H.C.; Philipsen, P.A. A Revised Action Spectrum for Vitamin D Synthesis by Suberythemal UV Radiation Exposure in Humans in Vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2015867118. [Google Scholar] [CrossRef]
- Panov, V.; Borisova-Papancheva, T. Application of Ultraviolet Light (UV) in Dental Medicdine. J. Med. Dent. Pract. 2015, 2, 194–200. [Google Scholar] [CrossRef]
- Agrawal, A.; Shindell, E.; Jordan, F.; Baeva, L.; Pfefer, J.; Godar, D.E. UV Radiation Increases Carcinogenic Risks for Oral Tissues Compared to Skin. Photochem. Photobiol. 2013, 89, 1193–1198. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Battistelli, M.; Baldassarri, V.; Maltarello, M.C.; Falcieri, E. Ultraviolet B (UVB) Irradiation-Induced Apoptosis in Various Cell Lineages In Vitro. Int. J. Mol. Sci. 2013, 14, 532–546. [Google Scholar] [CrossRef]
- Khalil, C. In Vitro UVB Induced Cellular Damage Assessment Using Primary Human Skin Derived Fibroblasts. MOJ Toxicol. 2015, 1, 138–143. [Google Scholar] [CrossRef]
- Fernandez, T.L.; Van Lonkhuyzen, D.R.; Dawson, R.A.; Kimlin, M.G.; Upton, Z. In Vitro Investigations on the Effect of Dermal Fibroblasts on Keratinocyte Responses to Ultraviolet B Radiation. Photochem. Photobiol. 2014, 90, 1332–1339. [Google Scholar] [CrossRef]
- Khalil, C. Combining Three In Vitro Assays for Detecting Early Signs of UVB Cytotoxicity in Cultured Human Skin Fibroblasts. WIT Trans. Biomed. Health 2006, 10, 349–359. [Google Scholar] [CrossRef]
- Khalil, C.; Shebaby, W. UVB Damage Onset and Progression 24 h Post Exposure in Human-Derived Skin Cells. Toxicol. Rep. 2017, 4, 441–449. [Google Scholar] [CrossRef]
- Boza, Y.; Yefi, R.; Rudolph, M.I.; Smith, P.C.; Oberyszyn, T.M.; Tober, K.L.; Rojas, I.G. Single Exposure of Human Oral Mucosa Fibroblasts to Ultraviolet B Radiation Reduces Proliferation and Induces COX-2 Expression and Activation. Rev. Clínica De Periodoncia Implantol. Y Rehabil. Oral 2010, 3, 123–127. [Google Scholar] [CrossRef]
- Straface, E.; Vona, R.; Ascione, B.; Matarrese, P.; Strudthoff, T.; Franconi, F.; Malorni, W. Single Exposure of Human Fibroblasts (WI-38) to a Sub-Cytotoxic Dose of UVB Induces Premature Senescence. FEBS Lett. 2007, 581, 4342–4348. [Google Scholar] [CrossRef]
- Chainiaux, F.; Magalhaes, J.-P.; Eliaers, F.; Remacle, J.; Toussaint, O. UVB-Induced Premature Senescence of Human Diploid Skin Fibroblasts. Int. J. Biochem. Cell Biol. 2002, 34, 1331–1339. [Google Scholar] [CrossRef]
- Cavinato, M.; Koziel, R.; Romani, N.; Weinmüllner, R.; Jenewein, B.; Hermann, M.; Dubrac, S.; Ratzinger, G.; Grillari, J.; Schmuth, M.; et al. UVB-Induced Senescence of Human Dermal Fibroblasts Involves Impairment of Proteasome and Enhanced Autophagic Activity. J. Gerontol. Ser. A 2017, 72, 632–639. [Google Scholar] [CrossRef]
- Kimura, H.; Lee, C.; Hayashi, K.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Bouvet, M.; Hoffman, R.M. UV Light Killing Efficacy of Fluorescent Protein-Expressing Cancer Cells In Vitro and In Vivo. J. Cell. Biochem. 2010, 110, 1439–1446. [Google Scholar] [CrossRef]
- Granados-López, A.J.; Manzanares-Acuña, E.; López-Hernández, Y.; Castañeda-Delgado, J.E.; Fraire-Soto, I.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; López, J.A. UVB Inhibits Proliferation, Cell Cycle and Induces Apoptosis via P53, E2F1 and Microtubules System in Cervical Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 5197. [Google Scholar] [CrossRef]
- Wang, H.-M.; Yang, H.-L.; Thiyagarajan, V.; Huang, T.-H.; Huang, P.-J.; Chen, S.-C.; Liu, J.-Y.; Hsu, L.-S.; Chang, H.-W.; Hseu, Y.-C. Coenzyme Q(0) Enhances Ultraviolet B-Induced Apoptosis in Human Estrogen Receptor-Positive Breast (MCF-7) Cancer Cells. Integr. Cancer Ther. 2017, 16, 385–396. [Google Scholar] [CrossRef]
- Sarkar, S.; Rajput, S.; Tripathi, A.K.; Mandal, M. Targeted Therapy against EGFR and VEGFR Using ZD6474 Enhances the Therapeutic Potential of UV-B Phototherapy in Breast Cancer Cells. Mol. Cancer 2013, 12, 122. [Google Scholar] [CrossRef]
- Bottone, M.G.; Santin, G.; Aredia, F.; Bernocchi, G.; Pellicciari, C.; Scovassi, A.I. Morphological Features of Organelles during Apoptosis: An Overview. Cells 2013, 2, 294–305. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Uncoupling Cell Shrinkage from Apoptosis Reveals That Na+ Influx Is Required for Volume Loss during Programmed Cell Death*. J. Biol. Chem. 2003, 278, 39176–39184. [Google Scholar] [CrossRef]
- Doonan, F.; Cotter, T.G. Morphological Assessment of Apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef]
- González-Juanatey, J.R.; Piñeiro, R.; Iglesias, M.J.; Gualillo, O.; Kelly, P.A.; Diéguez, C.; Lago, F. GH Prevents Apostosis in Cardiomyocytes Cultured In Vitro through a Calcineurin-Dependent Mechanism. J. Endocrinol. 2004, 180, 325–335. [Google Scholar] [CrossRef]
- Toné, S.; Sugimoto, K.; Tanda, K.; Suda, T.; Uehira, K.; Kanouchi, H.; Samejima, K.; Minatogawa, Y.; Earnshaw, W.C. Three Distinct Stages of Apoptotic Nuclear Condensation Revealed by Time-Lapse Imaging, Biochemical and Electron Microscopy Analysis of Cell-Free Apoptosis. Exp. Cell Res. 2007, 313, 3635–3644. [Google Scholar] [CrossRef]
- Chen, Q.; Kang, J.; Fu, C. The Independence of and Associations among Apoptosis, Autophagy, and Necrosis. Signal. Transduct. Target. Ther. 2018, 3, 18. [Google Scholar] [CrossRef]
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The Nuclear Envelope: Target and Mediator of the Apoptotic Process. Cell Death Discov. 2020, 6, 29. [Google Scholar] [CrossRef]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. News Physiol. Sci. 2004, 19, 124–128. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; De La Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Bouchier-Hayes, L.; Green, D.R. Mitochondrial Outer Membrane Permeabilization during Apoptosis: The Innocent Bystander Scenario. Cell Death Differ. 2006, 13, 1396–1402. [Google Scholar] [CrossRef]
- Lee, C.-H.; Yu, C.-L.; Liao, W.-T.; Kao, Y.-H.; Chai, C.-Y.; Chen, G.-S.; Yu, H.-S. Effects and Interactions of Low Doses of Arsenic and UVB on Keratinocyte Apoptosis. Chem. Res. Toxicol. 2004, 17, 1199–1205. [Google Scholar] [CrossRef]
- Trabosh, V.A.; Daher, A.; Divito, K.A.; Amin, K.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S. UVB Upregulates the Bax Promoter in Immortalized Human Keratinocytes via ROS Induction of Id3. Exp. Dermatol. 2009, 18, 387–395. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kim, H.J.; Kim, D.S.; Kim, S.D.; Han, W.S.; Kim, K.H.; Chung, J.H.; Park, K.C. Up-Regulation and Redistribution of Bax in Ultraviolet B-Irradiated Melanocytes. Pigment. Cell Res. 2000, 13, 352–357. [Google Scholar] [CrossRef]
- Caricchio, R.; McPhie, L.; Cohen, P.L. Ultraviolet B Radiation-Induced Cell Death: Critical Role of Ultraviolet Dose in Inflammation and Lupus Autoantigen Redistribution 1. J. Immunol. 2003, 171, 5778–5786. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, D.; Liu, L.; Gao, B. Regulation of Bax Activation and Apoptotic Response to UV Irradiation by P53 Transcription-Dependent and -Independent Pathways. Cancer Lett. 2008, 271, 231–239. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular Biology of Bax and Bak Activation and Action. Biochim. Biophys. Acta. Mol. Cell Res. 2011, 1813, 521–531. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 Family Proteins and Caspases in the Regulation of Apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Shi, Y. Caspase Activation, Inhibition, and Reactivation: A Mechanistic View. Protein Sci. 2004, 13, 1979–1987. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Miret, S.; De Groene, E.M.; Klaffke, W. Comparison of In Vitro Assays of Cellular Toxicity in the Human Hepatic Cell Line HepG2. J. Biomol. Screen. 2006, 11, 184–193. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-Da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef]
- Daher, A.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S. Apoptosis Induced by Ultraviolet B in HPV-Immortalized Human Keratinocytes Requires Caspase-9 and Is Death Receptor Independent. Exp. Dermatol. 2006, 15, 23–34. [Google Scholar] [CrossRef]
- Dunkern, T.R.; Fritz, G.; Kaina, B. Ultraviolet Light-Induced DNA Damage Triggers Apoptosis in Nucleotide Excision Repair-Deficient Cells via Bcl-2 Decline and Caspase-3/-8 Activation. Oncogene 2001, 20, 6026–6038. [Google Scholar] [CrossRef]
- Park, Y.-K.; Jang, B.-C. UVB-Induced Anti-Survival and pro-Apoptotic Effects on HaCaT Human Keratinocytes via Caspase- and PKC-Dependent Downregulation of PKB, HIAP-1, Mcl-1, XIAP and ER Stress. Int. J. Mol. Med. 2014, 33, 695–702. [Google Scholar] [CrossRef]
- Xu, H.; Yan, Y.; Li, L.; Peng, S.; Qu, T.; Wang, B. Ultraviolet B-Induced Apoptosis of Human Skin Fibroblasts Involves Activation of Caspase-8 and -3 with Increased Expression of Vimentin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 198–204. [Google Scholar] [CrossRef]
- Takasawa, R.; Nakamura, H.; Mori, T.; Tanuma, S. Differential Apoptotic Pathways in Human Keratinocyte HaCaT Cells Exposed to UVB and UVC. Apoptosis 2005, 10, 1121–1130. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gag, O.; Macasoi, I.; Pinzaru, I.; Dinu, S.; Popovici, R.; Cosoroaba, M.-R.; Buzatu, R.; Cabuta, M.; Chiriac, S.D. In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells. Photonics 2023, 10, 464. https://doi.org/10.3390/photonics10040464
Gag O, Macasoi I, Pinzaru I, Dinu S, Popovici R, Cosoroaba M-R, Buzatu R, Cabuta M, Chiriac SD. In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells. Photonics. 2023; 10(4):464. https://doi.org/10.3390/photonics10040464
Chicago/Turabian StyleGag, Otilia, Ioana Macasoi, Iulia Pinzaru, Stefania Dinu, Ramona Popovici, Mioara-Raluca Cosoroaba, Roxana Buzatu, Madalina Cabuta, and Sorin Dan Chiriac. 2023. "In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells" Photonics 10, no. 4: 464. https://doi.org/10.3390/photonics10040464
APA StyleGag, O., Macasoi, I., Pinzaru, I., Dinu, S., Popovici, R., Cosoroaba, M.-R., Buzatu, R., Cabuta, M., & Chiriac, S. D. (2023). In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells. Photonics, 10(4), 464. https://doi.org/10.3390/photonics10040464






