Abstract
An unstable bilirubin level in the human blood causes many dangerous health problems, such as jaundice, coronary artery disease, ulcerative colitis, and brain lesions. Therefore, the accurate and early detection of bilirubin concentrations in the blood is mandatory. In this work, a highly sensitive biosensor based on photonic crystal fiber (PCF) for monitoring bilirubin levels is proposed and analyzed. The sensor parameters, including relative sensitivity, effective mode area, confinement loss, and effective material loss, are calculated. The geometrical parameters are studied, and a modal analysis of the suggested sensor is carried out using the full-vectorial finite element method (FEM). The fabrication tolerance of the geometrical parameters is also studied to ensure the fabrication feasibility of the reported design. High sensitivities of 95% and 98% are obtained for the x-polarized and y-polarized modes, respectively. Furthermore, a small material loss of 0.00193 cm−1, a small confinement loss of 2.03 × 10−14 dB/cm, and a large effective mode area of 0.046 mm2 are achieved for the y-polarized mode. It is believed that the presented sensor will be helpful in health care and in the early detection of bilirubin levels in the blood.
1. Introduction
The terahertz (THz) range covers the gap between the far-infrared and microwave spectrum, which spans from 0.1 to 10 THz. Furthermore, the molecular resonance of most chemical and biological materials lies in the THz region. Therefore, the THz regime has attracted the interest of researchers all over the world for different applications, including waveguiding [1] and sensing [2,3] devices. Photonic crystals have attracted attention because of their wide range of application, including logic gates [4,5] and sensors [6,7,8,9,10]. Photonic crystal fiber (PCF) [11] is a new kind of optical fiber that has the advantages of single-mode guidance [12], a large degree of design freedom, and a large effective mode area. Over the last decade, PCF in the THz and IR regions has been increasingly used for chemical, gas, and biomedical sensing [13,14]; communications [15,16]; and time-domain spectroscopy [17]. The geometrical parameters of PCF sensors can be controlled to achieve a sensitivity that is higher than that of traditional optical fiber [18]. In this context, Rahaman et al. [19] proposed a liquid sensor based on a hollow-core PCF with asymmetric rectangular air holes. The presented sensor had relative sensitivities of 91.42%, 92.55%, and 94.03% for water, acetic acid, and chloroform, respectively. Bulbul et al. [20] suggested a blood component sensor with suspended circular PCFs, with four air fragments. This sensor had relative sensitivities of 96.19% for RBC, 95.57% for WBC, 95.89% for HB, 95.01% for water, and 95.39% for plasma. Another terahertz PCF sensor for blood components with square holes was presented by Islam et al. [21], with sensitivities of 99.39%, 99.27%, 99.12%, 99.03%, and 98.79% for RBC, hemoglobin, WBC, plasma, and water, respectively. Moreover, Bulbul et al. [18] proposed a hollow-core PCF sensor for determining the kerosene ratio in petrol and diesel, with a sensitivity of 98.68–98.83% for the different kerosene–petrol mixture ratios, while a sensitivity of 98.83–98.90% was achieved for the different kerosene–diesel mixture ratios. Ramachandran et al. [22] also reported a chemical sensor based on dual steering-wheel-shaped PCFs with a large noncircular air hole. The sensor [22] showed a relative sensitivity of 94.9% for water, 95.4% for ethanol, and 95.6% for benzene. Furthermore, Jibon et al. [23] introduced a THz nicotine sensor using a square-shaped core PCF, with a cladding of circular air holes arranged in a square manner, and a relative sensitivity of 83%. Podder et al. [24] presented a THz PCF for camel milk and buffalo milk sensing, with a relative sensitivity of 94.69% for camel milk and a relative sensitivity of 94.75% for buffalo milk.
Bilirubin is a yellowish pigment found in bile, a fluid made by the liver. A high level of bilirubin in the blood (˃51.3 µmol/L) can cause jaundice [25], where the skin and the whites of the eyes appear yellow. This is an indicator of hemolytic anemia, low liver activity, and gallstones [26]. However, low levels (˂5.47 µmol/L) can cause coronary artery disease [27], ulcerative colitis [28], and mental disabilities [29]. It has been reported that middle- and low-income countries suffer more from serious hyperbilirubinemia and kernicterus compared to other countries in the world [30]. It has been found that kernicterus is the most common reason for growth delay in children in Egypt [31]. Additionally, neonatal jaundice has been found to be one of the main significant contributing factors to cerebral palsy in children [32]. Therefore, there is a high need for a susceptible sensor for the early detection of liver activity with a cost-effective, short-time response and a portable, compact size.
In this work, a highly sensitive THz PCF biosensor was proposed for detecting bilirubin levels in the blood. The effective index (neff), relative sensitivity (RS), effective mode area (Aeff), confinement loss (CL), effective material loss (EML), and confined power fractions (η) were studied and analyzed. The proposed sensor had a circular-sectored cladding and a rectangular-shaped core with high-resistivity silicon (HRS) as the background material. A modal analysis was carried out using the full-vectorial finite element method [33] with the COMSOL Multiphysics software package [34]. To the best of our knowledge, this is the first time that a biosensor has been designed to monitor the bilirubin concentrations in the blood in the THz region, achieving a high analyte refractive index (≈3.21) and absorption coefficient (≈280 cm−1) with very low losses. High sensitivities of 95% and 98% were obtained for the x-polarized and y-polarized modes, respectively. Furthermore, a small material loss of 0.00213 cm−1, a small confinement loss of 7.741 × 10−14 dB/cm, and a large effective mode area of 0.0483 were achieved. Furthermore, the suggested sensor can be fabricated using currently available fabrication techniques.
2. Design Considerations and Sensing Mechanism
Figure 1 shows a 2D cross-section and 3D view of the suggested sensor with a diameter of 1.959 mm. The cladding region had two symmetrical air fragments. The core region consisted of 9 × 5 identical rectangular holes with the length Lc and width Wc. A large central rectangle was used to house the blood sample with the length La and width Wa. The total length (L) and width (W) of the core were 780 and 650 µm, respectively. The core region was connected with the outer shell of the fiber using a column of width h that had rectangular air holes (to reduce the material loss) with a length of 50 µm and width of 20 µm. Recently, due to the outstanding properties of the high-resistivity silicon (HRS) in the THz regime, it has been widely used as a background material in photonic crystal fibers [35]. The HRS material has an average high refractive index of 3.417 and an average absorption coefficient of 0.015 cm−1 at a frequency range from 0.5 to 1.1 THz [36]. The refractive index and absorption coefficient of the blood as a function of bilirubin concentrations from 4 to 12 µmol/L are given by the experimental measurements in [37], where the glucose concentration values were variated from 5.0 to 5.5 mmol/L to keep the blood samples at constant conditions. In these measurements, the refractive index and the absorption coefficient of the blood samples decreased as the bilirubin concentration increased. Furthermore, the human bilirubin contains 96% as nonpolar insoluble bilirubin that is attached to albumin. The remaining 4% are combined with glucuronic acid. Therefore, an increase in the bilirubin concentration accompanies an increase in the concentration of albumin, and this decreases the blood absorption coefficient. Thus, the refractive index of blood also decreases with the increase in the bilirubin concentration [37]. Moreover, THz spectroscopy determines the concentration of polar water molecules that are combined with bilirubin in the blood, which examines only the effect of the bilirubin level on the refractive index and absorption coefficient. Moreover, it has been reported that the other blood outlier factors (hemoglobin, white blood cells, and red blood cells) have no effect on the THz wave absorption and the refractive index of the whole blood at a glucose concentration range of 105.8–136.9 mg/dL (corresponding to 5.87–7.6 mmol/L) and at a frequency near 0.5 THz [38]. The glucose concentration in our study was in the same range reported in [38]. Therefore, the main factor affecting the refractive index and absorption coefficient of the whole blood is the bilirubin only.
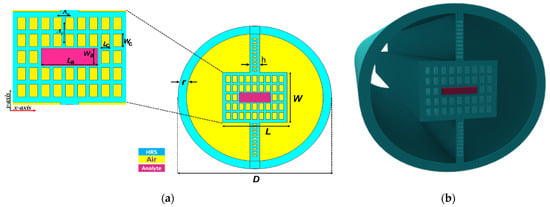
Figure 1.
(a) 2D cross-sectional diagram; (b) 3D view of the suggested biosensor.
Compared to previously reported THz slot PCFs, it is believed that the fabrication of the proposed structure is possible through the conventional stack-and-draw technique [39]. It is also possible to fabricate the rectangular air hole using the extrusion technique [40]. The sample can be infiltrated within the central rectangle using the selective infiltration technique [41]. The rectangular core air holes had an initial length of Lc = 75 µm and initial width Wc = 45 µm, and they were placed in a square lattice with a lattice constant Λx = 80 µm in the x-direction and Λy = 120 µm in the y-direction. The diameter (D) of the cladding air fragment was equal to 1856 µm. Furthermore, the length (La) and the width (Wa) of the central rectangle were equal to 365 and 75 µm, respectively. In addition, the structure width (h) of the proposed PCF was taken as 120 μm, and the thickness of the fiber’s wall (r) was taken as 103 µm. Table 1 shows the value of the geometrical parameter for the initial biosensor design. These initial parameters were chosen for the fabrication feasibility of the proposed design.

Table 1.
The initial geometrical parameters of the proposed biosensor design.
Although the proposed work was based on simulations, a schematic diagram for the sensing setup is presented in Figure 2. An ultrafast femtosecond laser source was used to produce the laser beam, which was incident onto a beam splitter. Then, the two beams, called probe and pump beams, were generated [24].
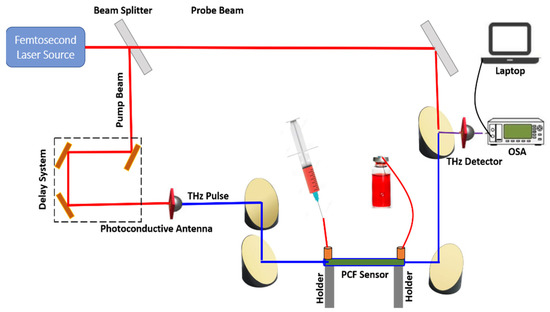
Figure 2.
Schematic diagram of the experimental setup of the proposed sensor for practical sensing application.
Secondly, the pump beam was incident on a photoconductive antenna (THz emitter) to emit the THz pulse. A system of parabolic mirrors was used to align and synchronize the THz beam onto the PCF core region, where two irises were used to fix the sensor. The blood samples with different bilirubin concentrations could be selectively infiltrated inside the core region using a hypodermic syringe based on the capillary action. The syringe was used to infiltrate the analyte into the core through the face opposite to the blocked end. The solution could be slowly fed from the hypodermic syringe to ensure that the analyte sample did not enter the fiber cladding [42]. The syringe did not affect the core’s refractive index, because it was removed immediately after the filling process. Injection of the UV-cured polymer was used to block the cladding holes and keep the central core hole open [43]. In addition, femtosecond laser micromachining could be used to block all the air holes using a section of conventional single mode fiber. Then, femtosecond laser direct drilling was used to open the selected holes through the blocked end [43]. In addition, the cladding holes could be blocked by selective sealing with molten uncured polymer. Then, analyte was infiltrated into the unsealed holes via capillary effects [44]. The transmitted THz beam could be realigned and refocused onto the photoconductive THz detector. The probe beam met the detector to measure the incident electric field with amplitude attenuation and time delay based on the THz pulse absorption of bilirubin. A delay unit was used to offset the pump and probe pulses and helped in the THz temporal analysis. A Fourier transform was then used to convert the time-domain spectroscopy into a frequency domain spectrum. The blood refractive index variation modulated the intensity of the transmitted optical power. The intensity modulation could be analyzed by the optical spectrum analyzer (OSA), and the output could be investigated using the computer to obtain the performance parameters, such as sensitivity and CL. The computer represented the data both numerically and graphically. The cutback method and external lenses were used to eliminate the coupling loss that resulted from the lateral misalignment of the system [45]. Currently, modern technologies overcome the THz gap in the sources, detectors, and coupling devices by developing an efficient device [46].
3. Numerical Results and Discussion
The modal characteristics of the supported x- and y-polarized modes were studied using the full vectorial finite element method (FEM) [47,48]. A numerical analysis was performed using COMSOL Multiphysics software, with a minimum element size of 0.1 µm, a maximum triangular element size of 20 µm, and an element growth rate of 1.1. Furthermore, the curvature factor was fixed at 0.2. Hence, the number of degrees of freedom was 241,055, and the obtained error from the convergence study was of the order 10−21. Moreover, a circular scattering boundary condition was used to absorb the outgoing waves. The fundamental mode electric field distributions for the two polarized modes using the initial parameters are shown in Figure 3, at 0.75 THz. It was revealed that the field intensity was maximum at the central region, with good confinement within the analyte core region for both polarization modes. Hence, a good light-analyte interaction could be achieved. It was also evident from Figure 3 that the confinement through the core region decreased by increasing the bilirubin concentrations. This was due to the refractive index of the blood decreasing as the bilirubin concentration increased. Therefore, the contrast in the refractive index between the cladding and core regions decreased. Subsequently, the mode field tried to dissipate towards the cladding.
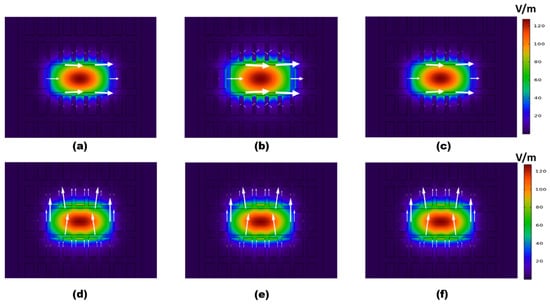
Figure 3.
The electric field distribution of the fundamental mode for the (a–c) x-polarized mode and the (d–f) y-polarized mode at f = 0.75 THz, C = 4, 8, and 12 µmol/L, respectively. The initial dimensions shown in Table 1 are used.
Figure 4 shows the variation of the effective index with the frequency for both the x- and y-polarized modes at different bilirubin concentrations. It may be seen that the effective index increased as the operating frequency increased. This was due to the good confinement of the mode through the high index core at large frequencies. Moreover, as the concentration of bilirubin increased, the refractive index of the analyte sample decreased. Therefore, the effective index of the supported modes decreased by increasing the bilirubin concentration [49]. The difference in the effective index between the x- and y-polarized modes was due to the asymmetrical structure of the core rectangular air hole [50,51].
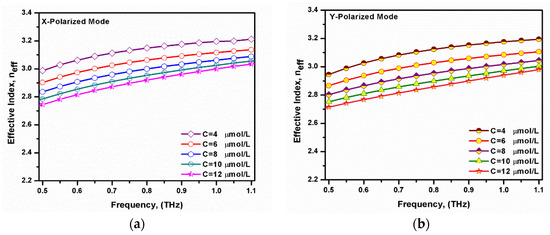
Figure 4.
Variation of the frequency-dependent effective index of the fundamental (a) x-polarized mode and (b) y-polarized mode at different bilirubin concentrations.
The effective material loss (EML) of the PCF was a limiting factor for the sensor’s performance. This loss mechanism occurred due to the absorption of the fiber background materials. Therefore, rectangular air holes were used to reduce the material loss. The EML can be calculated as [52,53,54,55,56,57]:
where EML is measured in cm−1, εo is the relative permittivity, and µo is the relative permeability of the free space. Moreover, nHRS and αHRS are the refractive index and absorption coefficient of the HRS material, respectively, while Sz denotes the z-component of the Poynting vector. The variations in the EML with the frequency for the two polarized modes at different bilirubin concentrations are shown in Figure 5. As the concentration of the bilirubin increased, the refractive index of the analyte sample decreased. The refractive index range of the infiltrated analyte within the core region was 2.666–3.213, corresponding to the analyte concentration of 12–4 µmol/L. However, the refractive index of the cladding material was equal to 3.417. It should be noted that the air hole slots in the background material decreased the effective refractive index of the cladding region. Furthermore, the index difference between the core region and the cladding region decreased by increasing the analyte’s concentration and, hence, the confinement of the supported modes will decrease. This will increase the interaction of the x- and y-polarized modes with the HRS material with increased EML [58], as shown in Figure 5. The proposed sensor had low EML values of 0.0069 cm−1 and 0.0065 cm−1 for the x- and y-polarized modes, respectively, at f = 0.75 THz. The obtained EML values were lower than those reported in [23,24].
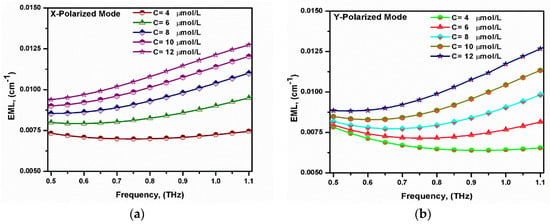
Figure 5.
The variation of the EML with the frequency for the fundamental (a) x-polarized mode and (b) y-polarized mode at different bilirubin concentrations.
The analyte sensing area of the PCF sensor is provided by calculating the effective mode area (Aeff) of the fundamental mode through the core region, as given in [18]:
where E denotes the transverse electric field intensity. Figure 6 shows the variation in the effective mode area with the frequency at different bilirubin concentrations. It may be seen that the effective mode area decreased with the increasing operating frequency. This was due to the electric field being more confined through the analyte by increasing the frequency [59,60]. It was also noted that the effective mode area increased with a decreasing refractive index of the analyte (i.e., increasing the bilirubin concentration). For lower indexed analyte, the light confinement through the core region will be reduced [61]. The obtained effective mode areas were 0.0526 mm2 and 0.0551 mm2 for the x-polarized mode and y-polarized mode, respectively, at f = 0.75 THz.
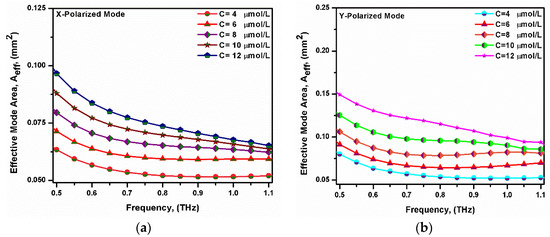
Figure 6.
Variation in the effective mode area with the frequency for the fundamental (a) x-polarized mode and (b) y-polarized mode at different bilirubin concentrations.
The confinement loss (CL) is another loss property that affects the sensor’s performance. It can be calculated using the imaginary part of the effective index as given by [62]:
where f, c, and neff are the operating frequency, speed of light in a vacuum, and the effective index of the studied mode, respectively. Figure 7 shows the variation in the confinement loss of the suggested sensor with the operating frequency. It was noted that the confinement loss decreased with the increasing frequency. Moreover, the confinement loss decreased by decreasing the bilirubin concentrations. This was owing to the well confinement through the core region at higher frequencies. As the bilirubin concentration increases, the analyte refractive index will decrease. Therefore, a low index contrast between the core and cladding region occurs with reduced light confinement in the core region. The proposed sensor had very low confinement loss values of 2.05 × 10−14 dB/cm and 8.204 × 10−15 dB/cm for the x- and y-polarized modes, respectively, at f = 0.75 THz.
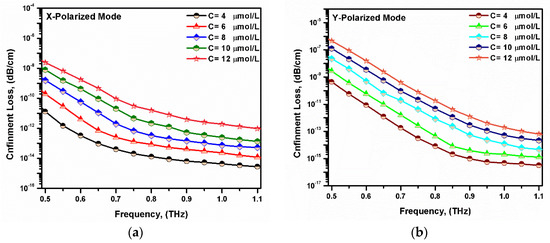
Figure 7.
Variation in the confinement loss with the frequency for the fundamental (a) x-polarized mode and (b) y-polarized mode at different bilirubin concentrations.
The core power fraction (η) is a very important property and represents the percentage of the propagated light power inside the core region to the total power through the PCF. It can be calculated by the following equation [63]:
where η is the power fraction, and Sz is the z-component of the Poynting vector. Figure 8 shows the variation in the analyte power fraction versus the frequency. It was noted that the power fraction in the analyte region increased with the increase in the operating frequency to 0.6 THz and then started to decrease. The reason behind that is the light with a higher frequency tries to propagate through the higher refractive indexed material. Therefore, some light leaks through the background material [52,64,65]. The power fraction also increased as the bilirubin concentration decreased, which increased the analyte refractive index [61]. This will increase the index contrast seen by the two polarized modes with better confinement in the core region. The maximum power fraction within the analyte region of the x- and y-polarized modes were 54.82% and 59.74%, respectively, at f = 0.75 THz.
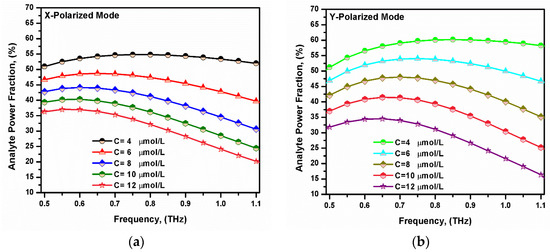
Figure 8.
The variation in the power fraction with the frequency for the fundamental (a) x-polarized mode and (b) y-polarized mode at different bilirubin concentrations.
The relative sensitivity is used to realize the sensing performance of the PCF sensor and can be calculated by the following [66]:
where nr is the analyte refractive index, neff is the effective mode index, and η is the analyte power fraction given by Equation (4). Figure 9 shows the variation in the relative sensitivity with the operating frequency for both the x- and y-polarized modes. It was revealed that the relative sensitivity increased with the increase in the operating frequency to 0.6 THz and then started to decrease. This can be attributed to the behavior of the core power fraction. Furthermore, the relative sensitivity of the y-polarized mode was higher than that of the x-polarized mode. Since the core had a greater geometrical dimension in the y-direction, hence, it provided a larger core power fraction [61]. The maximum sensitivity obtained for the proposed sensor was approximately 56.29% and 61.88% for the x- and y-polarized modes, respectively, at f = 0.75 THz.
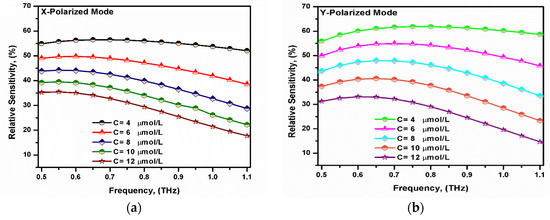
Figure 9.
Variation in the relative sensitivity with the frequency for the fundamental (a) x-polarized mode and (b) y-polarized mode at different bilirubin concentrations.
In order to maximize the sensitivity of the proposed sensor, the optimization of the different geometrical parameters was performed. The variation in the relative sensitivity of both polarized modes with the core length (La) is first presented in Figure 10a, while the other parameters were kept constant at their initial values. However, the variation in the relative sensitivity of both polarized modes with the core width (Wa) is shown in Figure 10b at f = 0.75 THz and C = 4 µmol/L. It is revealed from Figure 10 that the relative sensitivities of the two polarized modes increased by increasing the studied parameters. This was due to the increase in the analyte area, which increased the core power fraction [60,66]. It is also worth noting that the geometrical parameters were chosen for the ease of fabrication. Therefore, La = 377 µm and Wa =124 µm were chosen for the suggested design. It is also evident that the relative sensitivity of the y-polarized mode was better than that of the x-polarized mode. This was due to the core power fraction of the y-polarized mode being greater than that of the x-polarized mode [58,61].
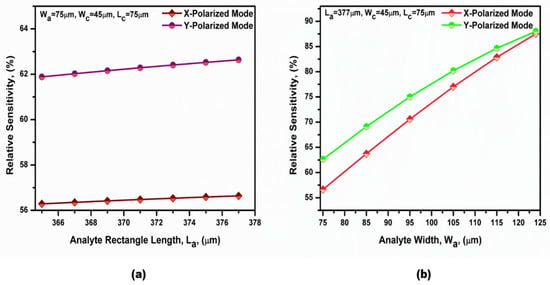
Figure 10.
Dependence of the relative sensitivity on (a) the analyte rectangle length (La) and (b) the analyte rectangle width (Wa) at a bilirubin concentration equal to 4 µmol/L and f = 0.75 THz.
The variation in the relative sensitivity of both polarized modes with the cladding rectangular hole width (Wc) is presented in Figure 11a, at Lc = 75 µm. Furthermore, the variation in the relative sensitivity of both polarized modes with the cladding rectangular hole length (Lc) is shown in Figure 11b, at Wc = 55 µm. As the studied parameters increased, the air cladding regions increased, which increased the index contrast between the core and cladding regions. Therefore, the filed confinement of the two polarized modes in the core region increased. Hence, the relative sensitivity was increased by increasing the studied parameters, as shown in Figure 12. In the next study, Wc = 55 µm and Lc = 90 µm were taken for the subsequent simulations. A maximum relative sensitivity of 95% and 98% were obtained for the x-polarized and y-polarized modes at the optimized geometrical parameters listed in Table 2. It should be noted that during the fabrication process, the minimum distance between the holes must not be smaller than 4 µm [53,67] due to the possible fluctuations of the geometrical parameters during the fabrication process.
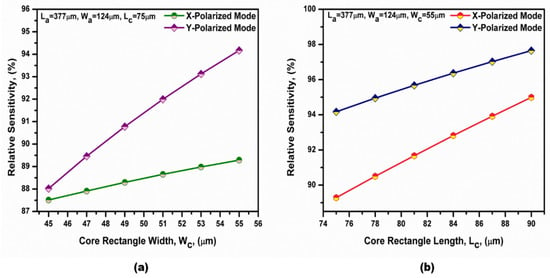
Figure 11.
Dependence of the relative sensitivity on (a) the cladding rectangular hole width (Wc) and (b) the cladding rectangular hole length (Lc) at a bilirubin concentration equal to 4 µmol/L and f = 0.75 THz.
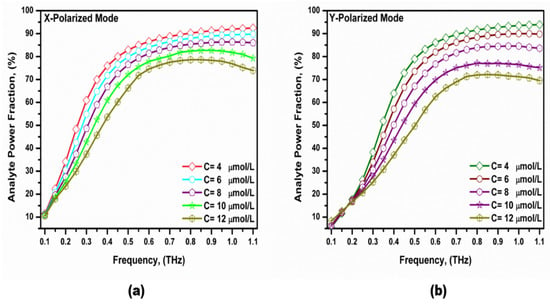
Figure 12.
The variation in the analyte power fraction with the frequency for the fundamental (a) x-polarized mode and (b) y-polarized mode at different bilirubin concentrations for the optimized design.

Table 2.
The geometrical parameter values for the optimized biosensor.
Table 3 presents the fabrication tolerance effect on the different PCF sensor parameters to consider the fabrication faults. The effect of the analyte rectangle length (La) and width (Wa) and cladding rectangular air hole length (Lc) and width (Wc) were studied with a tolerance of and , relative to their optimized values at f = 0.75 THz for the y-polarized mode. It was revealed that a minimum relative sensitivity of 96.15% and a maximum value of the relative sensitivity of 98.76% was obtained within a tolerance of −4% in the width and tolerance of +4% in the length of the analyte rectangle, which ensures the feasibility of the proposed design.

Table 3.
Fabrication tolerance of the length and width of the analyte rectangle and the cladding rectangular holes of the proposed terahertz biosensor.
Table 4 shows a comparison between the proposed design and some of those recently reported in the literature. Our sensor shows a higher sensitivity than that presented in [19,20,22,23,24,51,53,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] and is comparable with that reported in [18]. Furthermore, the suggested sensor showed lower confinement loss than that suggested in [19,63,68,69,70,72], and it is comparable with that proposed in [18,20,53]. In addition, the introduced sensor had a lower EML than all of those reported in Table 4. Moreover, the suggested sensor was compared with the corresponding silica-based PCF in the wavelength range of 0.8 to 2 µm [83,84,85,86,87]. It was revealed that the silica-based PCF sensors had low sensitivity and high confinement loss compared to the proposed sensor. Furthermore, it may be seen that the proposed sensor achieved very high sensitivity compared with silica-based PCF in the THz region, as reported in [64]. In addition, in THz region, the sensor can easily be fabricated compared to those in the optical regime. Additionally, the molecular resonances occurred in the terahertz range, making it an ideal candidate for chemical and biological sensing. Moreover, the PCF sensors worked outstandingly well, especially in the THz region, with a higher sensitivity and lower confinement loss than conventional PCF at an optical wavelength of 1.55 µm, as shown in Table 4. Considering all of the guiding and efficiency parameters, our sensor showed a remarkable performance in bilirubin sensing. Furthermore, there are different methods that have been developed for the measurement of bilirubin concentrations in medical samples. Direct spectroscopic measurement is widely used as a fast and simple method to detect bilirubin concentrations [88], but it has some drawbacks, such as the result being pH dependent [89], altered by other hemeproteins, and having low sensitivity [90]. A fluorometric biosensor is another detection method that is based on measuring the fluorescence of bilirubin [91]. It is observed that the bilirubin is very weakly fluorescent. Therefore, a special environment is needed to detect the bilirubin and enhance its emission, such as the presence of albumin bounded with bilirubin to enhance its fluorescence [92]. Highly emissive metallic nanoparticles can also be used due to the fact of their emissions being dependent on bilirubin concentrations [91]. In addition, an electrochemical biosensor can measure the oxidation potential of bilirubin into biliverdin. The demerits of the electrochemical sensor are that oxidation reaction relies on the pH of the environment; the measurement could interfere with other biological substances, such as glucose, ascorbic acid, uric acid, and glutathione [93]; and it has a very long response time. Moreover, an optical fiber-based sensor has been developed for measuring the absorption of the bilirubin in a cuvette [94]; this method needs flat reflecting surfaces [92].

Table 4.
Comparison between the guiding parameters of the suggested biosensor with the sensors in the literature. NA: Not Available.
Currently, there are great developments in the field of optical sensors. Some of these sensors are based on the edge topological defect state in a 1D photonic crystal [95]. However, these sensors are not easy to fabricate due to the lattice mismatch at the interface between the two photonic crystal layers [96] and due to the fact of their very small dimensions compared to our design. Additionally, new optical sensors have been implemented based on the excitation of high wave vector modes in metamaterials [97]. However, metamaterial sensors suffer from a complicated fabrication process with expensive equipment where prism or grating coupling configurations are needed [98].
4. Conclusions
In this paper, a highly sensitive THz-PCF biosensor was numerically analyzed for measuring bilirubin concentrations in the blood. The HRS material was used to construct the suggested PCF sensor due to the fact of its index contrast relative to the bilirubin with a low absorption coefficient in the THz region. The numerical simulation results showed a high relative sensitivity of 95% and 98% for the x-polarized and y-polarized modes, respectively, at f = 0.75 THz. Moreover, the suggested THz-PCF donated a low confinement loss of 2.64 × 10−15 dB/cm for the x-polarized mode and 2.03 × 10−14 dB/cm for the y-polarized mode at f = 0.75 THz. Furthermore, a low EML of 0.00131 cm−1 was achieved for the x-polarized mode and 0.00193 cm−1 for the y-polarized mode. This sensor was aimed at helping in the effectively earlier detection of diseases that are based on the disruption of bilirubin concentrations in the blood; hence, it will help in health care.
Author Contributions
Conceptualization, M.F.O.H. and S.S.A.O.; methodology, M.F.O.H. and M.S.S.I.; software, M.F.O.H., A.R.E., and M.S.S.I.; validation, A.R.E. and M.S.S.I.; data curation, M.F.O.H., A.R.E., and M.S.S.I.; writing—original draft preparation, A.R.E. and M.S.S.I.; writing—review and editing, M.F.O.H. and A.R.E.; visualization, M.F.O.H. and S.S.A.O.; supervision, M.F.O.H., S.S.A.O., A.N.Z.R., and A.E.-N.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsuruda, K.; Fujita, M.; Nagatsuma, T. Extremely low-loss terahertz waveguide based on photonic crystal slab. Opt. Express 2015, 23, 31977–31990. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Anower, S.; Islam, N. Terahertz sensing based on photonic crystal fibers. In Terahertz Technology; You, B., Lu, J.U., Eds.; Intech Open: London, UK, 2021. [Google Scholar] [CrossRef]
- Saadeldin, A.S.; Hameed, M.F.O.; Elkaramany, E.M.A.; Obayya, S.S.A. Highly Sensitive Terahertz Metamate-rial Sensor. IEEE Sens. J. 2019, 19, 7993–7999. [Google Scholar] [CrossRef]
- Areed, N.F.F.; Fakharany, A.E.; Hameed, M.F.O.; Obayya, S.S.A. Controlled optical photonic crystal AND gate using nematic liquid crystal layers. Opt Quant Electron. 2017, 49, 45. [Google Scholar] [CrossRef]
- Ibrahim, M.S.S.; El-Okr, M.M.; Hamed, M.K.G.; Obayya, S.S.A.; Hameed, M.F.O. Ultracompact tunable bifunctional XOR and XNOR photonic crystal logic gates. Opt. Eng. 2020, 59, 027106. [Google Scholar] [CrossRef]
- Parandin, F.; Heidari, F.; Rahimi, Z.; Olyaee, S. Two-Dimensional photonic crystal Biosensors: A review. Opt. Laser Technology. 2021, 144, 107397. [Google Scholar] [CrossRef]
- Parandin, F.; Heidari, F.; Aslinezhad, M.; Parandin, M.M.; Roshani, S.; Roshani, S. Design of 2D photonic crystal biosensor to detect blood components. Opt Quant. Electron. 2022, 54, 618. [Google Scholar] [CrossRef]
- Olyaee, S.; Najafgholinezhad, S. A High Quality Factor and Wide Measurement Range Biosensor Based on Photonic Crystal Nanocavity Resonator. Sens. Lett. 2013, 11, 483–488. [Google Scholar] [CrossRef]
- Ibrahim, M.S.S.; Hamed, M.K.G.; El-Okr, M.M.; Obayya, S.S.A.; Hameed, M.F.O. Highly sensitive photonic crystal gamma ray dosimeter. Opt. Quantum Electron. 2021, 53, 348. [Google Scholar] [CrossRef]
- Ibrahim, M.S.S.; Tarek, M.; Obayya, S.S.A.; Hameed, M.F.O. Highly Sensitive 1D Photonic Crystal Biosensor. In Proceedings of the 2021 International Applied Computational Electromagnetics Society Symposium (ACES), Hamilton, ON, Canada, 1–5 August 2021. [Google Scholar]
- Azzam, S.I.; Hameed, M.F.O.; Shehata, R.E.A.; Heikal, A.M.; Obayya, S.S.A. Multichannel photonic crystal fiber surface plasmon resonance-based sensor. Opt. Quantum Electron. 2016, 48, 142. [Google Scholar] [CrossRef]
- Fundamentals and Applications of Nanophotonics, 1st ed.; Woodhead Publishing: Sawston, UK, 2016. [CrossRef]
- Hossain, M.B.; Podder, E. Design and investigation of PCF-based blood components sensor in terahertz regime. Appl. Phys. A 2019, 125, 861. [Google Scholar] [CrossRef]
- Rabeea, A.S.H.; Hameed, M.F.O.; Heikal, A.M.; Obayya, S.S.A. Highly sensitive photonic crystal fiber gas sen-sor. Optik 2019, 188, 78–86. [Google Scholar] [CrossRef]
- Azab, M.Y.; Hameed, M.F.O.; El-Hefnawy, S.M.; Obayya, S.S.A. Ultra-compact liquid crystal dual core photonic crystal fibre multiplexer- demultiplexer. IET Optoelectron. 2015, 10, 21–27. [Google Scholar] [CrossRef]
- Hameed, M.F.O.; Heikal, A.M.; Younis, B.M.; Abdelrazzak, M.; Obayya, S.S.A. Ultra-high tunable liquid crystal-plasmonic photonic crystal fiber polarization filter. Opt. Express 2015, 23, 7007–7020. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yu, L.; Jiaoqi, H.; Guorong, H.; Yang, Z.; Weiling, F. Application of terahertz spectroscopy in biomolecule detection. Front. Lab. Med. 2018, 2, 127–133. [Google Scholar] [CrossRef]
- Bulbul, A.A.M.; Rashed, A.N.Z.; El-Hageen, H.M.; Alatwi, A.M. Design and numerical analysis of an extremely sensitive PCF-based sensor for detecting kerosene adulteration in petrol and diesel. Alex. Eng. J. 2021, 60, 5419–5430. [Google Scholar] [CrossRef]
- Ekhlasur Rahaman, M.; Bellal Hossain, M.; Shekhar Mondal, H.; Saha, R.; Mahbub Hossain, M.; Shamim Ahsan, M. Highly sensitive photonic crystal fiber liquid sensor in terahertz frequency range. Mater. Today Proc. 2020, 43, 3815–3820. [Google Scholar] [CrossRef]
- Bulbul, A.A.M.; Jibon, R.H.; Biswas, S.; Pasha, S.T.; Sayeed, M.A. Photonic crystal fiber-based blood components detection in THz regime: Design and simulation. Sens. Int. 2021, 2, 100081. [Google Scholar] [CrossRef]
- Islam, M.R.; Iftekher, A.N.M.; Mou, F.A.; Rahman, M.M.; Bhuiyan, M.I.H. Design of a Topas-based ultrahigh-sensitive PCF biosensor for blood component detection. Appl. Phys. A 2021, 127, 109. [Google Scholar] [CrossRef]
- Ramachandran, A.; Babu, P.R.; Senthilnathan, K. Design of a terahertz chemical sensor using a dual steering-wheel microstructured photonic crystal fiber. Photonics Nanostruct. Fundam. Appl. 2021, 46, 100952. [Google Scholar] [CrossRef]
- Jibon, R.H.; Ahmed, M.; Rahaman, M.E.; Hasan, M.K.; Shaikh, M.M.; Tooshil, A. Nicotine Sensing by Photonic Crystal Fiber in THz Regime. In Proceedings of the International Conference on Robotics, Electrical and Signal Processing Techniques, Dhaka, Bangladesh, 5–7 January 2021. [Google Scholar] [CrossRef]
- Podder, E.; Hossain, M.B.; Ahmed, K. Photonic crystal fiber for milk sensing. Sens. Bio.-Sens. Res. 2022, 38, 100534. [Google Scholar] [CrossRef]
- Porter, M.L.; Dennis, B.L. Hyperbilirubinemia in the term newborn. Am. Fam. Phys. 2022, 65, 599–606. [Google Scholar]
- Rocher, S.P.; Kobos, R. Jaundice in the Adult Patient. Am. Fam. Phys. 2004, 69, 299–304. [Google Scholar]
- Suh, S.; Cho, Y.R.; Park, M.K.; Kim, D.K.; Cho, N.H.; Lee, M.K. Relationship between serum bilirubin levels and cardiovascular disease. PLoS ONE 2018, 13, e0193041. [Google Scholar] [CrossRef] [PubMed]
- Schieffer, K.M.; Bruffy, S.M.; Rauscher, R.; Koltun, W.A.; Yochum, G.S.; Gallagher, C.J. Reduced total serum bilirubin levels are associated with ulcerative colitis. PLoS ONE 2017, 12, e0179267. [Google Scholar] [CrossRef] [PubMed]
- Wusthoff, C.J.; Leo, I.M. Impact of bilirubin-induced neurologic dysfunction on neurodevelopmental outcomes. Semin. Fetal Neonatal Med. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Arnolda, G.; Boo, N.Y.; Iskander, I.F.; Okolo, A.A.; Rohsiswatmo, R.; Coda Zabetta, C.D. Neonatal Jaundice in Low- and Middle-Income Countries: Lessons and Future Directions from the 2015 Don Ostrow Trieste Yellow Retreat. Neonatology 2016, 110, 172–180. [Google Scholar] [CrossRef]
- Mostafa, M.A.; Kamal, N.M.; Eltaher, S.; Hamed, Y.; Abdelaziz, H.; Abdelghany, W.; Sherief, L.M. Knowledge of Neonatal Hyperbilirubinemia Among Primary Health Care Physicians: A Single-Center Experience. Clin. Med. Insights Pediatr. 2019, 13, 1179556518824375. [Google Scholar] [CrossRef]
- El Meliegy, E.H.K.; El Sabbagh, M.H. Etiology of developmental delay in Egyptian children. Int. J. Child Neuropsychiatr. 2004, 1, 2919–2940. [Google Scholar]
- Obayya, S.S.A. Efficient finite-element-based time-domain beam propagation analysis of optical integrated circuits. IEEE J. Quantum Electron 2004, 40, 591–595. [Google Scholar] [CrossRef]
- COMSOL Multiphysics 6.1; COMSOL Inc.: Stockholm, Sweden, 2022; Available online: https://www.comsol.com (accessed on 1 January 2021).
- Yang, T.; Ding, C.; Ziolkowski, R.W.; Guo, Y.J. A terahertz (THz) single-polarization-single-mode (SPSM) photonic crystal fiber (PCF). Materials 2019, 12, 2442. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, J.; Zhang, W.; Grischkowsky, D. Terahertz time-domain spectroscopy characterization of the far-infrared absorption and index of refraction of high-resistivity, float-zone silicon. J. Opt. Soc. Am. B 2004, 21, 1379. [Google Scholar] [CrossRef]
- Zhang, T.; Demchenko, P.; Khodzitsky, M.K.; Kononova, Y.; Babenko, A.; Grineva, E. The Influence of Bilirubin and Creatinine on the Refractive Index of Whole Blood in Terahertz Frequency Range: A Qualitative Analysis. In Proceedings of the Optics in Health Care and Biomedical Optics VIII, Beijing, China, 23 October 2018. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Ma, S.; Wu, X.; Yang, W.; Zhang, W.; Li, X. Quantify Glucose Level in Freshly Diabetic’s Blood by Terahertz Time-Domain Spectroscopy. J. Infrared Milli Terahz Waves 2018, 39, 399–408. [Google Scholar] [CrossRef]
- Bao, H.; Nielsen, K.; Rasmussen, H.K.; Jepsen, P.U.; Bang, O. Fabrication and characterization of porous-core honeycomb bandgap THz fibers. Opt. Express 2012, 20, 29507–29517. [Google Scholar] [CrossRef]
- Katyba, G.M.; Zaytsev, K.I.; Chernomyrdin, N.V.; Shikunova, I.A.; Komandin, G.A.; Anzin, V.B.; Lebedev, S.P.; Spektor, I.E.; Karasik, V.E.; Yurchenko, S.O.; et al. Sapphire Photonic Crystal Waveguides for Terahertz Sensing in Aggressive Environments. Adv. Opt. Mater. 2018, 6, 1800573. [Google Scholar] [CrossRef]
- Cordeiro, C.M.B.; Santos, E.M.D.; Cruz, C.H.B.; Matos, C.G.D.; Daniel, S.; Ferreira, D.S. Lateral access to the holes of photonic crystal fibers–selective filling and sensing applications. Opt. Express 2006, 14, 8403–8412. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Al Naser, A.M.; Jaba, F.Z.; Anzum, F.; Iftekher, A.N.M.; Khan, M.R.H.; Nishat, M.M. Design of a hexagonal outlined porous cladding with vacant core photonic crystal fibre biosensor for cyanide detection at THz regime. IET Optoelectron. 2022, 13, 160–173. [Google Scholar] [CrossRef]
- Huang, Y.; Xua, Y.; Yariv, A. Fabrication of functional microstructured optical fibers through a selective-filling technique. Appl. Phys. Lett. 2004, 85, 5182–5184. [Google Scholar] [CrossRef]
- Kuo, S.M.; Huang, Y.W.; Yeh, S.M.; Cheng, W.H.; Lin, C.H. Liquid Crystal Modified Photonic Crystal Fiber (LC-PCF) Fabricated with an SU-8 Photoresist Sealing Technique for Electrical Flux Measurement. In Proceedings of the 2010 IEEE 23rd International Conference on Micro Electro Mechanical Systems (MEMS), Hong Kong, China, 24–28 January 2010. [Google Scholar] [CrossRef]
- Bowden, B.; Harrington, J.A.; Mitrofanov, O. Silver/Polystyrene-coated hollow glass waveguides for the transmission of terahertz radiation. Opt. Lett. 2007, 32, 2945–2947. [Google Scholar] [CrossRef]
- Salamin, Y.; Chelmus, I.C.; Fedoryshyn, Y.; Heni, W.; Elder, D.L.; Dalton, L.R.; Faist, J.; Leuthold, J. Compact and ultra-efficient broadband plasmonic terahertz field detector. Nat. Commun. 2009, 10, 5550. [Google Scholar] [CrossRef]
- Rahman, B.M.A.; Agrawal, A. Finite Element Modeling Methods for Photonics, 1st ed.; Artech House: Boston, MA, USA, 2013. [Google Scholar]
- Obayya, S.S.A.; Azizur Rahman, B.M.; El-Mikati, H.A. New Full-Vectorial Numerically Efficient Propagation Algorithm Based on the Finite Element Method. J. Light. Technol. 2000, 18, 409–415. [Google Scholar] [CrossRef]
- Islam, A.; Rakibul, M.; Ahmed, I.; Al, M.; Fariha, N.; Fatema, A.; Jaba, Z. Square structured photonic crystal fiber based THz sensor design for human body protein detection. J. Comput Electron. 2020, 20, 377–386. [Google Scholar] [CrossRef]
- Sultana, J.; Islam, S.; Faisal, M.; Rakibul, M.; Ng, B.W.; Ebendorff-heidepriem, H.; Abbott, D. Highly birefringent elliptical core photonic crystal fiber for terahertz application. Opt. Commun. 2018, 407, 92–96. [Google Scholar] [CrossRef]
- Mou, F.A.; Rahman, M.M.; Islam, M.R.; Bhuiyan, M.I.H. Development of a photonic crystal fiber for THz wave guidance and environmental pollutants detection. Sens. Bio-Sens. Res. 2020, 29, 100346. [Google Scholar] [CrossRef]
- Habib, A.; Anower, S.; Abdulrazak, L.F.; Reza, S. Hollow core photonic crystal fiber for chemical identification in terahertz regime. Opt. Fiber Technol. 2019, 52, 101933. [Google Scholar] [CrossRef]
- Tahhan, S.R.; Aljobouri, H.K. Sensing of Illegal Drugs by Using Photonic Crystal Fiber in Terahertz Regime. J. Opt. Commun. 2020. [Google Scholar] [CrossRef]
- Hasanuzzaman, G.K.M.; Rana, S.; Habib, S. A Novel Low Loss, Highly Birefringent Photonic Crystal Fiber in THz Regime. IEEE Photonics Technol. Lett. 2016, 28, 899–902. [Google Scholar] [CrossRef]
- Liang, J.; Ren, L.; Chen, N.; Zhou, C. Broadband, low-loss, dispersion flattened porous-core photonic bandgap fiber for terahertz (THz)-wave propagation. Opt. Commun. 2013, 295, 257–261. [Google Scholar] [CrossRef]
- Habib, A.; Anower, S.; Haque, I. Highly sensitive hollow core spiral fiber for chemical spectroscopic applications. Sens. Int. 2020, 1, 100011. [Google Scholar] [CrossRef]
- Arif, M.F.H.; Ahmed, K.; Asaduzzaman, S.; Azad, M. Design and optimization of photonic crystal fiber for liquid sensing applications. Photonic Sens. 2016, 6, 279–288. [Google Scholar] [CrossRef]
- Hossain, B.; Podder, E.; Bulbul, A.A.; Mondal, H.S. Optical Fiber Technology Bane chemicals detection through photonic crystal fiber in THz regime. Opt. Fiber Technol. 2020, 54, 102102. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sen, S.; Hossain, M.M. Design of a chemical sensing circular photonic crystal fiber with high relative sensitivity and low confinement loss for terahertz (THz) regime. Optik. 2020, 222, 165359. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mou, F.A.; Bhuiyan, M.I.H.; Islam, M.R. Refractometric THz Sensing of Blood Components in a Photonic Crystal Fiber Platform. Braz. J. Phys. 2022, 52, 47. [Google Scholar] [CrossRef]
- Podder, E.; Hossain, B.; Rahaman, E.; Bulbul, A.A.; Ahmed, K. Ensing and Bio-Sensing Research Design and optimization of terahertz blood components sensor using photonic crystal fiber. Sens. Bio.-Sens. Res. 2020, 30, 100386. [Google Scholar] [CrossRef]
- Habib, M.A.; Vera, E.R.; Aristizabal, J.V.; Anower, M.S. Numerical Modeling of a Rectangular Hollow-Core Waveguide for the Detection of Fuel Adulteration in Terahertz Region. Fibers 2020, 8, 63. [Google Scholar] [CrossRef]
- Jibon, R.H.; Biswas, S.; Nira, N.F.I. Poisonous chemical detection in the THz regime using PCF: Design and numerical investigation. J. Opt. 2021, 50, 671–680. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sen, S. Design and Performance Improvement of Optical Chemical Sensor Based Photonic Crystal Fiber (PCF) in the Terahertz (THz) Wave Propagation. Silicon 2020, 13, 3879–3887. [Google Scholar] [CrossRef]
- Sultana, J.; Islam, M.S.; Dinovitser, A.; Brian, W.-H.N.; Abbott, D. A novel Zeonex based oligoporous-core photonic crystal fiber for polarization preserving terahertz applications. Opt. Commun. 2018, 413, 242–248. [Google Scholar] [CrossRef]
- Ultana, J.A.S.; Slam, M.D.S.A.I. Terahertz detection of alcohol using a photonic crystal fiber sensor. Appl. Opt. 2018, 57, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.M.D.; Ng, A.K.L.; Heidepriem, H. E.Ultrasimplifed Single-Step Fabrication of Microstructured Optical Fiber. Sci. Rep. 2020, 10, 9678. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.E.; Jibon, R.H.; Mondal, H.S.; Hossain, M.B.; Bulbul, A.A.M.; Saha, R. Design and optimization of a PCF-based chemical sensor in THz regime. Sens. Bio-Sens. Res. 2021, 32, 100422. [Google Scholar] [CrossRef]
- Hossain, M.S.; Kamruzzaman, M.M.; Sen, S.; Azad, M.M.; Hossain Mollah, M.S. Hexahedron core with sensor based photonic crystal fiber: An approach of design and performance analysis. Sens. Bio-Sens. Res. 2021, 32, 100426. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, P.; Jindal, P. Decagonal solid core PCF based refractive index sensor for blood cells detection in terahertz regime. Opt. Quantum Electron 2021, 53, 165. [Google Scholar] [CrossRef]
- Islam, M.A.; Islam, M.R.; Siraz, S.; Rahman, M.; Anzum, M.S.; Noor, F. Wheel structured Zeonex-based photonic crystal fiber sensor in THz regime for sensing milk. Appl. Phys. A 2021, 127, 311. [Google Scholar] [CrossRef] [PubMed]
- Jibon, R.H.; Rahaman, M.K.; Alahe, M.A. Detection of primary chemical analytes in the THz regime with photonic crystal fiber. Sens. Bio-Sens. Res. 2021, 33, 100427. [Google Scholar] [CrossRef]
- Jibon, R.H.; Ahmed, M.; Abd-Elnaby, M.; Rashed, A.N.Z.; Eid, M.M.A. Design mechanism and performance evaluation of photonic crystal fiber (PCF)-based sensor in the THz regime for sensing noxious chemical substrates of poultry feed. Appl. Phys. A 2022, 128, 169. [Google Scholar] [CrossRef]
- Bulbul, A.A.M.; Hossain, M.B.; Dutta, R.; Hassan, M. Zeonex-based Tetra-rectangular Core-photonic Crystal Fiber for NaCl Detection. Nanosci. Nanotechnol. Asia 2021, 11, 112–120. [Google Scholar] [CrossRef]
- Hossain, M.S.; Faruq, M.A.; Rana, M.M.; Sen, S.; Haque, M.D.; Azad, M.M. Sensitivity analysis for detecting chemi-cals by the optical chemical sensor based Photonic Crystal Fiber (PCF) in the Terahertz (THz) regime. Phys Scr. 2021, 96, 125121. [Google Scholar] [CrossRef]
- Suhaimi, N.A.N.B.; Yakasai, I.K.; Abas, E.; Kaijage, S.; Begum, F. Modelling and simulation of novel liquid-infiltrated PCF biosensor in Terahertz frequencies. IET Optoelectron. 2020, 14, 411–416. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hussain, N.; Hossain, Z.; Zaman, M.S.; Rangon, M.N.H.; Al-Shafi, M.A.; Sen, S.; Azad, M.M. Performance analysis of alcohols sensing with optical sensor procedure using circular photonic crystal fiber (C-PCF) in the terahertz regime. Sens. Bio-Sens. Res. 2022, 35, 100469. [Google Scholar] [CrossRef]
- Luo, W.; Jiang, P.; Xu, Q.; Cao, L.; Jones, A.; Li, K.; Copner, N.; Gong, Y. Terahertz Sensor via Ultralow-Loss Dispersion-Flattened Polymer Optical Fiber: Design and Analysis. Materials 2021, 14, 4921. [Google Scholar] [CrossRef]
- Lu Xue, L.; Yani, Z.; Yuyu, Z. Terahertz Detection of Chemical Analytes Using a Hollow-Core Photonic Crystal Fiber Sensor. In Proceedings of the 2019 International Conference on Optical Instruments and Technology: IRMMW-THz Technologies and Applications, Beijing, China, 12 March 2020. [Google Scholar] [CrossRef]
- Bulbul, A.A.M.; Jibon, R.H.; Das, S.K.; Roy, T.; Saha, A.; Hossain, M.B. PCF Based Formalin Detection by Exploring the Optical Properties in THz Regime. Nanosci. Nanotechnol. Asia 2020, 11, 314–321. [Google Scholar] [CrossRef]
- Ahmed, K.; Paul, B.K.; Ahmed, F.; Jabin, M.A.; Uddin, M.S. Numerical demonstration of triangular shaped photonic crystal fibre-based biosensor in the Terahertz range. IET. Opt. Electron. 2021, 15, 1. [Google Scholar] [CrossRef]
- Sen, S.; Al-Shafi, A.; Sikder, A.S.; Hossain, S.; Azad, M.M. Zeonex based decagonal photonic crystal fiber (D-PCF) in the terahertz (THz) band for chemical sensing applications. Sens. Bio-Sens. Res. 2021, 31, 100393. [Google Scholar] [CrossRef]
- Asaduzzaman, S.; Ahmed, K. Microarray-core based circular photonic crystal fiber for high chemical sensing capacity with low confinement loss. Opt. Appl. 2017, 47, 41–49. [Google Scholar] [CrossRef]
- Anik, H.K.; Mahmud, S.; Chakma, P.; Talukder, H.; Chakrabarti, K.; Biswa, S.K. A Highly Sensitive and Ultra-Low Loss Photonic Crystal Fiber-Based Gas and Chemical Sensor. In Proceedings of the 3rd International Conference on Communication, Devices and Computing, 1st ed.; Skdar, B., Maity, S.P., Samanta, J., Roy, A., Eds.; Springer: Singapore, 2021; Volume 851, pp. 33–34. [Google Scholar]
- Abbaszadeh, A.; Makouei, S.; Meshgini, S. Highly sensitive triangular photonic crystal fiber sensor design applicable for gas detection. Adv. Electromagn. 2021, 10, 1–5. [Google Scholar] [CrossRef]
- Leon, M.J.B.M.; Abedin, S.; Kabir, M.A. A photonic crystal fiber for liquid sensing application with high sensitivity, birefringence and low confinement loss. Sens. Int. 2021, 2, 100061. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Makouei, S.; Meshgini, S. New hybrid photonic crystal fiber gas sensor with high sensitivity for ammonia gas detection. Can. J. Phys. 2022, 100, 129–137. [Google Scholar] [CrossRef]
- Doumas, B.T.; Perry, B.W.; Sasse, E.A.; Straumfjord, J.V. Standardization in Bilirubin Assays: Evaluation of Selected Methods and Stability of Bilirubin Solutions. Clin. Chem. 1973, 19, 984–993. [Google Scholar] [CrossRef]
- Li, X.; Rosenzweig, Z. A fiber optic sensor for rapid analysis of bilirubin in serum. Anal. Chim. Acta 1997, 353, 263–273. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Horder, M.; Rej, R. Approved recommendations on IFCC methods of measurement of catalytic concentration of enzymes. J. Clin. Chem. Clin. Biochem. 1986, 24, 481–489. [Google Scholar]
- Jayasree, M.; Aparna, R.S.; Anjana, R.R.; Devi, J.S.A.; John, N.; Abha, K.; Manikandan, A.; George, S. Fluorescence turn on detection of bilirubin using Fe (III) modulated BSA stabilized copper nanocluster; A mechanistic perception. Anal. Chim. Acta 2018, 1031, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Narwal, V.; Batra, B.; Kalra, V.; Jalandra, R.; Ahlawat, J.; Hooda, R.; Sharma, M.; Rana, J.S. Bilirubin detection by different methods with special emphasis on biosensing: A review. Sens. Bio-Sens. Res. 2021, 33, 100436. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Gabriel, W.E.; Santschi, C.; Martin, O.J.F. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, M.; Manap, H. The optimal absorption of bilirubin using an optical fiber sensor. ARPN J. Eng. Appl. Sci. 2015, 10, 8762–8764. [Google Scholar]
- Guo, Z.; Zhang, T.; Song, J.; Jiang, H.; Chen, H. Sensitivity of topological edge states in a non-Hermitian dimer chain. Photonics Res. 2021, 9, 574–582. [Google Scholar] [CrossRef]
- Gabrys, P.A.; Seo, S.E.; Wang, M.X.; Oh, E.; Macfarlane, R.J.; Mirkin, C.A. Lattice Mismatch in Crystalline Nanoparticle Thin Films. Nano Lett. 2018, 18, 579–585. [Google Scholar] [CrossRef]
- Guo, Z.; Long, Y.; Jiang, H.; Ren, J.; Chen, H. Anomalous unidirectional excitation of high-k hyperbolic modes using all-electric metasources. Adv. Photonics 2021, 3, 360011–3600110. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Chen, L.; Zheng, H.; Azeman, N.H.; Liu, M.X.; Liu, G.S.; Luo, Y.; Chen, Z. High-performance fiber plasmonic sensor by engineering the dispersion of hyperbolic metamaterials composed of Ag/TiO2. Opt. Express 2020, 28, 25562–25573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).