Long-Term Eutrophication in Mesotrophic–Eutrophic Lake Kawaguchi, Japan, Based on Observations of the Horizontal Distribution of Profundal Chironomid Larvae and Oligochaetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Methods
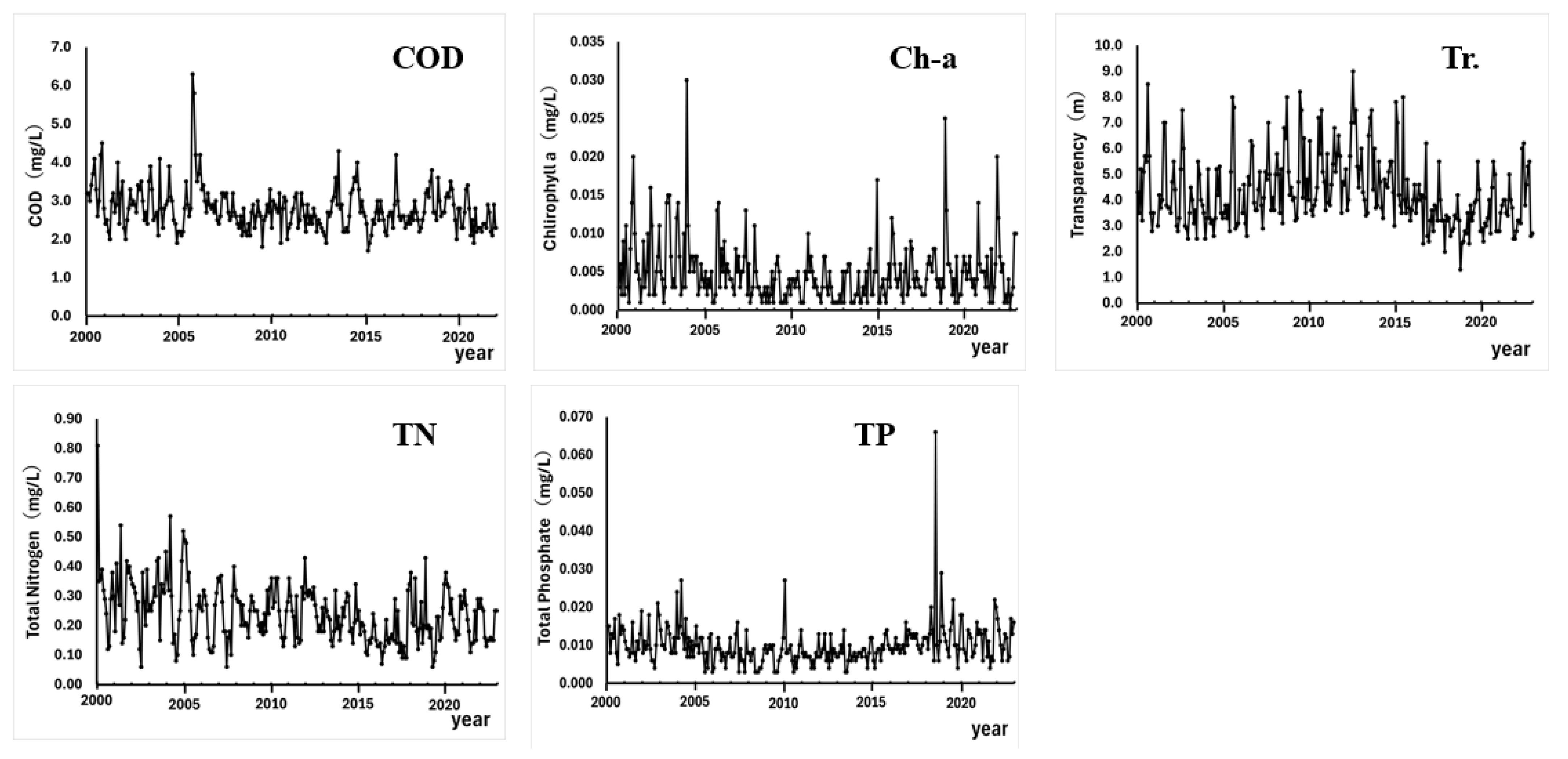
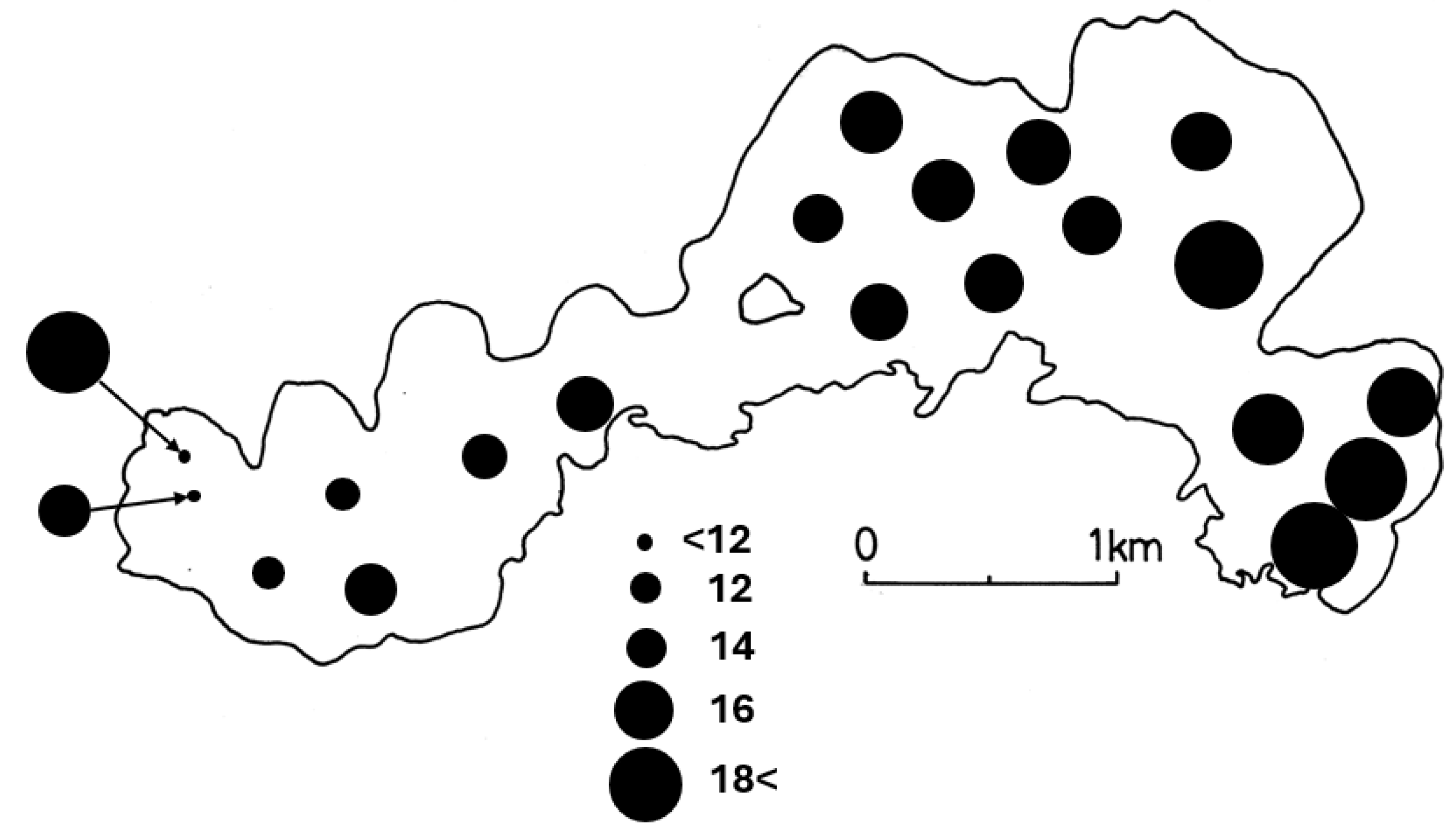
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrates species in freshwater ecosystems: Zoobenthic species influence energy flows and nutrient cycling. BioScience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, B.; Song, Z.; Xie, Z. Do functional traits chironomid assemblages respond more readily to eutrophication than taxonomic composition in Chinese floodplain lake? Ecol. Indic. 2019, 103, 355–362. [Google Scholar] [CrossRef]
- Ji, L.; Wang, Q.; Cui, S.; Chen, W.; Zhang, B.; Chu, J.; Ding, Y.; Shi, H.; Cao, Z.; Wang, L.; et al. Different responses f taxonomic and functional trait structure of benthic macroinvertebrate assemblages to eutrophication in large Chinese freshwater lake. Environ. Sci. Pollut. Res. 2024, 31, 9732–9744. [Google Scholar] [CrossRef] [PubMed]
- Martel-Cea, A.; Astorga, G.; Hernandez, M.; Caputo, L.; Abarzua, M.A. Modern chironomids (Diptera: Chironomidae) and the environmental variable that influence their distribution in the Araucanian lakes, south-central Chile. Hydrobiologia 2021, 848, 2551–2568. [Google Scholar] [CrossRef]
- Donohue, I.; Donhue, L.A.; Ainin, B.N.; Irvine, K. Assessment of eutrophication pressure on lakes using littoral invertebrates. Hydrobiologia 2009, 633, 105–122. [Google Scholar] [CrossRef]
- Bazzanti, M.; Mastrantuono, L.; Solimini, A.G. Selecting macroinvertebrate taxa and metrics to assess eutrophication in different depth zones of Mediterrancan lakes. Fundam. Appl. Limnol. 2012, 180, 133–143. [Google Scholar] [CrossRef]
- Li, D.; Erickson, R.A.; Tang, S.; Zhang, Y.; Niu, Z.; Liu, H.; Yu, H. Structure and spatial patterns of microbenthic community in Tai Lake, a large shallow lake, China. Ecol. Indic. 2016, 61, 179–187. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Jiang, X.; Song, Z.; Xie, Z. Effects of human-induce eutrophication on macroinvertebrate spatiotemporal dynamics in Lake Dianchi, a large shallow plateau lake in China. Environ. Sci. Pollut. Res. 2020, 27, 13066–13080. [Google Scholar] [CrossRef]
- Perera, G.A.C.D.; Chandrasekara, W.U. Microbenthic diversity and its bioindicator potential in urban reservoirs: A Sri Lanka case study. Lakes Reserv. 2022, 27, e12416. [Google Scholar] [CrossRef]
- Yamanashi Prefecture. The Water Quality of the Fuji Five Lakes over 21 Years; Yamanashi Prefecture: Kofu, Japan, 1993; pp. 1–138. (In Japanese) [Google Scholar]
- Aizaki, M.; Otsuki, A.; Fukushima, T.; Kawai, T.; Hosomi, M.; Muraoka, M. Application of modified Carlson’s trophic state index to Japanese lakes and its relationships to other parameters related to trophic state. Res. Rep. Nat. Inst. Environ. Stud. Jpn. 1981, 23, 13–31. [Google Scholar]
- Yoshizawa, K.; Watanabe, M.; Ariizumi, K. Picoplankyons observed in Fuji Five Lakes (Apr. 2001–Mar. 2002). Ann. Rep. Yamanashi Insti. Public Health. 2002, 45, 51–53. [Google Scholar]
- Hirabayashi, K.; Yoshizawa, K.; Yoshida, N.; Ariizumi, K.; Kazama, F. Long-term dynamics of freshwater red tide in shallow lake in central Japan. Environ. Health Prev. Med. 2007, 12, 33–39. [Google Scholar] [CrossRef]
- Nakamura, S.; Uejima, T.; Watanabe, H.; Matsuyama-Serisawa, K.; Serisawa, Y. Seasonal changes and long-term fluctuations of water quality in the Fuji Five Lakes, Central Japan. Fujisan Kenkyu. 2016, 10, 31–40. [Google Scholar]
- Terao, S. Study on Leptodora in Lake Kawaguchi. Jpn. J. Zool. 1912, 24, 650–651. [Google Scholar]
- Lindegaard, C. Classification of water-bodies and pollution. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 385–404. [Google Scholar]
- Yamanashi Prefecture. Water Quality Data in the Fuji Five Lakes. Available online: https://www.pref.yamanashi.jp/taiki-sui/sokutei.html (accessed on 14 October 2025).
- Yamagishi, H.; Fukuhara, H. Vertical migration of Spaniotoma akamusi larvae (Diptera: Chironomidae) through the bottom deposits of Lake Suwa. Jpn. J. Ecol. 1972, 22, 226–227. [Google Scholar]
- Iwakuma, T.; Yasuno, M. Chironomid productions in highly eutrophic Lake Kasumigaura. Verh. Internat. Verein. Limnol. 1981, 21, 664–674. [Google Scholar]
- Cranston, P.S. A Key to the Larvae of the British Orthocladiinae (Chironomidae); Freshwater biological association scientific publication; Freshwater Biological Association: Cumbria, UK, 1982; Volume 45, pp. 1–152. [Google Scholar]
- Wiederholm, T. Chironomidae of Holarctic region. keys and diagnoses. Part 1. larvae. Entmol. Scand. Suppl. 1983, 19, 1–457. [Google Scholar]
- Orendt, C.; Spies, M. Chironomus Meigen (Diptera: Chironomidae). In Key to the Larvae of Importance to Biological Water Analysis in Germany and Adjacent Area; Ehnert and Blankenburg GmbH: Leipzig, Germany, 2012; pp. 1–24. [Google Scholar]
- Sasa, M. Taxonomical and biological notes on Tokunagayusurika akamusi (Tokunaga), with description of immature stages (Diptera, Chironomidae). Jpn. J. Sani. Zool. 1978, 29, 93–101. [Google Scholar] [CrossRef]
- Brinkhurst, R.O. Distribution and abundance of tubificid (Oligochaeta) species in Toronto Harbour, Lake Ontario. J. Fish. Res. Board Can. 1970, 27, 1961–1969. [Google Scholar] [CrossRef]
- Miyadi, D. Studies on the bottom fauna of Japanese lakes. X. Regional characteristics and system of Japanese Lakes based on the bottom fauna. Jpn. J. Zool. 1933, 4, 417–437. [Google Scholar]
- Kitagawa, N. A classification of Japanese lakes based on hypolimnetic oxygen and benthonic fauna. Jpn. J. Limnol. 1978, 39, 1–8. [Google Scholar] [CrossRef]
- Yasuno, M.; Iwakuma, T.; Sugaya, Y.; Sasa, M. Zoobenthos of Japanese lakes of different trophic status, with special reference to Chironomidae. Res. Rep. Spec. Res. Proj. Environ. Sci. 1983, 182, 21–48. [Google Scholar]
- Iwakuma, T.; Yasuno, M.; Sugaya, Y.; Sasa, M. Three large species of Chironomidae (Diptera) as biological indicators of lake eutrophication. In Biological Monitoring of Environmental Pollution; Yasuno, M., Whitton, A.B., Eds.; Tokai University Press: Tokyo, Japan, 1988; pp. 101–113. [Google Scholar]
- Miyadi, D. Studies on the bottom fauna of Japanese lakes. 5. Five Lakes at the north foot of Mt. Hudi and Lake Asi. Jpn. J. Zool. 1932, 4, 81–125. [Google Scholar]
- Kitagawa, N. Studies on the bottom fauna of The Fuji Five Lakes and Lake Ashino. Rikusui Fueiyouka No Kisotekikenkyu 1973, 2, 32–37. [Google Scholar]
- Hirabayashi, K.; Yoshizawa, K.; Horiuchi, M. Horizontal distribution of benthic macroinvertebrates in Lake Kawaguchi, Japan. Rep. Suwa Hydrobiol. 1995, 9, 121–129. [Google Scholar]
- Hirabayashi, K.; Yoshizawa, K.; Oga, K.; Yoshida, N.; Ariizumi, K.; Kazama, F. Change of chironomid fauna (Diptera: Chironomidae) in eutrophic Lake Kawaguchi, Japan. Bol. Mus. Mun. Funchal Sup. 2008, 13, 109–117. [Google Scholar]
- Devai, G.; Moldovan, J. An attempt to trace eutrophication in a shallow lake (Balaton, Hungary) using chironomids. Hydrobiologia 1983, 103, 169–175. [Google Scholar] [CrossRef]
- Zou, W.; Cai, Y.; Tolonen, K.T.; Zhu, G.; Qin, B.; Peng, K.; Gong, Z. The adaptations to tube-dwelling life of Propsilocerus akamusi (Diptera: Chironomidae) larvae and its eutrophication-tolerant mechanisms. Limnologica 2019, 77, 125684. [Google Scholar] [CrossRef]


| Depth | WT | DO | IL | T-Ch | Cp | Pa | Oli | |
|---|---|---|---|---|---|---|---|---|
| Depth | - | −0.74 | −0.25 | −0.41 | 0.30 | 0.03 | 0.35 | −0.03 |
| WT | - | 0.04 | 0.41 | −0.24 | −0.27 | −0.16 | −0.11 | |
| DO | - | −0.08 | −0.41 | −0.02 | −0.48 | −0.08 | ||
| IL | - | 0.20 | −0.01 | 0.20 | −0.14 | |||
| T-Ch | - | 0.38 | 0.93 ** | 0.05 | ||||
| Cp | - | 0.06 | 0.27 | |||||
| Pa | - | −0.02 | ||||||
| Oli | - |
| Miyadi [29] | Kitagawa [30] | Hirabayashi et al. [31] | Hirabayashi et al. [32] | Present Study | |
|---|---|---|---|---|---|
| 2 May 1931 | 17 February 1973 | 5 March 1993 | 7 March 2006 | 7 March 2025 | |
| No. of sampling points | 16 | 12 | 22 | 22 | 20 |
| Mean depth (m) | 10.1 ± 1.3 | 10.9 ± 3.2 | 10.6 ± 2.1 | 9.9 ± 2.2 | 10.0 ± 1.9 |
| Ignition loss of sediment (%) | - | - | 10.4 ± 2.4 | 15.8 ± 3.2 | 16.4 ± 2.0 |
| Total Chironomid density (Ind./m2) | 429 ± 317 | 885 ± 384 | 1256 ± 661 | 474 ± 418 * | 816 ± 391 |
| C. plumosus (Ind./m2) | 75 ± 102 | 593 ± 258 | 341 ± 182 | 97 ± 96 * | 109 ± 114 |
| (%) | 17.4 | 66.9 | 27.2 | 20.5 * | 13.3 |
| P. akamusi (Ind./m2) | 0 | 259 ± 149 | 634 ± 280 | 335 ± 257 | 669 ± 358 |
| (%) | 0 | 29.2 | 50.5 | 70.7 | 82.0 |
| Oligochaeta (Ind./m2) | 1258 ± 500 | 139 ± 150 | 5489 ± 2769 | 1135 ± 717 | 2457 ± 1247 |
| (%) | 74.6 | 13.6 | 81.4 | 70.5 | 75.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirabayashi, K.; Takeda, M. Long-Term Eutrophication in Mesotrophic–Eutrophic Lake Kawaguchi, Japan, Based on Observations of the Horizontal Distribution of Profundal Chironomid Larvae and Oligochaetes. Limnol. Rev. 2025, 25, 53. https://doi.org/10.3390/limnolrev25040053
Hirabayashi K, Takeda M. Long-Term Eutrophication in Mesotrophic–Eutrophic Lake Kawaguchi, Japan, Based on Observations of the Horizontal Distribution of Profundal Chironomid Larvae and Oligochaetes. Limnological Review. 2025; 25(4):53. https://doi.org/10.3390/limnolrev25040053
Chicago/Turabian StyleHirabayashi, Kimio, and Masaaki Takeda. 2025. "Long-Term Eutrophication in Mesotrophic–Eutrophic Lake Kawaguchi, Japan, Based on Observations of the Horizontal Distribution of Profundal Chironomid Larvae and Oligochaetes" Limnological Review 25, no. 4: 53. https://doi.org/10.3390/limnolrev25040053
APA StyleHirabayashi, K., & Takeda, M. (2025). Long-Term Eutrophication in Mesotrophic–Eutrophic Lake Kawaguchi, Japan, Based on Observations of the Horizontal Distribution of Profundal Chironomid Larvae and Oligochaetes. Limnological Review, 25(4), 53. https://doi.org/10.3390/limnolrev25040053





