1. Introduction
Clean water is essential for life, yet billions lack access to it. This crisis is particularly severe in developing countries, where people rely on untreated surface water sources like rivers and dams. Unfortunately, these sources are often contaminated with pathogens and pollutants, causing gastrointestinal diseases like cholera, dysentery, and typhoid. According to the United Nations (UN), a staggering 2.1 billion people lack safe drinking water at home. This contamination disproportionately affects children under five, causing more than 700 deaths each day from diarrhea associated with unsafe water. The World Health Organization (WHO) estimates that nearly 159 million people depend on surface water sources, and at least two billion people consume water contaminated with fecal matter. This situation places a tremendous burden on public health resources in developing countries [
1].
For this reason, treatment plants play a crucial role in transforming this water into a safe resource for communities. Water treatment facilities use various processes, with coagulation–flocculation being a key step. This physicochemical treatment process removes particles and clarifies the water, preparing it for sedimentation, another essential treatment method [
2].
The most widely used coagulants and flocculants worldwide are inorganic chemicals, with aluminum- and iron-based compounds being the most common. The effectiveness of these chemicals is well known, but there are disadvantages associated with their use, such as high operating costs, negative impact on the environment, adverse effects on human health, and significant changes to the pH of treated water [
3].
Aluminum sulfate, a common coagulant, leaves traces of soluble aluminum in treated water. This form of aluminum can be absorbed by the human body, raising concerns about its potential long-term health effects [
4]. Some studies suggest a possible link between aluminum accumulation and nervous system disorders, including Alzheimer’s disease [
5], or neuropathological conditions [
6]. Aluminum can also accumulate in bones, causing excessive brittleness and softening. Other studies suggest a possible association between aluminum exposure and Amyotrophic Lateral Sclerosis (ALS) and Parkinsonism with dementia [
7].
In this regard, researchers are evaluating alternatives for water treatment, such as the use of natural coagulants, which are considered safe for human health, environmentally friendly, sustainable, and less polluting than chemicals due to their biodegradability. In developing countries, these coagulants are more accessible, and their organic nature allows the sludge produced to be safely applied as a fertilizer [
8].
A source of these natural coagulants are plant-based coagulants/flocculants obtained from natural sources such as the seeds, roots, peels, and leaves of various plants. They exhibit coagulation and flocculation properties due to the presence of active compounds such as proteins, polysaccharides, and tannins. With these coagulants, fruit waste has great potential. It is estimated that approximately 45% of the fruit produced worldwide is wasted, and this constitutes a cause of greenhouse gas emissions and provides a breeding ground for bacteria, pests, and mice in addition to significant financial losses. In 2022, 933 million tons of fresh fruit were produced worldwide [
9]. Waste occurs at various stages of the food supply chain, from production and harvesting to distribution, retail, processing, and home consumption. This waste represents an important raw material to produce natural coagulants.
Numerous positive studies on the application of natural coagulants derived from vegetables or plants for treating highly turbid water have been steadily drawing interest in bio-flocculation. For example, papaya seed coagulant removed 84.77% of the color in synthetic textile water [
10]; banana peel coagulant achieved 98.14% turbidity removal in synthetic water [
11]; nopal mucilage removed 70% of iron and 90% of manganese in river water [
12]; papaya seed removed 88% of turbidity from synthetic kaolin water [
5]; nopal cladode removed 77.8% of turbidity from dairy industry wastewater [
13]; pineapple leaves removed 88.40% of turbidity from polluted pond water [
14]; banana peel removed 85.20% of turbidity from simulated humic acid water [
15]; and mango peel removed 92.7% of turbidity from wastewater [
3]. Collectively, these findings indicate that natural coagulants can achieve removal efficiencies comparable to aluminum-based coagulants in reducing pollutants in wastewater.
This study proposes the use of papaya (
Carica papaya), both the peel and the seeds, which are thrown away, as sources to produce natural coagulants, because papaya is a plant widely harvested in Mexico. With an estimated production of 1.1 million tons per year, Mexico is the fifth largest producer of papayas in the world. Most of the production is destined for the domestic market and the rest for export, Mexico being the largest exporter worldwide, with 194,167 tons, representing 52% of global papaya exports in 2023 [
16]. Furthermore, papaya is one of the most produced tropical fruits in the world, it can be produced throughout the year, and the coagulants can be obtained from the seeds and peel, which are considered waste. Therefore, the production of coagulants does not affect or compete for raw materials with other markets. In this study, the Maradol variety has been considered, because it is the most common one in México.
This study explores the potential of Carica papaya (peel and seed) as a natural coagulant compared with aluminum sulfate, the most widely used inorganic coagulant for water purification. Although previous studies have shown the effectiveness of papaya peel and seed coagulants, the purpose of this study is to further understand how effectiveness changes with the initial turbidity, pH, and dose of the applied coagulant. This will allow the development of models that can be optimized to obtain the best values to maximize the reduction in turbidity and total suspended solids, as well as to obtain the best floc formation. Furthermore, these models will allow us to establish the basis for a subsequent study at pilot level considering the water conditions at which to set the treatment parameters to obtain optimal results.
In addition, this study analyzes how functional groups present in natural coagulants explain their coagulation and flocculation potential and the differences in their behavior. Finally, we evaluated the efficacy of papaya-based coagulants in treating surface water from the Valsequillo dam in Puebla, Mexico, which presents more extreme characteristics than those observed in the tested synthetic water.
2. Materials and Methods
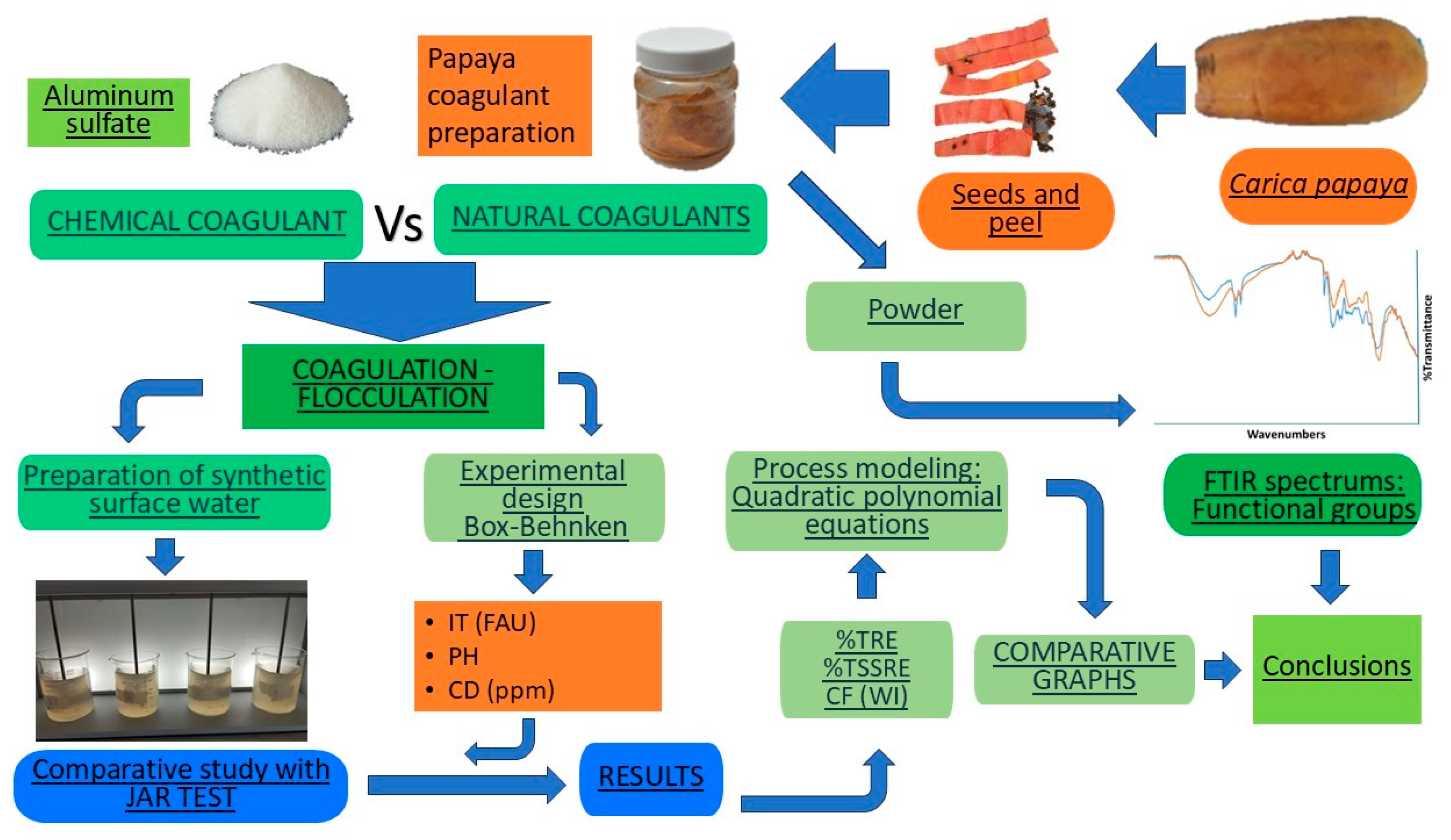
The overall methodology used in this study is described in
Figure 1.
2.1. Chemical Reagents
Aluminum sulfate coagulant (ASC) was supplied by the company Autotransportes TYP S.A. de C.V. (Jiutepec, Morelos, México), Sulfuric acid 0.1 mol and 0.1 N caustic soda were both obtained from Hycel Mexico (Hycel de México, S.A. de C.V., Zapopan, Jalisco, México) for pH adjustments.
2.2. Papaya Coagulant Preparation
The papaya (Carica papaya) was purchased in a local market in Cholula, Puebla. For the papaya peel coagulant (PPC), the papaya was peeled, and the pulp was removed. The peel was set to dry in the sun for 8 h to remove moisture. The dried peel was further dried at 60 °C for 24 h in a FELISA oven model FE-291AD (Feligneo Manufacturers, S.A. de C.V., Zapopan, Jalisco, México) and then crushed with a mortar until it was pulverized. The coagulant powder was milled down with a Beaika mill, model QL-002 (Yingqiang Enterprise Group, Baoding, Hebei, China) and sieved with a sifting device model W.S. Tyler Ro-Tap RX-29 (W.S. Tyler Company, OH, USA) to ensure a particle size below 75 µm (8″-FH-BR-BR-US-200 Sieve, VWR Company, West Chester, PA, USA). For the papaya seed coagulant (PSC), the seeds were removed and dried at 60 °C for 24 h and then crushed with a mortar and a mill until the material was pulverized. The seed coagulant powder was also sieved to ensure a particle size below 75 µm. The powders were kept dry until they were used to preserve their properties. Before use, solutions of the natural coagulants were prepared at 0.1% by weight with tap water.
2.3. Synthetic Surface Water Preparation
To simulate turbidity, a bentonite stock solution was prepared by mixing 10 g of bentonite clay (Materias Primas Xiloxoxtla, S.A. de C.V., Xiloxoxtla, Tlaxcala, México) with 1 L tap water. The suspension was stirred at 200 rpm for 1 h for complete mixing and dissolution. The solution was then left undisturbed for 1 h. The supernatant was recovered, and the precipitate was discarded. To simulate organics, a humic acids stock solution was prepared by adding 1 g of Leonardite powder (Grupo TCDN, S.A. de C.V., Puebla, Puebla, México) to one liter of tap water. The humic acids suspension was stirred at 200 rpm for 1 h for complete mixing and dissolution. The solution was then left undisturbed for 1 h. Afterwards the supernatant was recovered, and the precipitate was discarded. To obtain the synthetic surface water for the experiments, both stock solutions were mixed in a 2:1 (bentonite: humic acids) ratio and tap water was added to obtain the desired turbidity. The prepared synthetic water contained turbidity values between 40 ppm and 140 ppm, which are common for surface water.
2.4. Experimental Design
For this study, the final turbidity (FT), the total suspended solids (TSS), and the characteristics of the floc (CF) formed were considered outputs of the clarification process. The inputs or parameters of the coagulation process were initial turbidity (IT), coagulant dosage (CD), and pH.
For turbidity, the Formazine Attenuation Unit (FAU) was reported, which is equivalent to the Nephelometric Turbidity Unit (NTU). TSS was reported in mg/L and CD was reported in parts per million (ppm). To define CF, the Willcomb index (WI) was used, a scale where the characteristics of the floc were translated into the following numerical values: 0 (colloidal floc, no sign of agglutination), 2 (floc visible, but very small), 4 (dispersed, well-formed floc, uniformly distributed, but slow settling), 6 (floc clearly distinguishable, well formed, relatively large, but precipitates slowly), 8 (good floc which precipitates easily but not completely) and 10 (excellent floc, sediments completely, leaving the water crystal clear). The ranges of coagulation process variables used and their levels are presented in
Table 1.
After establishing the coagulation process parameters and levels, the design matrix was generated with Statgraphics Centurion Version 19.4.04 (32-bit) software. We used a randomized Box–Behnken design without repetitions. To anticipate all possibilities and select the optimal clarification process, 15 experiments in one block were required in this case. The design matrix is presented in
Table 2 which also includes the initial total suspended solids (ITSS) measured in the synthetic water.
2.5. Jar Test
Coagulation–flocculation experiments were carried out using synthetic surface water samples in a jar test apparatus Velp Scientifica model JLT100–240 V/50–60 Hz (Velp Scientifica Srl, Usmate Velate, Monza y Brianza, Italia). The jar test is a common laboratory procedure based on the American Society of Testing Materials (ASTM) standard, D2035 [
17], used to estimate the removal efficiency of total suspended solids and turbidity by varying the conditions, amount, and type of coagulant on a small scale to predict the performance of large-scale treatment operations.
Test water samples of 400 mL each were added to four 600 mL jars. Each coagulant was prepared with tap water at a concentration of 0.1% and was added according to the dose required in each test, as established in the design matrix in
Table 2.
Coagulation experiments were carried out as follows. After adding the coagulant, the solution was mixed at 200 rpm for 1 min, followed by a slow mix at 30 rpm for 15 min and sedimentation for an additional 30 min.
Experiments were repeated three times in these conditions, and the average values for final turbidity (FT) and total suspended solids (TSS) were calculated from a sample collected from the supernatant. The value of CF was determined by direct observation. This procedure was repeated for different dosages and combinations.
2.6. Analytical Methods
To evaluate the performance of the coagulants, the physicochemical characteristics of the water before and after the coagulation–flocculation process were determined for each run. The parameters that were measured in the water include pH, turbidity (FAU), and total suspended solids (mg/L). Turbidity and suspended solids were measured with a HACH DR/890 portable colorimeter (HACH Mexico, Reactivos y Equipo, S.A. de C.V, Naucalpan de Juárez, Estado de México, México) and pH was measured with a Thermo Scientific Orion Star A3255 Multi Parametric Meter (Thermo Fisher Scientific Inc., Waltham, MA, USA) calibrated before starting the tests.
2.7. Determination of Functional Groups in Carica papaya
To determine the chemical functional groups present in the papaya peel and seed coagulants, Fourier-transform infrared spectroscopy (FTIR) was used. The analysis was carried out at room temperature using an Agilent Cary 630 FTIR spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA) and all spectra were recorded from 4000 to 400
. The range chosen includes the mid-infrared region that consists of the “group frequency region”, 4000–1300
(2.5–8 µm), and the “fingerprints region”, 1300–650
(8.0–15.4 µm) [
18]. Natural coagulants were analyzed in their powder form.
2.8. Coagulant Test with Real Surface Water
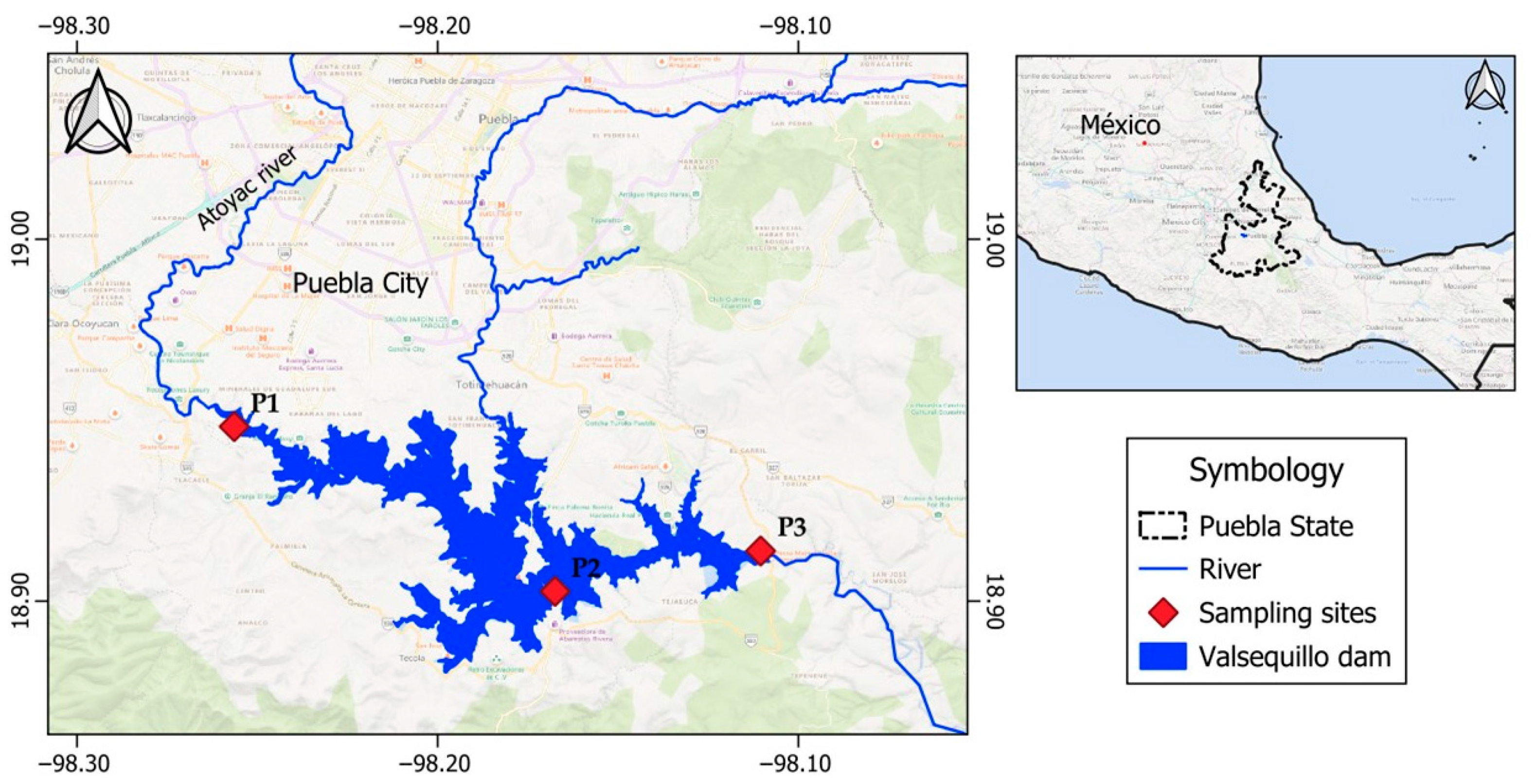
Finally, an additional test was performed with real surface water which, although it was outside the range of variables studied, as it was highly contaminated water, was intended to verify the effectiveness of the coagulants in these extreme cases. The water was taken from the Manuel Ávila Camacho dam, known as Valsequillo dam, located in the city of Puebla, Pue., Mexico. The Valsequillo dam is the largest surface water source in the area and is located at the mouth of the Atoyac River, which is heavily contaminated. Water samples were taken from three different points located at 18°56′53.3″ N 98°15′23.3″ W (point 1), 18°54′09.5″ N 98°10′03.2″ W (point 2) and 18°54′49.6″ N 98°06′38.1″ W (point 3), as shown in
Figure 2.
A proportional composite water sample was made with water collected from the three sites to test the removal efficiency of the coagulants.
4. Discussion
4.1. Models of Coagulation–Flocculation Processes and Graphs Relating Variables
Equations (5)–(7) represent the models and variable interactions. From the high values of the constants 3 and 8 in
Tables S2 and S3, the pH is of great importance in the behavior of the coagulants.
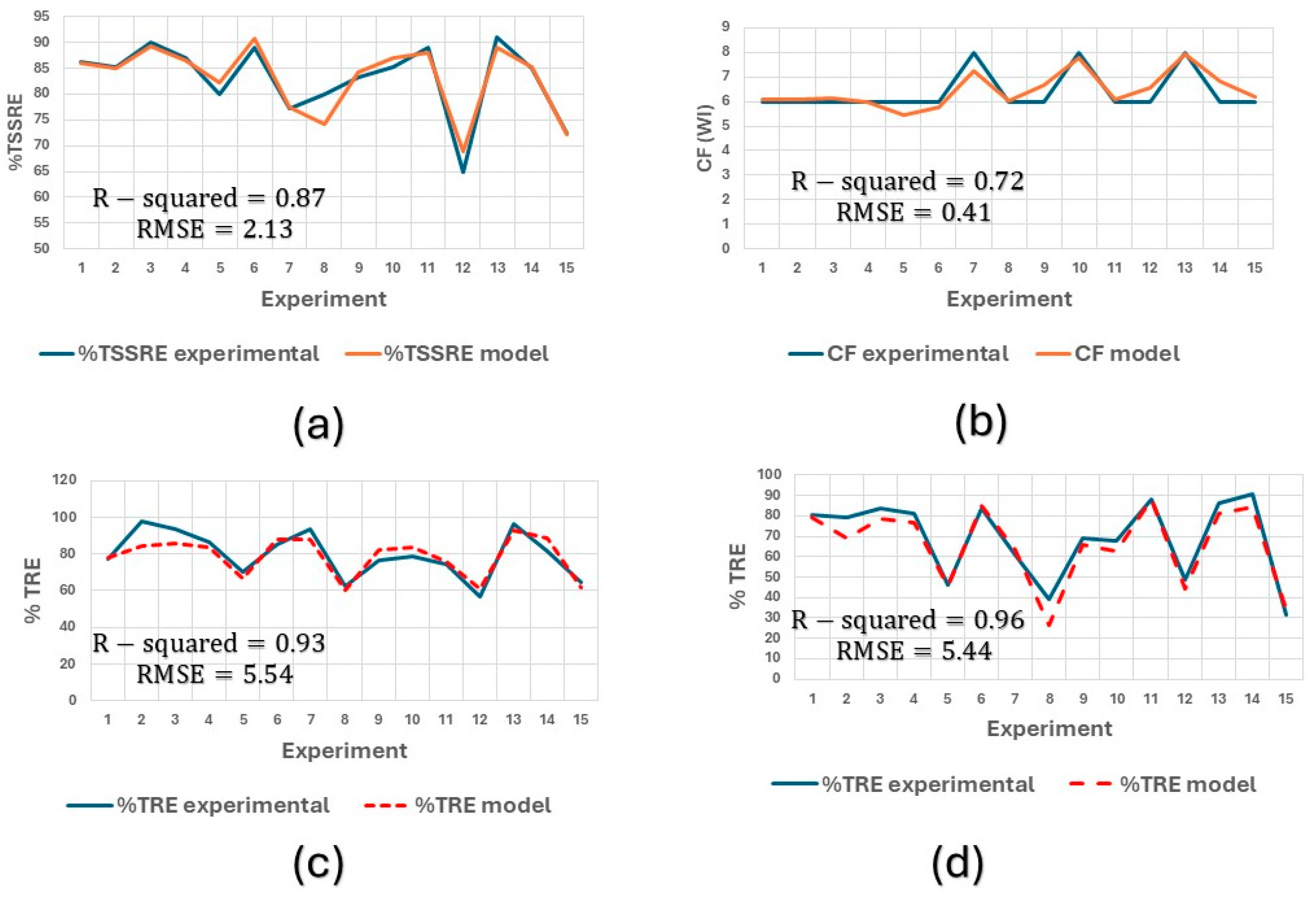
The models generated were compared with the experimental values as shown in
Figure 6, validating them in the ranges within which they worked and for the type of water treated.
4.2. Coagulation and Flocculation Activity
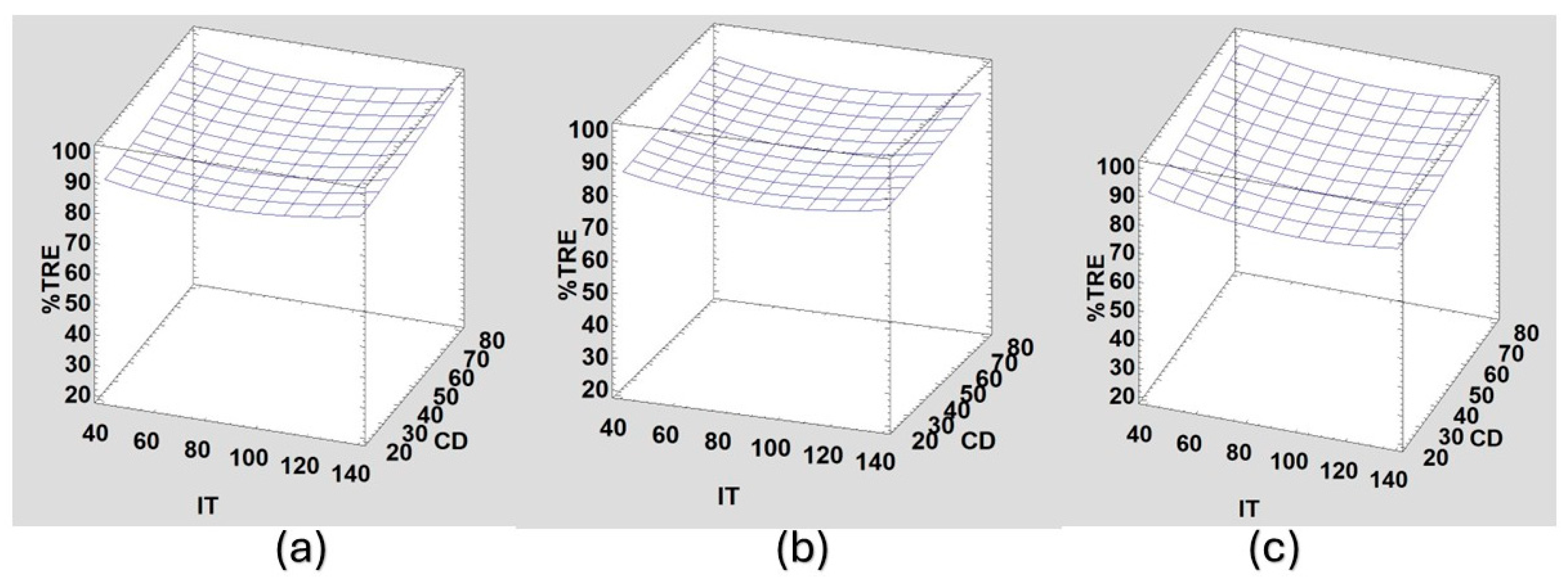
Response surface plots considering %TRE show the behavior of the coagulants.
Figure 3 shows that %TRE for ASC remains relatively consistent between 85% and 90% removal, with improvements at low IT and higher CD. There is also a performance improvement at low pH because under acidic conditions, aluminum sulfate dissolves, releasing aluminum ions (Al
3+). The optimal level is found at pH 5. This is a typical behavior of the charge neutralization mechanism.
For PPC,
Figure 4 shows that higher IT results in better removal due to the polymeric bridges formed by the functional groups trapping the suspended particles. The CD has little influence, so even at low CDs, removal rates are high at higher IT values. In this case, the best results are found at pH 7, probably due to a mixture of charge neutralization and the formation of polymeric bridges.
Like PPC, for PSC,
Figure 5 also shows higher IT values resulting in better removal rates due to the polymeric bridging mechanism. However, for PSC, lower CD values result in higher removal rates, and the best results are observed at pH 5. This might be because of the presence of carboxylic acids and amines at acidic pH levels, which aid the process. Amran et al. (2021) found comparable results with synthetic kaolin water using a bio-coagulant of deshelled
Carica papaya seeds, obtaining 88% turbidity removal [
5].
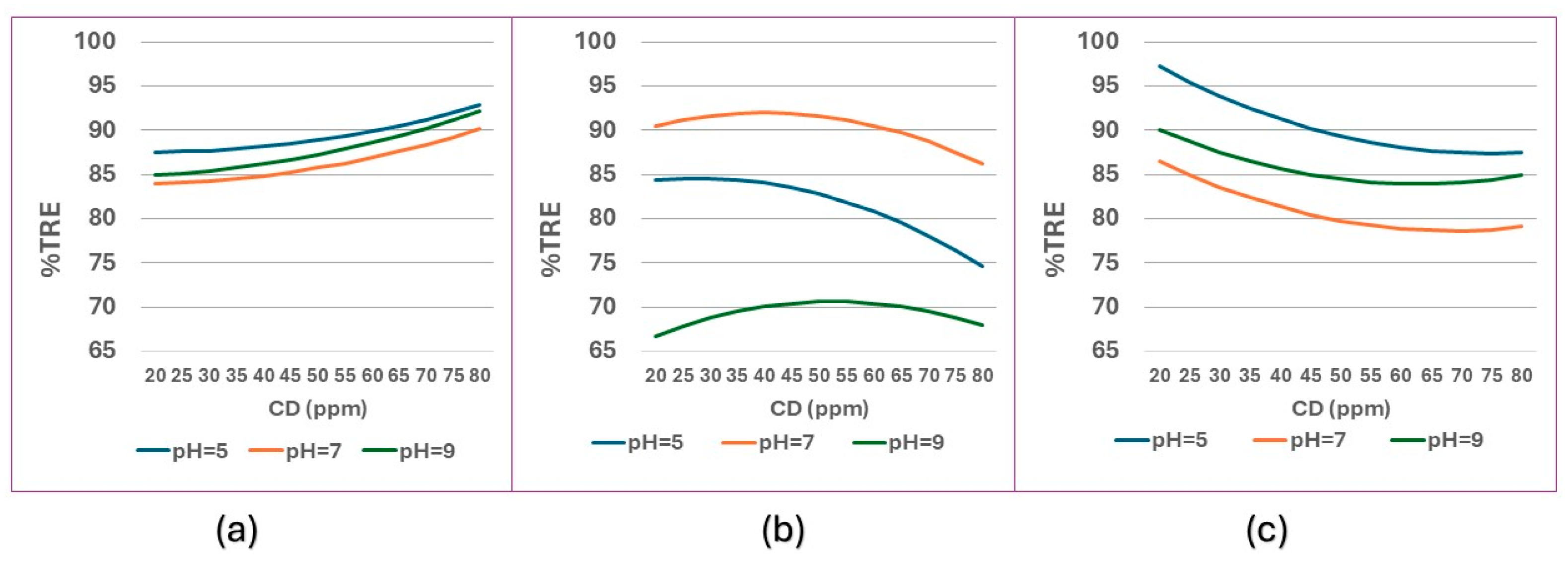
The above discussion can be better explained in
Figure 7. Comparing the three coagulants with an average turbidity (IT = 100 FAU), in
Figure 7a it can be observed that, at all pHs, the best results are obtained at higher CD. For PPC, in
Figure 7b, the best results are observed at neutral pH and CD values between 30 and 50 ppm. For PSC,
Figure 7c, the best results are observed at low CD values, at all pHs.
A %TRE vs. CD comparison at different pHs and IT= 100 FAU between the three coagulants can be observed in
Figure 8. This figure allows for a better understanding of the conditions and water characteristics under which PPC and PSC compare well with or can replace inorganic coagulants. In this figure, it can be observed that PSC performs better at low or high pH and low CD doses, whereas PPC performs better at neutral pH and CD values below 60 ppm. Finally, at all pHs, but pH = 9 in particular, ASC performs better at high CD values, indicating effectiveness but no opportunity for an optimal use of the coagulant quantity.
Considering the %TSSRE in
Figure 9, we find trends. However, PSC performs even better at all pHs and low CD values, probably due to higher proportions of pectin and polygalacturonic acid present in PSC (see
Figure 11), which allows it to better perform as a flocculant.
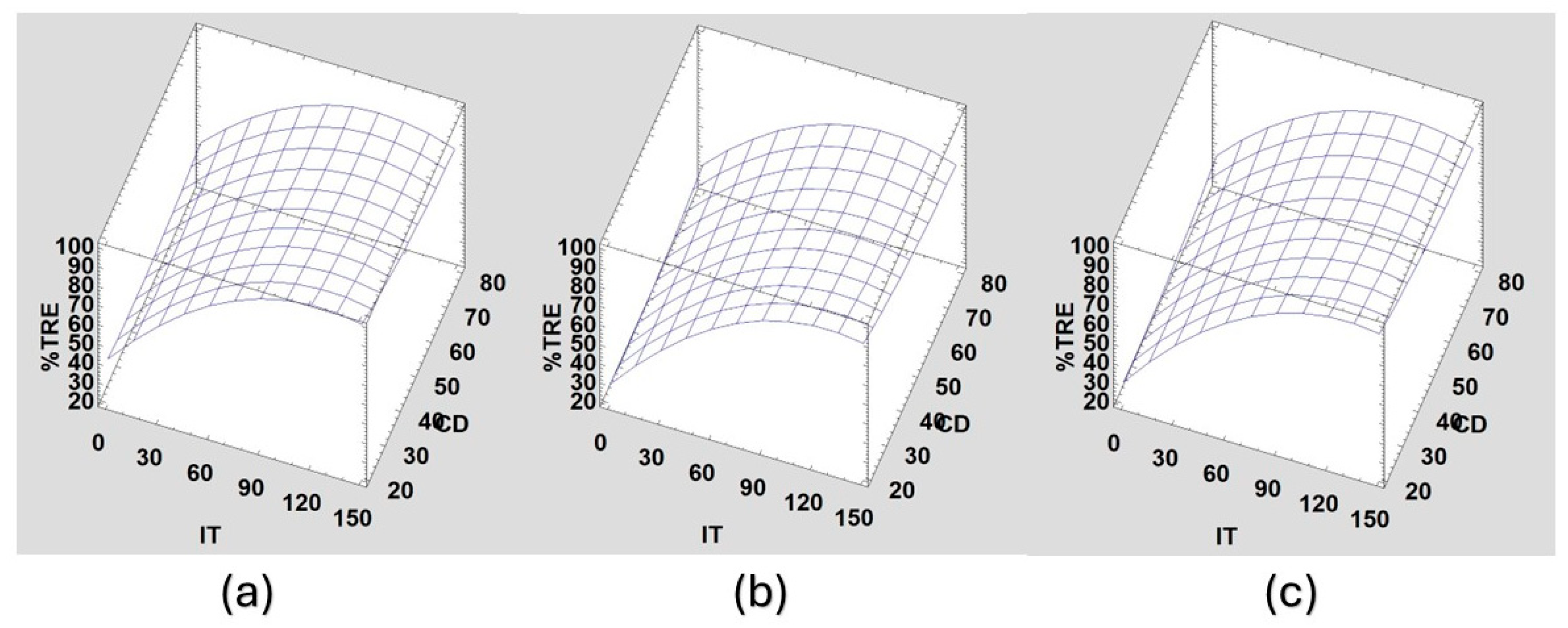
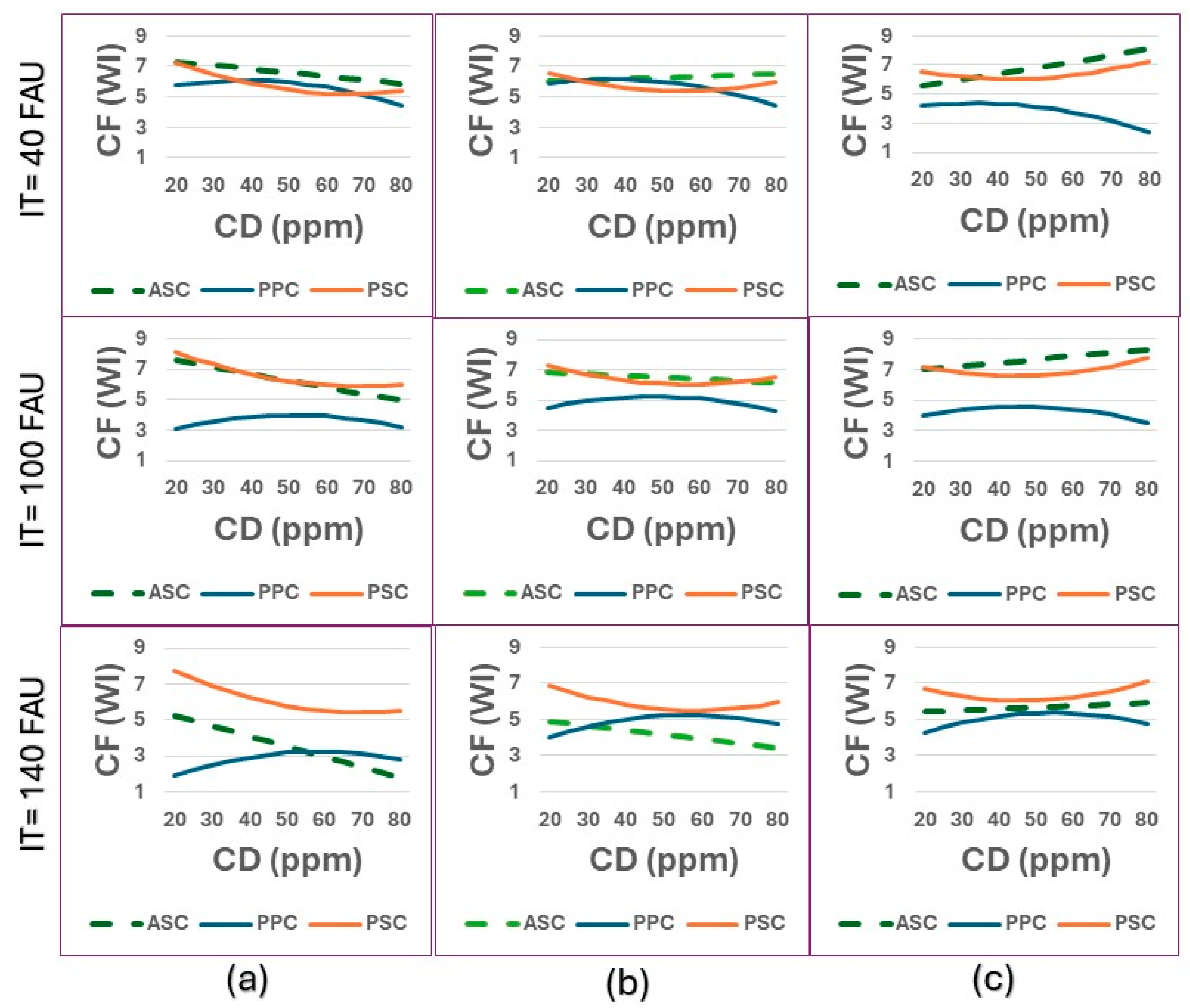
Finally, in
Figure 10 we observe CF and various interactions between IT and pH, to analyze which coagulant, parameters, and water characteristics lead to better floc formation. As can be seen, at all tested pHs, floc formation is similar between ASC and PSC at IT = 100 FAU and IT = 40 FAU. However, at higher IT values (140 FAU), floc formation is best for PSC at all pHs and CD values, indicating the potential for coagulant quantity use and optimization. The latter may indicate that ASC is not effective at high turbidity because its coagulation mechanism is charging neutralization, being unable to agglomerate higher concentrations of suspended particles.
4.3. Functional Groups in Coagulants
Coagulation and flocculation mechanisms are highly related to the functional groups and compounds present within the coagulant. For example, polysaccharides, protein groups, carbonyl, carboxyl, and hydroxyl groups can be responsible for adsorption and bridging mechanisms; amines, carboxyl, and hydroxyl groups are related to charge neutralization mechanisms; and amides, amines, carbonyl, and methylene groups can lead to charge neutralization and patch flocculation mechanisms [
12].
Peaks present in the FTIR spectra of the evaluated coagulants (
Figure 11) were assigned to various chemical groups and bonds in accordance with their respective wavenumbers (
). The strong peak observed between 3272 and 3284 cm
−1 corresponds to surface hydroxyl groups (-OH stretching group) and N-H stretching, related to charge neutralization and bridging. The peaks between 2852 and 2923 cm
−1 corresponds to aromatic C-H stretching related to charge neutralization and adsorption processes. Peaks between 1989 and 2175 cm
−1 correspond to C=O stretching vibration of methyl esters related to bridging processes. The peak between 1735 and 1745 cm
−1 corresponds to C=O stretching in carboxyl groups related to charge neutralization, adsorption and bridging. The peak between 1618 and 1637 cm
−1 corresponds to the presence of primary amide groups related to adsorption, bridging and charge neutralization. The peaks between 1235 and 1541 cm
−1 correspond to symmetrical stretching vibrations due to the COO- group of polygalacturonic acid, CH
2 bending of methylene bridge, C=C alkene, ether (R-O-R) and ring C-C bonds in pectin molecules, and O-H bending of the phenolic group, all of them related to bridging, adsorption, charge neutralization, and patch flocculation mechanisms in coagulation processes. Finally, the strong peak observed between 921 and 1142 cm
−1 is attributed to polygalacturonic acid in peptic polysaccharides, pyranose absorption vibrations, C
6–O
6H, C–O, C=O and C–C–O stretching, denoting phenols, alcohols, and esters related to bridging, charge neutralization and adsorption [
5,
19,
20].
Both natural coagulants evaluated contain, to different extent, the different peaks identified above, indicating charge neutralization, adsorption, bridging, and floc sweeping. However, in most cases, the peaks are presented with higher intensity in the PSC, explaining the higher efficiency of the latter as a coagulant and flocculant.
To better understand which functional groups or compounds present were more involved in the coagulation process, FTIR spectra were also collected after the coagulation process, as shown in
Figure 13.
The FTIR spectra of the PPC before the treatment was compared with the FTIR spectra after the treatment (floc;
Figure 13a) exhibiting before and after changes. In general, the decrease in intensity of all peaks in the floc confirms the participation of the different functional and chemical groups responsible for their coagulation process.
Similarly, in the case of PSC,
Figure 13b, changes between initial and final FTIR spectra can be seen. Again, the decrease in intensity of all peaks in the floc confirms the participation of several functional and chemical groups in the coagulation process. However, for PSC, after coagulation, the peaks have greater intensities, which could be indicative of the non-saturation of the functional groups that are still active, making it a more effective coagulant.
4.4. Comparisons of Tests with Real Surface Water
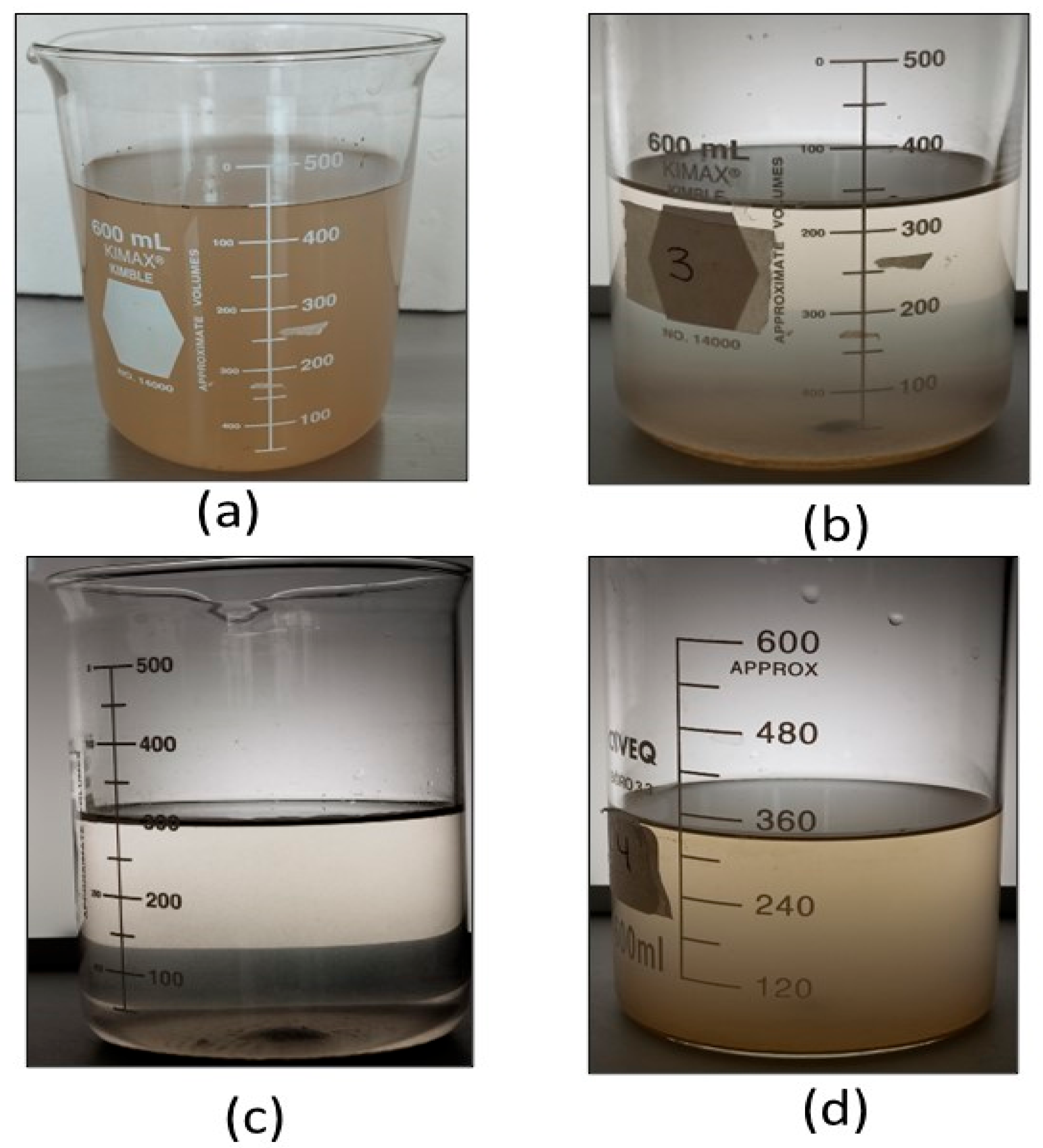
Table 4 shows the results for the Valsequillo water treatment. Visually, the initial raw water and final treated water are presented in
Figure 12. The best results are observed for PSC with a %TRE of 91%, followed by ASC with 78% and PPC with 69%. For the %TSSRE, the results are similar, with PSC obtaining 92% removal, being the best result of all. Due to the high IT (247 FAU), the results are not surprising, since PSC was seen to perform best in those conditions at any pH (See
Figure 10), and especially at low CD values. This result compares well with those presented by Yimer and Dame [
19], who tested the effectiveness of papaya seed extract for the removal of turbidity in raw water samples from the Tulte River in Ethiopia, reaching turbidity removal efficiencies between 82.64% and 87.19%.
Something very important to consider is that the optimal dosage for the PSC was 30 ppm, much lower than PPC which required 60 ppm and ASC which required 110 ppm.
4.5. Limitation to the Methodology
It is important to note that one limitation of the applied methodology is the need to use the coagulant solutions within five days after preparation, as chemical degradation alters their effectiveness over time. Additionally, papaya peels and seeds should not be used once putrefaction has begun and fungal growth is evident, since key polymeric structures begin to degrade during this process. Therefore, papaya waste should be used immediately or within one week to prepare coagulants. This period can be extended if the seeds and peel are sun-dried, as moisture removal stops the putrefaction process.
In addition, it is important to acknowledge that to analyze the formation of flocs we used WI values, which are not exact measurements, because they depend on the observer’s appreciation. For this study, the observers were trained, and we believe the values recorded provide a good understanding of how well the flocs were formed, but we acknowledge this limitation.
5. Conclusions
Papaya seed and papaya peel (Carica papaya) coagulants, obtained from the waste of this fruit, were evaluated alongside aluminum sulfate, a common inorganic coagulant, for their effectiveness in treating synthetic surface water. The processes of the three coagulants were modeled, developing the polynomials that allow predicting the expected removal efficiencies from the initial parameters.
All three coagulants had competitive results, reaching %TER levels above 90% for the cleaning of synthetic surface water. However, in equivalent tested ranges, natural coagulants required lower dosages and performed better with high initial water turbidity due to their polymeric bridging mechanisms through the action of their functional groups.
At neutral pH and medium doses between 30 and 60 ppm, PPC obtained %TER above 90%, while PSC had better removal rates at doses below 40 ppm and high IT values, outperforming ASC, primarily at pH 5.
ASC, at pH 9 and higher CD values, is superior to natural coagulants. %TER stands out, performing superiorly at low doses and at any pH, and achieving the best CF values in terms of floc formation. However, with increasing IT, natural coagulants are more effective due to the presence of functional and chemical groups that promote multiple coagulation mechanisms, including charge neutralization, polymeric bridging, adsorption processes, and sweep flocculation. Hydroxyl, carbonyl, methyl ester, amide, carboxyl, and alcohol groups, as well as polygalacturonic acid, pectin, polysaccharides, and phenolic groups are identified in the FTIR spectra of the natural coagulants.
An analysis of flocs after treatment for PPC and PSC indicated changes in the FTIR spectra, before and after the coagulation process, indicating the participation of several functional groups.
Additional testing with contaminated water from the Valsequillo dam confirms the use of these coagulants to treat water, with PSC yielding the best results and at lower doses, making it a competitive alternative.
It can be concluded that papaya-based coagulants obtained from waste can be used as an eco-friendly alternative to aluminum sulfate in physicochemical treatments to purify surface water for human consumption.