Phytoplankton Sampling: When the Method Shapes the Message
Abstract
1. Introduction
2. Material and Methods
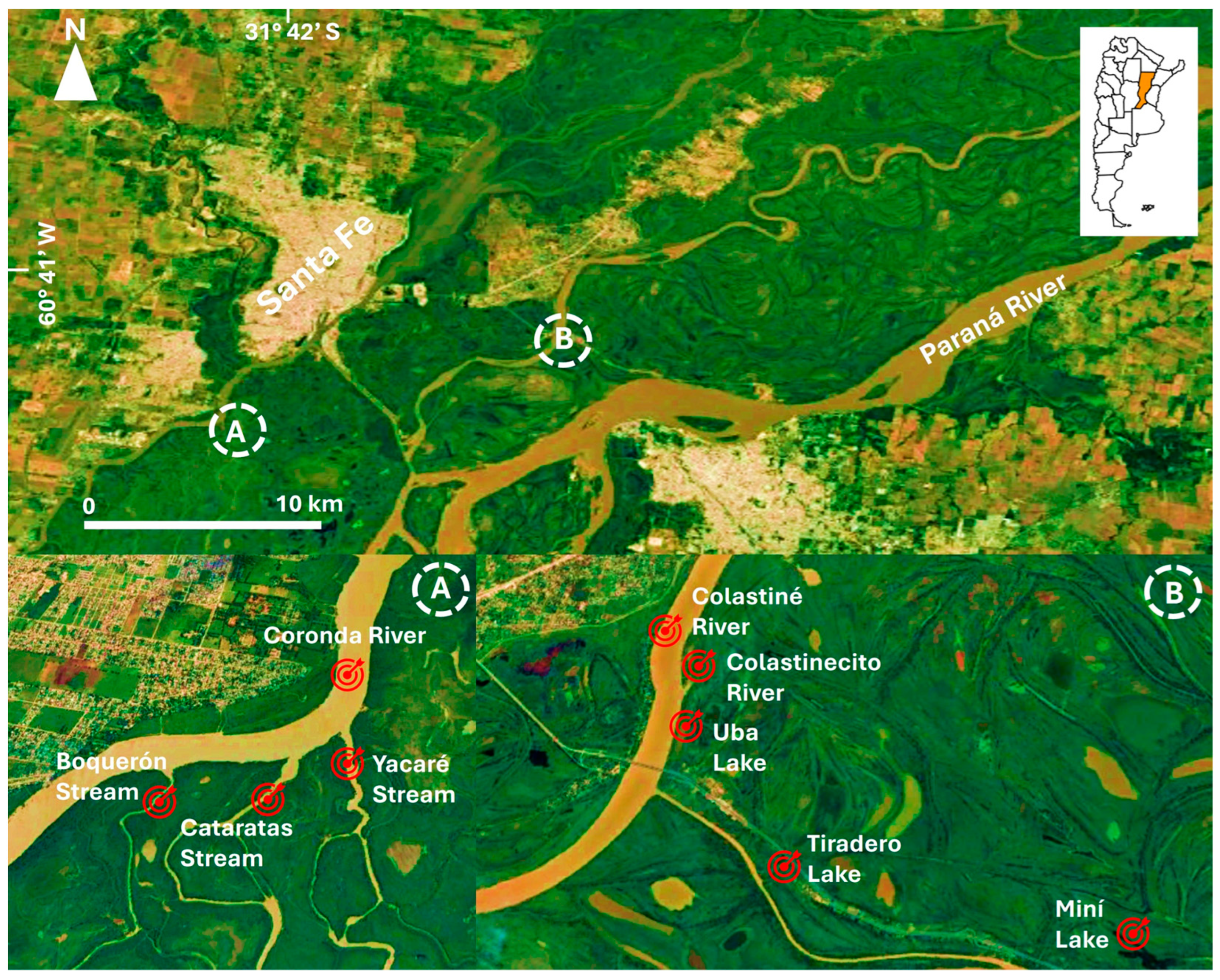
2.1. Study Area
2.2. Sampling and Counting Methods
2.3. Statistical Analyses
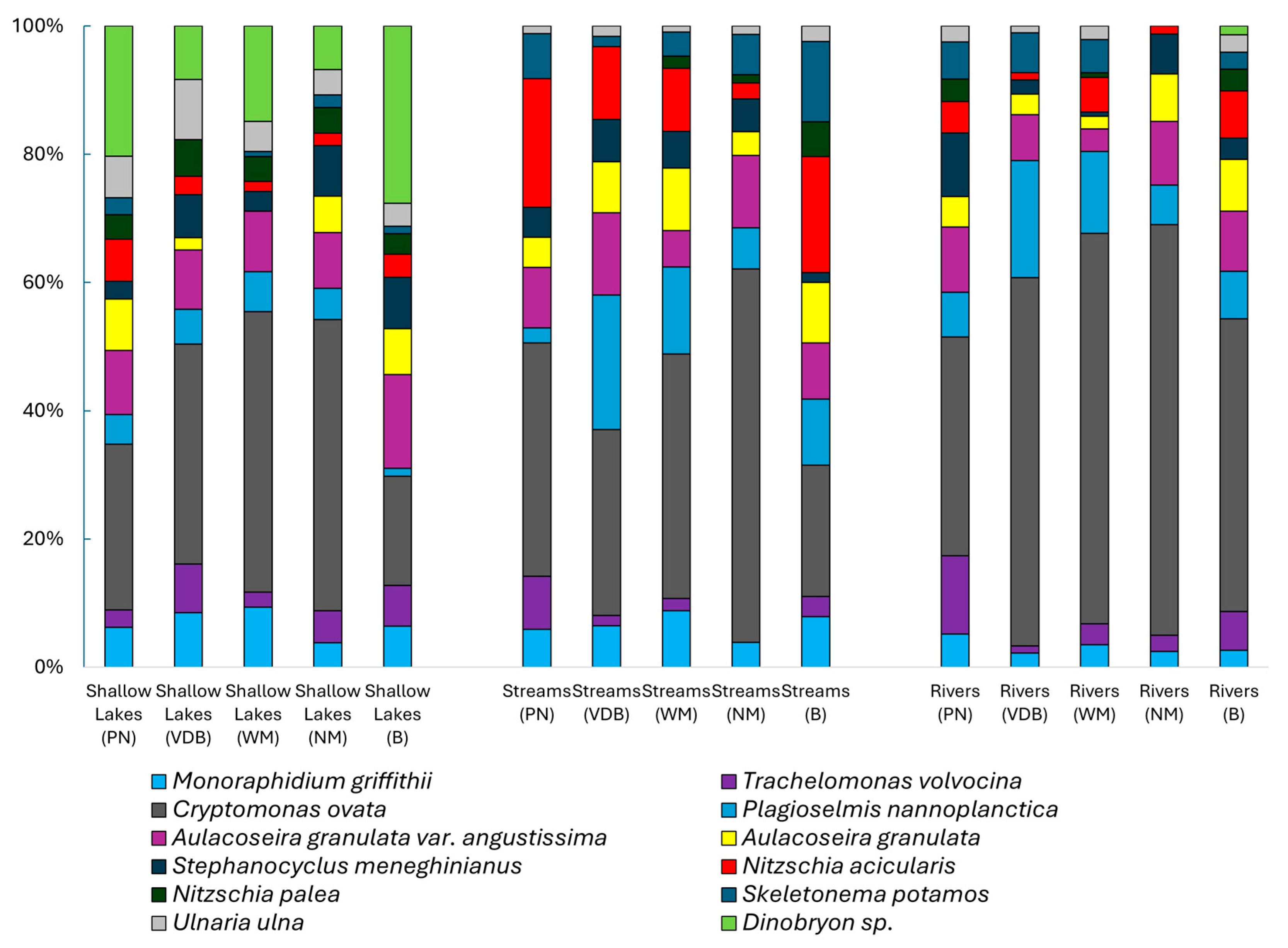
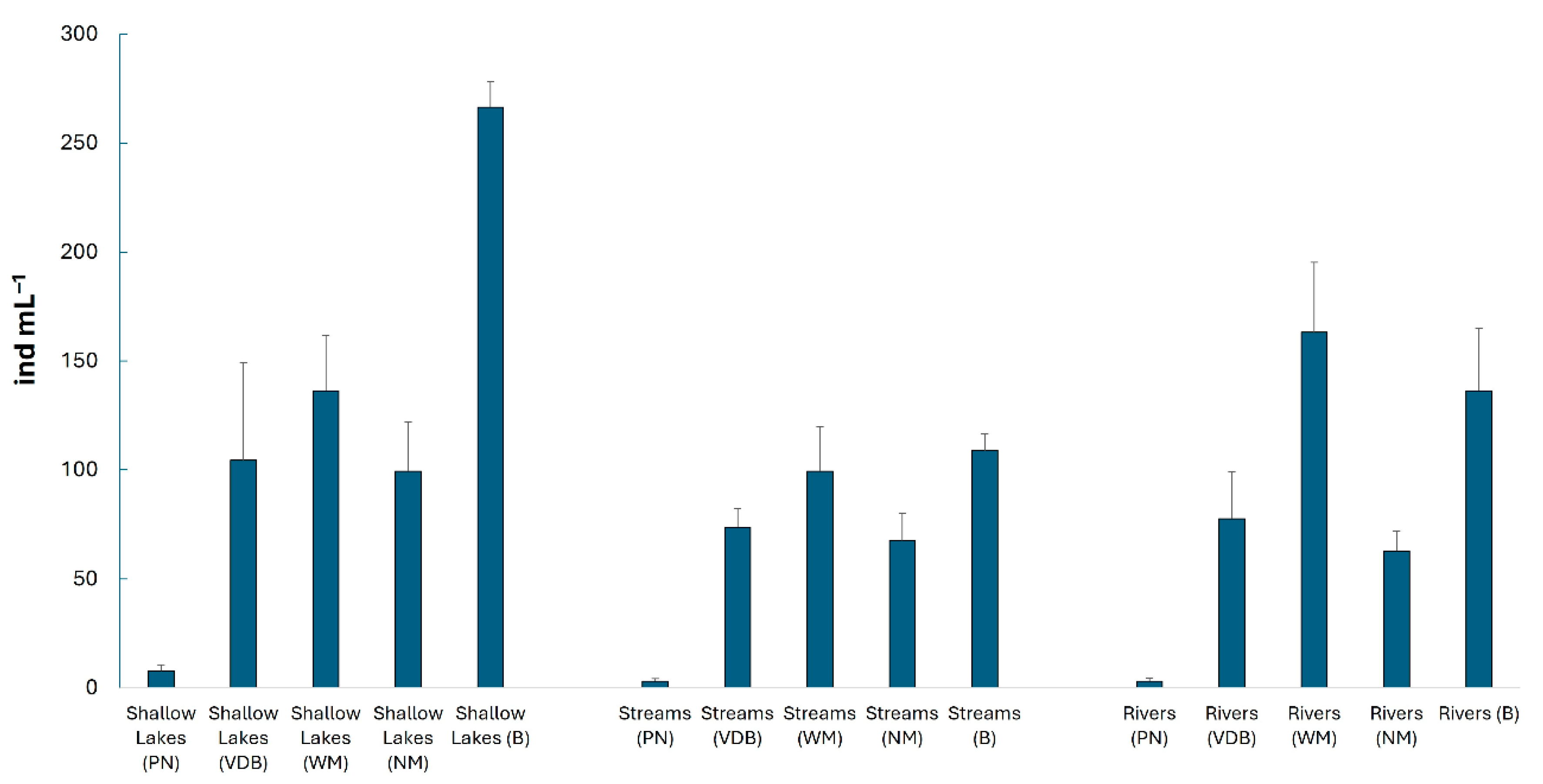
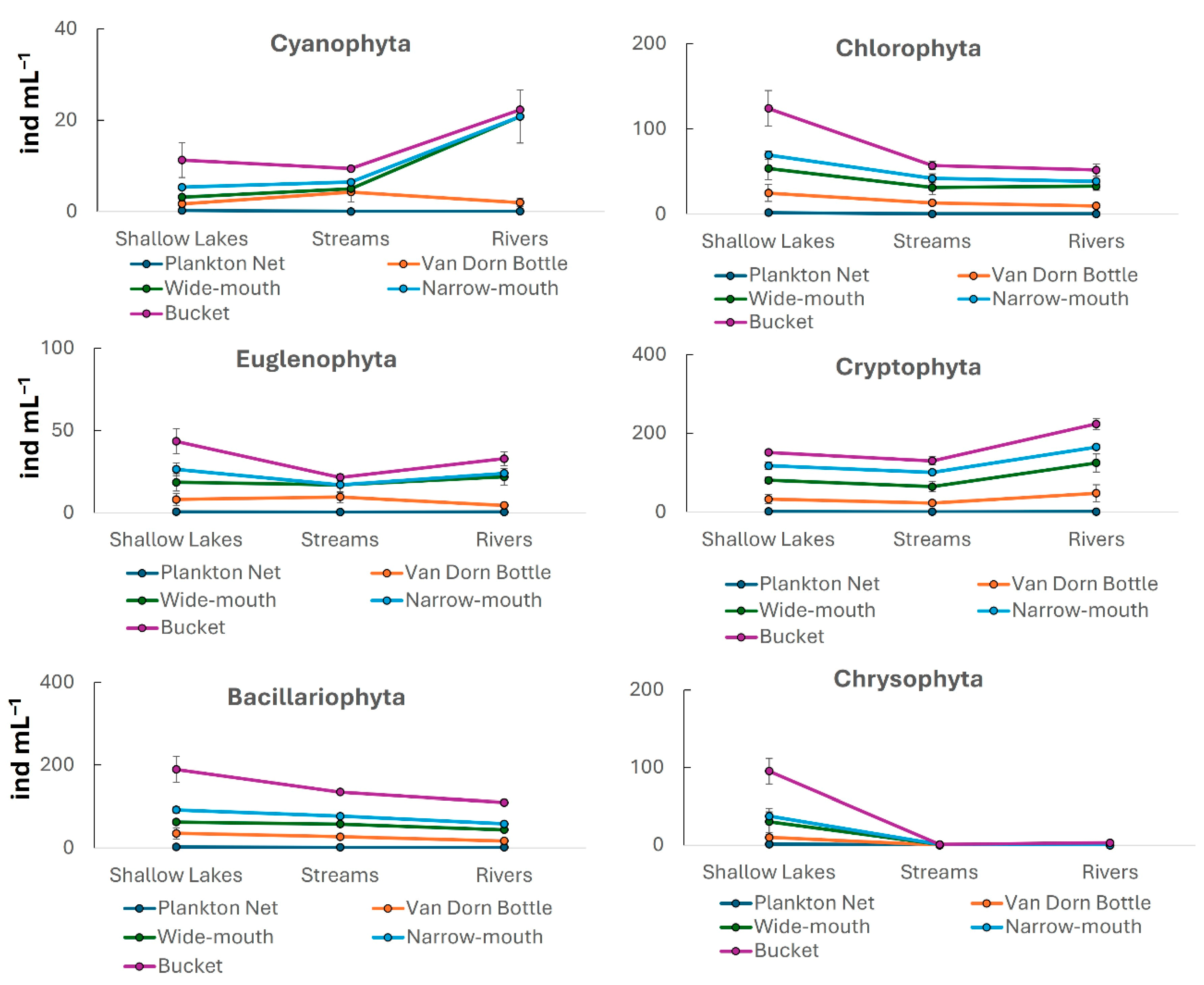
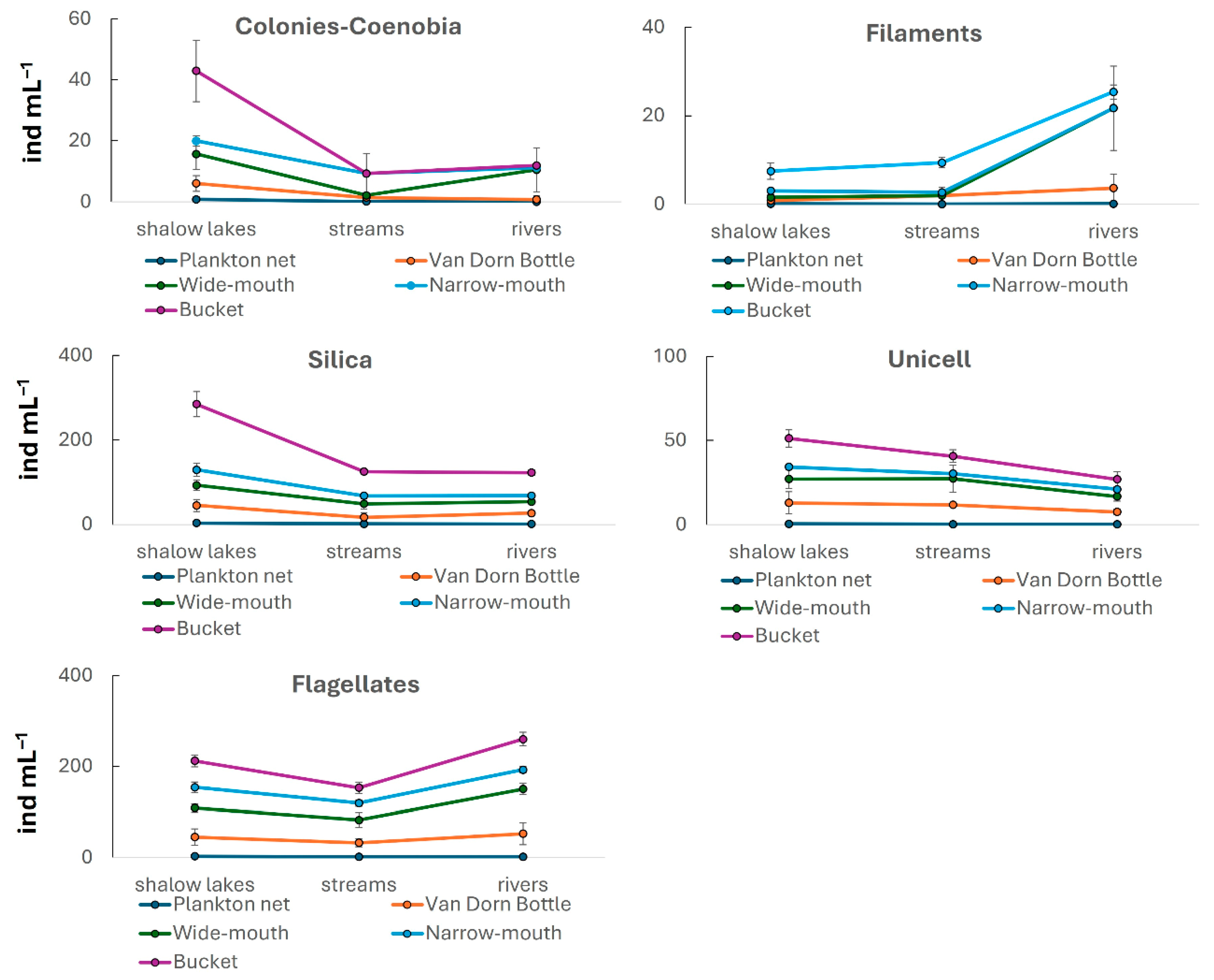
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salmaso, N.; Tolotti, M. Phytoplankton and anthropogenic changes in pelagic environments. Hydrobiologia 2021, 848, 251–284. [Google Scholar] [CrossRef]
- Edwards, K.F.; Litchman, E.; Klausmeier, C.A. Functional traits explain phytoplankton responses to environmental gradients across lakes of the United States. Ecology 2013, 94, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Naselli Flores, L.; Padisák, J. Analysis of morphological traits as a tool to identify the realized niche of phytoplankton populations: What do the shape of planktic microalgae, Anna Karenina and Vincent van Gogh have in common? Hydrobiologia 2024, 851, 733–749. [Google Scholar] [CrossRef]
- Borics, G.; Várbíró, G.; Grigorszky, I.; Krasznai, E.; Szabó, S.; Kiss, K. A new evaluation technique of potamoplankton for the assessment of the ecological status of rivers. Arch. Hydrobiol. 2007, 161, 465–486. [Google Scholar]
- Reavie, E.; Jicha, T.; Angradi, T.; Bolgrien, D.; Hill, B. Algal assemblages for large river monitoring: Comparison among biovolume, absolute and relative abundance metrics. Ecol. Indic. 2010, 10, 167–177. [Google Scholar] [CrossRef]
- Abonyi, A.; Leitão, M.; Lançon, A.M.; Padisák, J. Phytoplankton functional groups as indicators of human impacts along the River Loire (France). Hydrobiologia 2012, 698, 233–249. [Google Scholar] [CrossRef]
- Xie, C.; Hu, C.; Yang, W.; Wu, N.; Liu, Q.; Wei, J.; Wang, C. Phytoplankton species composition as bioindicator in the largest fragmented channel of the Pearl River. China. Environ. Monit. Assess. 2024, 196, 389. [Google Scholar] [CrossRef]
- Frau, D.; Gutierrez, M.F.; Regaldo, L.; Saigo, M.; Licursi, M. Plankton community responses in Pampean lowland streams linked to intensive agricultural pollution. Ecol. Indic. 2021, 120, 106934. [Google Scholar] [CrossRef]
- Frau, D.; Medrano, J.; Calvi, C.; Giorgi, A. Water quality assessment of a neotropical Pampean lowland stream using a phytoplankton functional trait approach. Environ. Monit. Assess. 2019, 191, 681–695. [Google Scholar] [CrossRef]
- Frau, D.; Pineda, A. Phytoplankton Species and Traits Response to a Gradient of Urbanization in Subtropical Lowland Streams. Ecohydrology 2024, 17, e2675. [Google Scholar] [CrossRef]
- Huisman, J.M.; Matthijs, H.C.P.; Visser, P.M. Harmful Cyanobacteria; (Aquatic Ecology Series); Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human exposure to cyanotoxins and their effects on health. Arch. Industr. Hyg. Toxicol. 2013, 64, 305–316. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from Cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- CENMA (Centro Nacional de Monitoreo Ambiental). Protocolo Para el Muestreo de Fitoplancton en Cuerpos de Agua Continentales; Ministerio de Ambiente y Desarrollo Sustentable: San Martín, Argentina, 2013. [Google Scholar]
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea, HELCOM Baltic Sea Environment Proceedings No. 106; Helsinki Commission: Helsinki, Finland, 2006. [Google Scholar]
- Hall, J.A.; Vincent, W.F.; Gibson, J.A.E. Phytoplankton ecology in Antarctic lakes. In Life Under Extreme Conditions; Laybourn-Parry, J., Laybourn, J.D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 25–42. [Google Scholar]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Hunter-Cevera, K.R.; Neubert, M.G.; Olson, R.J.; Solow, A.R.; Shalapyonok, A. Population ecology of a marine picoplankter: Seasonality and regulation at the edge of chaos. Limnol. Oceanogr. 2016, 61, 753–763. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). EPA Method 445.0: In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence; U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Venrick, E.L. How many cells to count? In Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 1978. [Google Scholar]
- Komárek, J.; Fott, B. Chlorophyceae: Chlorococcales. In Das Phytoplankton des Süsswassers; (Die Binnengewässer 16(5)); Huber-Pestalozzi, G., Ed.; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1983. [Google Scholar]
- Tell, G.; Conforti, V. Euglenophyta Pigmentadas de Argentina; (Bibliotheca Phycologica 75); Gebrüder Borntraeger Verlagsbuchhandlung: Stuttgart, Germany, 1986; pp. 1–301. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1991. [Google Scholar]
- Zalocar de Domitrovic, Y.; Maidana, N.I. Taxonomic and ecological studies of the Paraná River diatom flora (Argentina). In Bibliotheca Diatomologica; Kociolek, P., Lange-Bertalot, F., Eds.; J. Cramer: Berlin, Germany, 1997. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. Teil 1: Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Jena, Germany, 1998. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. Teil 2: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Elsevier: Berlin, Germany, 2005. [Google Scholar]
- Komárek, J. Cyanoprokaryota. Teil 3: Heterocytous genera. In Süßwasserflora von Mitteleuropa (Freshwater Flora of Central Europe); Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin, Germany, 2013. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST Palaeontological Statistics, version 3.18; University of Oslo: Oslo, Norway, 2018.
- Upreti, U.; Ram, R.N. Qualitative and Quantitative Estimation of Phytoplankton and their Monsoon-Post Monsoon Fluctuations in Different Water Bodies. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 2662–2669. [Google Scholar] [CrossRef]
- Soondur, S.A.; Appadoo, C.; Bhoyroo, V.R.; Joomun, N.; Bhagooli, R. Diversity, density and photo-physiology of micro-phytoplankton from degraded and non-degraded reefs around Rodrigues Island, Western Indian Ocean. Indo Pac. J. Ocean Life 2023, 7, 108–121. [Google Scholar] [CrossRef]
- Drago, E.C.E.; Amsler, M.L. Suspended sediment at a cross section of the Middle Paraná River: Concentration, granulometry and influence of the main tributaries. In: Sediment budgets. IAHS Publ. 1988, 174, 381–396. [Google Scholar]
- Graham, L.E.; Graham, J.M.; Wilcox, L.W. Algae, 2nd ed.; Benjamin Cummings: Boston, MA, USA, 2009. [Google Scholar]
- Bienfang, P.K. Sinking rates of phytoplankton assemblages in Hawaiian waters. J. Exp. Mar. Biol. Ecol. 1981, 54, 69–76. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Moss, B. Ecology of Freshwaters: Man and Medium, 3rd ed.; Blackwell Science: New York, NY, USA, 1998. [Google Scholar]
- Wetherbee, R.; Perica, D. Mechanisms of motility in algae: Insights from diatom gliding. Protist 1998, 149, 383–393. [Google Scholar]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Morsy, F.M. A simple approach to water and plankton sampling for water microbiological and physicochemical characterizations at various depths in aquatic ecosystems. Ann. Limnol. Int. J. Limnol. 2011, 47, 65–71. [Google Scholar] [CrossRef][Green Version]
- Raun, M. Improving Estimation of Phytoplankton Abundance and Distribution in Ballast Water Discharges. Doctoral Dissertation, Fachbibliothek TIB Hannover, Gottfried Wilhelm Leibniz Universität Hannover, Hannover, Germany, 2022. [Google Scholar][Green Version]
- White, F.M. Fluid Mechanics, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar][Green Version]
- Vogel, S. Life in Moving Fluids: The Physical Biology of Flow; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar][Green Version]
| Method (M) | Environment (E) | Interaction (M × E) | |
|---|---|---|---|
| Cyanophyta | F = 1.89 p = 0.13 | F = 0.01 p = 0.98 | F = 0.89 p = 0.53 |
| Chlorophyta | F = 15.3 p < 0.01 | F = 3.82 p = 0.03 | F = 0.52 p = 0.82 |
| Euglenophyta | F = 6.48 p < 0.01 | F = 1.93 p = 0.16 | F = 0.86 p = 0.55 |
| Cryptophyta | F = 40.17 p < 0.01 | F = 1.82 p = 0.17 | F = 0.21 p = 0.98 |
| Bacillariophyta | F = 30.33 p < 0.01 | F = 1.96 p = 0.15 | F = 0.36 p = 0.93 |
| Chrysophyta | F = 1.34 p = 0.27 | F = 6.16 p < 0.01 | F = 1.01 p = 0.45 |
| Colonies—coenobia | F = 0.60 p = 0.67 | F = 6.11 p = 0.02 | F = 0.27 p = 0.97 |
| Filaments | F = 3.43 p = 0.01 | F = 0.46 p = 0.63 | F = 1.16 p = 0.35 |
| Silica | F = 37.84 p < 0.01 | F = 4.83 p = 0.01 | F = 0.44 p = 0.88 |
| Unicell | F = 14.32 p < 0.01 | F = 1.83 p = 0.17 | F = 0.83 p = 0.58 |
| Flagellates | F = 40.8 p < 0.01 | F = 2.28 p = 0.11 | F = 0.34 p = 0.94 |
| Total phytoplankton | F = 56.31 p < 0.01 | F = 2.38 p = 0.10 | F = 0.49 p = 0.85 |
| Taxa | Av. Dissim | Contrib. % | Cumulative % | Plankton Net | Van Dorn | Wide-Mouth | Narrow-Mouth | Bucket |
|---|---|---|---|---|---|---|---|---|
| Cryptomonas ovata | 19.11 | 25.41 | 25.41 | 1 | 25.1 | 44.8 | 34.4 | 33.7 |
| Aulacoseira granulata var. angustissima | 5.041 | 6.702 | 32.11 | 0.326 | 5.78 | 5.16 | 6.16 | 15.2 |
| Plagioselmis nannoplanctica | 4.886 | 6.496 | 38.61 | 0.156 | 8.26 | 9.92 | 3.57 | 6.72 |
| Aulacoseira granulata | 3.688 | 4.903 | 43.51 | 0.21 | 2.34 | 3.18 | 3.51 | 10.3 |
| Nitzschia acicularis | 3.477 | 4.623 | 48.14 | 0.302 | 2.64 | 4.86 | 1.17 | 10.6 |
| Dinobryon sp. | 3.038 | 4.039 | 52.18 | 0.337 | 2.18 | 4.74 | 1.66 | 17.5 |
| Stephanodiscus meneghinianus | 2.971 | 3.949 | 56.13 | 0.169 | 3.19 | 2.69 | 4.09 | 6.66 |
| Monoraphidium griffithii | 2.901 | 3.857 | 59.98 | 0.193 | 3.64 | 6.43 | 2.15 | 7.4 |
| Skeletonema potamos | 2.525 | 3.357 | 63.34 | 0.149 | 1.52 | 3.01 | 1.67 | 5.66 |
| Euglena oblonga | 2.382 | 3.167 | 66.51 | 0.126 | 3.27 | 5.73 | 1.43 | 1.48 |
| Trachelomonas volvocina | 2.025 | 2.692 | 69.2 | 0.214 | 2.45 | 2.38 | 1.7 | 7.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frau, D. Phytoplankton Sampling: When the Method Shapes the Message. Limnol. Rev. 2025, 25, 45. https://doi.org/10.3390/limnolrev25030045
Frau D. Phytoplankton Sampling: When the Method Shapes the Message. Limnological Review. 2025; 25(3):45. https://doi.org/10.3390/limnolrev25030045
Chicago/Turabian StyleFrau, Diego. 2025. "Phytoplankton Sampling: When the Method Shapes the Message" Limnological Review 25, no. 3: 45. https://doi.org/10.3390/limnolrev25030045
APA StyleFrau, D. (2025). Phytoplankton Sampling: When the Method Shapes the Message. Limnological Review, 25(3), 45. https://doi.org/10.3390/limnolrev25030045







