Transforming Waste into Value: The Role of Physicochemical Treatments in Circular Water Management
Abstract
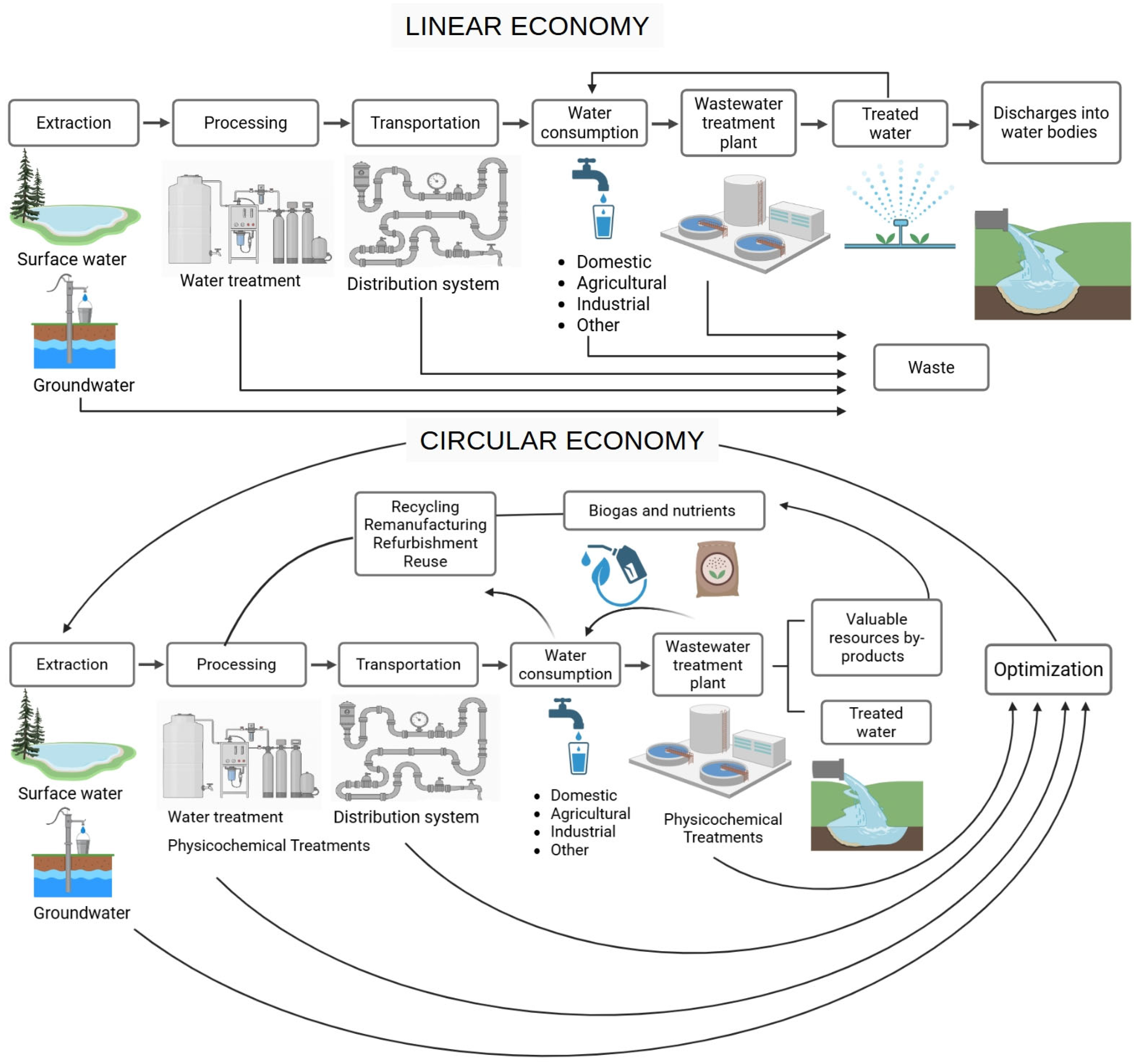
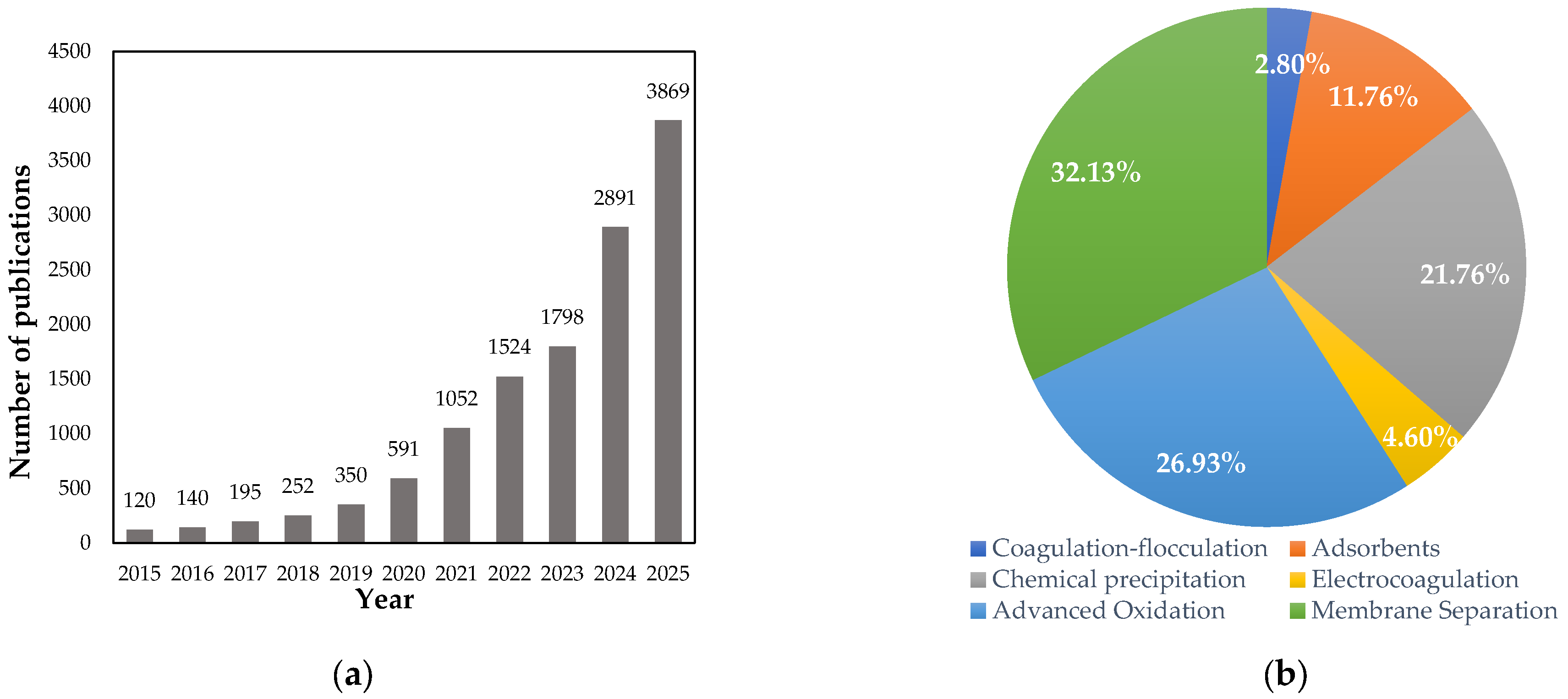
1. Introduction
2. Physicochemical Treatments in Circular Water Management
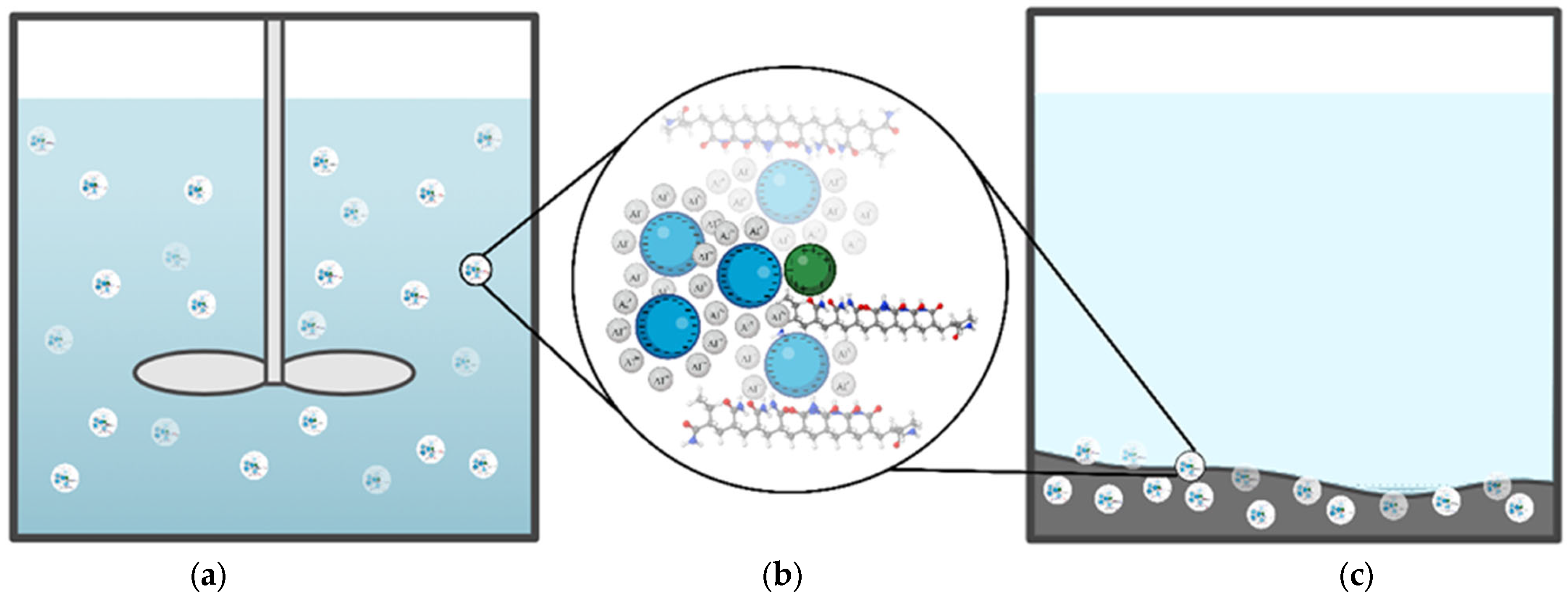
2.1. Coagulation–Flocculation, a Cornerstone in the Removal of Colloidal and Particulate Contaminants
2.2. From Conventional to Green Adsorbents
2.3. Chemical Precipitation of Valuable Resources in Water
2.4. Electrocoagulation as a Viable Candidate for Solar-Powered Water Treatment Processes
2.5. Advanced Oxidation Processes Within the Framework of the Circular Economy
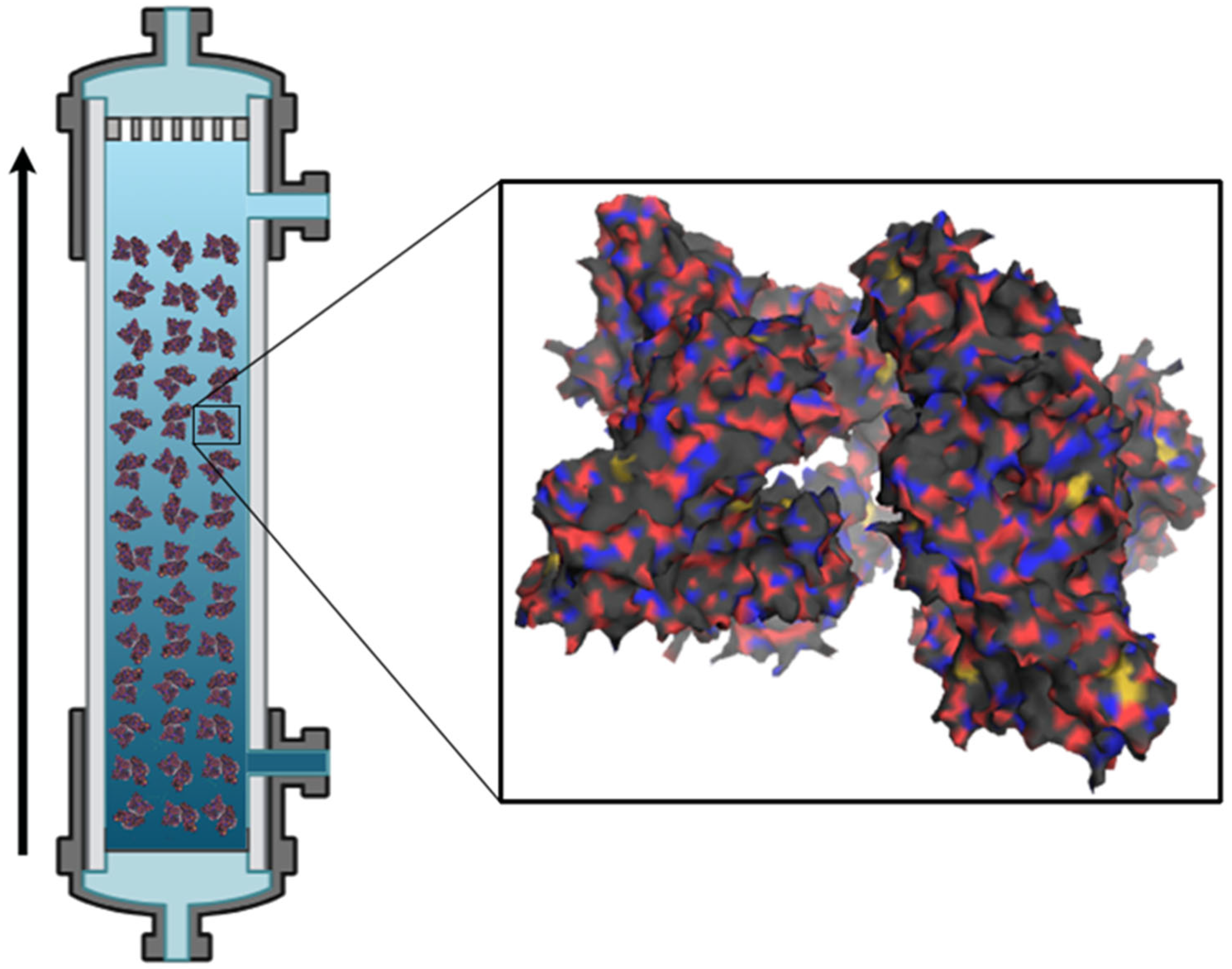
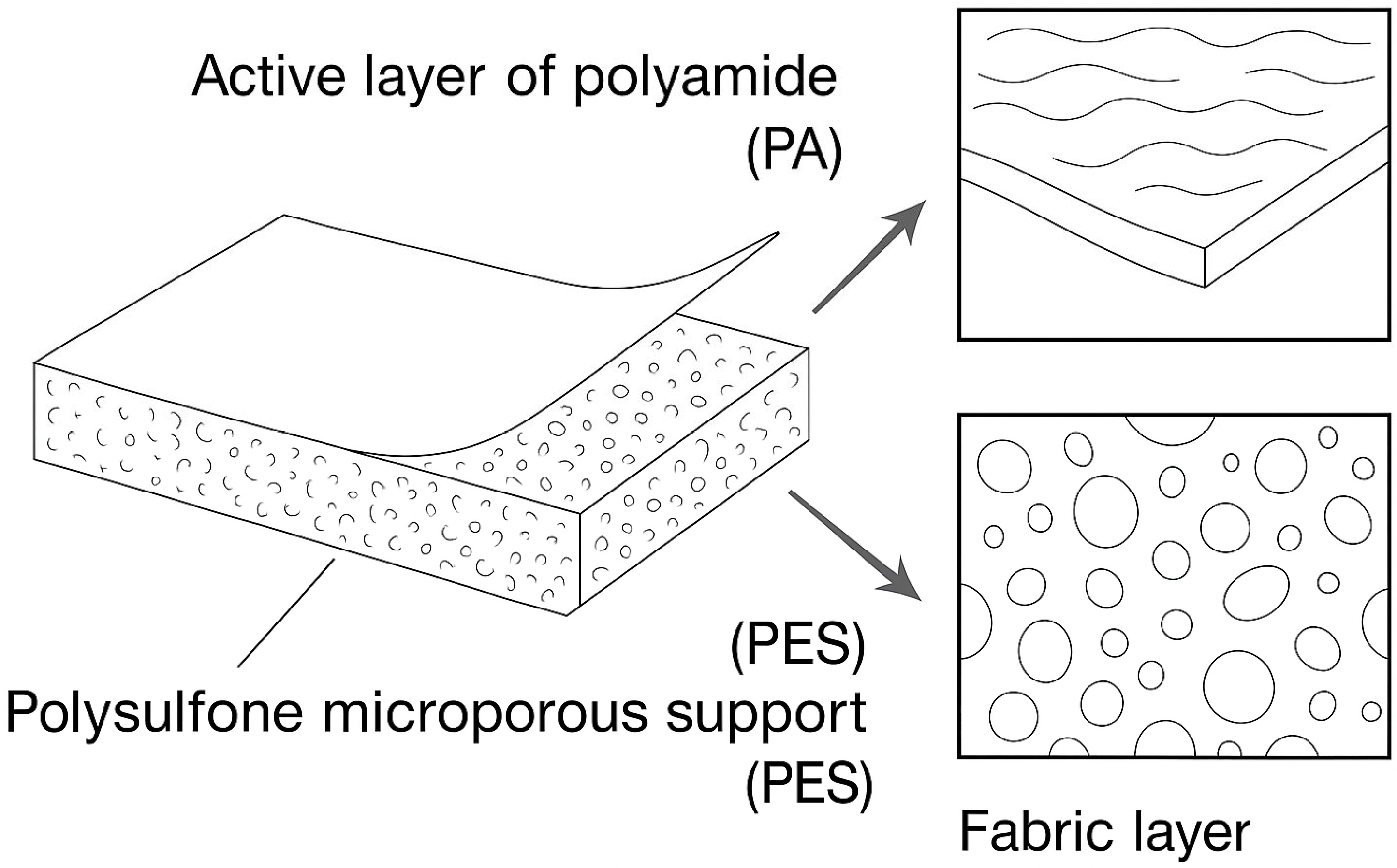
2.6. Recent Advances in Membrane Separation Processes for Circular Water Management
- -
- Microfiltration (UF): UF membranes are effective in removing large particles between 0.1 and 10 µm. The pores allow the retention of sediments, microorganisms, and some colloids. This process is characterized by its low energy consumption because it operates at relatively low pressures, which makes it an attractive option for various applications in wastewater treatment and for the food industry [94]. However, since it retains particles by size exclusion, it is not selective and only rejects larger suspended particles [90].
- -
- Ultrafiltration (UF): is a process that allows the size exclusion separation of macromolecules. The pores of these membranes are in a range of 2 to 100 nm, which allows the removal of colloidal dispersions of clays, sugars, proteins, and microorganisms present in contaminated waters [94]. Generally, these membranes are used in the pharmaceutical industry for the purification of biological products and concentration of solutions. Also, they are used in water treatment to eliminate organic contaminants and some microorganisms [95]. Among its advantages are low-cost, low-pressure requirements and high-water yield.
- -
- Nanofiltration (NF): NF membranes are capable of retaining small organic molecules and multivalent ions, thereby enabling the removal of specific contaminants in effluents, such as hardness, salts, and organic compounds. These membranes typically exhibit pore diameters in the range of 0.5–2 nm and operate under moderate pressures, offering higher water permeability compared to reverse osmosis (RO). The separation process is primarily governed by size exclusion, complemented by electrostatic interactions, the Donnan exclusion principle, and specific solute–membrane interactions. Beyond contaminant removal, NF membranes have demonstrated significant potential for the separation of industrially relevant compounds, as recently highlighted in the literature. Importantly, NF membranes exhibit higher selectivity towards divalent and polyvalent ions, while allowing partial passage of monovalent ions and small organic molecules. Their high solute–solute selectivity allows the fractionation of industrial wastewater. For instance, treating textile effluents recovers salts, organic compounds, and dyes, thereby promoting the valorization of by-products and reducing pollutant discharge. Furthermore, NF has been applied to solute–solute separations from brines, with particular emphasis on the selective extraction of lithium over magnesium, a strategic process for the sustainable exploitation of critical minerals [33,90].
- -
- Reverse osmosis (RO): RO membranes are semipermeable and, due to their very small effective pore size (~0.25 nm), they are capable of rejecting more than 98–99% of ions and molecules, allowing only water to permeate [37,96]. This technology has become a fundamental process for water desalination [97], and is considered the most efficient for converting saline water into high-quality drinking water, ranging from 1 to 10 g/L [22,98]. RO is also widely applied in the pharmaceutical and food industries for the purification of ingredients and final products, due to its high efficiency, low cost, and ease of operation.
3. Circular Economy in Physicochemical Treatments of Wastewater of Microelectronics
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater treatment: A short assessment on available techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Naciones Unidad. Naciones Unidad Paz, Dignidad e Egualdad en un Planeta Sano. Available online: https://www.un.org/sustainabledevelopment/es/2015/09/la-asamblea-general-adopta-la-agenda-2030-para-el-desarrollo-sostenible/ (accessed on 6 August 2025).
- Sulbarán-Rangel, B.; Guzmán González, C.A.; Romero Arellano, V.H. El Nexo Agua-Energía y los Objetivos de Desarrollo Sostenible. In Nexo Agua y Energía en la Implementación de los Objetivos del Desarrollo Sostenible Experiencias en Latinoamérica; Sulbarán-Rangel, B., Vega, C., Eds.; Prometeo Editores: Guadalajara, Mexico, 2022; pp. 7–25. [Google Scholar]
- Firozjaee, T.T.; Golbabaei Kootenaei, F.; Hasanlou, H.; Abdi, J. Water recycling, reuse, and sustainable development goals. In Water, the Environment, and the Sustainable Development Goals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 107–125. [Google Scholar] [CrossRef]
- Sperling, M. Wastewater Characteristics, Treatment and Disposal; Biological Wastewater Treatment Series; IWA Publishing: London, UK, 2007; p. 304. [Google Scholar]
- Gray, N.F. Chapter 14—Introduction to Wastewater Treatment. In Water Technology, 3rd ed.; Gray, N.F., Ed.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 425–459. [Google Scholar] [CrossRef]
- Riffat, R.; Husnain, T. Fundamentals of Wastewater Treatment and Engineering; CRC Press: London, UK, 2022; p. 430. [Google Scholar] [CrossRef]
- Gray, N.F. Chapter 20—Physico-chemical Treatment Processes. In Water Technology, 3rd ed.; Gray, N.F., Ed.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 605–644. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Mattsson, M.; Ulvenblad, P. Perception and awareness of circular economy within water-intensive and bio-based sectors: Understanding, benefits and barriers. J. Clean. Prod. 2024, 464, 142725. [Google Scholar] [CrossRef]
- Dereszewska, A.; Cytawa, S. Circular Economy in Wastewater Treatment Plants—Potential Opportunities for Biogenic Elements Recovery. Water 2023, 15, 3857. [Google Scholar] [CrossRef]
- Nesterov, D.; Barrera-Martínez, I.; Martínez-Sánchez, C.; Sandoval-González, A.; Bustos, E. Approaching the circular economy: Biological, physicochemical, and electrochemical methods to valorize agro-industrial residues, wastewater, and industrial wastes. J. Environ. Chem. Eng. 2024, 12, 113335. [Google Scholar] [CrossRef]
- Szołdrowska, D.; Włóka, D.; Smol, M. Circular economy of water: Rainwater harvesting (RWH) and reuse solutions. Desalination Water Treat. 2025, 323, 101257. [Google Scholar] [CrossRef]
- Stankiewicz, K.; Boroń, P.; Prajsnar, J.; Żelazny, M.; Heliasz, M.; Hunter, W.; Lenart-Boroń, A. Second life of water and wastewater in the context of circular economy—Do the membrane bioreactor technology and storage reservoirs make the recycled water safe for further use? Sci. Total Environ. 2024, 921, 170995. [Google Scholar] [CrossRef]
- Murrieta, M.F.; Cornejo, O.M.; Rivera, F.F.; Nava, J.L. Electrochemical recovery of inorganic value-added products from wastewater: Toward a circular economy model. Curr. Opin. Electrochem. 2024, 46, 101498. [Google Scholar] [CrossRef]
- Ramírez, Á.; Muñoz-Morales, M.; López-Fernández, E.; Fernández-Morales, F.J.; Llanos, J. Advancing circular economy: Critical insights into waste biomass derived carbon electrodes for (bio)electrochemical water treatment. Curr. Opin. Electrochem. 2024, 46, 101492. [Google Scholar] [CrossRef]
- Agnihotri, V.; Chandola, D.; Thathola, P. Grey water treatment and circular economy. Chemosphere 2025, 384, 144433. [Google Scholar] [CrossRef]
- Soo, A.; Kim, J.; Shon, H.K. Technologies for the wastewater circular economy—A review. Desalination Water Treat. 2024, 317, 100205. [Google Scholar] [CrossRef]
- Szołdrowska, D.; Smol, M. The current state of water resources in Poland—Possibilities of water reuse and management by the circular economy. Desalination Water Treat. 2025, 323, 101287. [Google Scholar] [CrossRef]
- Colella, M.; Ripa, M.; Cocozza, A.; Panfilo, C.; Ulgiati, S. Challenges and opportunities for more efficient water use and circular wastewater management: The case of Campania Region, Italy. J. Environ. Manag. 2021, 297, 113171. [Google Scholar] [CrossRef]
- Sharma, G.K.; Khanra, A.; Malla, F.A.; Khan, S.A.; Mishra, S.; Kumar, A.; Singh, A.; Yadav, K.K.; Jena, R.K.; Bhartii, R.K.; et al. Zero-waste perspective for circular bioeconomy of phycoremediation and life cycle assessment: Essentialities, development and challenges. Biomass Bioenergy 2025, 202, 108203. [Google Scholar] [CrossRef]
- Alirezazad, P.; Fazeli Sangani, M.; Khalili Rad, M.; Farhangi, M.B. Integrated treatment approach for recovering nutrients and reducing pollution load of landfill leachate: Targets towards a circular economy. J. Environ. Chem. Eng. 2025, 13, 115175. [Google Scholar] [CrossRef]
- El Aimani, S. Modeling of Reverse Osmosis Water Desalination Powered by Photovoltaic Solar Energy. Green Energy Environ. Technol. 2023, 2, 1–19. [Google Scholar] [CrossRef]
- Varma, J.V.; Veluru, S.; Gudapati, G.; Nikhil, S.; Pallavi, G.J.; Hamzah, H.T. Chapter 2—Life cycle assessment of gray and blackwater management within the circular economy. In Water Use Efficiency, Sustainability and The Circular Economy; Bandh, S.A., Malla, F.A., Halog, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 17–34. [Google Scholar] [CrossRef]
- Shahidi Nasab, M.H.; Hasani Zonoozi, M.; Ghasemi, M. Developing a robust indicator to evaluate circular economy through reuse strategy: A case study of using water treatment sludge as a coagulant for dewatering of iron ore tailings slurry. Miner. Eng. 2024, 219, 109062. [Google Scholar] [CrossRef]
- Idusuyi, N.; Adebayo, M.A.; Igwegbe, C.A.; Aghogho, O.T.; James, A.; Kazeem, R.A. A sustainable approach to dairy wastewater treatment through electrocoagulation: From beverage cans to clean water. Waste Manag. Bull. 2025, 3, 96–106. [Google Scholar] [CrossRef]
- Innocenzi, V.; Zueva, S.; Prisciandaro, M.; De Michelis, I.; Di Renzo, A.; Mazziotti di Celso, G.; Vegliò, F. Treatment of TMAH solutions from the microelectronics industry: A combined process scheme. J. Water Process Eng. 2019, 31, 100780. [Google Scholar] [CrossRef]
- Abo, L.D.; Jayakumar, M.; Jeyapaul, A.S.; Rangaraju, M.; Areti, H.A.; Assefa Adugna, A. Comprehensive review on co-integration of conventional systems and advanced oxidation processes for industrial and agricultural wastewater treatment applications. Environ. Adv. 2025, 20, 100638. [Google Scholar] [CrossRef]
- Koumaki, E.; Konomi, A.; Gkotsis, G.; Nika, M.-C.; Seintos, T.; Statiris, E.; Maragou, N.; Thomaidis, N.S.; Kouris, N.; Mamais, D.; et al. Circular water management in agriculture: Screening of contaminants of emerging concern in a real-world water-soil-crop system and human health risk assessment. J. Hazard. Mater. 2025, 492, 138167. [Google Scholar] [CrossRef]
- Baaloudj, O.; Chiron, S.; Zizzamia, A.R.; Trotta, V.; Buono, D.D.; Puglia, D.; Rallini, M.; Brienza, M. Efficient biochar regeneration for a circular economy: Removing emerging contaminants for sustainable water treatment. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135730. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pai, A.R.; Pasquini, D.; Shao-Yuan, L.; H.P.S, A.K.; Thomas, S. Nanomaterials—State of Art, New Challenges, and Opportunities. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Rodríguez-Alegre, R.; Pérez Megías, L.; Pérez-Moya, M.; García-Montaño, J.; Andecochea Saiz, C.; You, X. Improving efficiency and circularity of selective metals recovery from acid mine drainage. J. Environ. Chem. Eng. 2024, 12, 114655. [Google Scholar] [CrossRef]
- Chang, H.-F.; Lin, J.-Y.; Cheng, T.-M.; Lai, C.-H. Advanced absolute chemical precipitation for high-purity metal recovery in all-types of lithium-ion battery recycling. Sep. Purif. Technol. 2025, 361, 131454. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Nadzri, N.; Wang, R. Thin-film composite (TFC) membranes for sustainable desalination and water reuse: A perspective. Desalination 2025, 599, 118451. [Google Scholar] [CrossRef]
- Giwa, S.O.; Joseph, A.A.; Salis, A.; Abdulyekeen, K.A.; Giwa, A. Application of Circular Economy to Natural Coagulation/Flocculation of Surface Water: A Review. Path Sci. 2024, 10, 2001–2007. [Google Scholar] [CrossRef]
- Liu, M.; Zou, F.; Li, J.; Zhang, Q.; Wang, F.; Hong, G.; Yin, J. Sludge dewaterability improvement by the coagulation/flocculation based conditioning methods: Influencing factors, high performance, and future prospect. Bioresour. Technol. Rep. 2025, 31, 102174. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, D.H.; Eddy, M. Wastewater Engineering Treatment and Reuse; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Hailemariam, R.H.; Woo, Y.C.; Damtie, M.M.; Kim, B.C.; Park, K.D.; Choi, J.S. Reverse osmosis membrane fabrication and modification technologies and future trends: A review. Adv. Colloid Interface Sci. 2020, 276, 102100. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Y.; Fan, L.; Zheng, X.; Wang, J.; Jiang, C.; Xu, S.; Xu, H.; Wang, D. Coagulation effect of polyaluminum-titanium chloride coagulant and the effect of floc aging in fluoride removal: A mechanism analysis. Sep. Purif. Technol. 2023, 325, 124674. [Google Scholar] [CrossRef]
- Hendricks, D.W. Water Treatment Unit Processes; CRC Press: Boca Raton, FL, USA, 2018; p. 1266. [Google Scholar] [CrossRef]
- Lin, S.H.; Yang, C.R. Chemical and physical treatments of chemical mechanical polishing wastewater from semiconductor fabrication. J. Hazard. Mater. 2004, 108, 103–109. [Google Scholar] [CrossRef]
- Xu, X.; Zou, Z.; Guo, X.; Liang, S.; Yang, F.; Chen, S.; Yu, W.; Duan, H.; Yuan, S.; Yang, J. Comprehensive evaluation of bioavailable phosphorus in biochar synthesized by co-pyrolysis of sewage sludge and straw ash. Sci. Total Environ. 2024, 954, 176679. [Google Scholar] [CrossRef]
- Usman, I.M.T.; Ho, Y.-C.; Lam, M.-K.; Show, P.-L.; Sujarwo, W. Continuous-Flow Grafting of LENFLOCTM Coagulant for Water Treatment toward Circular Economy. Water 2023, 15, 2484. [Google Scholar] [CrossRef]
- Liu, Y.; Biswas, B.; Hassan, M.; Naidu, R. Green Adsorbents for Environmental Remediation: Synthesis Methods, Ecotoxicity, and Reusability Prospects. Processes 2024, 12, 1195. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, B.; Wang, T.; Yılmaz, M.; Sharma, G.; Kumar, A.; Shi, H. Multi-mechanism synergistic adsorption of lead and cadmium in water by structure-functionally adapted modified biochar: A review. Desalination Water Treat. 2025, 322, 101156. [Google Scholar] [CrossRef]
- Kumar, A.; Indhur, R.; Sheik, A.G.; Krishna, S.B.N.; Kumari, S.; Bux, F. A review on conventional and novel adsorbents to boost the sorption capacity of heavy metals: Current status, challenges and future outlook. Environ. Technol. Rev. 2024, 13, 521–543. [Google Scholar] [CrossRef]
- Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Aniagor, C.O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M.I.; López-Maldonado, E.A.; Ihara, I.; et al. Methods to prepare biosorbents and magnetic sorbents for water treatment: A review. Environ. Chem. Lett. 2023, 21, 2337–2398. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent advances on sustainable adsorbents for the remediation of noxious pollutants from water and wastewater: A critical review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Madeła, M.; Skuza, M. Towards a Circular Economy: Analysis of the Use of Biowaste as Biosorbent for the Removal of Heavy Metals. Energies 2021, 14, 5427. [Google Scholar] [CrossRef]
- Recio-Colmenares, C.L.; Flores-Gómez, J.; Morales Rivera, J.P.; Palacios Hinestroza, H.; Sulbarán-Rangel, B. Green Materials for Water and Wastewater Treatment: Mechanisms and Artificial Intelligence. Processes 2025, 13, 566. [Google Scholar] [CrossRef]
- Tsouko, E.; Pilafidis, S.; Kourmentza, K.; Gomes, H.I.; Sarris, G.; Koralli, P.; Papagiannopoulos, A.; Pispas, S.; Sarris, D. A sustainable bioprocess to produce bacterial cellulose (BC) using waste streams from wine distilleries and the biodiesel industry: Evaluation of BC for adsorption of phenolic compounds, dyes and metals. Biotechnol. Biofuels Bioprod. 2024, 17, 40. [Google Scholar] [CrossRef]
- Dahman, Y.; Deonanan, K.; Dontsos, T.; Iammatteo, A. Nanopolymers. In Nanotechnology and Functional Materials for Engineers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–144. [Google Scholar] [CrossRef]
- Djedidi, Z.; Khaled, J.B.; Cheikh, R.B.; Blais, J.-F.; Mercier, G.; Tyagi, R.D. Comparative study of dewatering characteristics of metal precipitates generated during treatment of monometallic solutions. Hydrometallurgy 2009, 95, 61–69. [Google Scholar] [CrossRef]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling—Closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Sniatala, B.; Al-Hazmi, H.E.; Sobotka, D.; Zhai, J.; Makinia, J. Advancing sustainable wastewater management: A comprehensive review of nutrient recovery products and their applications. Sci. Total Environ. 2024, 937, 173446. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Garcia, E.A.; Ballesteros, F.C.; Garcia-Segura, S.; Lu, M.-C. A review on chemical precipitation in carbon capture, utilization and storage. Sustain. Environ. Res. 2022, 32, 45. [Google Scholar] [CrossRef]
- Phu, T.K.C.; Nguyen, P.L.; Phung, T.V.B. Recent progress in highly effective electrocoagulation-coupled systems for advanced wastewater treatment. iScience 2025, 28, 111965. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process– a systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef]
- del Real-Olvera, J.; Morales-Rivera, J.; González-López, A.P.; Sulbarán-Rangel, B.; Zúñiga-Grajeda, V. Adsorption of Organic Pollutants from Cold Meat Industry Wastewater by Electrochemical Coagulation: Application of Artificial Neural Networks. Water 2020, 12, 3040. [Google Scholar] [CrossRef]
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Abbas, S.A. Electrocoagulation using a rotated anode: A novel reactor design for textile wastewater treatment. J. Environ. Manag. 2016, 176, 34–44. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A review on decontamination of arsenic-contained water by electrocoagulation: Reactor configurations and operating cost along with removal mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Kim, K.; Candeago, R.; Rim, G.; Raymond, D.; Park, A.-H.A.; Su, X. Electrochemical approaches for selective recovery of critical elements in hydrometallurgical processes of complex feedstocks. iScience 2021, 24, 102374. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Vasudevan, S.; Oturan, M.A. Electrochemistry: As cause and cure in water pollution—An overview. Environ. Chem. Lett. 2014, 12, 97–108. [Google Scholar] [CrossRef]
- Bajpai, M.; Seidu, I.; Gengec, E. Electrode design innovations in electrocoagulation: Passivation control, sludge valorization, and cost perspectives. J. Water Process Eng. 2025, 77, 108637. [Google Scholar] [CrossRef]
- Ammar, M.; Yousef, E.; Mahmoud, M.A.; Ashraf, S.; Baltrusaitis, J. A Comprehensive Review of the Developments in Electrocoagulation for the Removal of Contaminants from Wastewater. Separations 2023, 10, 337. [Google Scholar] [CrossRef]
- Etafo, N.O.; Adekanmi, D.G.; Awobifa, O.S.; Torres, J.R.P.; Herrera, L.A.I.; Awobifa, O.A. Clean and green: The multifaceted solution of the electrocoagulation technology in emerging contaminants in wastewater. Discov. Civ. Eng. 2025, 2, 103. [Google Scholar] [CrossRef]
- Graça, N.S.; Rodrigues, A.E. The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters. Clean Technol. 2022, 4, 1020–1053. [Google Scholar] [CrossRef]
- Sharma, G.; Choi, J.; Shon, H.K.; Phuntsho, S. Solar-powered electrocoagulation system for water and wastewater treatment. Desalination Water Treat. 2011, 32, 381–388. [Google Scholar] [CrossRef]
- Cetinkaya, A.Y. Integration of electrocoagulation and solar energy for sustainable wastewater treatment: A thermodynamic and life cycle assessment study. Environ. Monit. Assess. 2025, 197, 224. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Vallejo Rodríguez, R.; San Juan Farfán, R.E.; León Becerril, E.; Ojeda Castillo, V.; Osuna Laveaga, D.; Flores Payán, V. Proposal degradation pathway of BPA during ozone reaction. Nova Sci. 2023, 15, 1–15. [Google Scholar] [CrossRef]
- Momotko, M.; Koundle, P.; Latif, S.; Ilyas, M.; Samejo, B.A.; Imran, M.; Yin, Z.; Zhan, J.; Kong, L.; Boczkaj, G.; et al. Chapter 4—Advanced oxidation processes (AOPs) in wastewater treatments. In Advanced Technologies in Wastewater Treatment; Castro-Muñoz, R., Basile, A., Cassano, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 79–114. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, S.; Wang, D.; An, Q. Fenton-Like Reaction: Recent Advances and New Trends. Chem. A Eur. J. 2024, 30, e202304337. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Piya-areetham, P.; Shenchunthichai, K.; Hunsom, M. Application of electrooxidation process for treating concentrated wastewater from distillery industry with a voluminous electrode. Water Res. 2006, 40, 2857–2864. [Google Scholar] [CrossRef]
- Noman, E.A.; Ali Al-Gheethi, A.; Al-Sahari, M.; Yashni, G.; Mohamed, R.M.S.R.; Soon, C.F.; Nguyen, H.-H.T.; Vo, D.-V.N. An insight into microelectronics industry wastewater treatment, current challenges, and future perspectives: A critical review. Appl. Water Sci. 2024, 14, 64. [Google Scholar] [CrossRef]
- Wang, C.; Deng, S.-H.; You, N.; Bai, Y.; Jin, P.; Han, J. Pathways of wastewater treatment for resource recovery and energy minimization towards carbon neutrality and circular economy: Technological opinions. Front. Environ. Chem. 2023, 4, 1255092. [Google Scholar] [CrossRef]
- Bączkowska, E.; Pierpaoli, M.; Gamoń, F.; Luczkiewicz, A.; Fudala-Ksiazek, S.; Bray, R.; Szopińska, M. On-site medical wastewater treatment enabling sustainable water reclamation: Merged advanced oxidation process for disinfection, toxicity, and contaminants removal. J. Water Process Eng. 2025, 72, 107562. [Google Scholar] [CrossRef]
- Phillips, R.B.; James, R.R.; Magnuson, M.L. Functional categories of microbial toxicity resulting from three advanced oxidation process treatments during management and disposal of contaminated water. Chemosphere 2020, 238, 124550. [Google Scholar] [CrossRef] [PubMed]
- Dhamorikar, R.S.; Lade, V.G.; Kewalramani, P.V.; Bindwal, A.B. Review on integrated advanced oxidation processes for water and wastewater treatment. J. Ind. Eng. Chem. 2024, 138, 104–122. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Sabahat, S.; Shah, N.S.; Ajab, H.; Iqbal, J.; Ullah, F. Electrochemical Advanced Oxidation Processes as a feasible approach towards treatment of pesticides contaminated water and environmental sustainability: A review. J. Water Process Eng. 2025, 70, 107083. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Karim, M.A.H.; Hama, S. Biochar-based catalysts: An efficient and sustainable approach for water remediation from organic pollutants via advanced oxidation processes. J. Environ. Manag. 2025, 390, 126245. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.; Zhang, L.; Li, Y.; Li, Y.; Kubuki, S.; Wang, J.; Zhang, B. Revolutionizing water treatment: Polymerization pathways in advanced oxidation processes. Appl. Catal. B Environ. Energy 2025, 380, 125747. [Google Scholar] [CrossRef]
- Tayeh, Y.A. A comprehensive review of reverse osmosis desalination: Technology, water sources, membrane processes, fouling, and cleaning. Desalination Water Treat. 2024, 320, 100882. [Google Scholar] [CrossRef]
- Zuo, K.; Wang, K.; DuChanois, R.M.; Fang, Q.; Deemer, E.M.; Huang, X.; Xin, R.; Said, I.A.; He, Z.; Feng, Y.; et al. Selective membranes in water and wastewater treatment: Role of advanced materials. Mater. Today 2021, 50, 516–532. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Razi, F.; Sawada, I.; Takagi, R.; Ohmukai, Y.; Matsuyama, H. Improvement of antibiofouling performance of a reverse osmosis membrane through biocide release and adhesion resistance. Sep. Purif. Technol. 2013, 105, 106–113. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mahmoud, S.A.; Mohamed, A.A. Nanomaterials-modified reverse osmosis membranes: A comprehensive review. RSC Adv. 2024, 14, 18879–18906. [Google Scholar] [CrossRef]
- Chang, Z.H.; Sum, J.Y.; Lau, W.J.; Ang, W.L.; Teow, Y.H.; Ooi, B.S.; Yeap, S.P. Current State-of-the-Art of Non-Reverse Osmosis-like Forward Osmosis Technology. J. Membr. Sci. 2024, 711, 123209. [Google Scholar] [CrossRef]
- Solís-Carvajal, C.A.; Vélez Pasos, C.A.; Ramírez-Navas, J.S. Tecnología de membranas: Ultrafiltración. Entre Cienc. Ing. 2017, 11, 26–36. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, J.; Liu, F.; Xu, B.M.; Pan, Y. Recent Progress of Adsorptive Ultrafiltration Membranes in Water Treatment—A Mini Review. Membranes 2022, 12, 519. [Google Scholar] [CrossRef]
- Rodríguez, B.; Oztürk, D.; Rosales, M.; Flores, M.; García, A. Antibiofouling thin-film composite membranes (TFC) by in situ formation of Cu-(m-phenylenediamine) oligomer complex. J. Mater. Sci. 2018, 53, 6325–6338. [Google Scholar] [CrossRef]
- Valdes, H.; Saavedra, A.; Flores, M.; Vera-Puerto, I.; Avina, H.; Belmonte, M. Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies. Membranes 2021, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Shouman, L.A.; Afify, R.M.; Fadel, D.A.; Esawy, M.H. Fouling effect on Reverse Osmosis (RO) membranes performance in desalination plant. Desalination Water Treat. 2024, 319, 100502. [Google Scholar] [CrossRef]
- Naidu, M.; Zhou, S.; Zhang, G.; Manayil, J.C.; Wu, Z. Enhancing Nanofiltration in Thin Film Nanocomposite Membranes Using Bi-Metal Modified Biochar Nanofillers. Sep. Purif. Technol. 2025, 352, 128236. [Google Scholar] [CrossRef]
- Qin, Q.; Lu, H.; Zhu, Z.; Qiu, Y.; Liu, X.; Yin, D. Safety and security of household water purifiers against pathogenic microbial contamination and bio-risk evaluation of their microbial community structures. Sep. Purif. Technol. 2025, 357, 130012. [Google Scholar] [CrossRef]
- Sanchis-Perucho, P.; Aguado, D.; Ferrer, J.; Seco, A.; Robles, Á. A comprehensive review of the direct membrane filtration of municipal wastewater. Environ. Technol. Innov. 2024, 35, 103732. [Google Scholar] [CrossRef]
- Shetty Kodialbail, V.; Sophia, S. Concept of zero liquid dischare—Present scenario and new opportunities for economically viable solution. In Concept of Zero Liquid Discharge; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–31. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Choi, Y.; Lee, S. Treatment of Semiconductor Wastewater Containing Tetramethylammonium Hydroxide (TMAH) Using Nanofiltration, Reverse Osmosis, and Membrane Capacitive Deionization. Membranes 2023, 13, 336. [Google Scholar] [CrossRef]
- Strathmann, H. Chapter 6 Ion-Exchange Membrane Processes in Water Treatment. In Sustainability Science and Engineering; Escobar, I.C., Schäfer, A.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 141–199. [Google Scholar] [CrossRef]
- Alawa, B.; Galodiya, M.N.; Chakma, S. Source reduction, recycling, disposal, and treatment. In Hazardous Waste Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 67–88. [Google Scholar] [CrossRef]
- Salas Quintero, D.; Zapata, M.A.; Guerrero, J. Modelo de costos para el tratamiento de las aguas residuales en la región. Sci. Tech. 2007, XIII, 591–596. [Google Scholar]
| Type of Treated Stream | Physicochemical Technology | Circularity Potential | Reference |
|---|---|---|---|
| Landfill leachate | Adsorbents (natural zeolite), Coagulation–flocculation (polyferric sulfate), Chemical precipitation (struvite) | Recovery of N (89.4%), P (63.9%), and K (47.8%); sludge use as fertilizer | [21] |
| Dairy wastewater | Electrocoagulation (recycled Al electrodes) | COD * (91.67%) and BOD ** (95.36%) removal; treated water reuse; electrode recycling | [25] |
| Domestic greywater | Coagulation–flocculation, Adsorbents, Advanced oxidation, Membrane separation (MBR) | Reuse for irrigation and non-potable domestic purposes; use of solid residues | [16] |
| Textile wastewater | Coagulation–flocculation, Advanced oxidation (activated H2O2) | Color and aromatic compounds removal; process water recovery | [26] |
| Refinery wastewater | Dissolved air flotation and Advanced oxidation | Recovery of oils (>90%); treated water reuse in industrial processes | [23] |
| Agro-industrial wastewater | Advanced oxidation (ozone, photocatalysis) | Irrigation-grade water; pathogen reduction; valorization of residual biomass | [27] |
| Industrial brackish water | Membrane separation (electrodialysis) | Recovery of water and concentrated salts for reuse | [28] |
| Oily industrial effluents | Membrane separation (ceramic ultrafiltration) | Water reuse; oil recovery; high material durability | [13] |
| Mining wastewater | Adsorbents (modified industrial by-products) | Recovery of metals for reintegration into production processes | [29] |
| Hospital wastewater | Advanced oxidation (photocatalysis) and Filtration | Pharmaceuticals removal (>90%); reuse of water for non-potable purposes | [20,30] |
| Battery leachates | Chemical precipitation; selective adsorption; membrane separation | Recovery of critical metals (Li, Co, Ni) for battery manufacturing | [31,32] |
| Desalination brines | Nanofiltration; reverse osmosis; electrodialysis | Recovery of salts and critical minerals (Li, Mg, Sr, B) | [22,33] |
| Type of Wastewater | Main Contaminants | Removal Efficiency (%) | Technical Information | References |
|---|---|---|---|---|
| Textile | Dyes, COD, turbidity | 85–99 | 20–60 min; Al or Fe electrodes; pH 6–8; 10–25 mA/cm2 | [66] |
| Tannery | Chromium, dyes, organic matter | 80–98 | 30–90 min; Fe/Al electrodes; pH 4–7 | [64] |
| Mining/Metallurgy | Arsenic, Se, heavy metals | 85–99 | 15–40 min; Fe or Al electrodes; pH 6–8 | [67] |
| Food industry (dairy, oils) | Fats, oils, proteins | 80–95 | 30–60 min; Al/Fe electrodes; NaCl as supporting electrolyte | [68] |
| Municipal wastewater | Turbidity, pathogens, antibiotics | >99 | 10–30 min; Al/Fe electrodes; <20 mA/cm2 | [14,68] |
| Emerging contaminants | Micro-/nanoplastics, diclofenac, pesticides | 80–99 | 10–90 min; Al > Fe electrodes; neutral pH | [69,70] |
| Membrane Type | Permeate | Concentrate | Reference |
|---|---|---|---|
| Microfiltration (MF) | Rinse water or simple industrial processes. | Solids and microorganisms; composting or anaerobic digestion. | [37,95] |
| Ultrafiltration (UF) | Pretreated water for RO; use in food and pharmaceutical industries. | Proteins and polysaccharides; industrial or energetic valorization. | [95] |
| Nanofiltration (NF) | Agricultural irrigation or textile industry. | Salts, nutrients, and critical minerals (lithium, magnesium). | [22,33,92] |
| Reverse osmosis (RO) | Drinking, pharmaceutical, or food-grade water. | High salinity; crystallization and recovery of salts/minerals. | [22,37] |
| Technology | Advantages | Disadvantages | Economic Cost | Circular Economy Opportunities |
|---|---|---|---|---|
| Coagulation– Flocculation | Simple operation; effective for turbidity, colloids, and phosphorus removal | Generates large volumes of sludge; requires chemical inputs | Low | Sludge reuse (soil, construction) |
| Adsorption | High efficiency for organics and metals; regenerable; can use waste-based adsorbents | Adsorbent exhaustion and regeneration challenges; variable selectivity | Medium-High (depending on adsorbent) | Spent adsorbents → biochar/catalysts |
| Chemical Precipitation | High removal of metals and nutrients; enables recovery of struvite and salts | Produces chemical sludge; requires reagent addition | Low-Medium | Nutrient (struvite) and metal recovery |
| Electrocoagulation | No external coagulants; low-toxicity sludge; effective for diverse contaminants | Electrode consumption and passivation; energy demand | Medium | Metal recovery; H2 valorization |
| Advanced Oxidation Processes (AOPs) | Degrade refractory organics; non-selective; compatible with renewable energy | High energy and chemical use; potential formation of by-products (e.g., bromates) | High | Catalyst reuse; safe water reuse |
| Membrane Separation (MF/UF/NF/RO) | High selectivity; produces reusable permeate; concentrate valorization possible | Membrane fouling; concentrate management required | Medium-High | Permeate reuse; salt/mineral recovery |
| Pollutants | |||
|---|---|---|---|
| Organic | Inorganic | Heavy Metals | |
| 1-methyl-2-pyrrolidone | Ammonium | Aluminum | Silver |
| Acetic acid | Calcium fluoride | Arsenic | Tin |
| Acetone | Fluoride | Cadmium | Titanium |
| Ethyl lactate | Hydrogen peroxide | Chromium | Vanadium |
| Glycerol | Nitrates | Cobalt | Zinc |
| Organic solvents | Potassium hydroxide | Copper | |
| Perfluorooctanoic acid (PFOA) | Phosphate | Iron | |
| Phenol | Sulfates | Lead | |
| Phosphoric acid | Manganese | ||
| Propylene glycol methyl ether acetate | Mercury | ||
| Pyrazole | Nickel | ||
| Tetramethylammonium hydroxide (TMAH) | Platinum | ||
| Costs | Activity | |
|---|---|---|
| Inversion | Soil Studies | |
| Design and engineering | ||
| Construction | ||
| Land | ||
| Administrative, Legal and Financial Expenses | ||
| Replacement | ||
| Management | ||
| Operation | Operation and maintenance | Replacement |
| Reparations | ||
| Energy | ||
| Chemicals | ||
| Water quality monitoring | ||
| Operation and maintenance manpower | ||
| Sludge disposal | ||
| Administration | Equipment maintenance | |
| Administrative personal | ||
| Overheads | ||
| Environmental rates | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera-Rojas, J.; Muro-Medina, C.V.; Palacios-Hinestroza, H.; Flores-Payán, V.; Osuna-Laveaga, D.R.; Sulbarán-Rangel, B. Transforming Waste into Value: The Role of Physicochemical Treatments in Circular Water Management. Limnol. Rev. 2025, 25, 42. https://doi.org/10.3390/limnolrev25030042
Barrera-Rojas J, Muro-Medina CV, Palacios-Hinestroza H, Flores-Payán V, Osuna-Laveaga DR, Sulbarán-Rangel B. Transforming Waste into Value: The Role of Physicochemical Treatments in Circular Water Management. Limnological Review. 2025; 25(3):42. https://doi.org/10.3390/limnolrev25030042
Chicago/Turabian StyleBarrera-Rojas, Jesús, Carlos Vladimir Muro-Medina, Hasbleidy Palacios-Hinestroza, Valentín Flores-Payán, Daryl Rafael Osuna-Laveaga, and Belkis Sulbarán-Rangel. 2025. "Transforming Waste into Value: The Role of Physicochemical Treatments in Circular Water Management" Limnological Review 25, no. 3: 42. https://doi.org/10.3390/limnolrev25030042
APA StyleBarrera-Rojas, J., Muro-Medina, C. V., Palacios-Hinestroza, H., Flores-Payán, V., Osuna-Laveaga, D. R., & Sulbarán-Rangel, B. (2025). Transforming Waste into Value: The Role of Physicochemical Treatments in Circular Water Management. Limnological Review, 25(3), 42. https://doi.org/10.3390/limnolrev25030042








