Abstract
The study evaluated the vegetative growth and reproductive strategies of the macrophyte Pistia stratiotes under varying nutrient (carbon and nitrogen) and light (full sun and 70% shade) conditions, as well as its epiphytic association with heterocytous cyanobacteria. Plants were collected from a reservoir, transferred to a greenhouse, and subjected to six treatments, with sampling every 15 days. Clonal growth was higher in nitrogen-supplemented treatments, while flowering was more pronounced in carbon-supplemented treatments. Heterocyte production by epiphytic cyanobacteria on roots positively correlated with P. stratiotes total biomass, suggesting the macrophyte utilizes nitrogen fixed by periphytic cyanobacteria. The results highlight the importance of nitrogen and carbon for growth and reproduction, with distinct resource allocation for rosettes (nitrogen) and flowers (carbon). The P. stratiotes-cyanobacteria association may enhance macrophyte population expansion, underscoring the ecological role of these microorganisms.
1. Introduction
Clonal plants, such as Pistia stratiotes L., possess remarkable phenotypic plasticity, allowing them to modulate their allocation of photoassimilates in response to environmental conditions as a key survival strategy [1]. This macrophyte’s survival strategy involves dynamic reproductive switching—sexual reproduction under stress (ensuring genetic diversity) versus clonal expansion during resource abundance (rapid colonization) [1,2,3]. In nutrient-poor or unstable habitats, where survival is uncertain, P. stratiotes tends to prioritize sexual reproduction, producing seeds that remain dormant until conditions improve, thereby ensuring the long-term persistence of the species [2]. Conversely, in environments abundant in resources, this macrophyte predominantly invests in clonal growth, rapidly expanding through stolon elongation and ramet production, which facilitates the formation of dense floating mats [2,3,4]. This vegetative proliferation enhances competitive dominance by monopolizing light and nutrient resources, often leading to ecological imbalances. The capacity of P. stratiotes to dynamically adjust its reproductive strategies is indicative of its ecological versatility, thus contributing to its success in both natural and disturbed aquatic ecosystems. A comprehensive understanding of these mechanisms is imperative for predicting its invasive potential and managing its spread in eutrophic waters.
The presence of floating macrophytes has been demonstrated to create a microclimate conducive to the development of epiphytic microorganisms on their roots [5]. It is important to note the unique ability of heterocytic filamentous cyanobacteria [6] to fix atmospheric nitrogen through heterocytes [7]. This symbiotic relationship has been shown to be mutually beneficial for both the cyanobacteria and the host macrophytes. Environmental changes, such as variations in nutrient availability or physical conditions, have been demonstrated to modify these interactions, thereby affecting macrophyte growth and ecosystem dynamics [1]. It is imperative to comprehend these relationships in order to predict the behaviour of species, such as P. stratiotes, in diverse aquatic environments.
In the absence of nitrogen or in an environment enriched with carbon, filamentous cyanobacteria initiate a process of cellular differentiation, resulting in the conversion of vegetative cells into specialized heterocytes. This adaptive mechanism has been demonstrated to enhance biological nitrogen fixation (BNF), thereby compensating for N limitation while concomitantly effecting a rebalancing of the C/N ratio. The process is subject to regulation by hetR gene expression and patS peptide inhibition in response to metabolic demands [6,8]. The objective of this process is to rebalance the carbon/nitrogen (C/N) ratio through biological nitrogen fixation (BNF) [9,10,11], thereby releasing assimilable forms of N (e.g., ammonium) that can be utilized by macrophytes and other organisms [12]. Research has demonstrated that an increase in carbon within the medium has the capacity to stimulate the differentiation of heterocytes, whilst concurrently exerting an influence on reproductive allocation in aquatic plants [13,14]. Vanderhaeghe et al. [13] observed that elevated CO2; concentrations appear to both anticipate and intensify flowering in certain macrophytes, thus suggesting a strategic shift to sexual reproduction under conditions of excess carbon. This phenomenon may represent an adaptation to ensure genetic variability in environments where nutritional imbalance is prevalent, while the availability of nitrogen (N) favors clonal expansion [14]. Such mechanisms underscore the plasticity of macrophytes and their microbial interactions in regulating the dynamics of aquatic ecosystems.
In addition to the availability of nutrients, the intensity of light has been demonstrated to elicit discrete responses in macrophytes and the associated cyanobacteria, given the reliance of both on light for the process of photosynthesis [15,16]. It has been demonstrated that shaded environments may favor the proliferation of certain macrophytes [16] or induce stress responses in others [15]. This light variation has been demonstrated to influence resource allocation and may consequently alter the reproductive strategies of macrophytes and the production of heterocytes in cyanobacteria [17], given that these specialized cells require a significant amount of energy to perform nitrogen fixation (BNF) [17]. Consequently, light emerges as a pivotal regulator of these ecological interactions.
P. stratiotes is a free-floating clonal macrophyte which exhibits exceptional invasive potential. It has a rapid vegetative propagation, dual reproductive strategies (sexual and asexual), broad ecological tolerance, efficient nutrient accumulation and formation of dense mats [18,19]. It is notably recognised for forming mutualistic epiphytic associations with nitrogen-fixing cyanobacteria (particularly Anabaena and Nostoc spp.) on its root surfaces. This symbiosis has been shown to enhance the plant’s nitrogen acquisition through biological fixation, thereby conferring a competitive advantage in oligotrophic environments. In addition, this symbiosis provides the cyanobacteria with a stable habitat and photosynthetic products. The relevance of studies on this species is twofold: firstly, its economic importance as a raw material for biofuels and for causing blockages in hydroelectric reservoirs; secondly, its ecological importance as a primary source of energy, shelter for fauna, and as a bioremediation agent for polluted water bodies [18,19]. However, the interactions between macrophytes and epiphytic cyanobacteria remain under-explored in the extant literature, particularly with regard to the direct effects of this association on the physiology and ecology of plants [20]. In light of the observed discrepancy, the present study seeks to elucidate the plastic responses of P. stratiotes with respect to growth and reproduction under varying nutrient (N and C) and light conditions. Furthermore, it aims to investigate the causal relationship between plant development and the production of heterocytes by epiphytic cyanobacteria in its roots.
To this end, the following four hypotheses were tested: Firstly, rosettes exposed to elevated levels of nitrogen (N) and full light will allocate more resources to clonal growth (formation of stolons and ramets). Secondly, rosettes supplemented with carbon (C) will prioritize sexual reproduction (flowering). Thirdly, the combination of C and intense light will increase the production of heterocytes in cyanobacteria. Finally, the increase in heterocytes will increase plant biomass, suggesting transfer of fixed nitrogen (N) to the macrophyte.
These hypotheses represent a novel integration of the combined effects of light, nutrients, and microbial symbiosis on the invasiveness of P. stratiotes, thereby offering insights into the management of aquatic ecosystems.
2. Materials and Methods
2.1. Study Species
Pistia stratiotes, popularly referred to as water lettuce, is a pantropical floating macrophyte that thrives in freshwater systems across equatorial and warm temperate regions. This species demonstrates extraordinary ecological plasticity through its dual reproductive system, combining vigorous clonal spread via stolons with periodic sexual reproduction through monoecious flowers. Field studies have documented its capacity to dominate aquatic habitats through rapid vegetative multiplication, with single plants generating up to 12 daughter rosettes weekly under optimal nutrient conditions [3,21,22]. The plant’s stolons exhibit phototropic growth responses, extending up to 15 cm in 48 h to colonize new surface areas [23]. Concurrently, its sexual reproductive strategy ensures long-term persistence; each inflorescence produces 4–6 viable seeds that accumulate in benthic sediment, forming dense propagule banks exceeding 4000 seeds/m2 in Brazilian floodplains [24]. These seeds maintain 80% viability after 18 months submerged, enabling population recovery after seasonal droughts [24]. The species’ combined strategies—explosive clonal growth during eutrophic conditions and resilient seed banks for environmental stress periods—explain its global success as both an ecological pioneer and invasive nuisance across five continents [21].
2.2. Greenhouse Experiment
The samples of P. stratiotes used in this study were obtained from a reservoir in southeastern Brazil, with precise geographical coordinates of 21°8′36.45″ S, 45°2′11.12″ W. The rosettes of P. stratiotes were collected from a reservoir in southeastern Brazil (21°8′36.45″ S, 45°2′11.12″ W). Following collection, the plant specimens were transported to a greenhouse facility where they underwent a preparation protocol. This included careful cleaning to eliminate any residual substrate and non-viable tissue, followed by a 7-day acclimatization period under controlled conditions before experimental initiation. The study period spanned 60 days of continuous observation and measurement.
We placed rosettes of P. stratiotes of a similar size (diameter) and appearance in each 3L plastic tray, with one rosette in each tray. The trays were divided into six treatments, with four trays per treatment, giving a total of twenty-four trays. The treatments consisted of with carbon (WC) (addition of 2.2 g/L of ash from the burning of plant material from the species Citrus × limon (L.) Osbeck (Rutaceae), which had a high carbonate content in its constitution [25]) and reduced sun (RS) (70% shade, covered with a shade cloth) (WC/RS); with carbon and full sun (FS) (no shade cloth) (WC/FS); with nitrogen (WN) (half strength Hoagland’s solution [26]) and reduced sun (WN/RS); with nitrogen and full sun (WN/FS); control (CO) (with addition of 5% Hoagland’s solution) and reduced sun (CO/RS); and control and full sun (CO/FS). In the absence of nitrogen addition (WC and CO), 5% Hoagland’s nutrient solution was administered as a treatment. This dosage was determined through experimental testing, which revealed that P. stratiotes individuals could withstand the duration of the experiment at this minimal level. The pH was controlled in the treatments with carbon addition (WC) using HEPES buffer.
The experimental monitoring involved quarterly sampling (four collection events at 15-day intervals). During each sampling period, quantitative measurements were taken to assess both vegetative propagation (counting newly formed rosettes and measuring stolon elongation) and reproductive output (recording floral production). Upon completion of the 60-day trial, biometric analysis was performed by measuring and averaging the diameter of three representative rosettes per experimental unit. All harvested plant material (vegetative and reproductive structures) was subsequently processed through dehydration in a laboratory oven at 50 °C for a standardized 72 h period.
2.3. Cyanobacteria Observation and Heterocyte Count
To investigate the epiphytic cyanobacterial community and quantify heterocyte frequency, root fragments (2–3 cm apical segments) were aseptically collected from Pistia stratiotes at each sampling interval.
Aliquots (3.5 mL) of the processed suspensions were transferred to sterile Eppendorf tubes containing Transeau’s fixative (6:3:1 ethanol/water/acetic acid), with three technical replicates per sample. From each fixed aliquot, a 10 μL subsample was withdrawn using an automatic pipette (Eppendorf Research Plus) and deposited onto pre-cleaned microscope slides. A total of 6 replicate slides were prepared per sample (2 drops/slide, 5 μL/drop), totaling 576 slides across all treatments.
Microscopic analysis was performed under 1000× total magnification (Nikon Eclipse 200 MV R, Nikon Corporation, Tokyo, Japan) following systematic zigzag transects (10 fields/slide). Heterocyte enumeration employed an improved Neubauer chamber protocol, where all cells within 0.1 mm2 counting grids were tallied (minimum 200 counts/sample).
2.4. Data Analysis
The present study examined the influence of variations in nutrient availability and light conditions on the allocation of resources between clonal and reproductive structures in Pistia stratiotes. The experimental design incorporated two key environmental factors as categorical predictors. The first factor to be considered was that of nutrient supplementation regimes, and the second was that of light exposure levels. Six quantitative response variables were measured in order to evaluate plant performance, including vegetative expansion (stolon elongation and rosette production), morphological development (rosette diameter), and reproductive output (flower production and biomass allocation).
For the purpose of statistical analysis, a robust non-parametric approach was implemented, utilizing the Kruskal–Wallis test to accommodate the non-normal distribution of the dataset. Post hoc comparisons were conducted using sequential mean analysis (stepwise/stepdown methods) to establish treatment hierarchies. In order to test our hypothesis regarding the relationships between heterocyte biomass and treatments, we applied Spearman’s rank correlation coefficient. All computational procedures were executed in IBM SPSS Statistics (v.22), employing α = 0.05 as the significance threshold.
3. Results
All vegetative attributes were found to be influenced by the treatments. The stolon length was found to be significantly greater in the WC and WN treatments compared to CO, regardless of light (Figure 1A) (KW = 18,700; p = 0.002). Rosette production differed between the WN, WC, and CO treatments, regardless of light, with the highest production in WN and the lowest in CO (Figure 1B) (KW = 20,421; p = 0.001). Rosette diameter was greater in the WC and WN treatments, and, in relation to light intensity, rosette diameter was significantly greater in WC/RS than in WN/RS (Figure 1C) (KW = 19,248; p = 0.002). Vegetative biomass was higher in WN/FS compared to CO/FS, CO/RS, and WC/RS (Figure 1D) (KW = 19,160; p = 0.002).
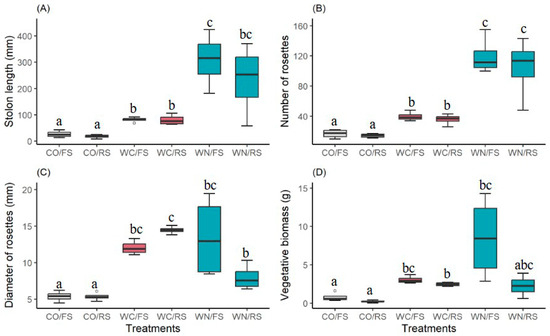
Figure 1.
Treatment-mediated effects on clonal propagation traits in P. stratiotes: control and full sun (CO/FS); control and reduced sun (CO/RS); with carbon addition and full sun (WC/FS); with carbon addition and reduced sun (WC/RS); with nitrogen addition and full sun (WN/FS); with nitrogen addition and reduced sun (WN/RS). (A) Stolon length, (B) vegetative biomass, (C) number of new rosettes, and (D) Diameter of the rosettes. The ranking procedure was achieved through a subsequent evaluation, based on stepwise and stepdown methods.
There was no flower production in CO. The production of flowers was greater in WC/FS (KW = 20,878; p = 0.004) and WC/RS (KW = 20,878; p = 0.002), regardless of the quantity of light (Figure 2A), while flower biomass was higher in WC/RS and WC/FS compared to WN/RS (Figure 2B) (KW = 20,070; p = 0.01; p = 0.01). A total of 796 heterocytes were produced in CO (CO/FS = 372 heterocytes and CO/RS = 424), while in WC, a total of 92,335 heterocytes were produced (WC/FS = 79,462 heterocytes and WC/RS = 12,873). The production of heterocytes in WN was found to be insignificant, with a total of only 10 heterocytes produced. Consequently, these data were not included in the subsequent correlation analysis. The correlation between the number of heterocytes produced and the plant’s total biomass was positive (ρ = 0.856, p < 0.001), and the treatment with added carbon and in full sun was the one with the highest number of heterocytes and, consequently, the highest plant biomass (Figure 3).
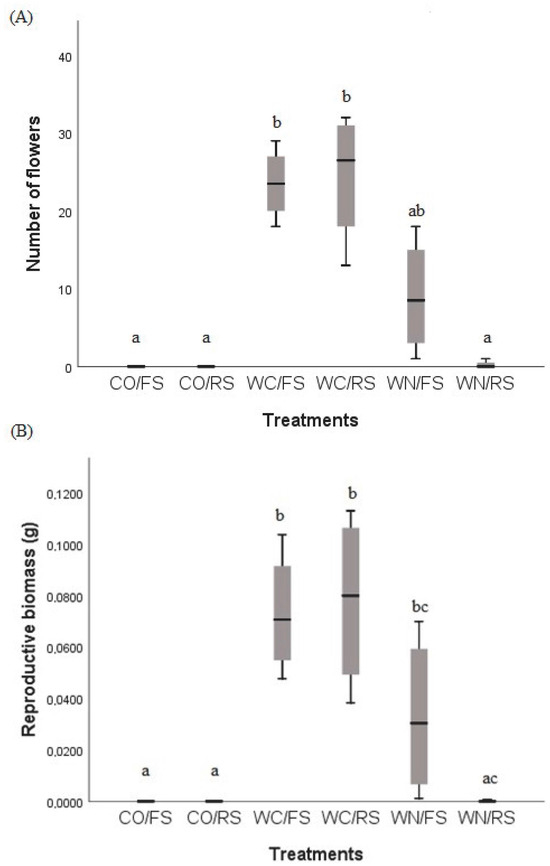
Figure 2.
Number of flowers and flower biomass according to the treatments: with carbon addition and full sun (WC/FS); with carbon addition and reduced sun (WC/RS); with nitrogen addition and full sun (WN/FS); with nitrogen addition and reduced sun (WN/RS); (A) relationship between number of flowers and treatment and (B) relationship between flower biomass and treatment. The ranking procedure was achieved through a subsequent evaluation, based on stepwise and stepdown methods.
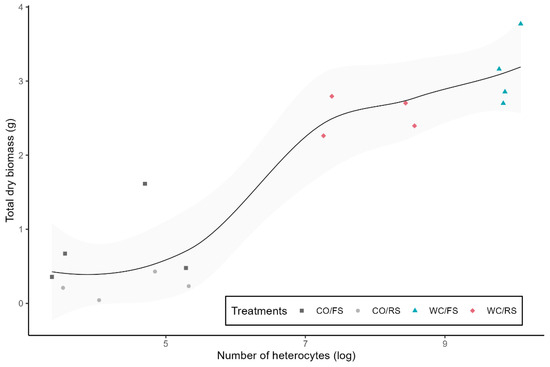
Figure 3.
Correlation between the total dry biomass of P. stratiotes as a logarithmic function of the number of heterocytes in treatments with the carbon addition in full sun and reduced sun (WC/FS and WC/RS) and control in full sun and reduced sun (CO/FS and CO/RS) groups.
4. Discussion
It is evident that plant species demonstrate considerable phenotypic plasticity in response to environmental variability, a trait that is particularly pronounced in aquatic ecosystems, where resource distribution is often heterogeneous [27,28]. This plasticity primarily manifests through the adaptive allocation of biomass between vegetative and reproductive structures, a strategy that optimizes fitness under fluctuating conditions. The experimental findings of this study demonstrate that P. stratiotes allocates resources to clonal growth in a preferential manner under elevated nitrogen (N) availability, as evidenced by significant stolon elongation (p < 0.05) and increased rosette production across all light treatments. This nitrogen-mediated vegetative expansion suggests a clear case of adaptive plasticity in resource partitioning, which is likely to have evolved to maximize the colonization of nutrient-rich habitats.
The observed response is consistent with the “foraging response” concept, a theory that has been extensively documented in clonal plants. In such environments, stolon elongation facilitates the exploitation of resources in patchy habitats [29]. In P. stratiotes, accelerated horizontal expansion under N-enrichment reflects an evolutionary adaptation to capitalise on localized nutrient pulses, a strategy analogous to that observed in Salvinia natans (L.) All [30]. Convergent adaptations among clonal aquatic plants emphasize the role of analogous selective pressures, particularly nutrient variability, in shaping similar phenotypic responses across phylogenetically distinct species. This plasticity is hypothesized to enhance ecological competitiveness in dynamic aquatic ecosystems, enabling the rapid colonization of resource-rich patches amid spatiotemporal heterogeneity in nutrient distribution and light availability [28,31].
The propensity for clonal proliferation under conditions of elevated nitrogen levels is not exclusive to aquatic systems. Terrestrial clonal species, such as Fragaria vesca (L.) and Glechoma hederacea (L.), similarly exhibit increased stolon production under N-enrichment [29,32]. This finding suggests the presence of a conserved adaptive strategy across different habitats, in which the availability of nitrogen (N) serves as a primary driver of vegetative expansion. In aquatic environments, however, the implications are magnified due to the floating life form of species, such as P. stratiotes. The formation of dense floating mats through stolon proliferation has been demonstrated to alter ecosystem dynamics by shading submerged vegetation, reducing oxygen levels, and modifying nutrient cycling [22,23].
Field observations have been shown to support the hypothesis that N-enriched waters often provide a conducive environment for the proliferation of P. stratiotes colonies, which, in turn, have been demonstrated to exert a detrimental effect on biodiversity by monopolizing light and space [23]. This competitive advantage is further reinforced by the species’ ability to store nitrogen (N) in vegetative tissues, thereby buffering against temporary nutrient shortages. This trait is shared with other invasive macrophytes, such as Eichhornia crassipes (Mart.) Solms [31]. The ecological consequences of such plasticity are profound, as rapid clonal expansion can lead to monoculture dominance, reducing habitat complexity and altering trophic interactions [22].
In contrast to N-driven clonal growth, the allocation of resources towards inflorescence development in P. stratiotes is strongly correlated with increased carbon (C) availability. This finding is consistent with the broader phenomenon of angiosperm phenological adaptations, which have evolved to optimize reproductive success under favorable conditions for carbon assimilation [33]. For instance, studies on Arabidopsis thaliana (L.) Heynh. demonstrate that C nanoparticles bioaccumulate in tissues, thereby triggering precocious flowering via gene expression modifications [14]. In addition, our experimental investigations into the use of ash-derived C supplementation revealed consistent enhancements in floral density and individual flower biomass across a range of light regimes. This finding suggests that C may act as a cue for reproductive investment.
The transition towards floral production under conditions of high C/N ratios stands in marked contrast to the N-mediated vegetative growth, thereby underscoring a tradeoff that is contingent on available resources. This tradeoff is ecologically strategic: in nutrient-rich waters, P. stratiotes prioritizes clonal spread to exploit spatial resources, whereas under high C (often linked to light-exposed, photosynthetically active conditions), it invests in sexual reproduction to ensure genetic diversity and long-term persistence. Such conditional strategies have been observed in other clonal plants, including Hydrocotyle vulgaris (L.), where the ratio of carbon to nitrogen is a determining factor in the frequency of branching versus the initiation of flowers [13].
The light intensity further refines these allocation strategies. While N-supplemented P. stratiotes achieved maximal vegetative biomass under full sun, C-supplemented plants produced greater flower biomass under reduced light. This finding indicates that C-mediated reproductive allocation is less light-dependent than N-driven clonal growth, which may be attributable to the presence of stored carbohydrate reserves that compensate for reduced photosynthesis rates. A parallel phenomenon occurs in Lemna (L.), where shaded conditions accelerate flowering when soluble sugars are abundant [34].
P. stratiotes employs a dual reproductive system, combining rapid clonal expansion with substantial sexual output (six seeds per flower). This bet-hedging strategy is a form of resilience in variable environments: clonal growth is dominant under stable, high-N conditions, while seed banks (up to 4000 seeds/m2 [24]) facilitate recovery after disturbances.
A notable finding was the 30-fold increase in heterocyte formation under C supplementation in reduced light, which is likely to be due to C/N imbalance disrupting normal cell differentiation [35]. This response is analogous to cyanobacterial heterocyte formation under N limitation, suggesting a conserved mechanism across kingdoms where C excess reprograms developmental pathways.
5. Conclusions
In conclusion, the results obtained in this study underscore the pivotal role that the availability of nitrogen (N) and carbon (C) elements play in facilitating the clonal proliferation and reproductive capacity of P. stratiotes. It was observed that the incorporation of nitrogen led to a marked enhancement in clonal expansion, while the inclusion of carbon exhibited a more pronounced stimulant effect on sexual reproduction. Excessive population growth of aquatic organisms can be facilitated by nutrients in excess, which can have deleterious effects on the ecosystem in which they occur. Given the capacity of N to be supplied not only by human activities but also by biological nitrogen fixation by cyanobacteria, it can be concluded that the epiphytic association between P. stratiotes and heterocytous filamentous cyanobacteria can contribute to the clonal expansion of this macrophyte.
Finally, we propose that such epiphytic associations between cyanobacteria and P. stratiotes can represent a further factor contributing to the invasive potential of this plant. It would be advantageous for studies to be conducted that directly assess the nitrogen uptake process by P. stratiotes in the context of cyanobacteria. The value of such studies would be especially evident in the context of evaluating interactions under varying environmental conditions.
The plasticity of P. stratiotes is a salient factor in its invasiveness in eutrophic waters. Future studies could explore the molecular pathways and regulatory networks involved in carbon and nitrogen (C/N) nutrient sensing and signal transduction, including key genes, post-translational modifications, and crosstalk between metabolic pathways.
Subsequent research endeavors should accord priority to in situ experimental validations of resource allocation tradeoffs across natural environmental gradients (e.g., nutrient availability, light intensity, and hydrological regimes) under ecologically realistic conditions, with a particular focus on wetlands subject to seasonal fluctuations.
It is recommended that future studies place particular emphasis on the investigation of synergistic and antagonistic interactions between combined stressors (particularly heavy metal contamination concomitant with nutrient enrichment) on physiological performance, clonal propagation strategies, and reproductive allocation patterns in floating macrophytes. Conducting such research could elucidate threshold effects and adaptive responses in the context of realistic pollution scenarios.
Author Contributions
Conceptualization, M.B., F.d.F.C. and M.G.M.V.V.; methodology, M.B., L.L.P., G.P.L. and M.G.M.V.V.; validation, F.d.F.C.; formal analysis, M.B., L.L.P., G.C.R. and F.d.F.C.; investigation, M.B., L.L.P., G.P.L. and M.G.M.V.V.; writing—original draft preparation, M.B. and F.d.F.C.; writing—review and editing, M.B. and F.d.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) provided support grants to the first, second, third, and fourth authors. This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-APQ-01347-22).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the Laboratory of Plant Anatomy (Federal University of Lavras), for providing the necessary research equipment. This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-APQ-01347-22) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (grant numbers [PAPG-12938] and [PNPD-1638006]), whose assistance we gratefully acknowledge. MGMVV also thanks the Belgian Science Policy Office that is supporting the BCCM/ULC Collection.
Conflicts of Interest
Author Marcelo Gomes Marçal Vieira Vaz is from Biodiversita Tecnologia Microbiana company, the Biodiversita Tecnologia Microbiana company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The others authors declare no conflicts of interest.
References
- Stuefer, J.F.; Erschbamer, B.; Huber, H.; Suzuki, J.-I. Ecology and Evolutionary Biology of Clonal Plants; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Adomako, M.O.; Alpert, P.; Du, D.-L.; Yu, F.-H. Effects of clonal integration, nutrients and cadmium on growth of the aquatic macrophyte Pistia stratiotes. J. Plant Ecol. 2020, 13, 765–772. [Google Scholar] [CrossRef]
- Ali, E.F.; Galal, T.M.; Hassan, L.M.; Al-Yasi, H.M.; Dakhil, M.A.; Eid, E.M. Seasonal potential of Pistia stratiotes in nutrient removal to eliminate eutrophication in Al-Sero Drain (South Nile Delta, Egypt). J. Freshw. Ecol. 2021, 36, 173–187. [Google Scholar] [CrossRef]
- Adomako, M.O.; Zhang, Q.; Yu, F.H. Genotypic differences in response to different patterns of clonal fragmentation in the aquatic macrophyte Pistia stratiotes. J. Plant Ecol. 2022, 15, 1199–1212. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Pimenta, L.L.; Lima, G.P.; Biondi, M.; Vaz, M.G.M.V.; Coelho, F.d.F. Epiphytic cyanobacterial strains in the roots of Salvinia auriculata and the effect of light and nutrients on the production of heterocyst, akinete and hormogonia. Aquat. Ecol. 2021, 56, 543–553. [Google Scholar] [CrossRef]
- Adams, D.G.; Duggan, P.S. Tansley Review No. 107. Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 2003, 144, 3–33. [Google Scholar] [CrossRef]
- Saha, S.K.; Das, R.; Bora, K.N.; Uma, L. Biodiversity of epilithic cyanobacteria from freshwater streams of Kakoijana reserve forest, Assam, India. Indian J. Microbiol. 2007, 47, 219–232. [Google Scholar] [CrossRef]
- Latysheva, N.; Junker, V.L.; Palmer, W.J.; Codd, G.A.; Barker, D. The evolution of nitrogen fixation in cyanobacteria. Bioinformatics 2012, 28, 603–606. [Google Scholar] [CrossRef]
- Zhang, C.C.; Zhou, C.Z.; Burnap, R.L.; Peng, L. Carbon/nitrogen metabolic balance: Lessons from cyanobacteria. Trends Plant Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef]
- Forchhammer, K.; Selim, K.A. Carbon/nitrogen homeostasis control in cyanobacteria. FEMS Microbiol. Rev. 2019, 44, 33–53. [Google Scholar] [CrossRef]
- Kumar, U.; Nayak, A.K.; Panneerselvam, P.; Kumar, A.; Mohanty, S.; Shahid, M.; Sahoo, A.; Kaviraj, M.; Priya, H.; Jambhulkar, N.N.; et al. Cyanobiont diversity in six Azolla spp. and relation to Azolla-nutrient profiling. Planta 2019, 249, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghe, F.; Smolders, A.J.P.; Roelofs, J.G.M.; Hoffmann, M. Water table and species identity outweigh carbon and nitrogen availability in a softwater plant community. Acta Oecologica 2013, 47, 57–67. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Panigrahy, M.; Sahoo, P.K.; Panigrahi, K.C. Carbon nanoparticles influence photomorphogenesis and flowering time in Arabidopsis thaliana. Plant Cell Rep. 2018, 37, 901–912. [Google Scholar] [CrossRef]
- Zhu, B.; Ellis, M.; Fancher, K.L.; Rudstam, L.G. Shading as a control method for invasive European frogbit (Hydrocharis morsus-ranae L.). PLoS ONE 2014, 9, e98488. [Google Scholar] [CrossRef]
- Medeiros, J.C.C.; Silva, J.C.F.; Teodoro, G.S.; Coelho, F.F. Effects of shade on individual ramet growth and on clonal growth of the aquatic fern Salvinia auriculata (Salviniaceae). Am. Fern J. 2017, 107, 21–29. [Google Scholar] [CrossRef]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Jaklič, M.; Koren, Š.; Jogan, N. Alien water lettuce (Pistia stratiotes L.) outcompeted native macrophytes and altered the ecological conditions of a Sava oxbow lake (SE Slovenia). Acta Bot. Croat. 2020, 79, 35–42. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Hill, M.; Byrne, M.J.; Bownes, A. A review of the biological control programmes on Eichhornia crassipes (C.Mart.) Solms (Pontederiaceae), Salvinia molesta D.S. Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoides Lam. (Azollaceae) in South Africa. Afr. Entomol. 2011, 19, 451–468. [Google Scholar] [CrossRef]
- Bergman, B.; Rai, A.N.; Rasmussen, U. Cyanobacterial associations. In Associative and Endophytic Nitrogen-Fixing Bacteria and Cyanobacterial Associations; Springer: Berlin/Heidelberg, Germany, 2007; pp. 257–301. [Google Scholar] [CrossRef]
- Pott, V.J.; Pott, A. Plantas Aquáticas do Pantanal; EMBRAPA: Brasília, Brazil, 2000. [Google Scholar]
- Lallana, V.H. Aspectos reproductivos del repolito de agua (Pistia stratiotes L.) en ambientes leníticos del río Paraná medio. Iheringia 1989, 39, 37–54. [Google Scholar]
- Lemon, G.D.; Posluszny, U. Shoot development and evolution in Pistia stratiotes (Araceae). Int. J. Plant Sci. 2000, 161, 721–732. [Google Scholar] [CrossRef]
- Muniappan, R.; Reddy, G.V.; Raman, A. Biological Control of Tropical Weeds Using Arthropods; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Etiégni, L.; Campbell, A.G. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Cheplick, G.P. Life-history variation in a native perennial grass (Tridens flavus): Reproductive allocation, biomass partitioning, and allometry. Plant Ecol. 2020, 221, 103–115. [Google Scholar] [CrossRef]
- Wu, J.; Hou, X.Z.; Zhu, J.L.; Miao, R.H.; Adomako, M.O. Nitrogen addition and drought impose divergent effects on belowground bud banks of grassland community: A meta-analysis. Front. Plant Sci. 2025, 15, 1464973. [Google Scholar] [CrossRef]
- Ren, G.-Q.; Li, Q.; Li, Y.; Li, J.; Adomako, M.O.; Dai, Z.-C.; Li, G.-L.; Wan, L.-Y.; Zhang, B.; Zou, C.B.; et al. The enhancement of root biomass increases the competitiveness of an invasive plant against a co-occurring native plant under elevated nitrogen deposition. Flora 2019, 261, 151486. [Google Scholar] [CrossRef]
- Si, C.; Xue, W.; Lin, J.; Zhang, J.-F.; Hong, M.-M.; Wang, Y.-Y.; Zhang, L.-F.; Yu, F.-H. No evidence of greater biomass allocation to stolons at moderate resource levels in a floating plant. Aquat. Ecol. 2020, 54, 421–429. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Yao, S.-M.; Jin, Y.; Song, M.-H.; Lei, N.-F.; Chen, J.-S.; Yu, F.-H. Effects of clonal fragmentation and nutrient availability on the competitive ability of the floating plant Salvinia natans. Folia Geobot. 2020, 55, 63–71. [Google Scholar] [CrossRef]
- Zheng, Z.; Bai, W.; Zhang, W.H. Clonality-dependent dynamic change of plant community in temperate grasslands under nitrogen enrichment. Oecologia 2019, 189, 255–266. [Google Scholar] [CrossRef]
- Xie, Z.; Zhu, W.; Qiao, K.; Zhan, P.; Li, P. Seasonal differences in relationships between changes in spring phenology and dynamics of carbon cycle in grasslands. Ecosphere 2019, 10, e02733. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W., III. Growth and nutritional quality of Lemnaceae viewed comparatively in an ecological and evolutionary context. Plants 2022, 11, 145. [Google Scholar] [CrossRef]
- Fávaro, A.; Nascimento, A.G.D.; Coelho, F.d.F. Urban environmental influences on heterocyst investment in Leptogium cyanescens (Collemataceae). Nova Hedwig. 2021, 113, 259–277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).