Influence of the Level of the Middle River Negro in the Amazon, Brazil, on the Properties of the Blood of the Cururu Freshwater Stingray Potamotrygon wallacei
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Capture and Collection of Blood from Animals
2.3. Hematological Procedures
2.4. Water Analysis
2.5. Water Level of the Middle Negro River
2.6. Statistical Analysis
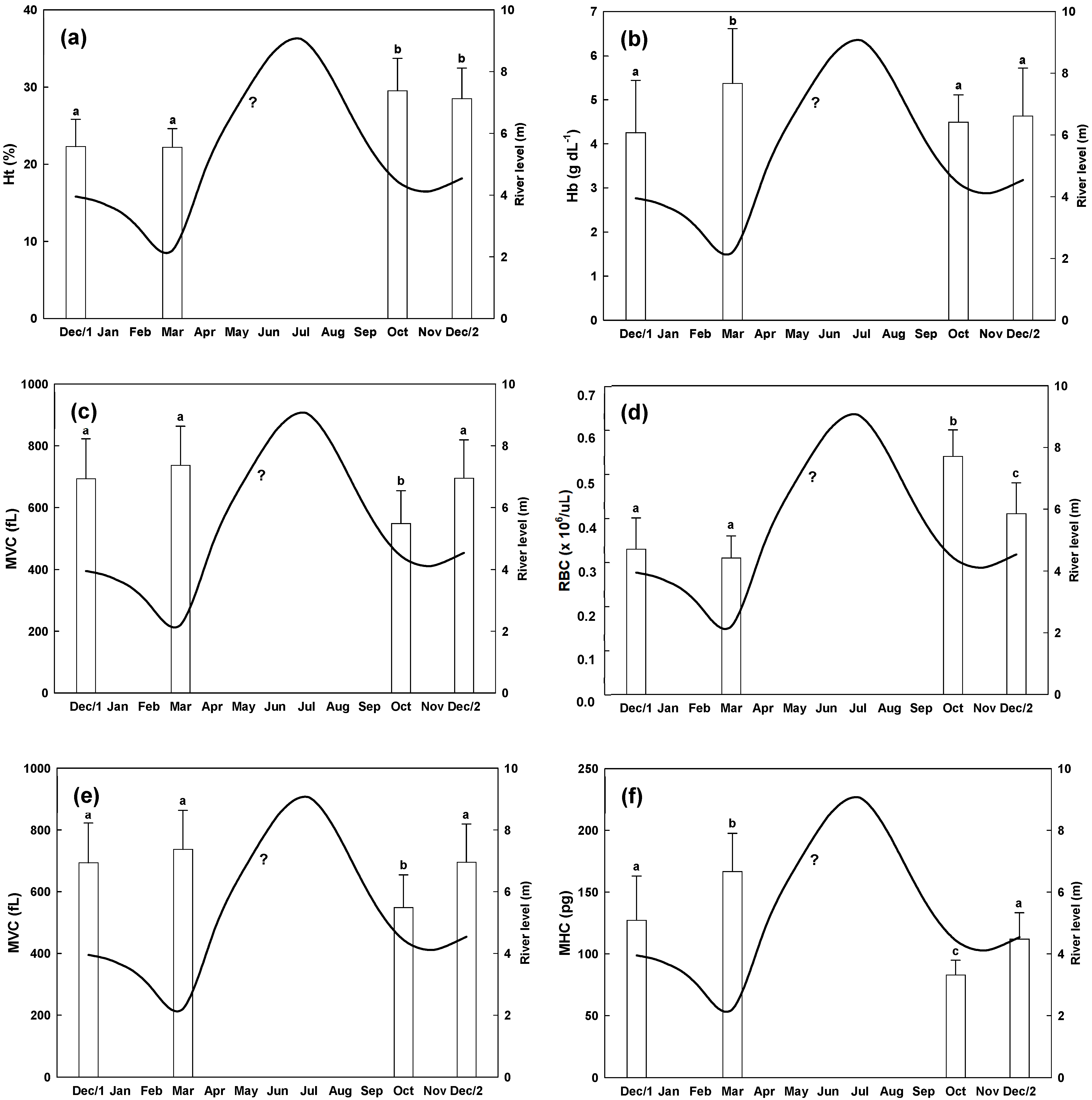
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latrubesse, E.M. Amazon lakes. In Lakes and Reservoirs; Hellström, T., Fairbridge, R.W., Bengtsson, L., Wohlfarth, B., Herschy, R.W., Hargeby, A., Blindow, I., Latrubesse, E.M., Hodgson, D.A., et al., Eds.; Springer: Berlin, Germany, 2012; pp. 13–26. [Google Scholar]
- Melack, J.M.; Hess, L.L. Remote sensing of the distribution and extent of wetlands in the Amazon Basin. In Amazonian Floodplain Forests; Junk, W.J., Piedade, M., Wittmann, F., Schönagart, J., Parolin, P., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Castro, P.D.S.; Ladislau, D.; Ribeiro, M.W.S.; Lopes, A.C.C.; Lavander, H.D.; Bassul, L.A.; Mattos, D.C.; Liebl, A.R.S.; Aride, P.H.R.; Oliveira, A.T. Hematological parameters of three species of tucunarés (Cichla spp.) from Lake Balbina, Presidente Figueiredo, Amazonas. Braz. J. Biol. 2020, 81, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Rosa, R.S.; Araújo, M.L. A new species of Neotropical freshwater stingray (Chondrichthyes: Potamotrygonidae) from the Rio Negro, Amazonas, Brazil: The smallest species of Potamotrygon. Zootaxa 2016, 4107, 566–586. [Google Scholar] [CrossRef]
- Oliveira, A.T.; Araújo, M.L.G.; Lemos, J.R.G.; Santos, M.Q.C.; Pantoja-Lima, J.; Aride, P.H.R.; Tavares-Dias, M.; Marcon, J.L. Ecophysiological interactions and water-related physicochemical parameters among freshwater stingrays. Braz. J. Biol. 2017, 77, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.T.; Santos, M.Q.C.; Araújo, M.L.G.; Lemos, J.R.G.; Sales, R.S.A.; Pantoja-Lima, J.; Tavares-Dias, M.; Marcon, J.L. Hematological Parameters of Three Freshwater Stingray Species (Chondrichthyes: Potamotrygonidae) in the Middle Rio Negro, Amazonas State. Biochem. Syst. Ecol. 2016, 69, 33–40. [Google Scholar] [CrossRef]
- Oliveira, A.T.; Lemos, J.R.G.; Santos, M.Q.C.; Pantoja-Lima, J.; Aride, P.H.R.; Araújo, M.L.G.; Tavares-Dias, M.; Marcon, J.L. Morphological, Cytochemical, and Ultrastructural Aspects of Blood Cells in Freshwater Stingray Species in the Middle Rio Negro Basin of Amazonian Brazil. Scient. Rep. 2021, 11, 15685. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.L.G. Reproductive Biology and Fisheries of Potamotrygon sp. (Chondrichthyes: Potamotrygonidae) in the Middle Rio Negro, Amazonas. Master’s Dissertation, National Institute for Amazonian Research, Manaus, Brazil, 1998. [Google Scholar]
- Charvet-Almeida, P.; Araújo, M.D.; Almeida, M.P.D. Reproductive aspects of freshwater stingrays (Chondrichthyes: Potamotrygonidae) in the Brazilian Amazon Basin. J. Northwest Atl. Fish. Sci. 2005, 35, 165–171. [Google Scholar] [CrossRef]
- Shibuya, A.; Araújo, M.L.G.; Zuanon, J. Analysis of stomach contents of freshwater stingrays (Elasmobranchii: Potamotrygonidae) from the middle Negro River, Amazonas, Brazil. Pan-Am. J. Aquat. Sci. 2009, 4, 466–475. [Google Scholar]
- Oliveira, A.T.; Araújo, M.L.G.; Pantoja-Lima, J.; Aride, P.H.R.; Tavares-Dias, M.; Brinn, R.P.; Marcon, J.L. Cyrilia sp. (Apicomplexa: Haemogregarinidae) in the Amazonian freshwater stingray Potamotrygon wallacei (cururu stingray) in different hydrological phases of the Rio Negro. Braz. J. Biol. 2017, 77, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.M.M.; Claudiano, G.S.; Yunis, J.; Mundim, A.V.; Tavares-Dias, M.; Viadanna, P.H.O.; Moraes, J.R.E.; Moraes, F.R. Hematology, biochemical profile, and thyroid hormones of four freshwater stingray species of the genus Potamotrygon. Braz. J. Vet. Res. Sci. 2015, 52, 249–256. [Google Scholar] [CrossRef]
- Santos, J.M.; Ribeiro-Neto, D.G.; Marques, E.E.; Seibert, C.S. Physiological adaptations of Potamotrygon rex (Potamotrygonidae) stingrays to seasonal environmental variations. Rev. Iberoam. Cienc. Ambient. 2020, 11, 409–423. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clarl, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Oliveira, A.T.; Lemos, J.R.G.; Santos, M.Q.C.; Araújo, M.L.G.; Tavares-Dias, M.; Marcon, J.L. Procedimentos de manuseio e de colheita de sangue em arraias de água doce. Embrapa Amapá-Doc. 2012, 77, 1–18. [Google Scholar]
- Oliveira, A.T.; Santos, M.Q.C.; Lemos, J.R.G.; Tavares-Dias, M.; Marcon, J.L. Comparison of the effects of anticoagulants used in blood collection to determine blood parameters of free-living stingrays from the Potamotrygon genus (Elasmobranchii: Potamotrygonidae). Biota Amazônia 2015, 5, 55–58. [Google Scholar]
- Oliveira, A.T.; Cruz, W.R.; Pantoja-Lima, J.; Araujo, S.B.; Araujo, M.L.G.; Marcon, J.L.; Tavares-Dias, M. Morphological and cytochemical characterization of thrombocytes and leukocytes in hatchlings of three species of Amazonian freshwater turtle. Veter. Arh. 2011, 81, 657–670. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Water Quality and Pond Soil Analyses for Aquaculture; Auburn University: Auburn, Alabama, 1992; p. 183. [Google Scholar]
- Santos, M.Q.C.; Aride, P.H.R.; Farias, F.D.F.; Oliveira, A.T. Hematological and Plasma Biochemical Profile of Two Species of Freshwater Stingrays from the Amazon. Vet. Res. Commun. 2024, 48, 2595–2610. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.P. Rayas de agua dulce (Potamotrygonidae) de Suramérica. Parte II: Colombia, Brasil, Perú, Bolivia, Paraguay, Uruguay y Argentina, 1st ed.; Instituto de Investigação de Recursos Biológicos Alexander von Humboldt: Bogotá, Colômbia, 2016; pp. 45–64. [Google Scholar]
- Ozretic, M.K.; Ozretic, B.; Petrovic, S.; Nikolic, T. Seasonal variations of some blood parameters in farmed sea bass (Dicentrarchus labrax L.). Per Biol. 2001, 103, 67–75. [Google Scholar]
- Rey Vázquez, G.; Lo Nostro, F. Changes in Hematological Parameters of Cichlasoma dimerus (Teleostei, Perciformes) Exposed to Sublethal Concentrations of 4-tert-Octylphenol. Arch. Environ. Contam. Toxicol. 2014, 66, 463–469. [Google Scholar] [CrossRef]
- Gomes, M.F.S.; Ribeiro, M.W.S.; Guimarães, C.C.; Nóbrega, T.C.; Pinto, T.L.; Paixão, R.M.; Santos, A.N.A.; Aride, P.H.R.; Oliveira, A.T. Physiological parameters of freshwater stingrays from the Uatumã River Basin, Amazon, Brazil. Ana Acad. Bras. Ciênc. 2025, 97, 1–8. [Google Scholar]
- Kubitza, F. Qualidade da Água no Cultivo de Peixes e Camarões; Acqua Supre Com. Suprim; Aqüicultura Ltda.: Jundiaí, Brazil, 2003; p. 229. [Google Scholar]
- Duncan, W.P.; Fernandes, M.N. Caracterização físico-química dos rios de águas brancas, pretas e claras da Bacia Amazônica e suas implicações na distribuição de arraias de água doce (Chondrichthyes: Potamotrygonidae). Pan-Am. J. Aquat. Sci. 2011, 5, 454–464. [Google Scholar]
- Wood, C.M.; Matsuo, A.Y.O.; Gonzalez, R.J.; Wilson, R.W.; Patrick, M.L.; Val, A.L. Mechanisms of Ion Transport in Potamotrygon, a Stenohaline Freshwater Elasmobranch Native to the Ion-Poor Blackwaters of the Rio Negro. J. Exp. Biol. 2002, 205, 3039–3054. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.P.; Machado, R.N.; Fernandes, M.N. Environmentally Induced Osmoregulation in Neotropical Freshwater Stingrays (Myliobatiformes: Potamotrygoninae) After Controlling for Phylogeny. Comp. Biochem. Phys. 2021, 262, 111076. [Google Scholar] [CrossRef] [PubMed]
- Thierney, K.B.; Farrell, A.P.; Kennedy, C.J. The differential landscape of four teleosts: Juvenile Oncorhynchus kisutch, Clupea pallasi, Culaea inconstans, and Pimephales promelas. J. Fish Biol. 2004, 65, 906–919. [Google Scholar] [CrossRef]
- Pavlidis, M.; Futter, W.C.; Kathario, P.; Divanach, P. Blood cells of six Mediterranean mariculture fish species. J. Appl. Ichthyol. 2007, 23, 70–73. [Google Scholar] [CrossRef]
| Parameters | December/1 | March | June | October | December/2 |
|---|---|---|---|---|---|
| River level (m) | 4.6 ± 0.03 a | 1.6 ± 00.5 b | 8.1 ± 0.09 c | 3.8 ± 0.14 d | 4.9 ± 7.2 e |
| Dissolved oxygen (mg L−1) | 4.5 ± 0.3 a | 4.6 ± 0.1 a | 3.7 ± 0.2 b | 4.0 ± 0.1 c | 4.2 ± 0.3 d |
| Temperature (°C) | 29.8 ± 0.9 a | 29.8 ± 0.2 ab | 26.5 ± 0.5 c | 27.6 ± 0.5 d | 29.4 ± 0.6 b |
| Conductivity (µS cm−1) | 15.1 ± 1.1 e | 16.9 ± 0.8 d | 11.1 ± 0.7 abc | 11.8 ± 0.6 b | 10.9 ± 0.6 c |
| pH | 4.2 ± 0.1 d | 4.2 ± 0.1 d | 4.0 ± 0.1 abc | 4.0 ± 0.0 b | 4.0 ± 0.1 c |
| Hardness (mg L−1) | 2.1 ± 0.8 b | 2.7 ± 0.3 b | 12.6 ± 2.7 a | 13.0 ± 7.6 a | 13.3 ± 8.6 a |
| Alkalinity (mg L−1) | 1.7 ± 1.1 c | 4.7 ± 0.4 b | 21.8 ± 2.8 a | 24.4 ± 5.3 a | 13.6 ± 5.6 d |
| Ammonia (mg L−1) | 0.03 ± 0.02 d | 0.04 ± 0.01 d | 0.73 ± 0.08 abc | 0.70 ± 0.06 b | 0.75 ± 0.09 c |
| Nitrite (mg L−1) | 0.015 ± 0.003 c | 0.008 ± 0.001 a | 0.009 ± 0.006 a | 0.003 ± 0.001 b | 0.004 ± 0.003 b |
| Na+ (mEq L−1) | 21.0 ± 6.9 c | 10.1 ± 0.7 b | 71.1 ± 10.9 a | 72.2 ± 16.1 a | 28.3 ± 13.8 c |
| K+ (mEq L−1) | 11.0 ± 3.3 a | 9.8 ± 1.1 a | 11.7 ± 1.7 a | 16.3 ± 3.2 b | 21.6 ± 4.1 c |
| Parameters | N | December/1 | N | March | N | October | N | December/2 |
|---|---|---|---|---|---|---|---|---|
| Glucose (mg dL−1) | 25 | 33.2 ± 12.0 a | 36 | 22.0 ± 9.3 b | 23 | 34.4 ± 8.6 a | 40 | 28.2 ± 9.9 a |
| Triglycerides (mg dL−1) | 23 | 56.9 ± 18.8 a | 36 | 57.1 ± 22.9 a | 22 | 53.4 ± 16.8 a | 34 | 53.2 ± 19.4 a |
| Cholesterol (mg dL−1) | 24 | 61.9 ± 25.9 a | 36 | 64.5 ± 26.4 a | 22 | 51.8 ± 18.5 ab | 36 | 42.1 ± 21.0 b |
| Total protein (g dL−1) | 25 | 1.0 ± 0.3 a | 36 | 1.1 ± 0.3 a | 21 | 1.1 ± 0.3 a | 34 | 1.0 ± 0.14 a |
| Urea (mg dL−1) | 22 | 4.9 ± 5.5 a | 36 | 5.0 ± 5.1 a | 22 | 4.2 ± 3.4 a | 32 | 3.0 ± 3.0 a |
| Chloride (mmol L−1) | 24 | 138.8 ± 16.5 ab | 30 | 144.7 ± 21.9 a | 20 | 129.3 ± 22.9 ab | 27 | 125.2 ± 18.5 b |
| Na+ (mmol L−1) | 24 | 179.7 ± 44.9 a | 37 | 191.9 ± 36.6 ab | 20 | 216.16 ± 45.8 b | 24 | 209.0 ± 48.7 ab |
| K+ (mmol/L) | 24 | 9.1 ± 2.9 ab | 37 | 7.4 ± 2.3 a | 20 | 9.5 ± 3.4 b | 25 | 9.3 ± 2.9 ab |
| Parameters (µL) | December/1 N = 25 | March N = 36 | October N = 26 | December/2 N = 40 |
|---|---|---|---|---|
| Thrombocytes | 1181 ± 602 a | 1492 ± 1629 a | 987 ± 1068 a | 1032 ± 986 a |
| Leukocytes | 5615 ± 3668 ab | 3367 ± 2110 a | 6426 ± 2657 b | 4499 ± 3443 ab |
| Lymphocytes | 2324 ± 1379 a | 1627 ± 1355 a | 2321 ± 1553 a | 1937 ± 1733 a |
| Monocytes | 1760 ± 1148 ac | 882 ± 626 a | 2308 ± 1184 b | 1231 ± 1008 bc |
| Heterophils | 735 ± 345 a | 738 ± 463 a | 1171 ± 539 a | 1082 ± 731 a |
| Basophils | 202 ± 153 a | 95 ± 135 a | 128 ± 165 a | 138 ± 141 a |
| Parameters | Glucose | Cholesterol | Chloride | Na+ | K+ | Leukocytes | Monocytes | Ht | Hb | RBC | MVC | MHC | MCHC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| River level | 0.099 | 0.012 * | 0.004 * | 0.918 | 0.708 | 0.283 | 0.364 | 0.016 * | 0.003 * | 0.001 * | 0.669 | 0.188 | 0.049 * |
| Dissolved oxygen | 0.372 | 0.520 | 0.535 | 0.899 | 0.249 | 0.823 | 0.333 | 0.091 | 0.771 | 0.531 | 0.383 | 0.903 | 0.183 |
| Conductivity | 0.198 | 0.216 | 0.027 * | 0.546 | 0.026 * | 0.001 * | 0.001 * | 0.004 * | 0.087 | 0.003 * | 0.683 | 0.930 | 0.798 |
| pH | 0.428 | 0.303 | 0.883 | 0.637 | 0.548 | 0.062 | 0.727 | 0.834 | 0.193 | 0.325 | 0.502 | 0.359 | 0.063 |
| Temperature | 0.097 | 0.843 | 0.170 | 0.606 | 0.838 | 0.993 | 0.259 | 0.477 | 0.296 | 0.047 * | 0.761 | 0.096 | 0.186 |
| Hardness | 0.503 | 0.110 | 0.336 | 0.101 | 0.895 | 0.044 * | 0.067 | 0.370 | 0.468 | 0.087 | 0.902 | 0.742 | 0.870 |
| Alkalinity | 0.001 * | 0.002 * | 0.060 | 0.954 | 0.021 * | 0.002 * | 0.005 * | 0.000 * | 0.326 | 0.078 | 0.471 | 0.661 | 0.412 |
| Ammonia | 0.301 | 0.101 | 0.663 | 0.970 | 0.107 | 0.005 * | 0.012 * | 0.586 | 0.294 | 0.017 * | 0.152 | 0.343 | 0.785 |
| Nitrite | 0.000 * | 0.022 * | 0.001 * | 0.202 | 0.130 | 0.021 * | 0.051 | 0.003 * | 0.660 | 0.001 * | 0.523 | 0.100 | 0.239 |
| Na+ | 0.064 | 0.136 | 0.001 * | 0.435 | 0.009 * | 0.346 | 0.324 | 0.001 * | 0.166 | 0.021 * | 0.001 * | 0.024 * | 0.846 |
| K+ | 0.080 | 0.050 | 0.098 | 0.658 | 0.326 | 0.792 | 0.994 | 0.174 | 0.587 | 0.750 | 0.346 | 0.503 | 0.284 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.T.d.; Liebl, A.R.d.S.; Gomes, M.F.d.S.; Ribeiro, M.W.S.; Paixão, R.M.; Paiva, A.J.V.; Santos, S.M.d.; Rufino, J.P.F.; Carvalho, J.R.; Aride, P.H.R. Influence of the Level of the Middle River Negro in the Amazon, Brazil, on the Properties of the Blood of the Cururu Freshwater Stingray Potamotrygon wallacei. Limnol. Rev. 2025, 25, 17. https://doi.org/10.3390/limnolrev25020017
Oliveira ATd, Liebl ARdS, Gomes MFdS, Ribeiro MWS, Paixão RM, Paiva AJV, Santos SMd, Rufino JPF, Carvalho JR, Aride PHR. Influence of the Level of the Middle River Negro in the Amazon, Brazil, on the Properties of the Blood of the Cururu Freshwater Stingray Potamotrygon wallacei. Limnological Review. 2025; 25(2):17. https://doi.org/10.3390/limnolrev25020017
Chicago/Turabian StyleOliveira, Adriano Teixeira de, Ariany Rabello da Silva Liebl, Maria Fernanda da Silva Gomes, Maiko Willas Soares Ribeiro, Rayana Melo Paixão, Antônia Jaqueline Vitor Paiva, Suelen Miranda dos Santos, João Paulo Ferreira Rufino, Junior Ribeiro Carvalho, and Paulo Henrique Rocha Aride. 2025. "Influence of the Level of the Middle River Negro in the Amazon, Brazil, on the Properties of the Blood of the Cururu Freshwater Stingray Potamotrygon wallacei" Limnological Review 25, no. 2: 17. https://doi.org/10.3390/limnolrev25020017
APA StyleOliveira, A. T. d., Liebl, A. R. d. S., Gomes, M. F. d. S., Ribeiro, M. W. S., Paixão, R. M., Paiva, A. J. V., Santos, S. M. d., Rufino, J. P. F., Carvalho, J. R., & Aride, P. H. R. (2025). Influence of the Level of the Middle River Negro in the Amazon, Brazil, on the Properties of the Blood of the Cururu Freshwater Stingray Potamotrygon wallacei. Limnological Review, 25(2), 17. https://doi.org/10.3390/limnolrev25020017







