Nutrient, Organic Matter and Shading Alter Planktonic Structure and Density of a Tropical Lake
Abstract
1. Introduction
2. Methods
2.1. Study Site
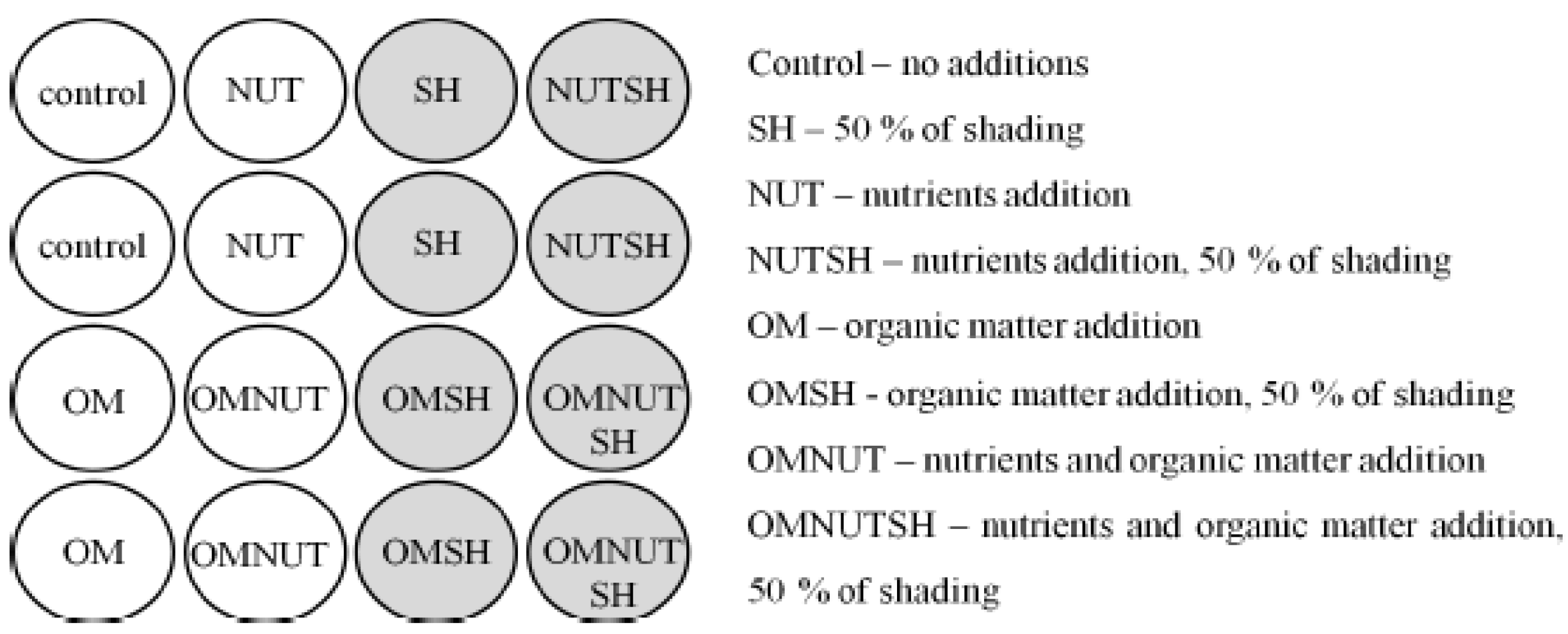
2.2. Experimental Design and Sampling
2.3. Nutrient Addition
2.4. Organic Matter Addition
2.5. Shading
2.6. Plankton Sampling and Analysis
2.7. Data Analysis
3. Results
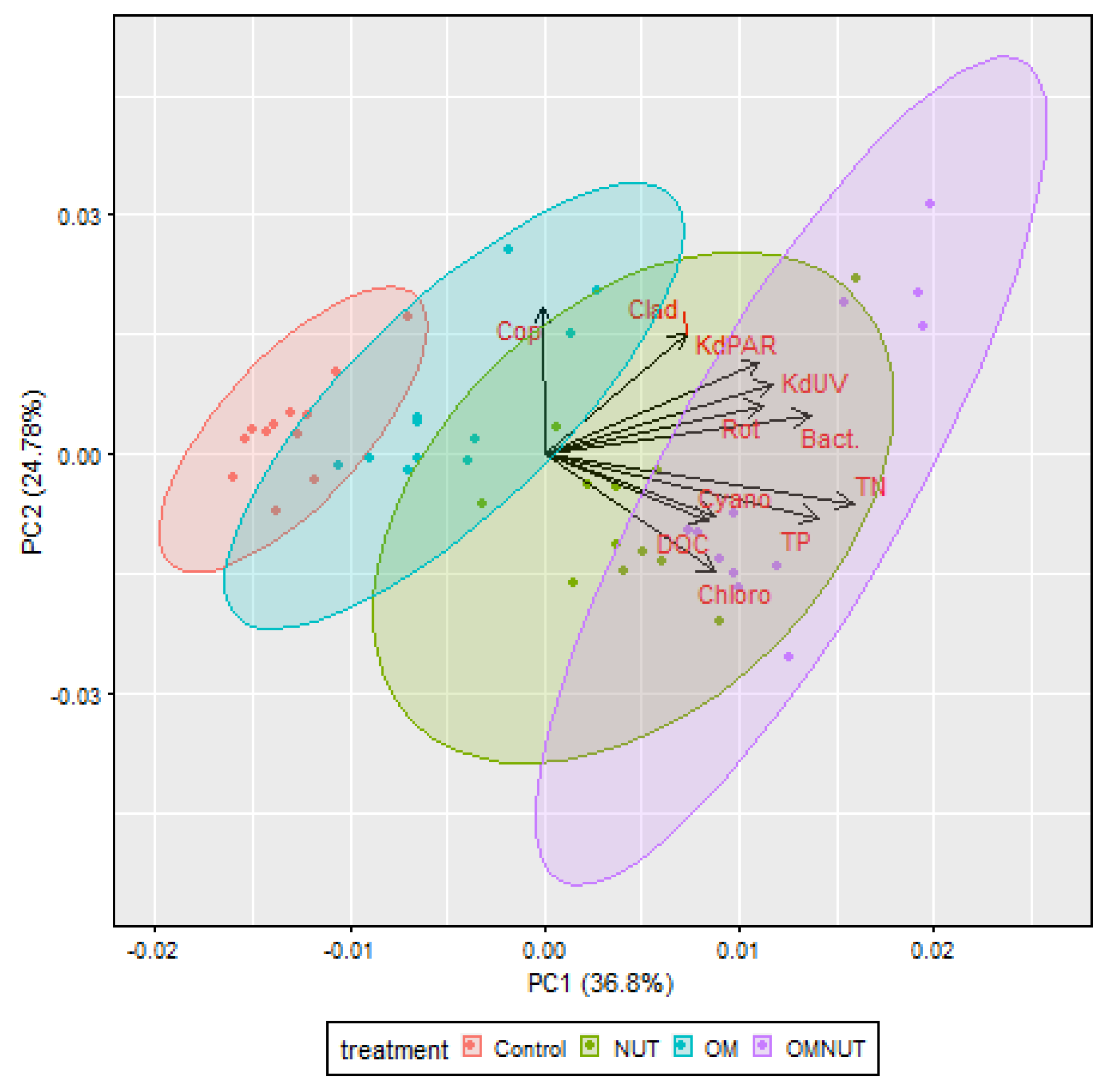
3.1. Environmental Variations Among Mesocosms
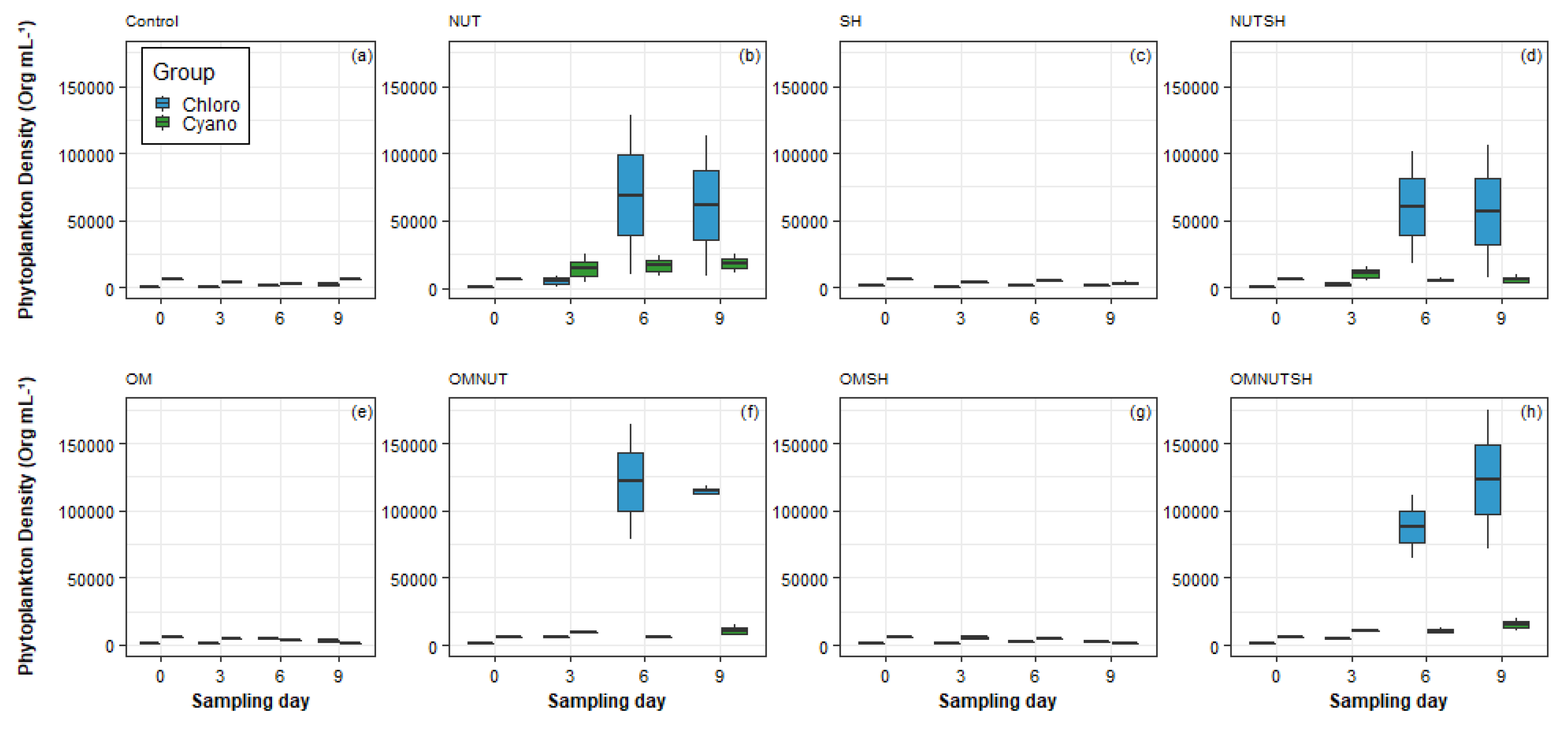
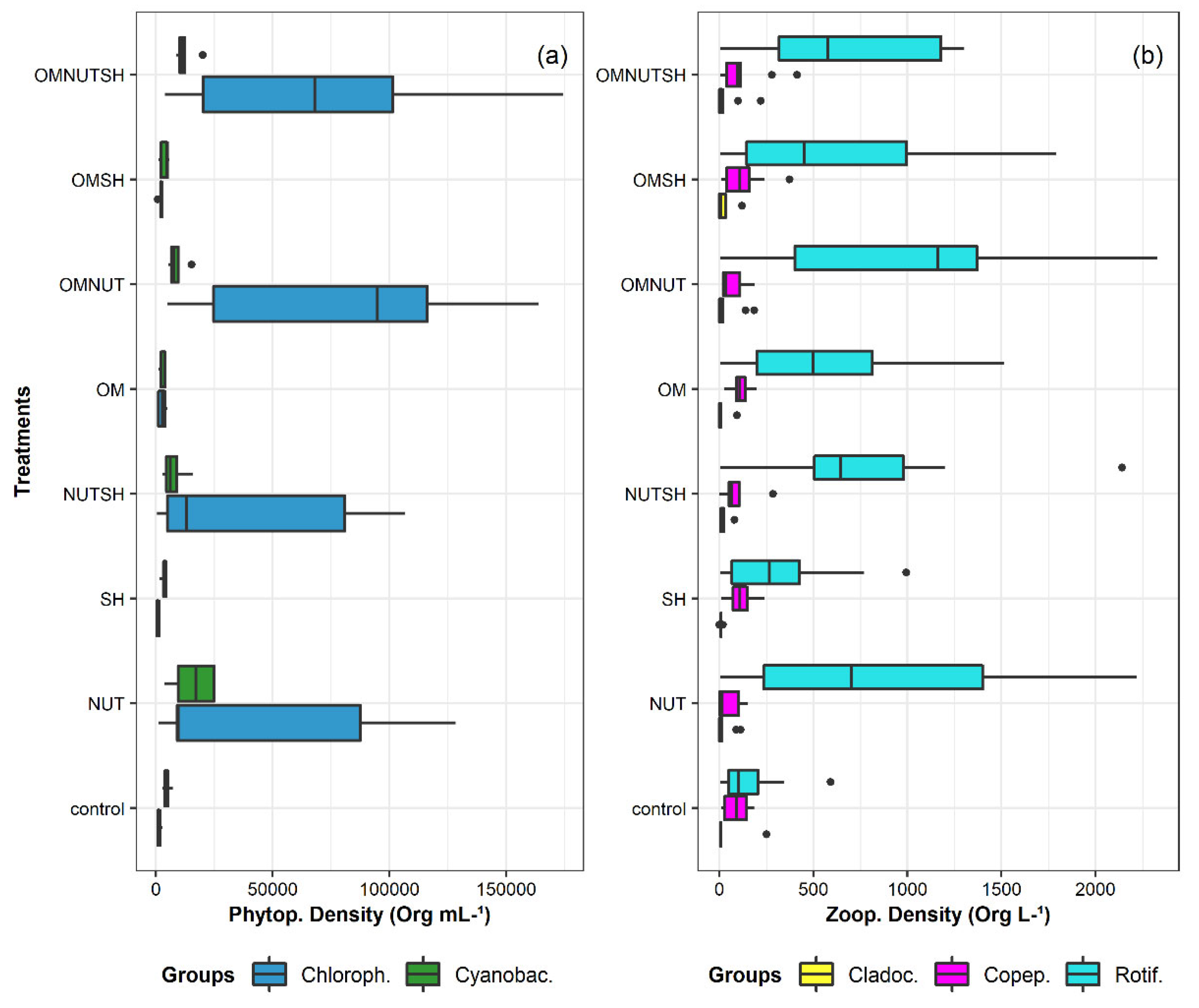
3.2. Plankton Distribution Between Mesocosms
3.3. Plankton Responses to Nutrients, Organic Matter and Shade Additions
4. Discussion
4.1. Phytoplankton Responses to Nutrients, OM, and Shading Addition
4.2. Zooplankton Responses to Nutrients, OM, and Shading Addition
4.3. The Food Chain Relationship and the Bottom–Up Effect
4.4. Ecological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef]
- Hosmani, S.P. Fresh Water Algae as Indicators of Water Quality. Univers. J. Environ. Res. Technol. 2013, 3, 473–482. [Google Scholar]
- Pålsson, C.; Granéli, W. Nutrient limitation of autotrophic and mixotrophic phytoplankton in a temperate and tropical humic lake gradient. J. Plankton Res. 2004, 26, 1005–1014. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F. (Eds.) The Trophic Cascade in Lakes; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Munawar, M.; Wilson, J.B. Phytoplankton-zooplankton associations in Lake Superior: A statistical approach. J. Great Lakes Res. 1978, 4, 497–504. [Google Scholar] [CrossRef]
- Henry, R.; Tundisi, J.G.; Curi, P.R. Effects of phosphorus and nitrogen enrichment on the phytoplankton in a tropical reservoir (Lobo Reservoir, Brazil). Hydrobiologia 1984, 118, 177–185. [Google Scholar] [CrossRef]
- Verburg, P.; Schallenberg, M.; Elliott, S.; McBride, C.G. Nutrient budgets in lakes. In Lake Restoration Handbook; Springer: Cham, Germany, 2018; pp. 129–163. [Google Scholar]
- Reynolds, C.S. Phytoplankton assemblages and their periodicity in stratifying lake systems. Ecography 1980, 3, 141–159. [Google Scholar] [CrossRef]
- Jansson, M. Nutrient limitation and bacteria—Phytoplankton interactions in humic lakes. In Aquatic Humic Substances; Springer: Berlin/Heidelberg, Germany, 1998; pp. 177–195. [Google Scholar]
- Carlsson, P.; Granéli, E. Availability of humic bound nitrogen for coastal phytoplankton. Estuar. Coast. Shelf Sci. 1993, 36, 433–447. [Google Scholar] [CrossRef]
- Vähätalo, A.V.; Salonen, K.; Münster, U.; Järvinen, M.; Wetzel, R.G. Photochemical transformation of allochthonous organic matter provides bioavailable nutrients in a humic lake. Arch. Für Hydrobiol. 2003, 156, 287–314. [Google Scholar] [CrossRef]
- Klug, J.L. Positive and negative effects of allochthonous dissolved organic matter and inorganic nutrients on phytoplankton growth. Can. J. Fish. Aquat. Sci. 2002, 59, 85–95. [Google Scholar] [CrossRef]
- Jurgens, K. Impact of Daphnia on planktonic. Mar. Microb. Food Webs 1994, 8, 295–324. [Google Scholar]
- de Melo, M.L.; Kothawala, D.N.; Bertilsson, S.; Amaral, J.H.; Forsberg, B.; Sarmento, H. Linking dissolved organic matter composition and bacterioplankton communities in an Amazon floodplain system. Limnol. Oceanogr. 2020, 65, 63–76. [Google Scholar] [CrossRef]
- Morris, D.P.; Zagarese, H.; Williamson, C.E.; Balseiro, E.G.; Hargreaves, B.R.; Modenutti, B.; Moeller, R.; Queimalinos, C. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol. Oceanogr. 1995, 40, 1381–1391. [Google Scholar] [CrossRef]
- Litchman, E. Competition and coexistence of phytoplankton under fluctuating light: Experiments with two cyanobacteria. Aquat. Microb. Ecol. 2003, 31, 241–248. [Google Scholar] [CrossRef]
- Cory, R.M.; Kling, G.W. Interactions between sunlight and microorganisms influence dissolved organic matter degradation along the aquatic continuum. Limnol. Oceanogr. Lett. 2018, 3, 102–116. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Smith, D.G. Turbidity suspended sediment, and water clarity: A review. JAWRA J. Am. Water Resour. Assoc. 2001, 37, 1085–1101. [Google Scholar] [CrossRef]
- Welch, E.B.; Cooke, G.D. Internal phosphorus loading in shallow lakes: Importance and control. Lake Reserv. Manag. 2005, 21, 209–217. [Google Scholar] [CrossRef]
- Qin, B.; Gao, G.; Zhu, G.; Zhang, Y.; Song, Y.; Tang, X.; Xu, H.; Deng, J. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2013, 58, 961–970. [Google Scholar] [CrossRef]
- Hagemann, S.; Chen, C.; Clark, D.B.; Folwell, S.; Gosling, S.N.; Haddeland, I.; Hanasaki, N.; Heinke, J.; Ludwig, F.; Voss, F.; et al. Climate change impact on available water resources obtained using multiple global climate and hydrology models. Earth Syst. Dyn. 2013, 4, 129–144. [Google Scholar] [CrossRef]
- Petrucio, M.M.; Barbosa, F.A.; Furtado, A.L. Bacterioplankton and phytoplankton production in seven lakes in the Middle Rio Doce basin, south-east Brazil. Limnologica 2006, 36, 192–203. [Google Scholar] [CrossRef]
- Gontijo, B.M.; Britto, C.Q. Identificação e classificação dos impactos ambientais no Parque Florestal Estadual do Rio Doce–MG. Geonomos 1997. [Google Scholar] [CrossRef][Green Version]
- Brandão, L.P.M.; Brighenti, L.S.; Staehr, P.A.; Barbosa, F.A.R.; Bezerra-Neto, J.F. Partitioning of the diffuse attenuation coefficient for photosynthetically available irradiance in a deep dendritic tropical lake. Ann. Braz. Acad. Sci. 2017, 89 (Suppl. 1), 469–489. [Google Scholar] [CrossRef]
- Gagliardi, L.M.; Brighenti, L.S.; Staehr, P.A.; Barbosa, F.A.R.; Bezerra-Neto, J.F. Reduced rainfall increases metabolic rates in upper mixed layers of tropical lakes. Ecosystems 2019, 22, 1406–1423. [Google Scholar] [CrossRef]
- Fiksen, Ø.; Giske, J. Vertical distribution and population dynamics of copepods by dynamic optimization. ICES J. Mar. Sci. 1995, 52, 483–503. [Google Scholar] [CrossRef]
- Brighenti, L.S.; Staehr, P.A.; Gagliardi, L.M.; Brandão, L.P.M.; Elias, E.C.; de Mello, N.A.S.T.; Barbosa, F.A.R.; Bezerra-Neto, J.F. Seasonal changes in metabolic rates of two tropical lakes in the Atlantic forest of Brazil. Ecosystems 2015, 18, 589–604. [Google Scholar] [CrossRef]
- Roland, F.; Huszar, V.L.M.; Farjalla, V.F.; Enrich-Prast, A.; Amado, A.M.; Ometto, J.P.H.B. Climate change in Brazil: Perspective on the biogeochemistry of inland waters. Braz. J. Biol. 2012, 72, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.A.; Alves, L.F.; Aidar, M.; Araújo, L.S.; Baker, T.; Batista, J.L.F.; Campos, M.C.; Camargo, P.B.; Chave, J.; Delitti, W.B.C.; et al. Estimation of biomass and carbon stocks: The case of the Atlantic Forest. Biota Neotrop. 2008, 8, 21–29. [Google Scholar] [CrossRef]
- Silva, L.H.S. Fitoplâncton de um reservatório eutrófico (Lago Monte Alegre), Ribeirão Preto, São Paulo, Brasil. Rev. Bras. Biol. 1999, 59, 281–303. [Google Scholar] [CrossRef]
- Tucci, A.; Sant’Anna, C.L.; Gentil, R.C.; Azevedo, M.D.P. Fitoplâncton do Lago das Garças, São Paulo, Brasil: Um reservatório urbano eutrófico. Hoehnea 2006, 33, 147–175. [Google Scholar]
- Pujoni, D.G.F. Padrões Espaço-Temporais da Comunidade Planctônica do Complexo Lacustre do Médio Rio Doce. Doctoral Dissertation, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2015. [Google Scholar]
- Bezerra-Neto, J.F.; Briguenti, L.S.; Pinto-Coelho, R.M. A new morphometric study of Carioca Lake, Parque Estadual do Rio Doce (PERD), Minas Gerais State, Brazil. Acta Sci. Biol. Sci. 2010, 32, 49–54. [Google Scholar] [CrossRef]
- Barbosa, F.A.; Padisák, J. The forgotten lake stratification pattern: Atelomixis, and its ecological importance. Int. Ver. Für Theor. Und Angew. Limnol. Verh. 2002, 28, 1385–1395. [Google Scholar] [CrossRef]
- Brandão, L.P.M.; Brighenti, L.S.; Staehr, P.A.; Asmala, E.; Massicotte, P.; Tonetta, D.; Barbosa, F.A.R.; Pujoni, D.; Bezerra-Neto, J.F. Distinctive effects of allochthonous and autochthonous organic matter on CDOM spectra in a tropical lake. Biogeosciences 2018, 15, 2931–2943. [Google Scholar] [CrossRef]
- Mackereth, F.J.H.; Heron, J.; Talling, J.F. Water analysis and some revised methods for limnologists. Freshw. Biol. Assoc. Sci. Pub. 1978, 36, 117. [Google Scholar]
- Ávila, M.P.; Brandão, L.P.; Brighenti, L.S.; Tonetta, D.; Reis, M.P.; Stæhr, P.A.; Asmala, E.; Amado, A.M.; Barbosa, F.A.; Bezerra-Neto, J.F.; et al. Linking shifts in bacterial community with changes in dissolved organic matter pool in a tropical lake. Sci. Total Environ. 2019, 672, 990–1003. [Google Scholar] [CrossRef]
- Staehr, P.A.; Sand-Jensen, K.A.J. Seasonal changes in temperature and nutrient control of photosynthesis, respiration and growth of natural phytoplankton communities. Freshw. Biol. 2006, 51, 249–262. [Google Scholar] [CrossRef]
- Jakobsen, H.H.; Blanda, E.; Staehr, P.A.; Højgård, J.K.; Rayner, T.A.; Pedersen, M.F.; Jepsen, P.M.; Hansen, B.W. Development of phytoplankton communities: Implications of nutrient injections on phytoplankton composition, pH and ecosystem production. J. Exp. Mar. Biol. Ecol. 2015, 473, 81–89. [Google Scholar] [CrossRef]
- Brandão, L.P.M.; Staehr, P.A.; Bezerra-Neto, J.F. Seasonal Changes in Optical Properties of Two Contrasting Tropical Freshwater Systems. J. Limnol. 2016, 75, 508–519. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Für Theor. Und Angew. Limnol. Mitt. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Villafañe, V.E.; Reid, F.M.H. Métodos de microscopía para la cuantificación del fitoplancton. In Manual de Métodos Ficológicos; Alveal, K., Ferreiro, M.E., Oliviera, E.C., Ferreiro, M.A., Eds.; Universidad de Concepción: Concepción, Chile, 1995; pp. 169–185. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Harrell, F.E. Hmisc: Harrell Miscellaneous; R package Version 4.0-1; with contributions from Charles Dupont and many others (2017); CRAN-R: Vienna, Austria, 2020. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Barros, C.F.A. Diversidade e Ecologia do Fitoplâncton em 18 Lagoas Naturais do Médio Rio Doce. Doctoral Dissertation, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2010. [Google Scholar]
- de J Magalhães, A.A.; da Luz, L.D.; de Aguiar Junior, T.R. Environmental factors driving the dominance of the harmful bloom-forming cyanobacteria Microcystis and Aphanocapsa in a tropical water supply reservoir. Water Environ. Res. 2019, 91, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Calijuri, M.D.C.; Dos Santos, A.C.A.; Jati, S. Temporal changes in the phytoplankton community structure in a tropical and eutrophic reservoir (Barra Bonita, SP—Brazil). J. Plankton Res. 2002, 24, 617–634. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Rocha, M.I.D.A.; Marinho, M.M.; Azevedo, S.M.; Branco, C.W.; Huszar, V.L. Changes in species composition during annual cyanobacterial dominance in a tropical reservoir: Physical factors, nutrients and grazing effects. Aquat. Microb. Ecol. 2009, 57, 137–149. [Google Scholar] [CrossRef]
- Reynolds, C.S. Non-determinism to probability, or N: P in the community ecology of phytoplankton. Arch. Für Hydrobiol. 1999, 146, 23–35. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Reynolds, C.S. Variability in the provision and function of mucilage in phytoplankton: Facultative responses to the environment. Hydrobiologia 2007, 578, 37–45. [Google Scholar] [CrossRef]
- Krichen, E.; Rapaport, A.; Le Floc’h, E.; Fouilland, E. Demonstration of facilitation between microalgae to face environmental stress. Sci. Rep. 2019, 9, 16076. [Google Scholar] [CrossRef]
- Bittencourt-Oliveira, M.D.C.; Moura, A.D.N.; De Oliveira, M.C.; Massola, N.S., Jr. Geitlerinema species (Oscillatoriales, Cyanobacteria) revealed by cellular morphology, ultrastructure, and DNA sequencing (1). J. Phycol. 2009, 45, 716–725. [Google Scholar] [CrossRef]
- Tranvik, L.J. Bacterioplankton growth on fractions of dissolved organic carbon of different molecular weights from humic and clear waters. Appl. Environ. Microbiol. 1990, 56, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, W.; Huang, L.; Zhang, Y.; Qin, J.; Li, K.; Chen, L. Spatial and temporal variability in water transparency in Yunnan Plateau lakes, China. Aquat. Sci. 2019, 81, 36. [Google Scholar] [CrossRef]
- Brighenti, L.S.; Staehr, P.A.; Brandão, L.P.M.; Barbosa, F.A.; Bezerra-Neto, J.F. Importance of nutrients, organic matter and light availability on epilimnetic metabolic rates in a mesotrophic tropical lake. Freshw. Biol. 2018, 63, 1143–1160. [Google Scholar] [CrossRef]
- Faria, G.R.; Paes, C.R.P.S.; Castro, D.J.F.A.; Tinoco, N.A.; Barbarino, E.; Lourenço, S.O. Effects of the availability of CO2 on growth, nutrient uptake, and chemical composition of the marine microalgae Chlorella sp. and Nannochloropsis oculata, two potentially useful strains for biofuel production. Int. Res. J. Biotechnol. 2012, 3, 65–75. [Google Scholar]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Rosa, A.P.C.D.; Moraes, L.; Morais, E.G.D.; Costa, J.A.V. Fatty Acid Biosynthesis from Chlorella in Autotrophic and Mixotrophic Cultivation. Braz. Arch. Biol. Technol. 2020, 63, e20180534. [Google Scholar] [CrossRef]
- Jansson, M.; Bergström, A.K.; Blomqvist, P.; Drakare, S. Allochthonous organic carbon and phytoplankton/bacterioplankton production relationships in lakes. Ecology 2000, 81, 3250–3255. [Google Scholar] [CrossRef]
- Rodríguez, M.P.; Matsumura-Tundisi, T. Variation of density, species composition and dominance of rotifers at a shallow tropical reservoir (Broa Reservoir, SP, Brazil) in a short scale time. Rev. Bras. Biol. 2000, 60, 01–09. [Google Scholar] [CrossRef]
- Gilbert, J.J.; Bogdan, K.G. 5. Rotifer Grazing. Trophic Interact. Within Aquat. Ecosyst. 1984, 85, 97. [Google Scholar]
- Schmid-Araya, J.M.; Schmid, P.E. Trophic relationships: Integrating meiofauna into a realistic benthic food web. Freshw. Biol. 2000, 44, 149–163. [Google Scholar] [CrossRef]
- Porter, K.G. Integrating the microbial loop and the classic food chain into a realistic planktonic food web. In Food Webs; Springer: Boston, MA, USA, 1996; pp. 51–59. [Google Scholar]
- Hambright, K.D.; Zohary, T.; Güde, H. Microzooplankton dominate carbon flow and nutrient cycling in a warm subtropical freshwater lake. Limnol. Oceanogr. 2007, 52, 1018–1025. [Google Scholar] [CrossRef]
- Perbiche-Neves, G.; Serafim-Júnior, M.; Ghidini, A.R.; Brito, L.D. Spatial and temporal distribution of Copepoda (Cyclopoida and Calanoida) of an eutrophic reservoir in the basin of upper Iguaçu River, Paraná, Brazil. Acta Limnol. Bras. 2007, 19, 393–406. [Google Scholar]
- Fontanarrosa, M.S.; Chaparro, G.; de Tezanos Pinto, P.; Rodriguez, P.; O’Farrell, I. Zooplankton response to shading effects of free-floating plants in shallow warm temperate lakes: A field mesocosm experiment. Hydrobiologia 2010, 646, 231–242. [Google Scholar] [CrossRef]
- Gannon, J.E.; Stemberger, R.S. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 16–35. [Google Scholar] [CrossRef]
- Work, K.A.; Havens, K.E. Zooplankton grazing on bacteria and cyanobacteria in a eutrophic lake. J. Plankton Res. 2003, 25, 1301–1306. [Google Scholar] [CrossRef]
- Yoshida, T.; Hairston, N.G., Jr.; Ellner, S.P. Evolutionary trade–off between defence against grazing and competitive ability in a simple unicellular alga, Chlorella vulgaris. Proc. R. Soc. London. Ser. B Biol. Sci. 2004, 271, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Stanković, N.; Kostić, I.; Jovanović, B.; Savić-Zdravković, D.; Matić, S.; Bašić, J.; Cvetković, T.; Simeunović, J.; Milošević, D. Can phytoplankton blooming be harmful to benthic organisms? The toxic influence of Anabaena sp. and Chlorella sp. on Chironomus riparius larvae. Sci. Total Environ. 2020, 729, 138666. [Google Scholar] [CrossRef]
- Reynolds, C.S. Hydrodynamics and mixing in lakes, reservoirs, wetlands and rivers. In Biogeochemistry of Inland Waters: A Derivative of Encyclopedia of Inland Waters; Cary Institute of Ecosystem Studies: Millbrook, NY, USA, 2009. [Google Scholar]
- Antunes, D.A.; Cupolillo, F. Análise do balanço hídrico climatológico decendial do Parque Estadual do Rio Doce—PERD e entorno: Climatologia de 2005–2015 comparada com o ano anômalo 2013–2014. Rev. Bras. Climatol. 2018, 1. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Yule, C.M.; Mathooko, J.M.; Pringle, C.M. Organic matter processing in tropical streams. In Tropical Stream Ecology; Academic Press: New York, NY, USA, 2008; pp. 43–64. [Google Scholar]

| Random Effects | Sources of Variation | DF | Sum Sq | F Value | p-Value | |
|---|---|---|---|---|---|---|
| log(sumphyto) | 1|~days | C | 1 | 1.90 | 4.00 | 0.05 |
| N | 1 | 47.41 | 99.66 *** | 0.00 | ||
| SH | 1 | 0.29 | 0.60 | 0.45 | ||
| C:N | 1 | 0.53 | 1.12 | 0.30 | ||
| C:SH | 1 | 0.29 | 0.61 | 0.44 | ||
| N:SH | 1 | 0.04 | 0.08 | 0.78 | ||
| C:N:SH | 1 | 0.00 | 0.00 | 0.99 | ||
| log(Chlorophyceae) | 1|~days | C | 1 | 9.91 | 13.76 *** | 0.00 |
| N | 1 | 98.68 | 137.02 *** | 0.00 | ||
| SH | 1 | 0.81 | 1.12 | 0.30 | ||
| C:N | 1 | 0.17 | 0.24 | 0.63 | ||
| C:SH | 1 | 0.36 | 0.50 | 0.49 | ||
| N:SH | 1 | 0.04 | 0.05 | 0.82 | ||
| C:N:SH | 1 | 0.28 | 0.39 | 0.54 | ||
| log(Cyanobacteria) | 1|~days | C | 1 | 0.11 | 0.38 | 0.54 |
| N | 1 | 12.50 | 42.63 *** | 0.00 | ||
| SH | 1 | 0.17 | 0.57 | 0.45 | ||
| C:N | 1 | 0.34 | 1.15 | 0.29 | ||
| C:SH | 1 | 1.72 | 5.88 * | 0.02 | ||
| N:SH | 1 | 0.05 | 0.17 | 0.68 | ||
| C:N:SH | 1 | 0.35 | 1.20 | 0.28 | ||
| log(sumzoo) | 1|~days | C | 1 | 1.83 | 5.03 * | 0.03 |
| N | 1 | 2.50 | 6.85 * | 0.01 | ||
| SH | 1 | 1.09 | 2.99 | 0.09 | ||
| C:N | 1 | 0.67 | 1.85 | 0.18 | ||
| C:SH | 1 | 0.16 | 0.44 | 0.51 | ||
| N:SH | 1 | 1.19 | 3.27 | 0.08 | ||
| C:N:SH | 1 | 0.05 | 0.13 | 0.73 | ||
| log(Rotifera) | 1|~days | C | 1 | 2.52 | 6.70 * | 0.01 |
| N | 1 | 5.04 | 13.41 *** | 0.00 | ||
| SH | 1 | 1.15 | 3.07 | 0.09 | ||
| C:N | 1 | 1.38 | 3.68 | 0.06 | ||
| C:SH | 1 | 0.39 | 1.05 | 0.31 | ||
| N:SH | 1 | 2.65 | 7.07 * | 0.01 | ||
| C:N:SH | 1 | 0.01 | 0.02 | 0.90 | ||
| Copepoda | 1|~days | C | 1 | 19,444.78 | 4.01 | 0.05 |
| N | 1 | 38,936.72 | 8.02 ** | 0.01 | ||
| SH | 1 | 19,711.36 | 4.06 | 0.05 | ||
| C:N | 1 | 5648.51 | 1.16 | 0.29 | ||
| C:SH | 1 | 5218.75 | 1.08 | 0.31 | ||
| N:SH | 1 | 22.28 | 0.00 | 0.95 | ||
| C:N:SH | 1 | 2306.03 | 0.48 | 0.50 | ||
| Cladocera | 1|~days | C | 1 | 2658.16 | 0.85 | 0.79 |
| N | 1 | 2405.50 | 0.77 | 0.41 | ||
| SH | 1 | 1559.52 | 0.50 | 0.85 | ||
| C:N | 1 | 1561.80 | 0.50 | 0.58 | ||
| C:SH | 1 | 1559.52 | 0.50 | 0.79 | ||
| N:SH | 1 | 386.47 | 0.12 | 0.86 | ||
| C:N:SH | 1 | 750.50 | 0.24 | 0.47 |
| Rot | Clad | Cop | Cyano | Chloro | Bact | SumPhyto | SumZoo | |
|---|---|---|---|---|---|---|---|---|
| TN | 0.43 ** | 0.59 *** | 0.58 *** | 0.53 *** | 0.62 *** | 0.41 ** | ||
| TP | 0.38 ** | −0.32 * | 0.57 *** | 0.54 *** | 0.50 *** | 0.59 *** | 0.34 * | |
| Chl-a | 0.47 *** | 0.40 ** | 0.61 *** | 0.66 *** | 0.64 *** | 0.41 ** | ||
| KdPAR | 0.38 ** | 0.57 *** | 0.42 ** | 0.41 ** | 0.43 ** | |||
| KdUV | 0.31 * | 0.46 *** | 0.36 * | 0.51 *** | 0.36 * | |||
| DOC | 0.59 *** | 0.37 * | 0.59 *** | |||||
| Rot | 0.35 * | 0.62 *** | 0.97 *** | |||||
| Clad | 0.48 *** | 0.38 ** | 0.47 *** | |||||
| Cop | −0.50 *** | −0.51 *** | 0.35 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.I.B.d.; Brandão, L.P.M.; Brighenti, L.S.; Staehr, P.A.U.; Barros, C.F.d.A.; Barbosa, F.A.R.; Bezerra-Neto, J.F. Nutrient, Organic Matter and Shading Alter Planktonic Structure and Density of a Tropical Lake. Limnol. Rev. 2025, 25, 16. https://doi.org/10.3390/limnolrev25020016
Silva MIBd, Brandão LPM, Brighenti LS, Staehr PAU, Barros CFdA, Barbosa FAR, Bezerra-Neto JF. Nutrient, Organic Matter and Shading Alter Planktonic Structure and Density of a Tropical Lake. Limnological Review. 2025; 25(2):16. https://doi.org/10.3390/limnolrev25020016
Chicago/Turabian StyleSilva, Marina Isabela Bessa da, Luciana Pena Mello Brandão, Ludmila Silva Brighenti, Peter A. U. Staehr, Cristiane Freitas de Azevedo Barros, Francisco Antônio Rodrigues Barbosa, and José Fernandes Bezerra-Neto. 2025. "Nutrient, Organic Matter and Shading Alter Planktonic Structure and Density of a Tropical Lake" Limnological Review 25, no. 2: 16. https://doi.org/10.3390/limnolrev25020016
APA StyleSilva, M. I. B. d., Brandão, L. P. M., Brighenti, L. S., Staehr, P. A. U., Barros, C. F. d. A., Barbosa, F. A. R., & Bezerra-Neto, J. F. (2025). Nutrient, Organic Matter and Shading Alter Planktonic Structure and Density of a Tropical Lake. Limnological Review, 25(2), 16. https://doi.org/10.3390/limnolrev25020016







