2.1. Comparison of Live and Preserved Zooplankton
To evaluate the suitability of formalin-preserved animals for stable isotope analyses, live zooplankton were collected from Baseline Lake, MI, USA (43.425 N, 83.895 W) by vertical net tows on 20 and 28 June 2017. The lake was one of the first sites for the application of egg ratio analysis to calculate birth and death rates in the study of population dynamics of
Daphnia and
Leptodora [
19]. The samples were transported to the laboratory within 2 h of collection. On 20 June, the live sample was immediately concentrated on Nitex netting, and individuals of
D. pulicaria and
L. kindti were picked by watchmaker forceps and placed in tared tin capsules, with five replicates of 40
Daphnia or 10
Leptodora per capsule. The samples were dried for 72 h at 55 °C, reweighed, and prepared for stable C and N isotope analysis. Additionally, residual
Leptodora from the live sample were resuspended in lake water and then preserved with 5% sucrose formalin. After 72 h, those preserved animals were rinsed in reverse osmosis water and treated for stable isotope analysis in identical fashion to the live samples.
On 28 June, the live sample was split into two equal subsamples. One subsample was preserved with 5% sucrose-formalin and was set aside. The other (live) subsample was concentrated on Nitex netting from which five replicates of 20 D. pulicaria and five replicates of 10 L. kindti were picked by watchmaker forceps, followed by being treated as on 20 June. After 72 h, the formalin-preserved animals were rinsed in reverse osmosis water. Five replicates of 20 D. pulicaria and five replicates of 10 L. kindti were picked by watchmaker forceps and then treated the same as the live samples.
The samples were submitted to the Laboratory for Biotechnology and Bioanalysis at Washington State University, Pullman, WA, USA for C and N isotope analysis. As our results will demonstrate, 15N data proved suitable but 13C data did not. Further results were confined to nitrogen isotopes alone.
2.2. Analysis of Plankton Samples from the Great Lakes
All Great Lakes zooplankton samples were collected by 1 m diameter Puget Sound closing nets of 130 µm net apertures while the vessel lay at anchor. The samples were processed and analyzed identically with the methods used by [
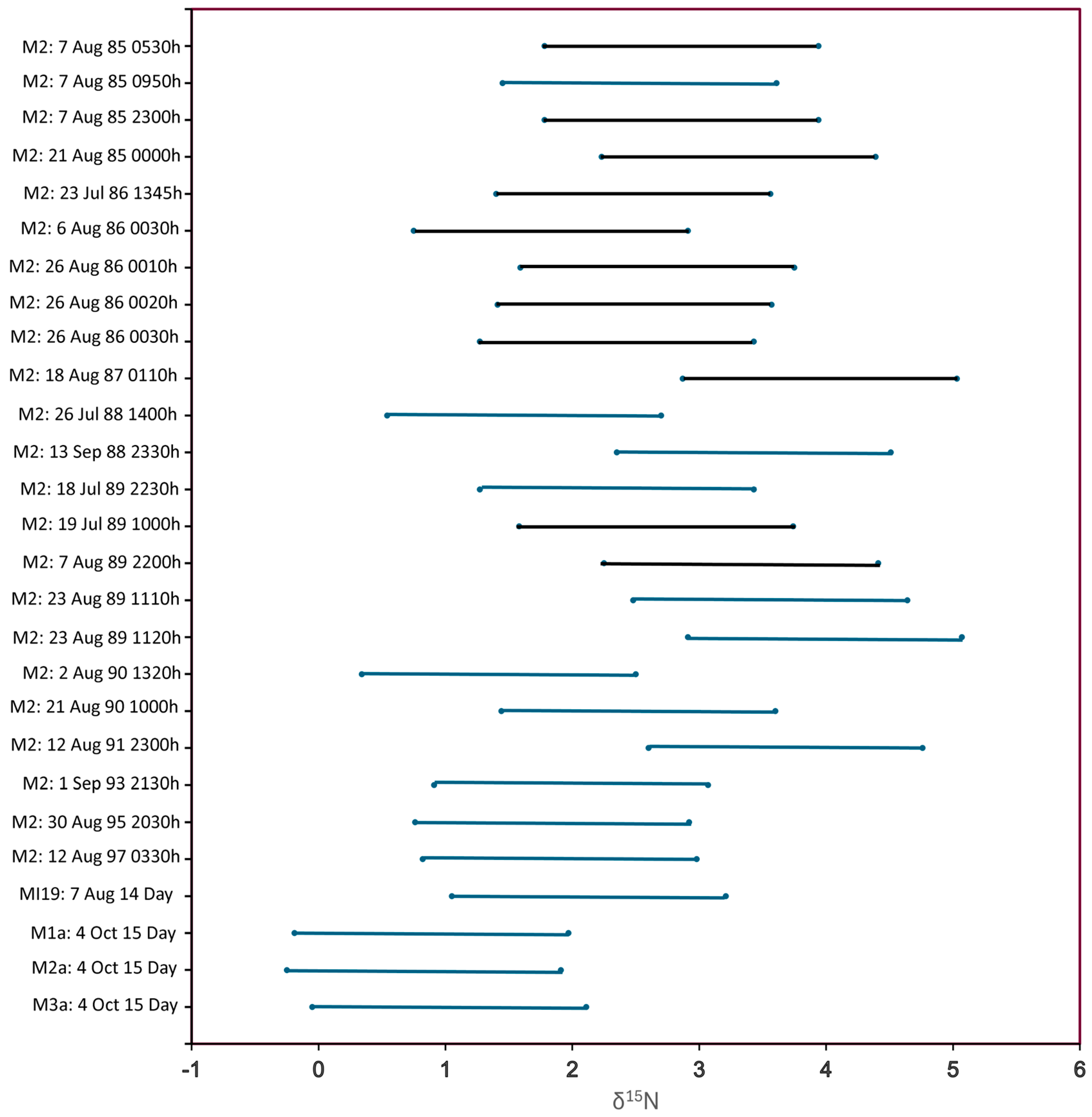
16]. We assembled a multi-year (1985 to 1997) time series of analyses for
D. mendotae from a single reference station in southern Lake Michigan (Station M2: 43.000 N, 86.667 W, 100 m station depth) and examined the sample means for evidence of variation with time of day or long-term temporal trends. We supplemented this reference time series with additional samples of opportunity collected in 2009, 2013, 2014 and 2015 at other sites in Lakes Huron, Michigan and Superior.
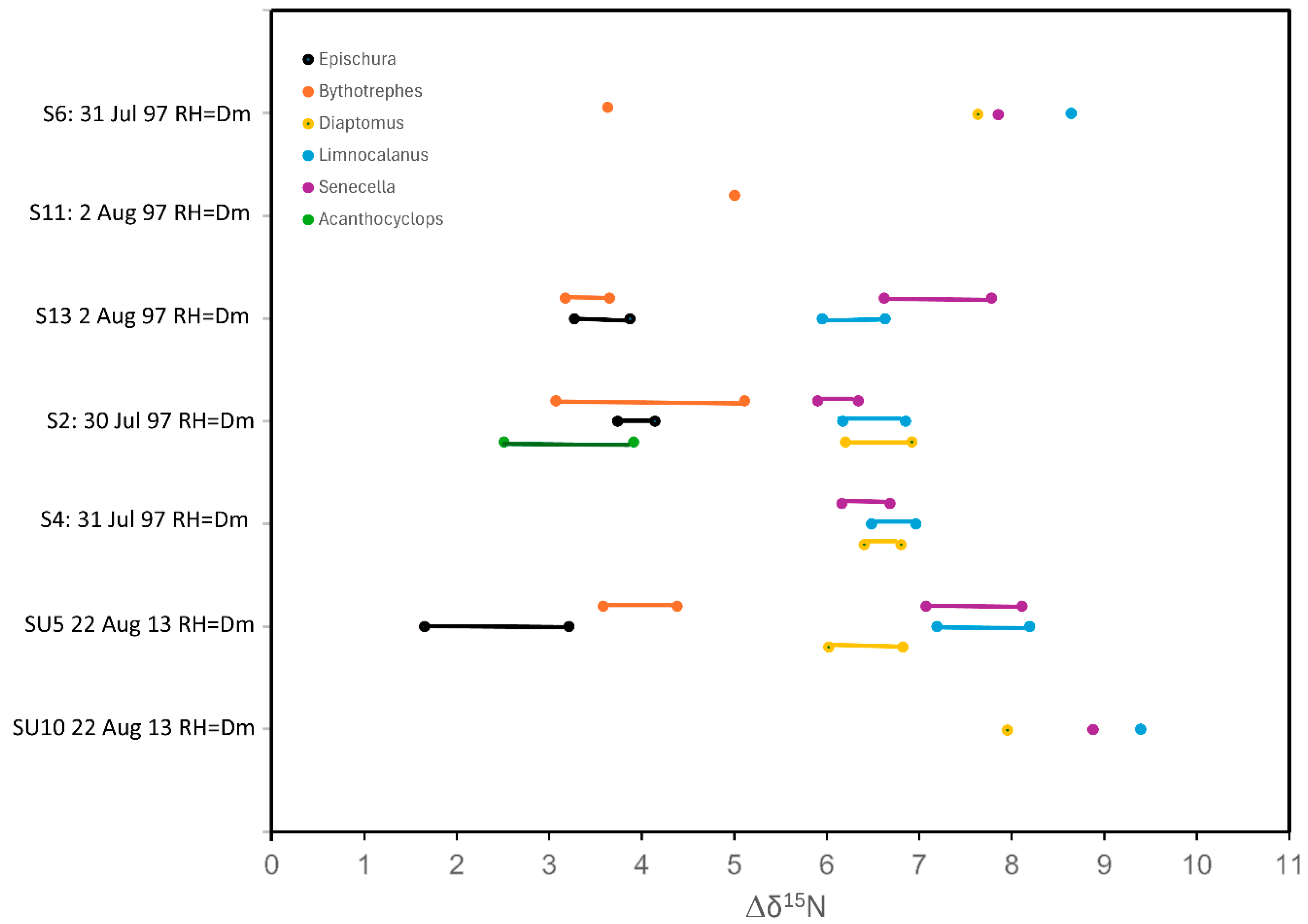
Our preferred reference herbivore was Daphnia mendotae, but we also measured D. pulicaria, D. retrocurva and the non-daphnids Bosmina longirostris, Holopedium gibberum and veliger larvae. For trophic-level comparisons, we used mainly Epischura lacustris, Diaptomus (syn. Leptodiaptomus) sicilis, Bythotrephes cederstroemi and Limnocalanus macrurus plus occasional occurrences of Senecella calanoides, Acanthocyclops vernalis, Mesocyclops edax, Polyphemus pediculus, Cercopagis pengoi, Leptodora kindti and Mysis relicta.
In addition to stable isotopes, we quantified the mean dry mass and %N of our samples. We quantified the variability typical of the data to help judge the likely ecological significance of any statistical tests. When large numbers of comparisons are based on relatively small numbers of replicates, there is always a possibility of spurious conclusions. Standard deviations (SDs) or coefficients of variation (CVs: SD/mean) among replicate determinations were ln-transformed, and single factor analysis of variance (AOV) was applied to determine whether there were statistically significant differences among SDs or CVs based on two, three or four replicates. We next tested for an a priori expected negative correlation between CVs or SDs and the number of animals that were pooled to produce each replicate analysis across the observed range of pool sizes from 2 to 500.
We quantified the diurnal variability observed on 7 August 85 at three separate sampling times and compared it with the temporal variability observed across the full time series exclusive of 7 August 85. The mean values observed at each date and sampling time were ln-transformed prior to statistical comparisons. We also examined whether differences might exist among daphnid taxa drawn from different vertical strata on the same date and time. We used samples collected by the Puget Sound closing net (Research Nets, Inc., Bellevue, WA, USA) from 15–0 m, 40–15 m and 90–40 m shortly after midnight on 26 August 1986.
We continued the practice adopted by [
16] of using
D. mendotae as the preferred reference herbivore for imputing trophic-level comparisons whenever possible.
We next performed a statistical power analysis to define our criteria for ascribing ecological significance to statistically significant analytical differences between replicated samples. We established a balance between Type I and Type II errors by setting α = 0.1 and β = 0.75. The object was to hold Type I errors reasonably low, while seeking a credible level of power to detect environmental differences if they indeed exist.
We estimated generic thresholds for ecologically significant differences between sample means (ΔX) as
where Zα and Zβ are values of the standard normal cumulative distribution under the specified power assumptions (1.28 and −0.67, respectively), σ is the standard deviation of the distribution of the variable being investigated and n is the sample size. For σ, we used the 90th percentile values for the SD of δ
15N (
Table 1) to ensure that the variance would be well constrained and conservatively overestimated in most cases. For comparisons made between samples with three replicates each (85% of the cases), the mean differences between samples for δ
15N would have to exceed 0.54‰ to be regarded as statistically significant according to our power criteria. We reasoned that, otherwise, statistical vagaries stemming from small sample sizes and large numbers of comparisons could produce spurious instances of presumed statistical significance.
For calculating lower and upper 95% confidence limits of SDs, we used conventional Excel™ functions:
For comparisons of trophic position among taxa across years and lakes, we subtracted the mean δ
15N of the putative herbivore, usually
D. mendotae, from the δ
15N of each replicate alternative taxon, e.g.,
L. macrurus, to produce a Δδ
15N statistic following the method of [
15]. The Δδ
15N values were compared by one-way AOV followed by post hoc Tukey’s Honestly Significant Difference (HSD) test.
Geographic coordinates and station depths as well as all the original Great Lakes data used in this paper are included in a
Supplemental Data File. Station locations are illustrated in
Figure 1. Additional data have been deposited in an open access dataset as part of the University of Michigan Deep Blue data archive project [
20].