Exploring the Antioxidant and Bioinsecticidal Activity of Spontaneous Flora Vegetal Extracts for Plant Protection and Prevention of Soil Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents
2.3. Extraction Procedure
2.4. Evaluation of the Antioxidant Activity
2.4.1. Total Polyphenols Content
2.4.2. Total Flavonoids Content
2.4.3. DPPH Free Radical Scavenging Activity
2.5. FTIR Analysis
2.6. Evaluation of Bioinsecticidal Activity
2.7. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.1.1. Total Polyphenols and Flavonoids Assay
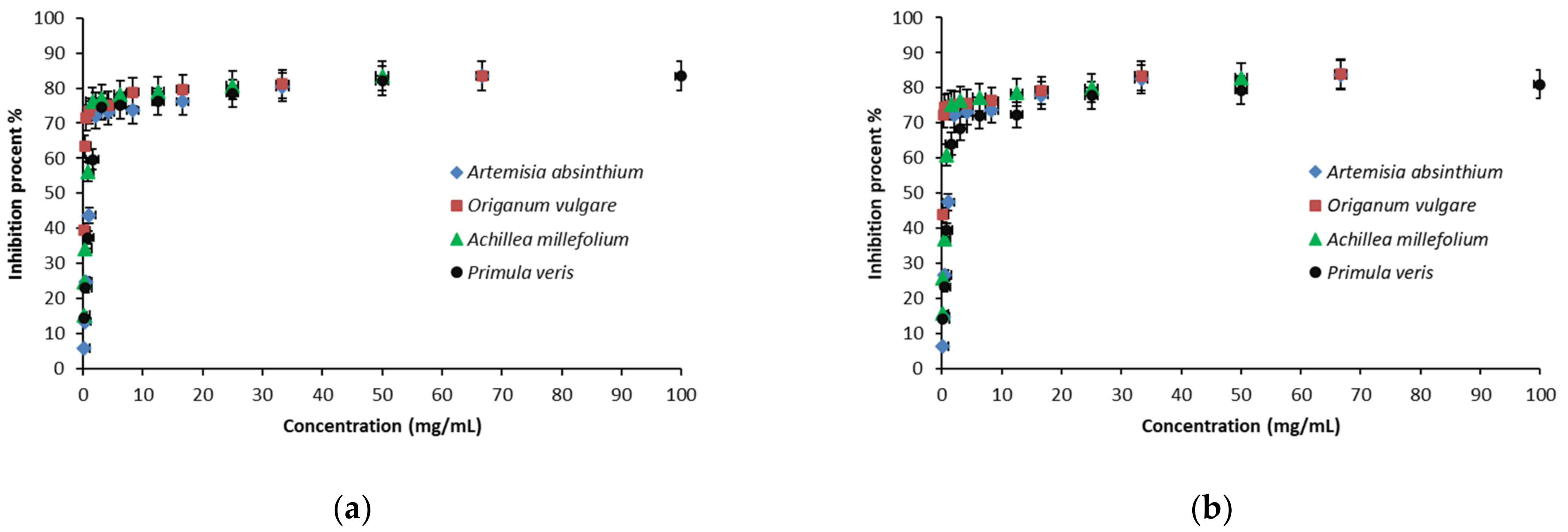
3.1.2. DPPH Free Radical Scavenging Assay
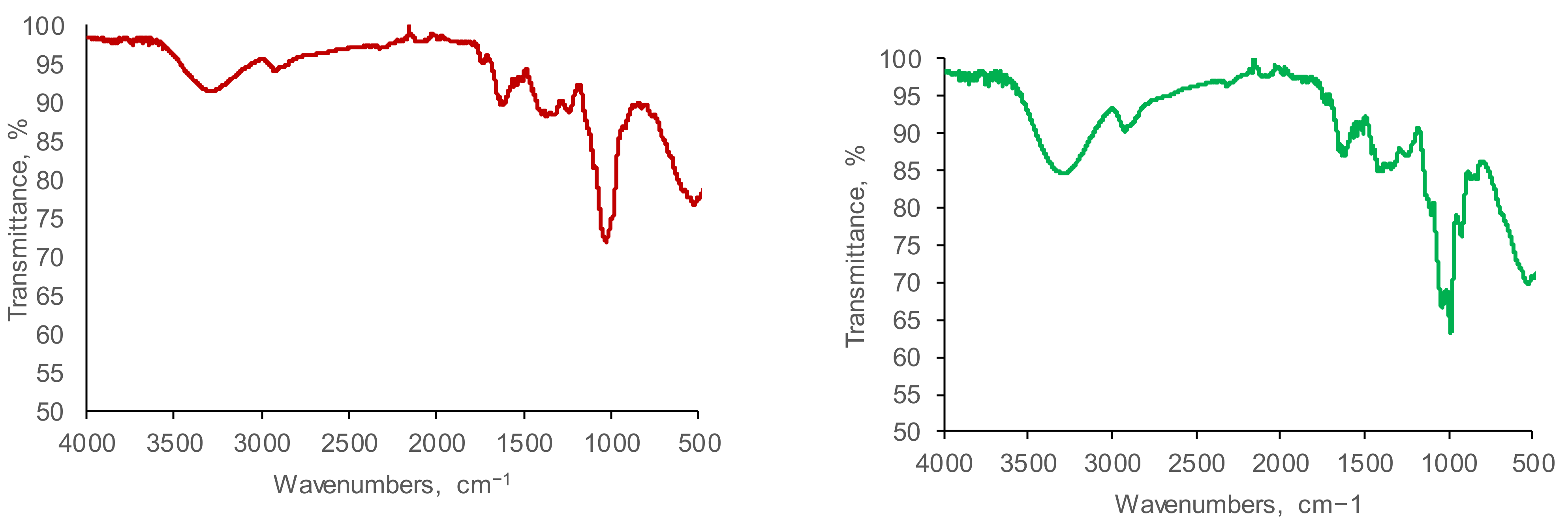
3.2. FTIR Analysis
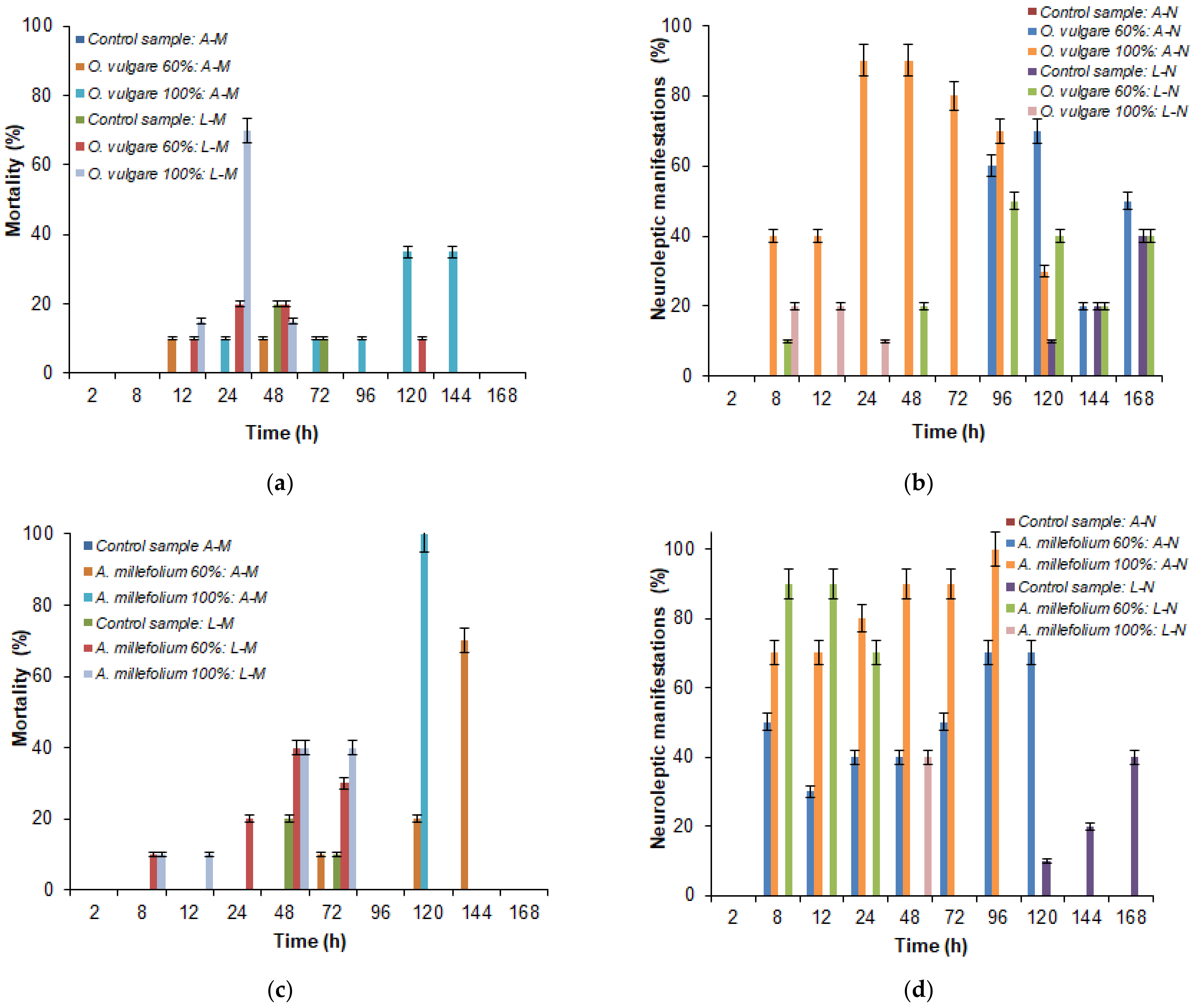
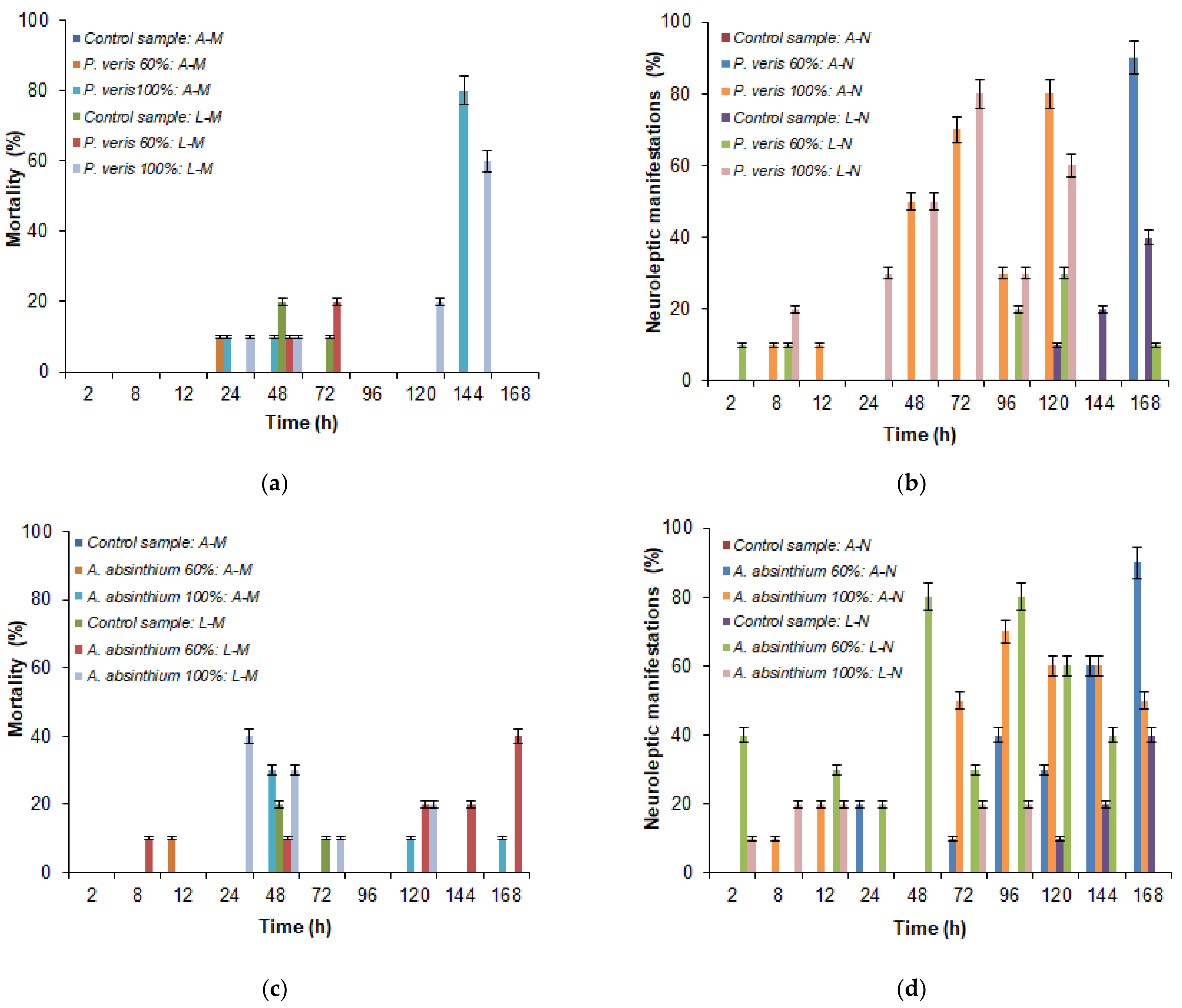
3.3. Assessing the Bioinsecticidal Activity of Plant Extracts
3.4. Statistical Analysis the Bioinsecticidal Activity of Plant Extracts
4. Discussions
4.1. Antioxidant Activity
4.1.1. Total Polyphenols and Flavonoids Assay
4.1.2. DPPH Free Radical Scavenging Assay
4.2. FTIR Analysis
4.3. Assessing the Bioinsecticidal Activity of Plant Extracts
4.4. Statistical Analysis of the Bioinsecticidal Activity of Plant Extracts
4.5. Insecticidal and Repellent Effect of Plant Compounds and Their Mechanism of Action
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grzywacz, D.; Stevenson, P.C.; Mushobozi, W.L.; Belmain, S.; Wilson, K. The Use of Indigenous Ecological Resources for Pest Control in Africa. Food Secur. 2014, 6, 71–86. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Mark Tatchell, G.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, L.; Mazourek, M. A Sustainable Agricultural Future Relies on the Transition to Organic Agroecological Pest Management. Sustainability 2018, 10, 2023. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Liu, Y.; Rahman, M.M.; Dharmarajan, R.; Duan, L.; Uddin, A.F.M.J.; Naidu, R. Nanobiopesticides: Composition and Preparation Methods; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128158296. [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural Products in Crop Protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Gupta, S.; Dikshit, A.K. Biopesticides: An Ecofriendly Approach for Pest Control. J. Biopestic. 2010, 3, 186–188. [Google Scholar]
- Cothran, R.D.; Brown, J.M.; Relyea, R.A. Proximity to Agriculture Is Correlated with Pesticide Tolerance: Evidence for the Evolution of Amphibian Resistance to Modern Pesticides. Evol. Appl. 2013, 6, 832–841. [Google Scholar] [CrossRef]
- Kaleeswaran, G.; Firake, D.M.; Sanjukta, R.; Behere, G.T.; Ngachan, S. V Bamboo-Leaf Prickly Ash Extract: A Potential Bio-Pesticide against Oriental Leaf Worm, Spodoptera Litura (Fabricius) (Lepidoptera: Noctuidae). J. Environ. Manag. 2018, 208, 46–55. [Google Scholar] [CrossRef]
- Afonso, C.; Pastorinho, M.R.; Palmeira-de-oliveira, A.; Ana, C.; Sousa, A. Ecotoxicity of Plant Extracts and Essential Oils: A Review ☆. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef]
- Bakshi, L.; Ghosh, R. Marigold Biopesticide as an Alternative to Conventional Chemical Pesticides. J. Adv. Sci. Res. 2022, 13, 26–33. [Google Scholar] [CrossRef]
- Lahlali, R.; El Hamss, H.; Mediouni-Ben Jemâa, J.; Barka, E.A. Editorial: The Use of Plant Extracts and Essential Oils as Biopesticides. Front. Agron. 2022, 4, 1–3. [Google Scholar] [CrossRef]
- Lopes, A.I.F.; Monteiro, M.; Ara, A.R.L.; Rodrigues, A.R.O.; Castanheira, E.M.S.; Pereira, D.M.; Olim, P.; Fortes, A.G.; Gonçalves, M.S.T. Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides. Molecules 2020, 25, 5855. [Google Scholar] [CrossRef]
- Dhakal, R.; Singh, D.N. Biopesticides: A Key to Sustainable Agriculture. Int. J. Pure Appl. Biosci. 2019, 7, 391–396. [Google Scholar] [CrossRef]
- Giraldo-Rivera, A.-I.; Guerrero-Álvarez, G.-E. Botanical Biopesticides: Research and Development Trends, a Focus on the Annonaceae Family Biopesticidas de Origen Botánico: Tendencias En Investigación y Desarrollo, Un Enfoque En La Familia Annonaceae. Rev. Colomb. Ciencias Hortícolas 2019, 13, 371–383. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.; Crivoi, F.; Rosu, C.; Corciovă, A. Secondary Metabolites from Artemisia Genus as Biopesticides. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Esmaeili, H.; Parvin, N.; Mojoudi, F.; Fateh, M.; Fateh, H.; Babapoor, A.; Mazraedoost, S.; Zarei, M. Investigating the Activity of Antioxidants Activities Content in Apiaceae and to Study Antimicrobial and Insecticidal Activity of Antioxidant by Using SPME Fiber Assembly Carboxen/Polydimethylsiloxane (CAR/PDMS). J. Environ. Treat. Tech. 2020, 8, 214–224. [Google Scholar]
- Chuang, K.J.; Chen, Z.J.; Cheng, C.L.; Hong, G.B. Investigation of the Antioxidant Capacity, Insecticidal Ability and Oxidation Stability of Chenopodium Formosanum Seed Extract. Int. J. Mol. Sci. 2018, 19, 2726. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Zheljazkov, V.D.; Radoukova, T.; Dincheva, I.; Piperkova, N.; Maneva, V.; Astatkie, T.; Kačániová, M. Biological Activity of Essential Oils of Four Juniper Species and Their Potential as Biopesticides. Molecules 2021, 26, 6358. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sandín-España, P.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides from Natural Products: Current Development, Legislative Framework, and Future Trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Comparative and Synergistic Activity of Rosmarinus Officinalis L. Essential Oil Constituents against the Larvae and an Ovarian Cell Line of the Cabbage Looper, Trichoplusia Ni (Lepidoptera: Noctuidae). Pest Manag. Sci. 2016, 72, 474–480. [Google Scholar] [CrossRef]
- Aryani, D.S.; Auamcharoen, W. Repellency and Contact Toxicity of Crude Extracts from Three Thai Plants (Zingiberaceae) against Maize Grain Weevil, Sitophilus Zeamais (Motschlusky) (Coleoptera: Curculionidae). J. Biopestic. 2016, 9, 52–62. [Google Scholar]
- Loko, L.Y.; Alagbe, O.; Dannon, E.A.; Datinon, B.; Orobiyi, A.; Thomas-Odjo, A.; Dansi, A.; Tamò, M. Repellent Effect and Insecticidal Activities of Bridelia Ferruginea, Blighia Sapida, and Khaya Senegalensis Leaves Powders and Extracts against Dinoderus Porcellus in Infested Dried Yam Chips. Psyche A J. EÈntomol. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Sattar, E.; Zaitoon, A.; Sayed, A.M.E.; Bakhashwain, A.A. Evaluation of Some Medicinal Plants in Controlling Culex Pipiens. J. Egypt. Soc. Parasitol. 2014, 44, 771–778. [Google Scholar] [PubMed]
- Handa, S.S.; Khanuja, S.P.S.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants; International Centre for Science and High Technology: Trieste, Italy, 2008; ISBN 9772081415. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Pirvu, L.; Armatu, A.; Bubueanu, C.; Pintilie, G.; Nita, S. Obtaining and Chemical Characterization of Some Vegetal Extracts with Corrosion-Scaling Inhibition Properties. Part I. Fagus Sylvatica and Alii Cepae Bulbus Extracts. Rom. Biotechnol. Lett. 2010, 15, 5683–5689. [Google Scholar]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y.; Bedong-semeling, J.; Nasi, B.A. Extraction, Isolation and Characterization of Bioactive Compounds from Plants Extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Benković, M.; Belščak Cvitanović, A.; Valinger, D.; Jurina, T.; Gajdoš Kljusurić, J. Kinetics and Thermodynamics of the Solid-Liquid Extraction Process of Total Polyphenols, Antioxidants and Extraction Yield from Asteraceae Plants. Ind. Crop. Prod. 2016, 91, 205–214. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical Diversity in Leaf and Stem Essential Oils of Origanum Vulgare L. and Their Effects on Microbicidal Activities. AMB Express 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Rafi Shaik, M.; Ali, Z.J.Q.; Khan, M.; Kuniyil, M.; Assal, M.E.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, M.; Adil, S.F. Green Synthesis and Characterization of Palladium Nanoparticles Using Origanum Vulgare L. Extract and Their Catalytic Activity. Molecules 2017, 22, 165. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Abdullah, M.M.S.; Al-Wahaibi, L.H.; Alkhathlan, H.Z. Characterization of Secondary Metabolites of Leaf and Stem Essential Oils of Achillea Fragrantissima from Central Region of Saudi Arabia. Arab. J. Chem. 2020, 13, 5254–5261. [Google Scholar] [CrossRef]
- Tampe, J.; Parra, L.; Huaiquil, K.; Mutis, A.; Quiroz, A. Repellent Effect and Metabolite Volatile Profile of the Essential Oil of Achillea Millefolium Against Aegorhinus Nodipennis (Hope) (Coleoptera: Curculionidae). Neotrop. Entomol. 2015, 44, 279–285. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, L.; Hu, W.; Wang, K.; Zhou, Y.; Li, H.; Huang, C.; Chun, J.; Zhang, Z. Insecticidal, Fumigant, and Repellent Activities of Sweet Wormwood Oil and Its Individual Components against Red Imported Fire Ant Workers (Hymenoptera: Formicidae). J. Insect Sci. 2014, 14. [Google Scholar] [CrossRef]
- Fernandes, M.J.G.; Pereira, R.B.; Pereira, D.M.; Fortes, A.G.; Castanheira, E.M.S.; Gonçalves, M.S.T. New Eugenol Derivatives with Enhanced Insecticidal Activity. Int. J. Mol. Sci. 2020, 21, 9257. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V. Carvone (Mentha Spicata L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 309–316. ISBN 9780124166417. [Google Scholar]
- Alkan, M. Achillea Millefolium L. (Asteraceae) Uçucu Yağının Kimyasal Bileşimi ve Sitophilus Granarius (Coleoptera: Curculionidae) ve Rhyzopertha Dominica (Coleoptera: Bostrichidae)′ya Karşı Insektisidal Aktivitesi. Bitki Koruma Bülteni 2020, 60, 85–93. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines Third Edition; Pharmacutical Press: London, UK, 2007; ISBN 9780878936953. [Google Scholar]
- Bujor, O. Ghidul Plantelor Medicinale Si Aromatice de La A La Z; Fiat Lux: Bucuresti, Romania, 2003; ISBN 9739250688. [Google Scholar]
- Daraban, G.; Marinela, B.; Rusu, L.; Daniela, S. Biopesticides-a New Challenge in Assuring Food Quality and Sustainable Agriculture. Lucr. Stiint. Ser. Hortic. 2018, 61, 269–274. [Google Scholar]
- Cudalbeanu, M.; Ghinea, I.O.; Furdui, B.; Dah-Nouvlessounon, D.; Raclea, R.; Costache, T.; Cucolea, I.E.; Urlan, F.; Dinica, R.M. Exploring New Antioxidant and Mineral Compounds from Nymphaea Alba Wild-Grown in Danube Delta Biosphere. Molecules 2018, 23, 1247. [Google Scholar] [CrossRef]
- Lemberkovics, É.; Czinner, E.; Szentmihályi, K.; Balázs, A.; Szoke, É. Comparative Evaluation of Helichrysi Flos Herbal Extracts as Dietary Sources of Plant Polyphenols, and Macro- and Microelements. Food Chem. 2002, 78, 119–127. [Google Scholar] [CrossRef]
- Park, M.; Lee, K.G. Effect of Roasting Temperature and Time on Volatile Compounds, Total Polyphenols, Total Flavonoids, and Lignan of Omija (Schisandra Chinensis Baillon) Fruit Extract. Food Chem. 2021, 338, 127836. [Google Scholar] [CrossRef]
- Nirangkush, B.; Chandan, T. Ultrasensitive Pd Nano Catalyst as Peroxidase Mimetics for Colorimetric Sensing and Evaluation of Antioxidants and Total Polyphenols in Beverages and Fruit Juices. Talanta 2022, 238, 123000. [Google Scholar] [CrossRef]
- Ghinea, I.O.; Ionica Mihaila, M.D.; Blaga, G.V.; Avramescu, S.M.; Cudalbeanu, M.; Isticioaia, S.F.; Dinica, R.M.; Furdui, B. Hplc-Dad Polyphenolic Profiling and Antioxidant Activities of Sorghum Bicolor during Germination. Agronomy 2021, 11, 417. [Google Scholar] [CrossRef]
- Silva, R.A.D.; Quintela, E.D.; Mascarin, G.M.; Barrigossi, J.A.F.; Lião, L.M. Compatibility of Agrochemicals with M. Anisopliae Scientia Agricola. Sci. Agric. 2013, 70, 152–160. [Google Scholar] [CrossRef]
- Busuioc, A.C.; Botezatu, A.V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant Activity Index (AAI) by the 2,2-Diphenyl-1-Picrylhydrazyl Method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Asawalam, E.F.; Emosairue, S.O.; Hassanali, A. Bioactivity of Xylopia Aethiopica (Dunal) a Rich Essential Oil Constituents on Maize Weevil Sitophilus Zeamais Motch. (Coleoptera: Curculionidae). Electron. J. Environ. Agric. Food Chem. 2006, 5, 1195–1204. [Google Scholar]
- Daraban, G.M.; Badeanu, M.; Rusu, L.; Zaharia, C.; Suteu, D. Vegetal Extract from Spontaneous Romanian Flora with Bioinsecticidal Action. Res. J. Agric. Sci. 2020, 52, 183–188. [Google Scholar]
- Daraban, G.M.; Badeanu, M.; Suteu, D. Repellent and Insecticide Activities of Plants Extracts from Spontaneous Flora Using Conventional and Innovative Assisted Extraction Techniques. Res. J. Agric. Sci. 2021, 53, 85–92. [Google Scholar]
- Doughari, J.H. Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 1–34. [Google Scholar]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR Spectroscopy, Total Phenolic Content, Antioxidant Activity and Anti-Amylase Activity of Extracts and Different Tea Forms of Garcinia Schomburgkiana Leaves. LWT Food Sci. Tehnol. 2020, 134, 110005. [Google Scholar] [CrossRef]
- Seixas, P.T.L.; Demuner, A.J.; Alvarenga, E.S.; Barbosa, L.C.A.; Marques, A.; Farias, E.d.S.; Picanço, M.C. Bioactivity of Essential Oils from Artemisia against Diaphania Hyalinata and Its Selectivity to Beneficial Insects. Sci. Agric. 2018, 75, 519–525. [Google Scholar] [CrossRef]
- Dancewicz, K.; Gabryś, B. Effect of Extracts of Garlic (Allium sativum L.), Wormwood (Artemisia absinthium L.) and Tansy (Tanaceum vulgare L.) on the Behaviour of the Peach Potato Aphid Myzus Persicae (Sulz.) during the Settling on Plants. Pestycydy 2008, 3–4, 93–99. [Google Scholar]
- Chaieb, I.; Hamouda, A.B.; Tayeb, W.; Zarrad, K.; Bouslema, T.; Laarif, A. The Tunisian Artemisia Essential Oil for Reducing Contamination of Stored Cereals by Tribolium Castaneum. Food Technol. Biotechnol. 2018, 56, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Magierowicz, K.; Górska-Drabik, E.; Golan, K. Effects of Plant Extracts and Essential Oils on the Behavior of Acrobasis Advenella (Zinck.) Caterpillars and Females. J. Plant Dis. Prot. 2020, 127, 63–71. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Saber, M.; Bagheri, M.; Mahdavinia, G.R. Achillea Millefolium Essential Oil and Chitosan Nanocapsules with Enhanced Activity against Tetranychus Urticae. J. Pest Sci. 2018, 91, 837–848. [Google Scholar] [CrossRef]
- Nasr, M.; Jalali Sendi, J.; Moharramipour, S.; Zibaee, A. Evaluation of Origanum Vulgare L. Essential Oil as a Source of Toxicant and an Inhibitor of Physiological Parameters in Diamondback Moth, Plutella Xylustella L. (Lepidoptera: Pyralidae). J. Saudi Soc. Agric. Sci. 2017, 16, 184–190. [Google Scholar] [CrossRef]
- Yazdani, E.; Sendi, J.J.; Hajizadeh, J. Effect of Thymus Vulgaris L. and Origanum Vulgare L. Essential Oils on Toxicity, Food Consumption, and Biochemical Properties of Lesser Mulberry Pyralid Glyphodes Pyloalis Walker (Lepidoptera: Pyralidae). J. Plant Prot. Res. 2014, 54, 53–61. [Google Scholar] [CrossRef]
- Khalfi, O.; Sahraoui, N.; Bentahar, F.; Boutekedjiret, C. Chemical Composition and Insecticidal Properties of Origanum Glandulosum (Desf.) Essential Oil from Algeria. J. Sci. Food Agric. 2008, 88, 1562–1566. [Google Scholar] [CrossRef]
- Yan, T.K.; Asari, A.; Abdullah, S.; Ismail, M.; Azmi, W.A. The Dataset for Antifeedant Activity of Eugenol Derived Compounds against Red Palm Weevil (Rhynchophorus Ferrugineus, Olivier) Larvae. Data Brief 2019, 25, 104227. [Google Scholar] [CrossRef]
- Babendreier, D.; Koku Agboyi, L.; Beseh, P.; Osae, M.; Nboyine, J.; Ofori, S.E.K.; Frimpong, J.O.; Attuquaye Clottey, V.; Kenis, M. The Efficacy of Alternative, Environmentally Friendly Plant Protection Measures for Control of Fall Armyworm, Spodoptera Frugiperda, in Maize. Insects 2020, 11, 240. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical Composition, Phytotoxic, Antimicrobial and Insecticidal Activity of the Essential Oils of Dracocephalum Integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef]
- Andrianjafinandrasana, S.N.; Andrianoelisoa, H.S.; Jeanson, M.L.; Ramonta, I.R.; Danthu, P. Allelopathic Effects of Volatile Compounds of Essential Oil from Ravensara Aromatic Sonnerat Chemotypes. Allelopath. J. 2013, 31, 333–344. [Google Scholar]
- Wang, C.F.; Yang, K.; Zhang, H.M.; Cao, J.; Fang, R.; Liu, Z.L.; Du, S.S.; Wang, Y.Y.; Deng, Z.W.; Zhou, L. Components and Insecticidal Activity against the Maize Weevils of Zanthoxylum Schinifolium Fruits and Leaves. Molecules 2011, 16, 3077–3088. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.E.I.; El-arami, S.A.A. Fumigant and Contact Toxicities of Monoterpenes to Sitophilus Oryzae (L.) and Tribolium Castaneum (Herbst) and Their Inhibitory Effects on Acetylcholinesterase Activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular and Pharmacological Analysis of an Octopamine Receptor From American Cockroach and Fruit Fly in Response to Plant Essential Oils. Arch. Insect Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a Constituent of Thyme Essential Oil, Is a Positive Allosteric Modulator of Human GABA A Receptors and a Homo-Oligomeric GABA Receptor from Drosophila Melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef]
- Tarapatskyy, M.; Gumienna, A.; Sowa, P.; Kapusta, I.; Puchalski, C. Bioactive Phenolic Compounds from Primula Veris l: Influence of the Extraction Conditions and Purification. Molecules 2021, 26, 997. [Google Scholar] [CrossRef]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are Naringenin and Quercetin Useful Chemicals in Pest-Management Strategies? J. Pest Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef] [Green Version]

| Vegetal Extract | Polyphenols, mg g−1 (Dilution 1:1) | Flavonoids, mg g−1 | ||||
|---|---|---|---|---|---|---|
| C gallic acid | C tanic acid | Total | C quercetin | C rutin | Total | |
| Oregano (Origanum vulgare) | 12.7624 ± 0.4477 a | 3.4511 a ± 0.1855 | 16.2134 ± 0.3974 a | 1.3858 ± 0.3434 b | 5.6211± 0.5608 b | 7.0068 ± 0.5531 b |
| Wormwood (Artemisia absinthium) | 3.2638± 0.2273 c | 0.8533 b,c ± 0.2919 | 4.1170 ± 0.2415 c | 1.4944 ± 0.3042 a,b | 6.0652± 0.5099 b | 7.5596 ± 0.4513 b |
| Yarrow (Achillea millefolium) | 4.1577± 0.3750 b | 1.0861 b ± 0.0954 | 5.2438± 0.4554 b | 2.0325 ± 0.2871 a | 8.2537 ± 0.5086 a | 10.2863 ± 0.7360 a |
| Cowslip (Primula veris) | 2.3807 ± 0.2077 d | 0.6267 c ± 0.0503 | 3.0073 ± 0.1704 d | 1.0544 ± 0.3074 b | 4.2852± 0.3999 c | 5.3396± 0.0943 c |
| Functional Group | Chemical Bond Vibration (Stretching/ Bending) | Range | Ethanolic Extracts | |||
|---|---|---|---|---|---|---|
| O. vulgare | A. absinthium | A. millefolium | P. veris | |||
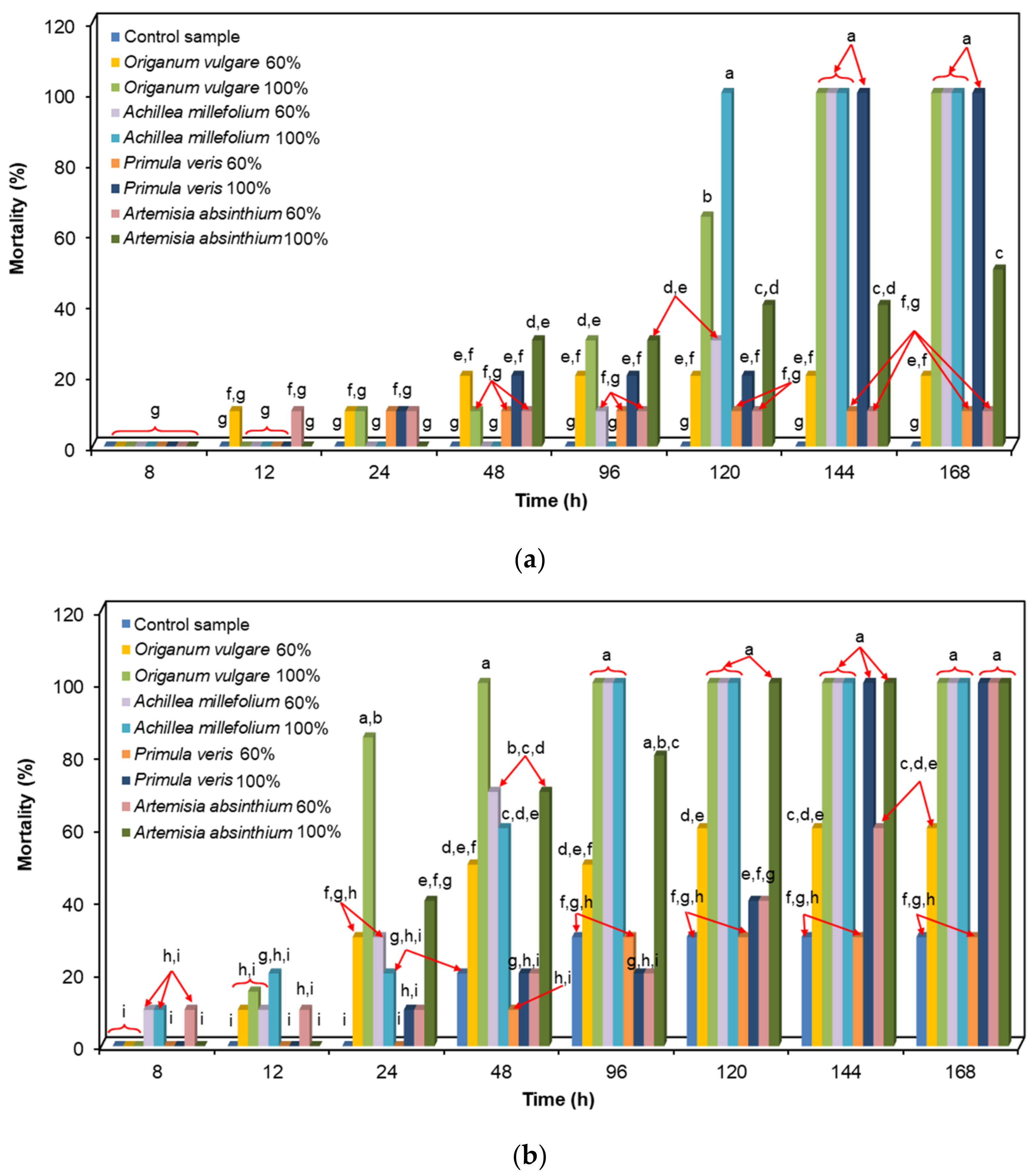
Alkyl moieties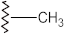 | C–H stretch C–H bend | 3000–2850 1500–1440 | + | + | + | + |
Olephinic moieties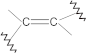 | C–H stretch C=C stretch | 3200–3000 1680–1600 | + | + | + | + |
Aromatic rings | C–H stretch C=C stretch | 3200–3000 1600–1400 | + | + | + | + |
Hydroxyl compounds | O–H stretch | 3600–3200 | + | + | + | + |
Carbonyl compounds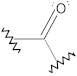 | C=O stretch | 1610–1550 | + | + | + | + |
Amino acids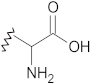 | C=O stretch | 1600–1660 | + | + | + | + |
Aldehydes  | C=O stretch C–H stretch of C=O | 1750–1625 2700–2850 | + | + | + | + |
Amines 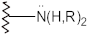 | N–H stretch N–H Bend | 3500–3100 1640–1550 | + | + | + | + |
Nitriles | CN stretch | 2500–2000 | + | + | + | + |
Amides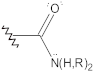 | N–H stretch C=O stretch N–H bend | 3500–3100 1670–1600 1640–1550 | + | + | + | + |
Ethers | C–O stretch | 1040–1260 | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daraban, G.M.; Rusu, L.; Dinica, R.M.; Roşca, M.; Badeanu, M.; Mihaila, M.D.I.; Suteu, D. Exploring the Antioxidant and Bioinsecticidal Activity of Spontaneous Flora Vegetal Extracts for Plant Protection and Prevention of Soil Contamination. Separations 2022, 9, 260. https://doi.org/10.3390/separations9090260
Daraban GM, Rusu L, Dinica RM, Roşca M, Badeanu M, Mihaila MDI, Suteu D. Exploring the Antioxidant and Bioinsecticidal Activity of Spontaneous Flora Vegetal Extracts for Plant Protection and Prevention of Soil Contamination. Separations. 2022; 9(9):260. https://doi.org/10.3390/separations9090260
Chicago/Turabian StyleDaraban, Gabriel Mihăiță, Lăcrămioara Rusu, Rodica Mihaela Dinica, Mihaela Roşca, Marinela Badeanu, Maria Daniela Ionica Mihaila, and Daniela Suteu. 2022. "Exploring the Antioxidant and Bioinsecticidal Activity of Spontaneous Flora Vegetal Extracts for Plant Protection and Prevention of Soil Contamination" Separations 9, no. 9: 260. https://doi.org/10.3390/separations9090260
APA StyleDaraban, G. M., Rusu, L., Dinica, R. M., Roşca, M., Badeanu, M., Mihaila, M. D. I., & Suteu, D. (2022). Exploring the Antioxidant and Bioinsecticidal Activity of Spontaneous Flora Vegetal Extracts for Plant Protection and Prevention of Soil Contamination. Separations, 9(9), 260. https://doi.org/10.3390/separations9090260










